Capsaicin: Emerging Pharmacological and Therapeutic Insights
Abstract
:1. Introduction
2. Materials and Methods
3. Pharmacokinetics and Pharmacodynamics of Capsaicin
4. Indications and Therapeutical Uses of Capsaicin
4.1. Capsaicin as a Local Agent
| Indication | Formulation | Effect | Action Mechanism | Type of Study | Year | References |
|---|---|---|---|---|---|---|
| Neuralgia associated with herpes zoster infection | Cream 0.025% 3–4 times/day for 2 days | Antalgic | Substance P depletion/prevention of reuptake | In vivo—human | 1988 | [161] |
| Neuralgia-associated periocular and facial pain | 15 mg of capsaicin cream 2 daily | Antalgic | Substance P depletion | In vivo—human | 1988 | [162] |
| Facial apocrine chromhidrosis | Cream 1–2 times/day | Antalgic and possible vasodilation inhibition | Substance P depletion/prevention of reuptake | In vivo—human | 1989 | [163] |
| Reflex sympathetic dystrophy | Cream 0.025% 1–2 times/day for 3 weeks | Antalgic | Substance P depletion | In vivo—human | 1990 | [164] |
| Diabetic neuropathy | 0.075% capsaicin cream for 8 weeks | Antalgic | Substance P depletion/desensitization of C nociceptal fibers | In vivo—human | 1991 | [165] |
| Chronic severe painful diabetic neuropathy unresponsive or intolerant to conventional therapy. | Cream 0.075% 4 times/day for 8 weeks | Antalgic | Substance P depletion/desensitization of warm nociceptors, polymodal nociceptors and nociceptive afferents | In vivo—human | 1992 | [166] |
| Osteoarthritis | Cream 0.075% | Antalgic | Unknown | In vivo—human | 1992 | [167] |
| Postmastectomy pain syndrome | 0.075% capsaicin 4–5 times/day for 4–6 weeks | Antalgic | Unknown | In vivo—human | 1992 | [57] |
| Notalgia paresthetica | Cream 0.025 percent for four months | Antalgic, antipruritic | Uncertain | In vivo—human | 1992 | [168] |
| Haemodialysis-induced pruritus | Cream 0.025% 4 times/day for 6 weeks | Anti-pruritic | Substance P prevention of reuptake/depletion/desensitization of unmyelinated c fibers of cutaneous nerves | In vivo—human | 1992 | [67] |
| Chronic postherpetic neuralgia | 0.075% cream | Antalgic | Possible desensitisation of nociceptors | In vivo—human | 1993 | [169] |
| Pruritic psoriasis | 0.025% cream 4 times/day for 6 weeks | Antipruritic | Substance P depletion | In vivo—human | 1993 | [170] |
| Post-mastectomy pain syndrome | 0.025% cream 3 times/day for 2 months. | Antalgic | Substance P depletion | In vivo—human | 1993 | [171] |
| Cluster headache | Intranasal 3% camphor in 0.025% capsaicin cream for 7 days | Antalgic | Substance P depletion | In vivo—human | 1993 | [172] |
| Aquagenic pruritus | Cream 0.025%, 0.5% or 1.0% 3 times/day for 4 weeks | Antipruritic | Substance P depletion | In vivo—human | 1994 | [173] |
| Erythromelalgia | Cream 0.025% every 12 h for 2 months | Antalgic | Substance P depletion | In vivo—human | 1994 | [174] |
| Trigeminal neuralgia manifesting as intraoral pain | Cream 0.025% 4 times/day for 4 weeks | Antalgic | Substance P depletion/desensitization of c nociceptors | In vivo—human | 1994 | [175] |
| Chronic neck pain | Cream 0.025% 4 times/day for 5 weeks | Antalgic | Substance P depletion | In vivo—human | 1995 | [176] |
| Meralgia paraesthetica | Cream 0.025% 5 times/day for 15 days | Antalgic | Substance P depletion/prevention of reuptake/desensitisation of C-polymodal nociceptors | In vivo—human | 1995 | [177] |
| Skin flap survival | Silicongel 0.025% | Increased flap survival | platelet disaggregation | In vivo—animal | 1996 | [178] |
| Haemodialysis-induced pruritus | Cream 0.025% 4 times/day | Antipruritic | Substance P depletion | In vivo—human | 1996 | [68] |
| Herpes zoster ophthalmicus neuralgia | Cream 0.025% 5 times/day for 4 weeks | Antalgic | Substance P depletion | In vivo—human | 1997 | [179] |
| Complex regional pain syndromes and neuropathic pain | Cream 7.5% | Antalgic | Desensitization of C-fiber nociceptors | In vivo—human | 1998 | [180] |
| Atopic eczema | Cream 0.05% 3 times/day for 5 days | Antipruritic | Substance P depletion/inhibition | In vivo—human | 1998 | [181] |
| Diffuse eosinophilic sinonasal polyposis | 3 days 0.5 mL 30 micromol/L capsaicin solution and on days 4 and 5, 100 micromol/L | Improved subjective and endoscopy scores | Possible neurotoxic effect | In vivo—human | 2000 | [182] |
| Pain of osteoarthritis | 0.025% capsaicin, 1.33% glyceryl trinitrate (one part 0.075% capsaicin, two parts 2% glyceryl trinitrate) | Antalgic | Nociceptive blocking/increase in perfusion of glyceryl trinitrate | In vivo—human | 2000 | [58] |
| Pain following spinal cord injury | Cream 0.025% 4 times/day | Antalgic | Substance P depletion/desensitization of unmyelinated afferent C fibers | In vivo—human | 2000 | [183] |
| Prurigo nodularis | Cream 0.025% to 0.3% 4 to 6 times daily for 2 weeks up to 10 months | Antipruritic | Substance P depletion | In vivo—human | 2001 | [184] |
| Complex regional painsyndrome type I | Cream 0.075% 2 times/day for 6 weeks | Antalgic | Desensitization of epidermal C fibers | In vivo—human | 2001 | [185] |
| Atopic dermatitis | Lotion 0.025% 2 times/day for 6 weeks | Antipruritic | Possible desensitization or neuroinhibition | In vivo—animal | 2002 | [186] |
| Abdominal wall scar pain | Cream 0.075% 3 times/day usually for 2 weeks and after that 2 times/day | Antalgic | Desensitization of vanilloid subtype 1 (VR1) receptors | In vivo—human | 2002 | [187] |
| Post-operative nausea and vomiting after abdominal hysterectomy | Capsicum plaster with 345.80 mg of powdered capsicum for at least 30 min before anesthesia and eight hours after surgery on the acupuncture point P6 or the Korean acupuncture point K-D2 | Antiemetic | Desensitization of K-D2 hand point zone | In vivo—human | 2002 | [64] |
| Haemodialysis related pruritus | Cream 0.05% liniment 3 times/day for 5 days | Antipruritic | Substance P depletion/desensitization of epidermal nerve fibers | In vivo—human | 2003 | [188] |
| Meningeal nociception and headache | 10 μM topical | Antalgic | Desensitization of afferent fibers | In vivo—animal | 2003 | [189] |
| Saphenous neuralgia | Cream 5 times/day for 2 months | Antalgic | Substance P depletion | In vivo—human | 2003 | [190] |
| Idiopathic intractable pruritus ani | Capsaicin ointment 0.006–0.012% (depending on dilution) for 4 weeks followed by a week washout and by 4 weeks of placebo (menthol 1%) | Antipruritic | Substance P depletion | In vivo—human | 2003 | [69] |
| Detrusor hyperreflexia | 10 mM topical for 3 months intravesical instillations | Improving continence and bladder function | Possible desensitization of sensory Aδ and unmyelined C fibers | In vivo—human | 2004 | [191] |
| Burning mouth syndrome | Oral capsaicin 0.25% 3 times/day for 1 month | Antalgic | Desensitisation of type-C pain receptors | In vivo—human | 2004 | [192] |
| Detrusor hyper-reflexia in spinal cord-injured patients | Intravesical instillation of 1 mmol/L CAP diluted in glucidic solvent for 3 months | Improving continence and bladder function | Desensitization or blocking of afferent C-nerve fibres | In vivo—human | 2004 | [73] |
| Prevention of post-operative sore throat | Capsicum plaster with powdered capsicum 345.8 mg on the Korean acupuncture point K-A20 | Antalgic | Unknown—presumably release of endogenous opioids | In vivo—human | 2004 | [66] |
| Post-operative nausea and vomiting after anaesthesia in middle ear surgery | Capsicum plaster withcapsicum oleoresin 1% w/w on acupuncture point P6 | Antiemetic | Release of endogenous opioids/modulation of neurotransmitters of the vestibular system | In vivo—human | 2005 | [65] |
| Post-operative nausea and vomiting after laparoscopic cholecystectomy | Capsaicin ointment with oleoresin capsicum equivalent to capsaicin 0.075% w/w and methyl salicylate I.P. 20% w/w on the Korean acupuncture point K-D2 | Antiemetic | Blocking of synthesis of substance-P from sensory C-fibers/desensitisation of afferent sensory nerves. | In vivo—human | 2005 | [63] |
| Acute lobular panniculitis | 0.075% capsaicin cream 5 times/day for 3 weeks | Antalgic, antithrombotic | Substance P depletion | In vivo—human | 2005 | [193] |
| Acute lipodermatosclerosis | 0.075% capsaicin cream for 1–2 weeks followed by a month of continuation | Antalgic, antithrombotic | Substance P depletion | In vivo—human | 2005 | |
| Post-abdominal hysterctomy pain | Plaster of capsaicin (0.046% w/w) mixture of powdered capsicum 345.80 mg and capsicum tincture 34.58 mg at ST36 acupuncture point | Antalgic, antiemetic | Release of endogenous opioids (possibly) | In vivo—human | 2006 | [194] |
| Skinmorphological changesin patients with growth hormone deficiency and in the elderly | Cream of 0.01% capsaicinoids (dihydrocapsaicin and nordihydrocapsaicin)/0.01% capsinoids (capsiate, dihydrocapsiate and nordihydrocapsiate) | Increased skin elasticity | Increased dermal IGF-I levels | In vivo—human | 2007 | [195] |
| Painful HIV-associated distal sensory polyneuropathy (DSP) | Patch 640 microg/cm2, 8% w/w 60 min 1 time/day for 12 weeks | Antalgic | Desensitization of cutaneous nociceptors | In vivo—human | 2008 | [196] |
| Post-operative pain after orthognathic surgery | Capsicum plaster with 345.80 mg powdered capsicum and 34.58 mg capsicum tincture applied on LI4 acupuncture point | Antalgic, antiemetic | Blocking of transport and synthesis of substance P from sensory C-fibers | In vivo—human | 2009 | [197] |
| Migraine pain | Capsaicin jelly with 0.1% capsaicin | Relief and prevention of mild migraines | Substance P depletion | In vivo—human | 2010 | [198] |
| Chronic soft tissue pain | 0.05% capsaicin cream | Antalgic | Substance P depletion/degeneration of epidermal nerve fibres | In vivo—human | 2010 | [199] |
| Haemodialysis-induced uremic pruritus | 0.03% capsaicin ointment 4 times/day for 4 weeks | Antipruritic | Substance P depletion | In vivo—human | 2010 | [70] |
| Cardiac ischemia | 5 mL of 0.1% capsaicin cream applied to abdomen; experimental conditions different per animal group | Remote cardioprotective | Release of blood-bornecardioprotective factors | In vivo—animal | 2012 | [200] |
| Trigeminal Postherpetic neuralgia | Capsaicin 8% patch; single 60 min application | Antalgic | Substance P depletion/defunctionalization of TRPV1 receptors on sensory nerve endings | In vivo—human | 2012 | [201] |
| Visceral obesity | 0.075% capsaicin cream for 7 + 7 weeks (pretreatment and post-treatment) | Antiinflammatory, antilipidemic, anti-diabetic | Increased adiponectin, PPARα, PPARγ, visfatin, adipsin and decreased TNF-α and IL-6 | In vivo—animal | 2013 | [202] |
