Injection of Adipose-Derived Mesenchymal Stem/Stromal Cells Suppresses Muscle Atrophy Markers and Adipogenic Markers in a Rat Fatty Muscle Degeneration Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Experimental Design
2.2. Establishment of Fatty Muscle Degeneration Model by Sciatic Nerve Injury
2.3. Injection of Mesenchymal Stem Cells
2.4. Gross and Histopathological Examination
2.5. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Statistical Analysis
3. Results
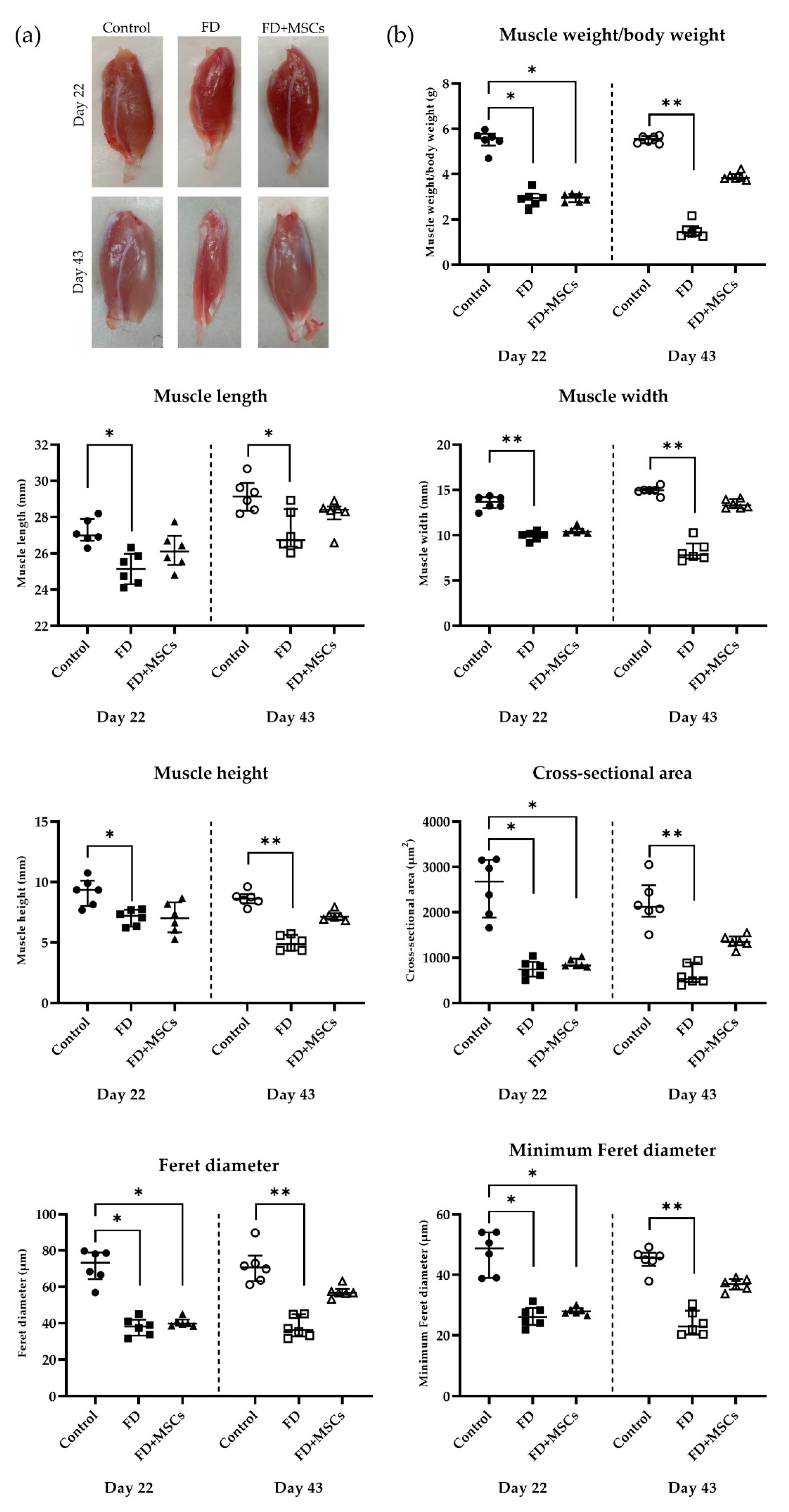
3.1. Gross and Histopathological Examination
3.2. Gene Expression Analysis by RT-qPCR
3.2.1. Muscle Atrophy Marker Gene Expression
3.2.2. Adipocyte and FAPs Marker Gene Expression
3.2.3. Myogenic and Satellite Cell Marker Gene Expression
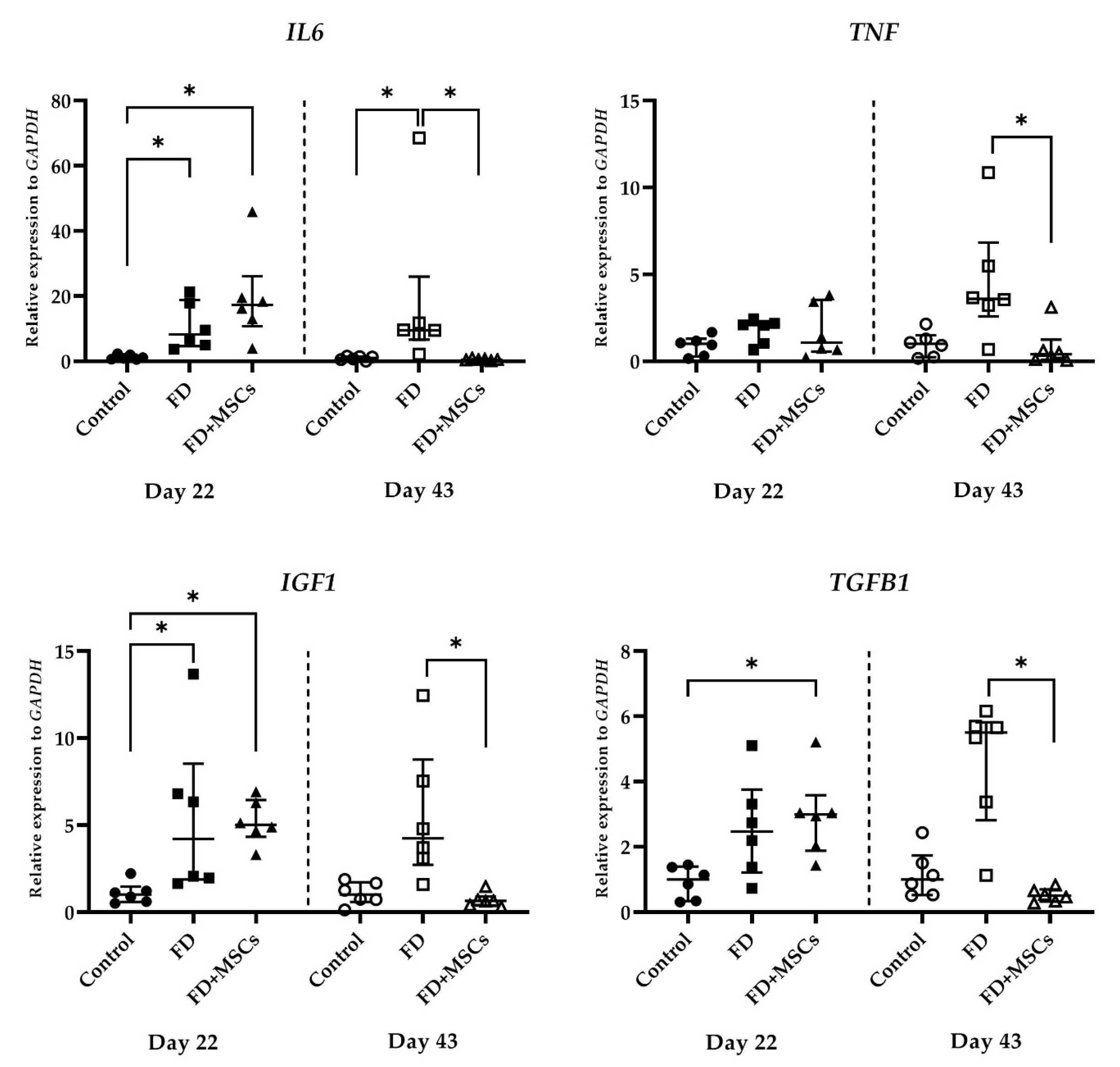
3.2.4. Immunomodulatory Marker Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Addison, O.; Marcus, R.L.; Lastayo, P.C.; Ryan, A.S. Intermuscular fat: A review of the consequences and causes. Int. J. Endocrinol. 2014, 2014, 309570. [Google Scholar] [CrossRef] [PubMed]
- Hairi, N.N.; Cumming, R.G.; Naganathan, V.; Handelsman, D.J.; Le Couteur, D.G.; Creasey, H.; Waite, L.M.; Seibel, M.J.; Sambrook, P.N. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: The Concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2010, 58, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.; Hamrick, M.W. Role of fibro-adipogenic progenitor cells in muscle atrophy and musculoskeletal diseases. Curr. Opin. Pharmacol. 2021, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C.; Nalls, M.A.; Goodpaster, B.H.; Ploutz-Snyder, L.L.; Harris, T.B. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am. J. Clin. Nutr. 2007, 85, 377–384. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Marques Bernabé, R.; de Souza Vieira, M.; Felício de Souza, V.; Gomes Fontana, L.; Albergaria, B.H.; Marques-Rocha, J.L.; Guandalini, V.R. Muscle strength is associated with fracture risk obtained by fracture risk assessment tool (FRAX) in women with breast cancer. BMC Cancer 2022, 22, 1115. [Google Scholar] [CrossRef] [PubMed]
- Osti, L.; Buda, M.; Del Buono, A. Fatty infiltration of the shoulder: Diagnosis and reversibility. Muscles Ligaments Tendons J. 2013, 3, 351–354. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Wolf, D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr. Diabetes 2004, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Theret, M.; Rossi, F.M.V.; Contreras, O. Evolving Roles of Muscle-Resident Fibro-Adipogenic Progenitors in Health, Regeneration, Neuromuscular Disorders, and Aging. Front. Physiol. 2021, 12, 673404. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, J.N.; Bishop, J.Y.; Lo, I.K.; Flatow, E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am. J. Sports Med. 2007, 35, 719–728. [Google Scholar] [CrossRef]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef]