| Fibromyalgia | 0.075% capsaicin cream 3 times/day for 6 weeks | Antalgic | Substance P depletion/desensitization of polymodal nociceptors | In vivo—human | 2013 | [203] |
| Peripheral neuropathic pain | 8% capsaicin cutaneous patch for 30 min to the feet and 60 min to other parts of the body | Antalgic | Probably substance P related | In vivo—human | 2014 | [204] |
| Posttraumatic neuropathic pain | 8% capsaicin cutaneous patch for 30 min for the feet and 60 min for other locations every 90 days | Antalgic, anti-inflammatory | Defunctionalisation of nociceptors | In vivo—human | 2014 | [205] |
| Arthritis and associated inflammo-musculoskeletal disorders | Topical ethosomal capsaicin | Antalgic, anti-inflammatory | Substance P inhibition | In vivo—animal | 2015 | [206] |
| Post-herpetic neuralgia | Liposomal non-ionic capsaicin cream (0.025%) 2–3 times/day for 6 weeks followed by a 2-week cessation | Antalgic | Unclear | In vivo—human | 2015 | [207] |
| Intraoral somatosensory sensitivity | 30 μL of 5% capsaicin on a paper disc for 15 min | Mechanical desensitization | Desensitization of C-nociceptors | In vivo—human | 2015 | [208] |
| Lichen amyloidosis | 8% capsaicin patch with 179 mg capsaicin for 60 min | Antipruritic | Defunctionalization of transient receptor potential ion channel vanilloid-1 | In vivo—human | 2016 | [209] |
| Cannabinoid hyperemesis syndrome | Capsaicin cream | Antiemetic | Substance P depletion | In vivo—human | 2017 | [210] |
| Burning mouth syndrome | 0.01% or 0.025% oral capsaicin gel 3 times/day for 14 days | Antalgic | Substance P depletion/desensitization of transient receptor potential ion channel vanilloid-1 | In vivo—human | 2017 | [211] |
| Neuropathic pain caused by lumbosacral radiculopathies | 8% capsaicin patch for 30 min for the feet and 60 min for other locations | Antalgic | Desensitization of lumbosacral spinal nerves | In vivo—human | 2017 | [212] |
| Histamine-induced pruritus on canine skin | 3 mL of 0.1% capsaicin solution 2 times/day for 8 days | Antipruritic | Desensitization of the sensory afferents | In vivo—animal | 2018 | [213] |
| Neurogenic inflammation | Topical 50 μM of capsaicin for 15 min after the topical application of 200 μM of capsazepine | Neutrophil leukocyte activation | Increased leukocyte rolling and adhesion, increased expression of E-selectin and ICAM-1 | In vivo—animal | 2018 | [214] |
| Myofascial pain syndrome | 10 g capsaicin cream 8%, for 30 min | Antalgic | Substance P depletion/inhibition (probably) | In vivo—human | 2019 | [215] |
| Acute musculoskeletal injuries | Capsaicin gel of 0.05% capsaicin 3 times/day for 72 h | Antalgic | Substance P depletion/inhibition (probable) | In vivo—human | 2020 | [216] |
| Hepatic staetosis, obesity, dislipidemia and high blood pressure associated with hypoestrogenism | 0.75 g/kg capsaicin cream | Anti-obesity, antilipidemic, antihypertensive | Activation of TRPV1 receptors in neurons of the digestive tract/ increased lipid mobilization and oxidation/reduced cholesterol shynthesis | In vivo—animal | 2020 | [217] |
| Type 2 diabetic patients with painful peripheral neuropathy | 0.075% capsaicin gel | Antalgic | Substance P depletion/defunctionalization of the C fiber nociceptors | In vivo—human | 2020 | [218] |
| Trigeminal neuropathic pain | 10 µg in 20 µL of vehicle subcutaneously injected | Antalgic | Capsaicin-induced ablation of TRPV1+ afferent terminals | In vivo—animal | 2020 | [219] |
| Psoriasis | 10 μg of Capzasin-HP cream (0.1% capsaicin) for 2 times/day for 8 days | Anti-inflammatory | Desensitization of TRPV1 nerves/denervation-induced inhibition of cutaneous inflammatory responses | In vivo—animal | 2021 | [220] |
| Sensory neuropathic cough | Spray of capsaicin 0.02% to 0.04% for 4 times/day for 2 weeks | Antitussive | Substance P depletion/defunctionalization of thermal, mechanical, chemical, and other sensory nerve endings | In vivo—human | 2021 | [221] |
| Cannabinoid-induced hyperemesis syndrome | Capsaicin cream 0.025% | Anti-emetic effect | Substance P depletion/defunctionalization of TRPV1 | In vivo—human | 2021 | [222] |
| UVB-induced cutaneous hyperalgesia | 8% transdermal patch or two vehicle patches | Antalgic | Substance P depletion/defunctionalisation of local nociceptors | In vivo—human | 2021 | [223] |
| Idiopathic rhinitis | Nasal spray of 0.01 mM capsaicin | Reduction of nasal symptoms | Substance P depletion | In vivo—human | 2021 | [224] |
| Hamartoma tumour syndrome | Patch 8% | Pain relief | Substance P depletion/inhibition | In vivo—human | 2022 | [225] |
| Acute trauma pain | 0.05% capsaicin gel for 3 times/day for 72 h after discharge from the hospital | Antalgic | Substance P depletion/inhibition (probable) | In vivo—human | 2022 | [226] |
| Improved dermal blood flow | Cream 8% | Improved skin oxigenation | Local vasodilation induced by TRPV1-mediated release of substance P, CGRP, and other vasoactive peptides | In vivo—human | 2022 | [227] |
| Chronic postsurgical pain | 8% capsaicin patch every 3 months | Antalgic | Defunctionalization of transient receptor potential vanilloid-1 (probable) | In vivo—human | 2022 | [228] |
| Pain during microfocused ultrasound with visualization (MFU-V) treatment | 0.025% capsaicin gel | Antalgic | Defunctionalization of transient receptor potential vanilloid-1 | In vivo—human | 2023 | [151] |
| Peripheral neuropathic pain | One topical high-concentration capsaicin application | Antalgic | Axon reflex vasodilatation associated with pain reduction | In vivo—human | 2023 | [229] |
4.2. Systemic Applications of Capsaicin
| Indication | Formulation | Effect | Action Mechanism | Type of Study | Year | References |
|---|---|---|---|---|---|---|
| Systemic anti-inflammatory effect of somatostatin | Μice: 30, 60 and 90 mg/kg on 3 consecutive days under anaesthesia; guinea pigs: 2% capsaicin solution perineural for 30 min | Anti-inflammatory | Somatostatin release | In vivo—animal | 2000 | [260] |
| Burning mouth syndrome | 3 capsules of capsaicin (50 mg of powder of red pepper with 0.25% capsaicin) a day for 1 month | Antalgic | Presumed inhibition of substance P | In vivo—human | 2003 | [261] |
| Possible protection against cancer, atherosclerosis and age-related diseases | 10 μM | Antioxidant | Decrease in malondialdehyde level and protein carbonyl group content | In vitro—erythrocytes | 2006 | [262] |
| Helicobacter pylori gastritis | 10 μg/mL | Anti-inflammatory | Inhibition of H. pylori-induced IL-8 production | In vitro—AGS or MKN45 cells | 2007 | [263] |
| Endometriosis | 1M solution | Inhibition of proliferation of endometriotic cells | Inhibition of NF-kB | In vitro-immortalized stromal-like and epithelial-like endometriotic cells | 2008 | [264] |
| Irritable bowel syndrome | Pills 0.50 mg of capsaicin, 4 pills per day, 6 weeks | Antalgic, antibloating | Desensitisation of nociceptive receptors, depletion of substance P | In vivo—human | 2011 | [265] |
| Cardiovascular and metabolic diseases | 1% red pepper powder which contains approximately 2.45 mg/g of capsaicin | Antilipidaemic, antiobesity | TRPV1 activation | In vivo—animal | 2012 | [266] |
| Chronic unexplained cough triggered by environmental irritants | 1 capsule with 0.4 mg pure capsaicin 2 times/day, for 2 weeks, followed by 2 capsules with 0.4 mg pure capsaicin 2 times/day for 2 weeks | Antitussive | Desensitisation of the cough-sensitive TRPV1 | In vivo—human | 2015 | [267] |
| Atherosclerosis | 10, 20, 30, 40, and 50 μM | Antioxidant | Caspase-3 mediated pathways suppression | In vitro—macrophage RAW 264.7 cells | 2015 | [268] |
| Anoxia/Reoxygenation injury | 10 μM to 40 μM | Cardioprotective | Upregulation of SIRT1 pathway | In vitro—rat cardiomyocytes | 2017 | [269] |
| Heart failure post myocardial infarction | 0.1% cream, 150 μL/25 g | Cardioprotective | Induction of nociceptor-induced conditioning | In vivo—animal | 2019 | [270] |
| Hyperlipidaemia, oxidative stress, atherosclerosis | 2.5, 5 and 10 mg/kg administered by gavage once daily | Anti-inflammatory, antioxidant, cardioprotective | Decreased total and LDL cholesterol, triglycerides, and apo B-100, and increased HDL cholesterol and SOD | In vivo—animal | 2019 | [271] |
| Hepatic steatosis | Cream 0.075%—8 week duration | Antilipidemic, antioxidative | Inhibition of β-oxidation, inhibition of hepatic lipogenesis | In vivo—animal | 2020 | [272] |
| Renovascular hypertension | 0.006% capsaicin diet for 6 weeks | Antihypertensive | Increased phosphorylation of protein kinase B and endothelial NO synthase | In vivo—animal | 2020 | [273] |
| Acute inflammatory demyelinating polyneuropathy (AIDP)/Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) | 10 μM | Antioxidative, immunomodulatory | Reduction of IFN gamma-induced MHC-II production and decreased TLR4 and ICAM-1 mRNA expression | In vitro—Schwann cells | 2020 | [274] |
| Pentylenetetrazole-Induced Seizures | 1 or 2 mg/kg | Anticonvulsant, neuroprotective | reduced glutathione (GSH), nitric oxide, and paraoxonase-1 (PON-1) | In vivo—animal | 2020 | [275] |
| Hypercholesterolemia | 200 µM | Hypolipidemic | Upregulation of LDLR and downregulation of PCSK9 expression | In vitro-HepG2 cells | 2022 | [276] |
4.3. Capsaicin as an Anti-Cancer Agent
5. Capsaicin in Traditional Medicine Therapies
6. Side Effects of Capsaicin
| System | TRPV1-Bearing Cell Types | Toxic Side-Effects | References |
|---|---|---|---|
| CNS | Cerebral neurons, sensory neurons of the dorsal root ganglion | Convulsions, excitement, disorientation, fear, loss of body motor control | [109,425,426,427] |
| Cardiovascular | Vascular smooth muscle cells, endothelial cells | Heart rate increase, blood pressure increase, hypertension, tachycardia, ventricular fibrillation, increased vascular contractility, atherosclerosis | [427,428] |
| Respiratory | Airway epithelial cells, T cells of the upper and lower airways | Bronchoconstriction, coughing, laryngeal oedema, pulmonary oedema, chemical pneumonitis, respiratory arrest | [114,433,434] |
| Gastrointestinal | Submucosal nerve plexus, myenteric nerve plexus, gastrointestinal mucosal cells, parietal and antral G cells | General irritation and pain, increased ulcer incidence | [117,275,435,437] |
| Integumentary | Unmyelinated type C and thin myelinated Aδ sensory nerve fibres, keratinocytes, mast cells, dermal blood vessels, fibroblasts, hair follicles, vascular smooth muscle cells, sebocytes and eccrine sweat glands | Skin erythema, non-blistering associated burning, “Hunan hand” (capsaicin-specific contact dermatitis) | [118,119,120,421,423,424] |
| Eyes | Corneal cells, retinal ganglion cells | Marked lacrimation, blepharospasm, conjunctivitis | [121,122,422] |
7. Discussion and Future Research Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordell, G.A.; Araujo, O.E. Capsaicin: Identification, nomenclature, and pharmacotherapy. Ann. Pharmacother. 1993, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.P.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Feng, L.; Feng, S.; Yan, Y.; Ge, Z.; Li, Z.; Chen, Z. Multiple quantitative structure-pungency correlations of capsaicinoids. Food Chem. 2019, 283, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Mózsik, G.; Past, T.; Abdel Salam, O.M.; Kuzma, M.; Perjési, P. Interdisciplinary review for correlation between the plant origin capsaicinoids, non-steroidal antiinflammatory drugs, gastrointestinal mucosal damage and prevention in animals and human beings. Inflammopharmacology 2009, 17, 113–150. [Google Scholar] [CrossRef] [PubMed]
- Chiou, K.L.; Hastorf, C.A.; Bonavia, D.; Dillehay, T.D. Documenting Cultural Selection Pressure Changes on Chile Pepper (Capsicum baccatum L.) Seed Size Through Time in Coastal Peru (7,600 B.P.–Present). Econ. Bot. 2014, 68, 190–202. [Google Scholar] [CrossRef]
- Nunn, N.; Qian, N. The Columbian exchange: A history of disease, food, and ideas. J. Econ. Perspect. 2010, 24, 163–188. [Google Scholar] [CrossRef]
- Cumo, C. The Ongoing Columbian Exchange: Stories of Biological and Economic Transfer in World History: Stories of Biological and Economic Transfer in World History; ABC-CLIO: New York, NY, USA, 2015. [Google Scholar]
- Williams, D.E. Agricultural Biodiversity and the Columbian Exchange. In Routledge Handbook of Agricultural Biodiversity; Routledge: Oxfordshire, UK, 2017; pp. 192–212. [Google Scholar]
- Herniter, I.A.; Muñoz-Amatriaín, M.; Close, T.J. Genetic, textual, and archeological evidence of the historical global spread of cowpea (Vigna unguiculata [L.] Walp.). Legume Sci. 2020, 2, e57. [Google Scholar] [CrossRef]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Manchego, C.; Haak, D.C.; Levey, D.J. Where did the chili get its spice? Biogeography of capsaicinoid production in ancestral wild chili species. J. Chem. Ecol. 2006, 32, 547–564. [Google Scholar] [CrossRef]
- Haak, D.C.; McGinnis, L.A.; Levey, D.J.; Tewksbury, J.J. Why are not all chilies hot? A trade-off limits pungency. Proc. Biol. Sci. 2012, 279, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, C. Chemische Untersuchung der trockenen reifen spanischen Pfeffers. Alm. Oder Taschenb. Scheidekünstler Apoth. 1816, 37, 1–30. [Google Scholar]
- King, J.; Felter, H.W. King’s American Dispensatory; Ohio Valley Company: Cincinnati, OH, USA, 1909; Volume 1. [Google Scholar]
- Thresh, J.C. Isolation of capsaicin. Pharm. J. Trans. 1876, 6, 941–947. [Google Scholar]
- Thresh, J.C. Capsaicin, the active principle of capsicum fruits. Pharm. J. Trans. 1876, 7, 259–260. [Google Scholar]
- Thresh, J.C. Capsaicin—The active principle of cayenne pepper. Analyst 1877, 2, 108. [Google Scholar] [CrossRef]
- Nelson, E. The constitution of capsaicin, the pungent principle of capsicum. J. Am. Chem. Soc. 1919, 41, 1115–1121. [Google Scholar] [CrossRef]
- Micko, K. Zur Kenntniss des Capsaïcins. Z. Unters. Nahr.-Und Genußmittel Sowie Gebrauchsgegenstände 1898, 1, 818–829. [Google Scholar] [CrossRef]
- Micko, K. Ueber den wirksamen Bestandtheil des Cayennepfeffers. Z. Unters. Nahr.-Und Genußmittel Sowie Gebrauchsgegenstände 1899, 2, 411–412. [Google Scholar] [CrossRef]
- Kosuge, S.; Furuta, M.; Oda, T. Studies on the Pungent Principles of Red Pepper. Part XIII On the pungent principles contents of Japanese red pepper. Nippon. Shokuhin Kogyo Gakkaishi 1967, 14, 407–410. [Google Scholar] [CrossRef]
- Gradinaru, T.C.; Petran, M.; Dragos, D.; Gilca, M. PlantMolecularTasteDB: A Database of Taste Active Phytochemicals. Front. Pharmacol. 2021, 12, 751712. [Google Scholar] [CrossRef]
- Wachtel, R.E. Capsaicin. Reg. Anesth. Pain. Med. 1999, 24, 361–363. [Google Scholar] [CrossRef]
- Alberti, A.; Galasso, V.; Kovac, B.; Modelli, A.; Pichierri, F. Probing the molecular and electronic structure of capsaicin: A spectroscopic and quantum mechanical study. J. Phys. Chem. A 2008, 112, 5700–5711. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Sung, Y.; Chang, Y.-Y.; Ni-Lun, T. Capsaicin biosynthesis in water-stressed hot pepper fruits. Bot. Bull. Acad. Sin. 2005, 46, 35–42. [Google Scholar]
- Arora, R.; Gill, N.; Chauhan, G.; Rana, A. An overview about versatile molecule capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286. [Google Scholar]
- Katsuragi, H.; Shimoda, K.; Yamamoto, R.; Ohara, T.; Hamada, H. Enzymatic synthesis of capsaicin 4-O-β-xylooligosaccharides by β-xylosidase from Aspergillus sp. Acta Biol. Hung. 2011, 62, 151–155. [Google Scholar] [CrossRef]
- Späth, E.; Darling, S.F. Synthese des capsaicins. Berichte Dtsch. Chem. Ges. (A B Ser.) 1930, 63, 737–743. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N.; Ramirez-Malagon, R. In vitro chili pepper biotechnology. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 701–729. [Google Scholar] [CrossRef]
- Castillo, E.; López-González, I.; De Regil-Hernández, R.; Reyes-Duarte, D.; Sánchez-Herrera, D.; López-Munguía, A.; Darszon, A. Enzymatic synthesis of capsaicin analogs and their effect on the T-type Ca2+ channels. Biochem. Biophys. Res. Commun. 2007, 356, 424–430. [Google Scholar] [CrossRef]
- Pandhair, V.; Gosal, S. Capsaicin production in cell suspension cultures derived from placenta of Capsicum annuum L. fruit. Indian J. Agric. Biochem. 2009, 22, 78–82. [Google Scholar]
- Akhtar, F.; Muhammad Sharif, H.; Arshad Mallick, M.; Zahoor, F.; Abdulmalik, A.; Baig, W.; Shujaat, N.; Gul, S.; Bibi, G.; Ramzan, R.; et al. Capsaicin: Its Biological Activities and In Silico Target Fishing. Acta Pol. Pharm. 2017, 74, 321–329. [Google Scholar] [PubMed]
- Moirangthem, S.S.; Gogoi, S.; Thongbam, P.D.; Ramya, K.T.; Fiyaz, R.A.; Pandey, D.S. Effect of sowing time and crop geometry on the Capsaicinoid content in Bhoot Jolokia (Capsicum chinense Jacq.). J. Food Sci. Technol. 2014, 51, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Gothandam, K.M.; Ranjan, V.; Shakya, A.; Pareek, S. Effect of drying methods (microwave vacuum, freeze, hot air and sun drying) on physical, chemical and nutritional attributes of five pepper (Capsicum annuum var. annuum) cultivars. J. Sci. Food Agric. 2018, 98, 3492–3500. [Google Scholar] [CrossRef]
- Krishnatreyya, H.; Hazarika, H.; Saha, A.; Chattopadhyay, P. Capsaicin, the primary constituent of pepper sprays and its pharmacological effects on mammalian ocular tissues. Eur. J. Pharmacol. 2018, 819, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Busker, R.W.; van Helden, H.P. Toxicologic evaluation of pepper spray as a possible weapon for the Dutch police force: Risk assessment and efficacy. Am. J. Forensic Med. Pathol. 1998, 19, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Payal, A.R.; Daly, M.K. Effects of tear gases on the eye. Surv. Ophthalmol. 2016, 61, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, R.D.; Wills, B.K. Tear Gas and Pepper Spray Toxicity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Quiroga-Garza, M.E.; Ruiz-Lozano, R.E.; Azar, N.S.; Mousa, H.M.; Komai, S.; Sevilla-Llorca, J.L.; Perez, V.L. Noxious effects of riot control agents on the ocular surface: Pathogenic mechanisms and management. Front. Toxicol. 2023, 5, 1118731. [Google Scholar] [CrossRef]
- Cowles, R.S.; Keller, J.E.; Miller, J.R. Pungent spices, ground red pepper, and synthetic capsaicin as onion fly ovipositional deterrents. J. Chem. Ecol. 1989, 15, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.T.; Shumake, S.A.; Gaddis, S.E.; Bourassa, J.B. Capsicum oleoresin: Development of an in-soil repellent for pocket gophers. Pest. Manag. Sci. 2005, 61, 1202–1208. [Google Scholar] [CrossRef]
- Kimball, B.A.; Taylor, J.; Perry, K.R.; Capelli, C. Deer responses to repellent stimuli. J. Chem. Ecol. 2009, 35, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Stock, B.; Haag-Wackernagel, D. Effectiveness of Gel Repellents on Feral Pigeons. Animals 2013, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Galves, C.; Racioni Goncalves, A.C.; Chen, J.; Fisk, I. Impact of capsaicin on aroma release: In vitro and in vivo analysis. Food Res. Int. 2020, 133, 109197. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ayed, C.; Chen, J.; Fisk, I.; Yang, N. The role of capsaicin stimulation on the physicochemical properties of saliva and aroma release in model aqueous and oil systems. Food Chem. 2022, 386, 132824. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Yang, Q.; Chen, J.; Fisk, I. Impact of capsaicin on aroma release and perception from flavoured solutions. LWT 2021, 138, 110613. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, J.; Wyszkowska, J.; Kletkiewicz, H.; Rogalska, J. Capsaicin-induced dysregulation of acid-base status in the American cockroach. J. Environ. Sci. Health B 2019, 54, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, P.; Wei, L.; Kang, R.; Chen, L.; Zhang, M.; Tan, E.K.; Liu, W. Capsaicin Functions as Drosophila Ovipositional Repellent and Causes Intestinal Dysplasia. Sci. Rep. 2020, 10, 9963. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yang, Z.; Hu, X.; Wen, Y. Synthesis, Antibacterial and Insecticidal Activities of Novel Capsaicin Derivatives Containing a Sulfonic Acid Esters Moiety. Front. Chem. 2022, 10, 929050. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.-F.; Wang, J.-W.; Li, H.-F.; Fang, R.; Yu, X.; Lu, Y.-J. Microencapsulation of Capsaicin in Chitosan Microcapsules: Characterization, Release Behavior, and Pesticidal Properties against Tribolium castaneum (Herbst). Insects 2023, 14, 27. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Chiu, C.C.; Liu, Y.L.; Chiu, W.C.; Chiu, C.H.; Chiu, Y.S.; Huang, C.C. Capsaicin Supplementation Reduces Physical Fatigue and Improves Exercise Performance in Mice. Nutrients 2016, 8, 648. [Google Scholar] [CrossRef]
- Adaszek, Ł.; Gadomska, D.; Mazurek, Ł.; Łyp, P.; Madany, J.; Winiarczyk, S. Properties of capsaicin and its utility in veterinary and human medicine. Res. Vet. Sci. 2019, 123, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Mustofa Helmi, E.; Faisal, F.; Muhammad Thohawi Elziyad, P. Role of Capsaicin in the Repair of Cellular Activity in Mice Liver. Pharmacogn. J. 2021, 13, 1573–1576. [Google Scholar]
- Watson, P.N.C.; Evans, R.J. The postmastectomy pain syndrome and topical capsaicin: A randomized trial. Pain 1992, 51, 375–379. [Google Scholar] [CrossRef] [PubMed]