- Farup, J.; Madaro, L.; Puri, P.L.; Mikkelsen, U.R. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015, 6, e1830. [Google Scholar] [CrossRef] [PubMed]
- Frechette, D.M.; Krishnamoorthy, D.; Adler, B.J.; Chan, M.E.; Rubin, C.T. Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. J. Appl. Physiol. 2015, 119, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Beasley, L.E.; Koster, A.; Newman, A.B.; Javaid, M.K.; Ferrucci, L.; Kritchevsky, S.B.; Kuller, L.H.; Pahor, M.; Schaap, L.A.; Visser, M.; et al. Inflammation and race and gender differences in computerized tomography-measured adipose depots. Obesity 2009, 17, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Rossi, A.; Di Francesco, V.; Sepe, A.; Olioso, D.; Pizzini, F.; Fantin, F.; Bosello, O.; Cominacini, L.; Harris, T.B.; et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: Relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Bitto, F.F.; Klumpp, D.; Lange, C.; Boos, A.M.; Arkudas, A.; Bleiziffer, O.; Horch, R.E.; Kneser, U.; Beier, J.P. Myogenic differentiation of mesenchymal stem cells in a newly developed neurotised AV-loop model. Biomed. Res. Int. 2013, 2013, 935046. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bayati, V.; Hashemitabar, M.; Gazor, R.; Nejatbakhsh, R.; Bijannejad, D. Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: A comparative study. Anat. Cell Biol. 2013, 46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Kulesza, A.; Burdzinska, A.; Szczepanska, I.; Zarychta-Wisniewska, W.; Pajak, B.; Bojarczuk, K.; Dybowski, B.; Paczek, L. The Mutual Interactions between Mesenchymal Stem Cells and Myoblasts in an Autologous Co-Culture Model. PLoS ONE 2016, 11, e0161693. [Google Scholar] [CrossRef]
- Pumberger, M.; Qazi, T.H.; Ehrentraut, M.C.; Textor, M.; Kueper, J.; Stoltenburg-Didinger, G.; Winkler, T.; von Roth, P.; Reinke, S.; Borselli, C.; et al. Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials 2016, 99, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Takegaki, J.; Sase, K.; Kono, Y.; Nakano, D.; Fujita, T.; Konishi, S.; Fujita, S. Intramuscular injection of mesenchymal stem cells activates anabolic and catabolic systems in mouse skeletal muscle. Sci. Rep. 2021, 11, 21224. [Google Scholar] [CrossRef] [PubMed]
- Heszele, M.F.C.; Price, S.R. Insulin-Like Growth Factor I: The Yin and Yang of Muscle Atrophy. Endocrinology 2004, 145, 4803–4805. [Google Scholar] [CrossRef] [PubMed]
- Husmann, I.; Soulet, L.; Gautron, J.; Martelly, I.; Barritault, D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor. Rev. 1996, 7, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Flück, M.; Kasper, S.; Benn, M.C.; Clement Frey, F.; von Rechenberg, B.; Giraud, M.N.; Meyer, D.C.; Wieser, K.; Gerber, C. Transplant of Autologous Mesenchymal Stem Cells Halts Fatty Atrophy of Detached Rotator Cuff Muscle After Tendon Repair: Molecular, Microscopic, and Macroscopic Results from an Ovine Model. Am. J. Sports Med. 2021, 49, 3970–3980. [Google Scholar] [CrossRef]
- Kim, H.; Shim, I.K.; Choi, J.H.; Lee, E.-S.; Lee, Y.N.; Jeon, I.-H.; Koh, K.H. Extracellular Vesicles Delivered by Injectable Collagen Promote Bone–Tendon Interface Healing and Prevent Fatty Degeneration of Rotator Cuff Muscle. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Brandão, A.G.M.; Consalter, A.; Leite, J.D.S.; Zamprogno, H.; Ferreira, A.M.R. Muscle regeneration through therapy with estromal stem cells in injury of infraespinhosus muscle of sheep. Acta Cir. Bras. 2018, 33, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Miyamoto, A.; Hiraoka, S.; Negoro, R.; Fujita, T. Mesenchymal Stem Cells Alter the Inflammatory Response of C2C12 Mouse Skeletal Muscle Cells. Biol. Pharm. Bull. 2020, 43, 1785–1791. [Google Scholar] [CrossRef]
- Shinohara, I.; Kataoka, T.; Mifune, Y.; Inui, A.; Sakata, R.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Yoshikawa, T.; Kato, T.; et al. Influence of adiponectin and inflammatory cytokines in fatty degenerative atrophic muscle. Sci. Rep. 2022, 12, 1557. [Google Scholar] [CrossRef]
- Koung Ngeun, S.; Shimizu, M.; Kaneda, M. Myogenic Differentiation and Immunomodulatory Properties of Rat Adipose-Derived Mesenchymal Stem/Stromal Cells. Biology 2024, 13, 72. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Chu, T.; Wang, L.; An, S.; Li, Y.; Hao, H.; Zhang, Z.; Yue, H. Frequent injections of high-dose human umbilical cord mesenchymal stem cells slightly aggravate arthritis and skeletal muscle cachexia in collagen-induced arthritic mice. Exp. Ther. Med. 2021, 22, 1272. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, J.; Cui, C.; Hu, H.; Zang, N.; Yang, M.; Yang, J.; Zou, Y.; Li, J.; Wang, L.; et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J. Cachexia Sarcopenia Muscle 2023, 14, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, T.J.; Smith, S.M.; DeLong, A.D.; Wang, H.; Unterman, T.G.; Esser, K.A. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am. J. Physiol. Cell Physiol. 2009, 297, C548–C555. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Kook, M.G.; Lee, S.; Shin, N.; Kong, D.; Kim, D.-H.; Kim, M.-S.; Kang, H.K.; Choi, S.W.; Kang, K.-S. Repeated intramuscular transplantations of hUCB-MSCs improves motor function and survival in the SOD1 G93A mice through activation of AMPK. Sci. Rep. 2020, 10, 1572. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zan, L.S.; Wang, H.B.; Cheng, G.; Du, M.; Jiang, Z.; Hausman, G.J.; McFarland, D.C.; Dodson, M.V. Adenovirus-mediated interference of FABP4 regulates mRNA expression of ADIPOQ, LEP and LEPR in bovine adipocytes. Genet. Mol. Res. 2013, 12, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Milne, K.J.; Hawke, T.J. Adiponectin-Consideration for its Role in Skeletal Muscle Health. Int. J. Mol. Sci. 2019, 20, 1528. [Google Scholar] [CrossRef]
- Goto, A.; Ohno, Y.; Ikuta, A.; Suzuki, M.; Ohira, T.; Egawa, T.; Sugiura, T.; Yoshioka, T.; Ohira, Y.; Goto, K. Up-regulation of adiponectin expression in antigravitational soleus muscle in response to unloading followed by reloading, and functional overloading in mice. PLoS ONE 2013, 8, e81929. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010, 12, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Chung, S.W.; Kim, S.H.; Chung, J.Y.; Kim, J.Y. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J. Shoulder Elb. Surg. 2014, 23, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, J.; Rando, T.A. Stem cells in postnatal myogenesis: Molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005, 15, 666–673. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S.; Relaix, F.; Nagata, Y.; Ruiz, A.P.; Collins, C.A.; Partridge, T.A.; Beauchamp, J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006, 119, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Jeong, J.A.; Hong, S.H.; Hwang, S.H.; Kim, S.W.; Yang, I.H.; Ahn, C.; Han, H.; Kim, H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells 2004, 22, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Bober, E.; Babinet, C.; Pournin, S.; Arnold, H.; Buckingham, M. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle. Dev. Dyn. 1996, 206, 291–300. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef]
- Ferrucci, L.; Penninx, B.W.; Volpato, S.; Harris, T.B.; Bandeen-Roche, K.; Balfour, J.; Leveille, S.G.; Fried, L.P.; Md, J.M. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J. Am. Geriatr. Soc. 2002, 50, 1947–1954. [Google Scholar] [CrossRef]