- McCleane, G. The analgesic efficacy of topical capsaicin is enhanced by glyceryl trinitrate in painful osteoarthritis: A randomized, double blind, placebo controlled study. Eur. J. Pain 2000, 4, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Grushka, M.; Epstein, J.B.; Gorsky, M. Burning mouth syndrome. Am. Fam. Physician 2002, 65, 615–620. [Google Scholar]
- Mason, L.; Moore, R.A.; Derry, S.; Edwards, J.E.; McQuay, H.J. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef] [PubMed]
- Saguil, A.; Kane, S.; Mercado, M.; Lauters, R. Herpes Zoster and Postherpetic Neuralgia: Prevention and Management. Am. Fam. Physician 2017, 96, 656–663. [Google Scholar]
- Hayman, M.; Kam, P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Agarwal, A.; Dhiraaj, S.; Tandon, M.; Singh, P.K.; Singh, U.; Pawar, S. Evaluation of capsaicin ointment at the Korean hand acupressure point K-D2 for prevention of postoperative nausea and vomiting. Anaesthesia 2005, 60, 1185–1188. [Google Scholar] [CrossRef]
- Kim, K.S.; Koo, M.S.; Jeon, J.W.; Park, H.S.; Seung, I.S. Capsicum plaster at the korean hand acupuncture point reduces postoperative nausea and vomiting after abdominal hysterectomy. Anesth. Analg. 2002, 95, 1103–1107. [Google Scholar] [CrossRef]
- Misra, M.N.; Pullani, A.J.; Mohamed, Z.U. Prevention of PONV by acustimulation with capsicum plaster is comparable to ondansetron after middle ear surgery. Can. J. Anaesth. 2005, 52, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, K.S.; Min, H.K.; Kim, D.W. Prevention of postoperative sore throat using capsicum plaster applied at the Korean hand acupuncture point. Anaesthesia 2004, 59, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Breneman, D.L.; Cardone, J.S.; Blumsack, R.F.; Lather, R.M.; Searle, E.A.; Pollack, V.E. Topical capsaicin for treatment of hemodialysis-related pruritus. J. Am. Acad. Dermatol. 1992, 26, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Tarng, D.C.; Cho, Y.L.; Liu, H.N.; Huang, T.P. Hemodialysis-related pruritus: A double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron 1996, 72, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Lysy, J.; Sistiery-Ittah, M.; Israelit, Y.; Shmueli, A.; Strauss-Liviatan, N.; Mindrul, V.; Keret, D.; Goldin, E. Topical capsaicin--a novel and effective treatment for idiopathic intractable pruritus ani: A randomised, placebo controlled, crossover study. Gut 2003, 52, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Makhlough, A.; Ala, S.; Haj-Heydari, Z.; Kashi, Z.; Bari, A. Topical capsaicin therapy for uremic pruritus in patients on hemodialysis. Iran. J. Kidney Dis. 2010, 4, 137–140. [Google Scholar] [PubMed]
- Gooding, S.M.; Canter, P.H.; Coelho, H.F.; Boddy, K.; Ernst, E. Systematic review of topical capsaicin in the treatment of pruritus. Int. J. Dermatol. 2010, 49, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F. Mechanisms involved in new therapies for overactive bladder. Urology 2004, 63, 65–73. [Google Scholar] [CrossRef] [PubMed]
- de Sèze, M.; Wiart, L.; de Sèze, M.P.; Soyeur, L.; Dosque, J.P.; Blajezewski, S.; Moore, N.; Brochet, B.; Mazaux, J.M.; Barat, M.; et al. Intravesical capsaicin versus resiniferatoxin for the treatment of detrusor hyperreflexia in spinal cord injured patients: A double-blind, randomized, controlled study. J. Urol. 2004, 171, 251–255. [Google Scholar] [CrossRef]
- Botonis, P.G.; Miliotis, P.G.; Kounalakis, S.N.; Koskolou, M.D.; Geladas, N.D. Effects of capsaicin application on the skin during resting exposure to temperate and warm conditions. Scand. J. Med. Sci. Sports 2019, 29, 171–179. [Google Scholar] [CrossRef]
- Rothenberger, J.; Wittwer, M.; Tschumi, C.; Constantinescu, M.A.; Daigeler, A.; Olariu, R. Quantitative impact analysis of remote ischemic conditioning and capsaicin application on human skin microcirculation. Clin. Hemorheol. Microcirc. 2019, 71, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Suzuki, T.; Takahashi, M.; Iwai, K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984, 72, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Crouch, D.J.; Yost, G.S.; Fatah, A.A. Determination of capsaicin, nonivamide, and dihydrocapsaicin in blood and tissue by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2002, 26, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ilie, M.A.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Negrei, C.; Ion, R.M.; Constantin, C.; Neagu, M.; Boda, D. Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions. Exp. Ther. Med. 2019, 18, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Huang, J.; Hu, Y.; Xu, Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2020, 45, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, Y.; Gong, Y.; Chen, J.; Xu, B.; Tang, L.; Guo, L.; Xie, J. Capsaicin metabolites and GSH-associated detoxification and biotransformation pathways in human liver microsomes revealed by LC-HRMS/MS with data-mining tools. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1133, 121843. [Google Scholar] [CrossRef]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Saba, A.B.; Azeez, O.I. Capsaicin: A novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J. Cancer 2010, 47, 53–58. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Reilly, C.A.; Yost, G.S. Metabolism of capsaicinoids by P450 enzymes: A review of recent findings on reaction mechanisms, bio-activation, and detoxification processes. Drug Metab. Rev. 2006, 38, 685–706. [Google Scholar] [CrossRef]
- van Eijl, S.; Zhu, Z.; Cupitt, J.; Gierula, M.; Götz, C.; Fritsche, E.; Edwards, R.J. Elucidation of xenobiotic metabolism pathways in human skin and human skin models by proteomic profiling. PLoS ONE 2012, 7, e41721. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Babbar, S.; Marier, J.F.; Mouksassi, M.S.; Beliveau, M.; Vanhove, G.F.; Chanda, S.; Bley, K. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther. Drug Monit. 2009, 31, 502–510. [Google Scholar] [CrossRef]
- Zak, A.; Siwinska, N.; Slowikowska, M.; Borowicz, H.; Szpot, P.; Zawadzki, M.; Niedzwiedz, A. The detection of capsaicin and dihydrocapsaicin in horse serum following long-term local administration. BMC Vet. Res. 2018, 14, 193. [Google Scholar] [CrossRef]
- Tian, K.; Zhu, J.; Li, M.; Qiu, X. Capsaicin is efficiently transformed by multiple cytochrome P450s from Capsicum fruit-feeding Helicoverpa armigera. Pestic. Biochem. Physiol. 2019, 156, 145–151. [Google Scholar] [CrossRef]
- Hanson, S.M.; Newstead, S.; Swartz, K.J.; Sansom, M.S.P. Capsaicin interaction with TRPV1 channels in a lipid bilayer: Molecular dynamics simulation. Biophys. J. 2015, 108, 1425–1434. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef]
- Cheng, Y. TRPV1 and Piezo: The 2021 Nobel Prize in Physiology or Medicine. IUCrJ 2022, 9, 4–5. [Google Scholar] [CrossRef]
- Earley, S.; Santana, L.F.; Lederer, W.J. The physiological sensor channels TRP and piezo: Nobel Prize in Physiology or Medicine 2021. Physiol. Rev. 2022, 102, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Reeh, P.W.; Fischer, M.J. Nobel somatosensations and pain. Pflügers Arch.-Eur. J. Physiol. 2022, 474, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.; Trafton, J.; Petersen-Zeitz, K.; Koltzenburg, M.; Basbaum, A.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.; Merighi, A. Capsaicin, Nociception and Pain. Molecules 2016, 21, 797. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef]
- Frey, E.; Karney-Grobe, S.; Krolak, T.; Milbrandt, J.; DiAntonio, A. TRPV1 Agonist, Capsaicin, Induces Axon Outgrowth after Injury via Ca(2+)/PKA Signaling. eNeuro 2018, 5, ENEURO.0095-18.2018. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.S.; Zhao, Z.Q. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacology 2001, 41, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, J.W.; Osaka, T.; Kobayashi, A.; Namba, Y.; Inoue, S.; Kimura, S. Lack of integrative control of body temperature after capsaicin administration. Korean J. Intern. Med. 2000, 15, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Binzen, U.; Treede, R.-D.; Greffrath, W. The capsaicin receptor TRPV1 is the first line defense protecting from acute non damaging heat: A translational approach. J. Transl. Med. 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflug. Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ye, L.; Zhang, Q.; Wu, F.; Wang, L. The role of TRPV1 channels in atherosclerosis. Channels 2020, 14, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Munjuluri, S.; Wilkerson, D.A.; Sooch, G.; Chen, X.; White, F.A.; Obukhov, A.G. Capsaicin and TRPV1 Channels in the Cardiovascular System: The Role of Inflammation. Cells 2021, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Hoebart, C.; Rojas-Galvan, N.S.; Ciotu, C.I.; Aykac, I.; Reissig, L.F.; Weninger, W.J.; Kiss, A.; Podesser, B.K.; Fischer, M.J.M.; Heber, S. No functional TRPA1 in cardiomyocytes. Acta Physiol. 2021, 232, e13659. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, K.; Rajendran, P.S.; Massoud, L.; Mistry, J.; Swid, M.A.; Wu, X.; Sallam, T.; Zhang, R.; Goldhaber, J.I.; Salavatian, S.; et al. Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight 2020, 5, e124477. [Google Scholar] [CrossRef]
- Kim, J.H. The Emerging Role of TRPV1 in Airway Inflammation. Allergy Asthma Immunol. Res. 2018, 10, 187–188. [Google Scholar] [CrossRef]
- Baxter, M.; Eltom, S.; Dekkak, B.; Yew-Booth, L.; Dubuis, E.D.; Maher, S.A.; Belvisi, M.G.; Birrell, M.A. Role of transient receptor potential and pannexin channels in cigarette smoke-triggered ATP release in the lung. Thorax 2014, 69, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, L.P.; Butler, C.A.; Stokesberry, S.; Polley, L.; McQuaid, S.; Abdullah, H.; Ashraf, S.; McGahon, M.K.; Curtis, T.M.; Arron, J.; et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J. Allergy Clin. Immunol. 2014, 133, 704–712.e4. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liao, Q.; Chen, C.; Yang, X.; Xie, R.; Xu, J. The Role of Transient Receptor Potential Vanilloid 1 in Common Diseases of the Digestive Tract and the Cardiovascular and Respiratory System. Front. Physiol. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, C.; Negrei, C.; Ilie Ghita, M.; Caruntu, A.; Badarau, B.; ioan Buraga, I.B.; Boda, D.; Albu, A.; Brănişteanu, D. Capsaicin, a hot topic in skin pharmacology and physiology. Farmacia 2015, 63, 487–491. [Google Scholar]
- Inoue, K.; Koizumi, S.; Fuziwara, S.; Denda, S.; Inoue, K.; Denda, M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2002, 291, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Moormann, C.; Schumacher, M.; Buddenkotte, J.; Artuc, M.; Shpacovitch, V.; Brzoska, T.; Lippert, U.; Henz, B.M.; Luger, T.A.; et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 2004, 13, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.J.; Yu, Y.; Yang, J.Y.; Li, J.J.; Zhu, J.Y.; Vieira, J.A.C.; Jiang, Q. Involvement of transient receptor potential channels in ocular diseases: A narrative review. Ann. Transl. Med. 2022, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Sappington, R.M.; Sidorova, T.; Long, D.J.; Calkins, D.J. TRPV1: Contribution to retinal ganglion cell apoptosis and increased intracellular Ca2+ with exposure to hydrostatic pressure. Investig. Ophthalmol. Vis. Sci. 2009, 50, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xue, Y.; Fu, L.; Wang, Y.; He, M.; Zhao, L.; Liao, X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5322–5348. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. Capsaicin: Effects on the Pathogenesis of Hepatocellular Carcinoma. Molecules 2019, 24, 2350. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef] [PubMed]
- Caballero, J. A new era for the design of TRPV1 antagonists and agonists with the use of structural information and molecular docking of capsaicin-like compounds. J. Enzym. Inhib. Med. Chem. 2022, 37, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Raisinghani, M.; Pabbidi, R.M.; Premkumar, L.S. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J. Physiol. 2005, 567, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Duarte, Y.; Cáceres, J.; Sepúlveda, R.V.; Arriagada, D.; Olivares, P.; Díaz-Franulic, I.; Stehberg, J.; González-Nilo, F. Novel TRPV1 Channel Agonists with Faster and More Potent Analgesic Properties Than Capsaicin. Front. Pharmacol. 2020, 11, 1040. [Google Scholar] [CrossRef]
- Căruntu, C.; Boda, D. Evaluation through in vivo reflectance confocal microscopy of the cutaneous neurogenic inflammatory reaction induced by capsaicin in human subjects. J. Biomed. Opt. 2012, 17, 085003. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Kang, H.J.; Moon, A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001, 165, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hail, N., Jr.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002, 94, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Park, W.H.; Park, J.Y.; Kang, J.H.; Kim, M.O.; Kawada, T.; Yoo, H.; Han, I.S.; Yu, R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J. Med. Food 2004, 7, 267–273. [Google Scholar] [CrossRef]
- Lo, Y.C.; Yang, Y.C.; Wu, I.C.; Kuo, F.C.; Liu, C.M.; Wang, H.W.; Kuo, C.H.; Wu, J.Y.; Wu, D.C. Capsaicin-induced cell death in a human gastric adenocarcinoma cell line. World J. Gastroenterol. 2005, 11, 6254–6257. [Google Scholar] [CrossRef]
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Smith, P.A.; Vakilpour, S.; Kumaran, N.M.; Turner, A.E.; Bagiokou, D.; Layfield, R.; Ray, D.E.; Westwell, A.D.; Alexander, S.P.; et al. Vanilloid receptor agonists and antagonists are mitochondrial inhibitors: How vanilloids cause non-vanilloid receptor mediated cell death. Biochem. Biophys. Res. Commun. 2007, 354, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xiong, Z.; Liu, C.; Liu, L. Inhibitory effects of capsaicin on voltage-gated potassium channels by TRPV1-independent pathway. Cell. Mol. Neurobiol. 2014, 34, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gamper, N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013, 11, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Howarth, F.C. Transient receptor potential vanilloid 1 (TRPV1)-independent actions of capsaicin on cellular excitability and ion transport. Med. Res. Rev. 2023, 43, 1038–1067. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef] [PubMed]
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.F.; Buck, S.H.; Miller, M.S. Mechanisms of depletion of substance P by capsaicin. Fed. Proc. 1985, 44, 2531–2534. [Google Scholar]
- Dray, A. Mechanism of action of capsaicin-like molecules on sensory neurons. Life Sci. 1992, 51, 1759–1765. [Google Scholar] [CrossRef]
- Ding, H.; Kiguchi, N.; Dobbins, M.; Romero-Sandoval, E.A.; Kishioka, S.; Ko, M.-C. Nociceptin Receptor-Related Agonists as Safe and Non-addictive Analgesics. Drugs 2023, 83, 771–793. [Google Scholar] [CrossRef]
- Liu, L.; Oortgiesen, M.; Li, L.; Simon, S.A. Capsaicin inhibits activation of voltage-gated sodium currents in capsaicin-sensitive trigeminal ganglion neurons. J. Neurophysiol. 2001, 85, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Pasierski, M.; Szulczyk, B. Capsaicin inhibits sodium currents and epileptiform activity in prefrontal cortex pyramidal neurons. Neurochem. Int. 2020, 135, 104709. [Google Scholar] [CrossRef]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Qiu, H.; Li, H.; Ma, D.; Guan, Y.; Wang, Y. The preemptive analgesic effect of capsaicin involves attenuations of epidermal keratinocytes proliferation and expression of pro-inflammatory mediators after plantar incision in rats. J. Pain. Res. 2023, 16, 141–149. [Google Scholar] [CrossRef]
- Goodwin, B.; Chiplunkar, M.; Salerno, R.; Coombs, K.; Sannoh, U.; Shah, V.; Averell, N.; Al-Shebab, U.; Janora, D. Topical capsaicin for the management of painful diabetic neuropathy: A narrative systematic review. Pain Manag. 2023, 13, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Vachiramon, V.; Tanratana, P.; Anuntrangsee, T.; Palakornkitti, P.; Yeesibsean, N.; Kungvalpivat, P.; Fabi, S. The role of topical capsaicin gel in pain management during microfocused ultrasound treatment for neck laxity. Skin Res. Technol. 2023, 29, e13240. [Google Scholar] [CrossRef]
- Peel, J.; John, K.; Page, J.; Jeffries, O.; Heffernan, S.M.; Tallent, J.; Waldron, M. Topical application of isolated menthol and combined menthol-capsaicin creams: Exercise tolerance, thermal perception, pain, attentional focus and thermoregulation in the heat. Eur. J. Sport. Sci. 2023, 23, 2038–2048. [Google Scholar] [CrossRef]
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Saberski, L.; Bartoshuk, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain. Symptom Manag. 1995, 10, 243–248. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Kalso, E.A.; Bell, R.F.; Aldington, D.; Phillips, T.; Gaskell, H.; Moore, R.A. Topical analgesics for acute and chronic pain in adults—An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 5, Cd008609. [Google Scholar] [CrossRef]
- Wang, X.R.; Gao, S.Q.; Niu, X.Q.; Li, L.J.; Ying, X.Y.; Hu, Z.J.; Gao, J.Q. Capsaicin-loaded nanolipoidal carriers for topical application: Design, characterization, and in vitro/in vivo evaluation. Int. J. Nanomed. 2017, 12, 3881–3898. [Google Scholar] [CrossRef] [PubMed]
- Anantaworasakul, P.; Anuchapreeda, S.; Yotsawimonwat, S.; Naksuriya, O.; Lekawanvijit, S.; Tovanabutra, N.; Anantaworasakul, P.; Wattanasri, W.; Buranapreecha, N.; Ampasavate, C. Nanomaterial Lipid-Based Carrier for Non-Invasive Capsaicin Delivery; Manufacturing Scale-Up and Human Irritation Assessment. Molecules 2020, 25, 5575. [Google Scholar] [CrossRef] [PubMed]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The use of nanoencapsulation to decrease human skin irritation caused by capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Mahajan, A.; Negi, P.; Bhatia, A.; Katare, O.P. Design and evaluation of flexible membrane vesicles (FMVs) for enhanced topical delivery of capsaicin. J. Drug Target. 2011, 19, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hudita, A.; Galateanu, B.; Costache, M.; Negrei, C.; Ion, R.M.; Iancu, L.; Ginghina, O. In Vitro Cytotoxic Protective Effect of Alginate-Encapsulated Capsaicin Might Improve Skin Side Effects Associated with the Topical Application of Capsaicin. Molecules 2021, 26, 1455. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Katare, O.P. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J. Liposome Res. 2014, 24, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Don, P.C. Topical capsaicin for treatment of neuralgia associated with herpes zoster infection. J. Am. Acad. Dermatol. 1988, 18, 1135–1136. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, N.S.; Rath, P.P.; Hirano, M. The treatment of periocular and facial pain with topical capsaicin. J. Neuroophthalmol. 1998, 18, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.G., Jr. Treatment of apocrine chromhidrosis with topical capsaicin. J. Am. Acad. Dermatol. 1989, 21, 418–420. [Google Scholar] [CrossRef]
- Cheshire, W.P.; Snyder, C.R. Treatment of reflex sympathetic dystrophy with topical capsaicin. Case report. Pain 1990, 42, 307–311. [Google Scholar] [CrossRef]
- Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. The Capsaicin Study Group. Arch. Intern. Med. 1991, 151, 2225–2229. [CrossRef]
- Tandan, R.; Lewis, G.A.; Krusinski, P.B.; Badger, G.B.; Fries, T.J. Topical capsaicin in painful diabetic neuropathy. Controlled study with long-term follow-up. Diabetes Care 1992, 15, 8–14. [Google Scholar] [CrossRef]
- McCarthy, G.M.; McCarty, D.J. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J. Rheumatol. 1992, 19, 604–607. [Google Scholar] [PubMed]
- Leibsohn, E. Treatment of notalgia paresthetica with capsaicin. Cutis 1992, 49, 335–336. [Google Scholar] [PubMed]
- Watson, C.P.; Tyler, K.L.; Bickers, D.R.; Millikan, L.E.; Smith, S.; Coleman, E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin. Ther. 1993, 15, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.N.; Berberian, B.; Sulica, V.I.; Dodd, W.A.; Jarratt, M.T.; Katz, H.I.; Prawer, S.; Krueger, G.; Rex, I.H., Jr.; Wolf, J.E. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J. Am. Acad. Dermatol. 1993, 29, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Dini, D.; Bertelli, G.; Gozza, A.; Forno, G.G. Treatment of the post-mastectomy pain syndrome with topical capsaicin. Pain 1993, 54, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.R.; Rapoport, A.; Padla, D.; Weeks, R.; Rosum, R.; Sheftell, F.; Arrowsmith, F. A double-blind placebo-controlled trial of intranasal capsaicin for cluster headache. Cephalalgia 1993, 13, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Teofoli, P.; Tsampau, D. Treatment of aquagenic pruritus with topical capsaicin cream. J. Am. Acad. Dermatol. 1994, 30, 232–235. [Google Scholar] [CrossRef]
- Muhiddin, K.A.; Gallen, I.W.; Harries, S.; Pearce, V.R. The use of capsaicin cream in a case of erythromelalgia. Postgrad. Med. J. 1994, 70, 841–843. [Google Scholar] [CrossRef]