- Penninx, B.W.; Kritchevsky, S.B.; Newman, A.B.; Nicklas, B.J.; Simonsick, E.M.; Rubin, S.; Nevitt, M.; Visser, M.; Harris, T.; Pahor, M. Inflammatory markers and incident mobility limitation in the elderly. J. Am. Geriatr. Soc. 2004, 52, 1105–1113. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Howard, E.E.; Pasiakos, S.M.; Blesso, C.N.; Fussell, M.A.; Rodriguez, N.R. Divergent Roles of Inflammation in Skeletal Muscle Recovery from Injury. Front. Physiol. 2020, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. 2), 120–122. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Bendall, S.C.; Stewart, M.H.; Menendez, P.; George, D.; Vijayaragavan, K.; Werbowetski-Ogilvie, T.; Ramos-Mejia, V.; Rouleau, A.; Yang, J.; Bossé, M.; et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 2007, 448, 1015–1021. [Google Scholar] [CrossRef]
- Lowe, W.L., Jr.; Lasky, S.R.; LeRoith, D.; Roberts, C.T., Jr. Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: Evidence for differential processing and regulation in liver. Mol. Endocrinol. 1988, 2, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Hirata, A.; Masuda, S.; Tamura, T.; Kai, K.; Ojima, K.; Fukase, A.; Motoyoshi, K.; Kamakura, K.; Miyagoe-Suzuki, Y.; Takeda, S. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection: A role for osteopontin. Am. J. Pathol. 2003, 163, 203–215. [Google Scholar] [CrossRef]
- Zimowska, M.; Duchesnay, A.; Dragun, P.; Oberbek, A.; Moraczewski, J.; Martelly, I. Immunoneutralization of TGFbeta1 Improves Skeletal Muscle Regeneration: Effects on Myoblast Differentiation and Glycosaminoglycan Content. Int. J. Cell Biol. 2009, 2009, 659372. [Google Scholar] [CrossRef] [PubMed]
- Delaney, K.; Kasprzycka, P.; Ciemerych, M.A.; Zimowska, M. The role of TGF-β1 during skeletal muscle regeneration. Cell Biol. Int. 2017, 41, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Conboy, M.J.; Morgenthaler, A.; Schlesinger, C.; Bugaj, L.; Paliwal, P.; Greer, C.; Conboy, I.M.; Schaffer, D. Systemic attenuation of the TGF-β pathway by a single drug simultaneously rejuvenates hippocampal neurogenesis and myogenesis in the same old mammal. Oncotarget 2015, 6, 11959–11978. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qiu, H.; Xue, M.; Zhang, S.; Zhang, X.; Xu, J.; Chen, J.; Yang, Y.; Xie, J. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res. Ther. 2019, 10, 345. [Google Scholar] [CrossRef]