- Epstein, J.B.; Marcoe, J.H. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mathias, B.J.; Dillingham, T.R.; Zeigler, D.N.; Chang, A.S.; Belandres, P.V. Topical capsaicin for chronic neck pain. A pilot study. Am. J. Phys. Med. Rehabil. 1995, 74, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Alegre, M.; de Moragas, J.M. Treatment of meralgia paraesthetica with topical capsaicin. Dermatology 1995, 191, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, T.; Sawada, Y. Topical application of capsaicin and flap survival. Br. J. Plast. Surg. 1996, 49, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Frucht-Pery, J.; Feldman, S.T.; Brown, S.I. The use of capsaicin in herpes zoster ophthalmicus neuralgia. Acta Ophthalmol. Scand. 1997, 75, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.R.; Staats, P.S.; Levine, J.; Fields, H.L.; Allen, R.W.; Campbell, J.N.; Pappagallo, M. Treatment of intractable pain with topical large-dose capsaicin: Preliminary report. Anesth. Analg. 1998, 86, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, E.; Heyer, G.; Forster, C.; Handwerker, H.O. Effect of topical capsaicin on the cutaneous reactions and itching to histamine in atopic eczema compared to healthy skin. Arch. Dermatol. Res. 1998, 290, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, T.; Kalogjera, L.; Hat, J. Capsaicin significantly reduces sinonasal polyps. Acta Otolaryngol. 2000, 120, 307–311. [Google Scholar] [CrossRef]
- Sandford, P.R.; Benes, P.S. Use of capsaicin in the treatment of radicular pain in spinal cord injury. J. Spinal Cord. Med. 2000, 23, 238–243. [Google Scholar] [CrossRef]
- Ständer, S.; Luger, T.; Metze, D. Treatment of prurigo nodularis with topical capsaicin. J. Am. Acad. Dermatol. 2001, 44, 471–478. [Google Scholar] [CrossRef]
- Ribbers, G.M.; Stam, H.J. Complex regional pain syndrome type I treated with topical capsaicin: A case report. Arch. Phys. Med. Rehabil. 2001, 82, 851–852. [Google Scholar] [CrossRef]
- Marsella, R.; Nicklin, C.F.; Melloy, C. The effects of capsaicin topical therapy in dogs with atopic dermatitis: A randomized, double-blinded, placebo-controlled, cross-over clinical trial. Vet. Dermatol. 2002, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M. Use of capsaicin cream for abdominal wall scar pain. Am. Fam. Physician 2002, 65, 2211; author reply 2212. [Google Scholar]
- Weisshaar, E.; Dunker, N.; Gollnick, H. Topical capsaicin therapy in humans with hemodialysis-related pruritus. Neurosci. Lett. 2003, 345, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Dux, M.; Sántha, P.; Jancsó, G. Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J. Physiol. 2003, 552, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Perkins, P.; Morgan, S.; Closs, S.P. Topical capsaicin for saphenous neuralgia. J. Pain. Symptom Manag. 2003, 26, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, M.; Spinelli, M.; Zanollo, A.; Turini, D. Intravesical vanilloids and neurogenic incontinence: Ten years experience. Urol. Int. 2004, 72, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, M.; Lauritano, D.; De Benedittis, M.; Baldoni, M.; Serpico, R. Systemic capsaicin for burning mouth syndrome: Short-term results of a pilot study. J. Oral. Pathol. Med. 2004, 33, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Mengesha, Y.; Facliaru, D.; David, M. Topical capsaicin for the treatment of acute lipodermatosclerosis and lobular panniculitis. J. Dermatol. Treat. 2005, 16, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Nam, Y.M. The analgesic effects of capsicum plaster at the Zusanli point after abdominal hysterectomy. Anesth. Analg. 2006, 103, 709–713. [Google Scholar] [CrossRef]
- Harada, N.; Okajima, K. Effect of topical application of capsaicin and its related compounds on dermal insulin-like growth factor-I levels in mice and on facial skin elasticity in humans. Growth Horm. IGF Res. 2007, 17, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Estanislao, L.; Brown, S.J.; Sampson, J. An open-label pilot study of high-concentration capsaicin patch in painful HIV neuropathy. J. Pain. Symptom Manag. 2008, 35, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, K.N.; Hwang, K.G.; Park, C.J. Capsicum plaster at the Hegu point reduces postoperative analgesic requirement after orthognathic surgery. Anesth. Analg. 2009, 108, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, C. Capsaicin jelly against migraine pain. Int. J. Clin. Pract. 2010, 64, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Weiser, T.; Beime, B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother. Res. 2010, 24, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Redington, K.L.; Disenhouse, T.; Strantzas, S.C.; Gladstone, R.; Wei, C.; Tropak, M.B.; Dai, X.; Manlhiot, C.; Li, J.; Redington, A.N. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic. Res. Cardiol. 2012, 107, 241. [Google Scholar] [CrossRef]
- Sayanlar, J.; Guleyupoglu, N.; Portenoy, R.; Ashina, S. Trigeminal postherpetic neuralgia responsive to treatment with capsaicin 8% topical patch: A case report. J. Headache Pain 2012, 13, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Shin, M.K.; Yoon, D.J.; Kim, A.R.; Yu, R.; Park, N.H.; Han, I.S. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 21, 115–122. [Google Scholar] [CrossRef]
- Casanueva, B.; Rodero, B.; Quintial, C.; Llorca, J.; González-Gay, M.A. Short-term efficacy of topical capsaicin therapy in severely affected fibromyalgia patients. Rheumatol. Int. 2013, 33, 2665–2670. [Google Scholar] [CrossRef]
- Maihöfner, C.G.; Heskamp, M.L. Treatment of peripheral neuropathic pain by topical capsaicin: Impact of pre-existing pain in the QUEPP-study. Eur. J. Pain 2014, 18, 671–679. [Google Scholar] [CrossRef]
- Zis, P.; Apsokardos, A.; Isaia, C.; Sykioti, P.; Vadalouca, A. Posttraumatic and postsurgical neuropathic pain responsive to treatment with capsaicin 8% topical patch. Pain. Physician 2014, 17, E213–E218. [Google Scholar]
- Kumar Sarwa, K.; Rudrapal, M.; Mazumder, B. Topical ethosomal capsaicin attenuates edema and nociception in arthritic rats. Drug Deliv. 2015, 22, 1043–1052. [Google Scholar] [CrossRef]
- Teixeira, M.J.; Menezes, L.M.; Silva, V.; Galhardoni, R.; Sasson, J.; Okada, M.; Duarte, K.P.; Yeng, L.T.; Andrade, D.C. Liposomal topical capsaicin in post-herpetic neuralgia: A safety pilot study. Arq. Neuropsiquiatr. 2015, 73, 237–240. [Google Scholar] [CrossRef]
- Lu, S.; Baad-Hansen, L.; Zhang, Z.; Svensson, P. Spatial and Temporal Effects of Capsaicin and Menthol on Intraoral Somatosensory Sensitivity. J. Oral. Facial Pain. Headache 2015, 29, 257–264. [Google Scholar] [CrossRef]
- Zeidler, C.; Metze, D.; Ständer, S. Successful treatment of lichen amyloidosis using capsaicin 8% patch. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Dezieck, L.; Hafez, Z.; Conicella, A.; Blohm, E.; O’Connor, M.J.; Schwarz, E.S.; Mullins, M.E. Resolution of cannabis hyperemesis syndrome with topical capsaicin in the emergency department: A case series. Clin. Toxicol. 2017, 55, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.R.; Pedersen, A.M. Analgesic effect of topical oral capsaicin gel in burning mouth syndrome. Acta Odontol. Scand. 2017, 75, 130–136. [Google Scholar] [CrossRef]
- Baron, R.; Treede, R.D.; Birklein, F.; Cegla, T.; Freynhagen, R.; Heskamp, M.L.; Kern, K.U.; Maier, C.; Rolke, R.; Seddigh, S.; et al. Treatment of painful radiculopathies with capsaicin 8% cutaneous patch. Curr. Med. Res. Opin. 2017, 33, 1401–1411. [Google Scholar] [CrossRef]
- Bae, S.; Yu, J.; Jeong, H.; Oh, T. Anti-pruritic effect of topical capsaicin against histamine-induced pruritus on canine skin. Pol. J. Vet. Sci. 2018, 21, 789–796. [Google Scholar] [CrossRef]
- Járomi, P.; Garab, D.; Hartmann, P.; Bodnár, D.; Nyíri, S.; Sántha, P.; Boros, M.; Jancsó, G.; Szabó, A. Capsaicin-induced rapid neutrophil leukocyte activation in the rat urinary bladder microcirculatory bed. Neurourol. Urodyn. 2018, 37, 690–698. [Google Scholar] [CrossRef]
- Romero, V.; Lara, J.R.; Otero-Espinar, F.; Salgado, M.H.; Modolo, N.S.P.; Barros, G.A.M. [Capsaicin topical cream (8%) for the treatment of myofascial pain syndrome]. Braz. J. Anesthesiol. 2019, 69, 432–438. [Google Scholar] [CrossRef]
- Kocak, A.O.; Dogruyol, S.; Akbas, I.; Menekse, T.S.; Gur, S.T.A.; Kocak, M.B.; Cekmen, B.; Orun, S.; Cakir, Z. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: A randomized double-blind trial. Am. J. Emerg. Med. 2020, 38, 1767–1771. [Google Scholar] [CrossRef]
- de Lourdes Medina-Contreras, J.M.; Mailloux-Salinas, P.; Colado-Velazquez, J.I.; Gómez-Viquez, N.; Velázquez-Espejel, R.; Del Carmen Susunaga-Notario, A.; Arias-Chávez, D.J.; Bravo, G. Topical capsaicin cream with moderate exercise protects against hepatic steatosis, dyslipidemia and increased blood pressure in hypoestrogenic obese rats. J. Sci. Food Agric. 2020, 100, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
- Agoons, B.B.; Dehayem Yefou, M.; Katte, J.C.; Etoa Etoga, M.C.; Agoons, D.D.; Yepnjio, F.; Boli, A.; Wasnyo, Y.; Sobngwi, E.; Mbanya, J.C. Effect of Topical Capsaicin on Painful Sensory Peripheral Neuropathy in Patients with Type 2 Diabetes: A Double-Blind Placebo-Controlled Randomised Clinical Trial. Cureus 2020, 12, e11147. [Google Scholar] [CrossRef]
- Wang, S.; Bian, C.; Yang, J.; Arora, V.; Gao, Y.; Wei, F.; Chung, M.K. Ablation of TRPV1+ Afferent Terminals by Capsaicin Mediates Long-Lasting Analgesia for Trigeminal Neuropathic Pain. eNeuro 2020, 7, ENEURO.0118-20.2020. [Google Scholar] [CrossRef]
- Chan, T.C.; Lee, M.S.; Huang, W.C.; Chang, W.Y.; Krueger, J.G.; Tsai, T.F. Capsaicin attenuates imiquimod-induced epidermal hyperplasia and cutaneous inflammation in a murine model of psoriasis. Biomed. Pharmacother. 2021, 141, 111950. [Google Scholar] [CrossRef] [PubMed]
- Hoesli, R.C.; Wingo, M.L.; Wajsberg, B.; Bastian, R.W. Topical Capsaicin for the Treatment of Sensory Neuropathic Cough. OTO Open 2021, 5, 2473974x211065668. [Google Scholar] [CrossRef] [PubMed]
- Kum, V.; Bell, A.; Fang, W.; VanWert, E. Efficacy of topical capsaicin for cannabinoid hyperemesis syndrome in a pediatric and adult emergency department. Am. J. Emerg. Med. 2021, 49, 343–351. [Google Scholar] [CrossRef]
- Lo Vecchio, S.; Andersen, H.H.; Elberling, J.; Arendt-Nielsen, L. Sensory defunctionalization induced by 8% topical capsaicin treatment in a model of ultraviolet-B-induced cutaneous hyperalgesia. Exp. Brain Res. 2021, 239, 2873–2886. [Google Scholar] [CrossRef]
- Van Gerven, L.; Steelant, B.; Cools, L.; Callebaut, I.; Backaert, W.; de Hoon, J.; Ampe, E.; Talavera, K.; Hellings, P.W. Low-dose capsaicin (0.01 mM) nasal spray is equally effective as the current standard treatment for idiopathic rhinitis: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2021, 147, 397–400.e4. [Google Scholar] [CrossRef]
- Silva-Clavería, F.; Bernabeu-Wittel, J.; Monserrat, M.T. Topical capsaicin patch for pain management in PTEN hamartoma tumor syndromes. J. Dtsch. Dermatol. Ges. 2022, 20, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Cleary, D.; Burton, S.; Cardon, B. Time to consider topical capsaicin for acute trauma pain? J. Fam. Pract. 2022, 71, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, R.C.; Sohn, A.; Kersten, A.; Amr, A.; Held, M.; Wenger, A. Quantification of Dermal Microcirculatory Changes after Topical Administration of Capsaicin: A Randomized Placebo-Controlled Study in 46 Subjects. J. Investig. Surg. 2022, 35, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Laude-Pagniez, E.; Leclerc, J.; Lok, C.; Chaby, G.; Arnault, J.P. Capsaicin 8% patch as therapy for neuropathic chronic postsurgical pain after melanoma excision surgery: A single center case series. JAAD Case Rep. 2022, 30, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sendel, M.; Dunst, A.; Forstenpointner, J.; Hüllemann, P.; Baron, R. Capsaicin treatment in neuropathic pain: Axon reflex vasodilatation after 4 weeks correlates with pain reduction. Pain 2023, 164, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ercan, N.; Uludag, M.O.; Agis, E.R.; Demirel-Yilmaz, E. The anti-inflammatory effect of diclofenac is considerably augmented by topical capsaicinoids-containing patch in carrageenan-induced paw oedema of rat. Inflammopharmacology 2013, 21, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Payan, D.G. Neuropeptides and inflammation: The role of substance P. Annu. Rev. Med. 1989, 40, 341–352. [Google Scholar] [CrossRef]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Bartold, P.; Kylstra, A.; Lawson, R. Substance P: An immunohistochemical and biochemical study in human gingival tissues. A role for neurogenic inflammation? J. Periodontol. 1994, 65, 1113–1121. [Google Scholar] [CrossRef]
- DeVane, C.L. Substance P: A new era, a new role. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 1061–1069. [Google Scholar] [CrossRef]
- Hägermark, O.; Hökfelt, T.; Pernow, B. Flare and itch induced by substance P in human skin. Nation 1978, 12, 13. [Google Scholar] [CrossRef]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: Correlation with sensory function. J. Neurosci. 1998, 18, 8947–8959. [Google Scholar] [CrossRef]
- Caruntu, C.; Boda, D.; Musat, S.; Caruntu, A.; Mandache, E. Stress-induced mast cell activation in glabrous and hairy skin. Mediat. Inflamm. 2014, 2014, 105950. [Google Scholar] [CrossRef]
- Brodin, E.; Nilsson, G. Concentration of substance P-like immunoreactivity (SPLI) in tissues of dog, rat and mouse. Acta Physiol. Scand. 1981, 112, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mistrova, E.; Kruzliak, P.; Dvorakova, M.C. Role of substance P in the cardiovascular system. Neuropeptides 2016, 58, 41–51. [Google Scholar] [CrossRef]
- Geppetti, P.; Bertrand, C.; Baker, J.; Yamawaki, I.; Piedimonte, G.; Nadel, J.A. Ruthenium red, but not capsazepine reduces plasma extravasation by cigarette smoke in rat airways. Br. J. Pharmacol. 1993, 108, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Figini, M.; Emanueli, C.; Bertrand, C.; Javdan, P.; Geppetti, P. Evidence that tachykinins relax the guinea-pig trachea via nitric oxide release and by stimulation of a septide-insensitive NK1 receptor. Br. J. Pharmacol. 1996, 117, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Kronberger, C.; Hacker, G.W.; Franz, P.; Albegger, K.; Dietze, O. CGRP and substance P in intraepithelial neuronal structures of the human upper respiratory system. Regul. Pept. 1997, 72, 79–85. [Google Scholar] [CrossRef]
- Barthó, L.; Holzer, P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience 1985, 16, 1–32. [Google Scholar] [CrossRef]
- Costa, M.; Furness, J.B.; Gibbins, I.L. Chapter 15 Chemical coding of enteric neurons. In Progress in Brain Research; Hökfelt, T., Fuxe, K., Pernow, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 68, pp. 217–239. [Google Scholar]
- Hökfelt, T.; Pernow, B.; Wahren, J. Substance P: A pioneer amongst neuropeptides. J. Intern. Med. 2001, 249, 27–40. [Google Scholar] [CrossRef]
- Watts, S.W.; Cohen, M.L. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides 1991, 12, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995, 45, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Rosendal-Helgesen, S.; Uddman, R. Substance P: Localization, concentration and release in cerebral arteries, choroid plexus and dura mater. Cell Tissue Res. 1983, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Petersson, J.; Zygmunt, P.M.; Brandt, L.; Högestätt, E.D. Substance P-induced relaxation and hyperpolarization in human cerebral arteries. Br. J. Pharmacol. 1995, 115, 889. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Mehboob, R.; Oehme, P.; Pfaff, G. The role of Substance P in the defense line of the respiratory tract and neurological manifestations post COVID-19 infection. Front. Neurol. 2023, 14, 1052811. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Wan, Q.; Fang, J.; Peng, L.; Li, Q.L.; Hu, J. The Src1-PGC1α-AP1 complex-dependent secretion of substance P induces inflammation and apoptosis in encephalomyocarditis virus-infected mice. Cytokine 2023, 165, 156186. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Vasconcelos, R.; Chiarelli-Neto, V.M.; Louzada, L.M.; Aveiro, E.; Alves-Silva, E.G.; de-Jesus-Soares, A.; Ferraz, C.C.R.; Almeida, J.F.A.; Marciano, M.A.; Pecorari, V.G.A.; et al. Quantitative analysis of culturable bacteria, levels of endotoxins, inflammatory mediators and substance P in teeth with symptomatic irreversible pulpitis and in teeth with vital normal pulp tissues. Int. Endod. J. 2023, 56, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Suptela, S.R.; Sipprell, S.E.; Marriott, I. Substance P Exacerbates the Inflammatory and Pro-osteoclastogenic Responses of Murine Osteoclasts and Osteoblasts to Staphylococcus aureus. Inflammation 2023, 46, 256–269. [Google Scholar] [CrossRef]
- Scheau, C.; Ilie Ghita, M.; Grigore, O.; Mihailescu, A.; Caruntu, A.; Mihai, L.; Bădărău, I.; Boda, D.; Caruntu, C. Modulation of capsaicin-induced neurogenic vasodilation by acute psychological stress. Farmacia 2021, 69, 778–784. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, X.; Zhang, T.; Wu, X.; Fan, D.; Hu, Y.; Ding, J.; Yang, X.; Lou, J.; Du, Q.; et al. Beneficial effects of dietary capsaicin in gastrointestinal health and disease. Exp. Cell Res. 2022, 417, 113227. [Google Scholar] [CrossRef] [PubMed]
- Patowary, P.; Pathak, M.P.; Zaman, K.; Raju, P.S.; Chattopadhyay, P. Research progress of capsaicin responses to various pharmacological challenges. Biomed. Pharmacother. 2017, 96, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Kunjiappan, S.; Sankaranarayanan, M.; Karan Kumar, B.; Pavadai, P.; Babkiewicz, E.; Maszczyk, P.; Glodkowska-Mrowka, E.; Arunachalam, S.; Ram Kumar Pandian, S.; Ravishankar, V.; et al. Capsaicin-loaded solid lipid nanoparticles: Design, biodistribution, in silico modeling and in vitro cytotoxicity evaluation. Nanotechnology 2021, 32, 095101. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, A.R.; Solouk, A.; Saber-Samandari, S.; Akbari, S.; Ghanbari, H.; Brycki, B.E. Capsaicin-loaded alginate nanoparticles embedded polycaprolactone-chitosan nanofibers as a controlled drug delivery nanoplatform for anticancer activity. J. Colloid. Interface Sci. 2023, 638, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Thán, M.; Németh, J.; Szilvássy, Z.; Pintér, E.; Helyes, Z.; Szolcsányi, J. Systemic anti-inflammatory effect of somatostatin released from capsaicin-sensitive vagal and sciatic sensory fibres of the rat and guinea-pig. Eur. J. Pharmacol. 2000, 399, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Petruzzi, M.; Baldoni, M. [Preliminary protocol for systemic administration of capsaicin for the treatment of the burning mouth syndrome]. Minerva Stomatol. 2003, 52, 273–278. [Google Scholar] [PubMed]
- Luqman, S.; Rizvi, S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006, 20, 303–306. [Google Scholar] [CrossRef]
- Lee, I.O.; Lee, K.H.; Pyo, J.H.; Kim, J.H.; Choi, Y.J.; Lee, Y.C. Anti-inflammatory Effect of Capsaicin in Helicobacter pylori-Infected Gastric Epithelial Cells. Helicobacter 2007, 12, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Starzinski-Powitz, A.; Guo, S.W. Capsaicin inhibits proliferation of endometriotic cells in vitro. Gynecol. Obstet. Investig. 2008, 66, 59–62. [Google Scholar] [CrossRef]
- Bortolotti, M.; Porta, S. Effect of red pepper on symptoms of irritable bowel syndrome: Preliminary study. Dig. Dis. Sci. 2011, 56, 3288–3295. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Yu, Y.; Li, Y.; Zhao, S.; Chen, Y.; Waqar, A.B.; Fan, J.; Liu, E. Expression of TRPV1 in rabbits and consuming hot pepper affects its body weight. Mol. Biol. Rep. 2012, 39, 7583–7589. [Google Scholar] [CrossRef]
- Ternesten-Hasséus, E.; Johansson, E.L.; Millqvist, E. Cough reduction using capsaicin. Respir. Med. 2015, 109, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Chen, P.N.; Hsieh, Y.S.; Lin, C.Y.; Lee, Y.H.; Chu, S.C. Capsaicin protects endothelial cells and macrophage against oxidized low-density lipoprotein-induced injury by direct antioxidant action. Chem. Biol. Interact. 2015, 228, 35–45. [Google Scholar] [CrossRef]
- He, H.; Zhou, Y.; Huang, J.; Wu, Z.; Liao, Z.; Liu, D.; Yin, D.; He, M. Capsaicin Protects Cardiomyocytes against Anoxia/Reoxygenation Injury via Preventing Mitochondrial Dysfunction Mediated by SIRT1. Oxid. Med. Cell. Longev. 2017, 2017, 1035702. [Google Scholar] [CrossRef]
- Ren, X.; Roessler, A.E.; Lynch, T.L., 4th; Haar, L.; Mallick, F.; Lui, Y.; Tranter, M.; Ren, M.H.; Xie, W.R.; Fan, G.-C.; et al. Cardioprotection via the skin: Nociceptor-induced conditioning against cardiac MI in the NIC of time. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H543–H553. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Meng, L.; Hu, X. Capsaicin is beneficial to hyperlipidemia, oxidative stress, endothelial dysfunction, and atherosclerosis in Guinea pigs fed on a high-fat diet. Chem. Biol. Interact. 2019, 297, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Yang, S.-M.; Han, I.-S. Capsaicin suppresses liver fat accumulation in high-fat diet-induced NAFLD mice. Anim. Cells Syst. 2020, 24, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Hashimoto, H.; Maruyama, S.; Shintani, M.; Ohno, H.; Nakai, Y.; Osera, T.; Kurihara, N. Dietary capsaicin-mediated attenuation of hypertension in a rat model of renovascular hypertension. Clin. Exp. Hypertens. 2020, 42, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Grüter, T.; Blusch, A.; Motte, J.; Sgodzai, M.; Bachir, H.; Klimas, R.; Ambrosius, B.; Gold, R.; Ellrichmann, G.; Pitarokoili, K. Immunomodulatory and anti-oxidative effect of the direct TRPV1 receptor agonist capsaicin on Schwann cells. J. Neuroinflammation 2020, 17, 145. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Sleem, A.A.; Sayed, M.; Youness, E.R.; Shaffie, N. Capsaicin Exerts Anti-convulsant and Neuroprotective Effects in Pentylenetetrazole-Induced Seizures. Neurochem. Res. 2020, 45, 1045–1061. [Google Scholar] [CrossRef]