- Yang, W.; Hu, P. Skeletal muscle regeneration is modulated by inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef]






| Gene Name | Direction | Primer Sequences (5′-3′) | |
|---|---|---|---|
| Housekeeping gene | GAPDH | Forward | CCTGTTCTAGAGACAGCCGC |
| Reverse | ATCCGTTCACACCGACCTTC | ||
| Immunomodulatory markers | IL6 | Forward | CCACCCACAACAGACCAGTA |
| Reverse | TCTGACAGTGCATCATCGCT | ||
| TNF | Forward | TCTTCAAGGGACAAGGCTGC | |
| Reverse | CGGAGAGGAGGCTGACTTTC | ||
| TGFB1 | Forward | ATGCCAACTTCTGTCTGGGG | |
| Reverse | GGTTGTAGAGGGCAAGGACC | ||
| IGF1 | Forward | TGGTGGACGCTCTTCAGTTC | |
| Reverse | TCCGGAAGCAACACTCATCC | ||
| Adipocyte and FAPs markers | PPARG | Forward | AGCTCTGTGGACCTCTCTGT |
| Reverse | GTCAGCTCTTGTGAACGGGA | ||
| PDGFRA | Forward | AGTGCTTGGTCGGATCTTGG | |
| Reverse | GAGCATCTTCACAGCCACCT | ||
| FABP4 | Forward | AACTGGGCGTGGAATTCGAT | |
| Reverse | CACATGTACCAGGACCCCAC | ||
| ADIPOQ | Forward | TAATTCAGAGCAGCCCGTAG | |
| Reverse | TGGGGATAACACTCAGAACC | ||
| Atrophy markers | FBXO32 | Forward | TGCTCCGTCTCACTTTCACC |
| Reverse | AGGGGCCTTCTGAAGTGTTG | ||
| TRIM63 | Forward | ACCAAGGAAAACAGCCACCA | |
| Reverse | GGATCAGGGCCTCGATGAAG | ||
| FOXO1 | Forward | AGCTGCATCCATGGACAACA | |
| Reverse | TCATCATTGCTGTGGGACCC | ||
| Myogenic and satellite cell markers | PAX7 | Forward | CAAGATGCTGGGACACTCGT |
| Reverse | ATGCCAGAGAGCCAGTTTCC | ||
| MYOD | Forward | CGACTCTTCAGGCTTGGGTT | |
| Reverse | TGTCGCAAAGGAGCAGAGAG | ||
| MYOG | Forward | GGCAATGCACTGGAGTTTGG | |
| Reverse | CGTAAGGGAGTGCAGGTTGT | ||
| MYF5 | Forward | ATGGACATGACGGACAGCTG | |
| Reverse | TGCGACTCTTGGCTCAAACT | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koung Ngeun, S.; Shimizu, M.; Kaneda, M. Injection of Adipose-Derived Mesenchymal Stem/Stromal Cells Suppresses Muscle Atrophy Markers and Adipogenic Markers in a Rat Fatty Muscle Degeneration Model. Curr. Issues Mol. Biol. 2024, 46, 7877-7894. https://doi.org/10.3390/cimb46080467
Koung Ngeun S, Shimizu M, Kaneda M. Injection of Adipose-Derived Mesenchymal Stem/Stromal Cells Suppresses Muscle Atrophy Markers and Adipogenic Markers in a Rat Fatty Muscle Degeneration Model. Current Issues in Molecular Biology. 2024; 46(8):7877-7894. https://doi.org/10.3390/cimb46080467
Chicago/Turabian StyleKoung Ngeun, Sai, Miki Shimizu, and Masahiro Kaneda. 2024. "Injection of Adipose-Derived Mesenchymal Stem/Stromal Cells Suppresses Muscle Atrophy Markers and Adipogenic Markers in a Rat Fatty Muscle Degeneration Model" Current Issues in Molecular Biology 46, no. 8: 7877-7894. https://doi.org/10.3390/cimb46080467