- Nawaka, N.; Wanmasae, S.; Makarasen, A.; Dechtrirat, D.; Techasakul, S.; Jeenduang, N. Allicin and Capsaicin Ameliorated Hypercholesterolemia by Upregulating LDLR and Downregulating PCSK9 Expression in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 14299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, P.; Xia, F.; Tang, H.; Chen, J.; Zhang, J.; Liu, D.; Zhu, Y.; Liu, Y.; Gu, L.; et al. Capsaicin ameliorates inflammation in a TRPV1-independent mechanism by inhibiting PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem. Biol. 2022, 29, 1248–1259.e6. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The metabolism of carcinoma cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. Ueber den stoffwechsel von tumoren im körper. Klin. Wochenschr. 1926, 5, 829–832. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Palsson-McDermott, E.M.; O’neill, L.A. The Warburg effect then and now: From cancer to inflammatory diseases. Bioessays 2013, 35, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Kozal, K.; Jóźwiak, P.; Krześlak, A. Contemporary perspectives on the Warburg effect inhibition in cancer therapy. Cancer Control. 2021, 28, 10732748211041243. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, C. COX-2 inhibitors. Lancet 1999, 353, 307–314. [Google Scholar] [CrossRef]
- Stichtenoth, D.O.; Frölich, J.C. The second generation of COX-2 inhibitors: What advantages do the newest offer? Drugs 2003, 63, 33–45. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. IJPR 2011, 10, 655. [Google Scholar]
- Movahed, P.; Jönsson, B.A.; Birnir, B.; Wingstrand, J.A.; Jørgensen, T.D.; Ermund, A.; Sterner, O.; Zygmunt, P.M.; Högestätt, E.D. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J. Biol. Chem. 2005, 280, 38496–38504. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Gharat, L.A.; Szallasi, A. Advances in the design and therapeutic use of capsaicin receptor TRPV1 agonists and antagonists. Expert. Opin. Ther. Pat. 2008, 18, 159–209. [Google Scholar] [CrossRef]
- Khairatkar-Joshi, N.; Szallasi, A. TRPV1 antagonists: The challenges for therapeutic targeting. Trends Mol. Med. 2009, 15, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kym, P.R.; Kort, M.E.; Hutchins, C.W. Analgesic potential of TRPV1 antagonists. Biochem. Pharmacol. 2009, 78, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kort, M.E.; Kym, P.R. TRPV1 antagonists: Clinical setbacks and prospects for future development. Prog. Med. Chem. 2012, 51, 57–70. [Google Scholar]
- Brandt, M.R.; Beyer, C.E.; Stahl, S.M. TRPV1 antagonists and chronic pain: Beyond thermal perception. Pharmaceuticals 2012, 5, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta 2007, 1773, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Park, T.; Lee, C.K.; Kang, M.K.; Park, M.S.; Kang, H.I.; Surh, Y.J.; Kim, O.H. Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2. Food Chem. Toxicol. 2007, 45, 708–715. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Vinodhkumar, R.; Devaki, T. Stabilization of pulmonary mitochondrial enzyme system by capsaicin during benzo(a)pyrene induced experimental lung cancer. Biomed. Pharmacother. 2008, 62, 390–394. [Google Scholar] [CrossRef]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS ONE 2010, 5, e10243. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, H.; Zhang, W.; Matkowskyj, K.A.; Liao, J.; Srivastava, S.K.; Yang, G.Y. Inhibition of chronic pancreatitis and pancreatic intraepithelial neoplasia (PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice. Carcinogenesis 2011, 32, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Chen, S.T.; Chien, S.Y.; Kuo, S.J.; Tsai, H.T.; Chen, D.R. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum. Exp. Toxicol. 2011, 30, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Lan, S.H.; Huang, A.C.; Yang, J.S.; Chen, Y.Y.; Huang, H.Y.; Lin, Z.P.; Hsu, Y.M.; Yang, M.D.; Chiu, C.F. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria-dependent and-independent pathways. Environ. Toxicol. 2012, 27, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of β-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, Z.; Wang, Y.; Zhu, G.; Wang, X. Capsaicin induces cycle arrest by inhibiting cyclin-dependent-kinase in bladder carcinoma cells. Int. J. Urol. 2012, 19, 662–668. [Google Scholar] [CrossRef]
- Lin, C.H.; Lu, W.C.; Wang, C.W.; Chan, Y.C.; Chen, M.K. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.Y.; Lee, S.M.; Jun, C.H.; Cho, S.B.; Park, C.H.; Joo, Y.E.; Kim, H.S.; Choi, S.K.; Rew, J.S. Capsaicin induces apoptosis and modulates MAPK signaling in human gastric cancer cells. Mol. Med. Rep. 2014, 9, 499–502. [Google Scholar] [CrossRef]
- Wutka, A.; Palagani, V.; Barat, S.; Chen, X.; El Khatib, M.; Götze, J.; Belahmer, H.; Zender, S.; Bozko, P.; Malek, N.P.; et al. Capsaicin treatment attenuates cholangiocarcinoma carcinogenesis. PLoS ONE 2014, 9, e95605. [Google Scholar] [CrossRef]
- Skrzypski, M.; Sassek, M.; Abdelmessih, S.; Mergler, S.; Grötzinger, C.; Metzke, D.; Wojciechowicz, T.; Nowak, K.W.; Strowski, M.Z. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell Signal. 2014, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gilardini Montani, M.S.; D’Eliseo, D.; Cirone, M.; Di Renzo, L.; Faggioni, A.; Santoni, A.; Velotti, F. Capsaicin-mediated apoptosis of human bladder cancer cells activates dendritic cells via CD91. Nutrition 2015, 31, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, J.; Ma, Z.; Liu, W.; Yang, F.; Yang, Z.; Wang, K.; Wang, X.; He, D.; Li, L. Capsaicin causes inactivation and degradation of the androgen receptor by inducing the restoration of miR-449a in prostate cancer. Oncol. Rep. 2015, 34, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Lee, Y.H.; Cheng, H.L.; Chen, H.Y.; Jhuang, F.H.; Chueh, P.J. Capsaicin Inhibits Multiple Bladder Cancer Cell Phenotypes by Inhibiting Tumor-Associated NADH Oxidase (tNOX) and Sirtuin1 (SIRT1). Molecules 2016, 21, 849. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, J.; Liu, D.; Zhao, T.; Lu, Z.; Zhu, L.; Cao, L.; Yang, J.; Jin, J.; Cai, Y. Capsaicin reactivates hMOF in gastric cancer cells and induces cell growth inhibition. Cancer Biol. Ther. 2016, 17, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Torres, Á.; Bort, A.; Morell, C.; Rodríguez-Henche, N.; Díaz-Laviada, I. The pepper’s natural ingredient capsaicin induces autophagy blockage in prostate cancer cells. Oncotarget 2016, 7, 1569–1583. [Google Scholar] [CrossRef]
- Liu, T.; Wang, G.; Tao, H.; Yang, Z.; Wang, Y.; Meng, Z.; Cao, R.; Xiao, Y.; Wang, X.; Zhou, J. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer 2016, 16, 790. [Google Scholar] [CrossRef]
- Lv, L.; Zhuang, Y.X.; Zhang, H.W.; Tian, N.N.; Dang, W.Z.; Wu, S.Y. Capsaicin-loaded folic acid-conjugated lipid nanoparticles for enhanced therapeutic efficacy in ovarian cancers. Biomed. Pharmacother. 2017, 91, 999–1005. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, H.C.; Hsu, Y.C.; Cho, C.L.; Yang, M.Y.; Chien, C.Y. Capsaicin Induces Autophagy and Apoptosis in Human Nasopharyngeal Carcinoma Cells by Downregulating the PI3K/AKT/mTOR Pathway. Int. J. Mol. Sci. 2017, 18, 1343. [Google Scholar] [CrossRef]
- Mao, X.; Zhu, H.; Luo, D.; Ye, L.; Yin, H.; Zhang, J.; Zhang, Y.; Zhang, Y. Capsaicin inhibits glycolysis in esophageal squamous cell carcinoma by regulating hexokinase-2 expression. Mol. Med. Rep. 2018, 17, 6116–6121. [Google Scholar] [CrossRef]
- Kamaruddin, M.F.; Hossain, M.Z.; Mohamed Alabsi, A.; Mohd Bakri, M. The Antiproliferative and Apoptotic Effects of Capsaicin on an Oral Squamous Cancer Cell Line of Asian Origin, ORL-48. Medicina 2019, 55, 322. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Dai, X.; Wang, P.; Tao, Y.; Chai, D. Capsaicin induces cytotoxicity in human osteosarcoma MG63 cells through TRPV1-dependent and-independent pathways. Cell Cycle 2019, 18, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jia, H.; Zhang, Z.; Li, S. Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020, 22, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yu, X.; Zheng, Z.; Huang, J.; Yang, X.; Shi, H. Capsaicin suppressed activity of prostate cancer stem cells by inhibition of Wnt/β-catenin pathway. Phytother. Res. 2020, 34, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Cang, S.; Ma, P.; Song, Z. Capsaicin: A “hot” KDM1A/LSD1 inhibitor from peppers. Bioorg Chem. 2020, 103, 104161. [Google Scholar] [CrossRef] [PubMed]
- Szoka, L.; Palka, J. Capsaicin up-regulates pro-apoptotic activity of thiazolidinediones in glioblastoma cell line. Biomed. Pharmacother. 2020, 132, 110741. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiao, C.; Jiang, W.; Yang, W.; Qin, Q.; Tan, Q.; Lian, B.; Liang, Z.; Wei, C. Capsaicin Inhibits Proliferation and Induces Apoptosis in Breast Cancer by Down-Regulating FBI-1-Mediated NF-κB Pathway. Drug Des. Dev. Ther. 2021, 15, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Park, M.K.; Nakamura, H.; Ban, H.S. Capsaicin inhibits HIF-1α accumulation through suppression of mitochondrial respiration in lung cancer cells. Biomed. Pharmacother. 2022, 146, 112500. [Google Scholar] [CrossRef]
- Que, T.; Ren, B.; Fan, Y.; Liu, T.; Hou, T.; Dan, W.; Liu, B.; Wei, Y.; Lei, Y.; Zeng, J.; et al. Capsaicin inhibits the migration, invasion and EMT of renal cancer cells by inducing AMPK/mTOR-mediated autophagy. Chem. Biol. Interact. 2022, 366, 110043. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wei, D.-G.; Li, R.-S. Capsaicin induces ferroptosis of NSCLC by regulating SLC7A11/GPX4 signaling in vitro. Sci. Rep. 2022, 12, 11996. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Li, H.X.; Hou, X.J.; Huang, M.Y.; Zhu, Z.M.; Wei, L.X.; Tang, C.X. Capsaicin suppresses hepatocarcinogenesis by inhibiting the stemness of hepatic progenitor cells via SIRT1/SOX2 signaling pathway. Cancer Med. 2022, 11, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, S.; Cheng, X.; Zhang, L.; Wang, Y.; Wu, J.; Bao, J.; Yu, H.; Lu, R. Capsaicin inhibits the stemness of anaplastic thyroid carcinoma cells by triggering autophagy-lysosome mediated OCT4A degradation. Phytother. Res. 2022, 36, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, C.; Boda, D.; Constantin, C.; Caruntu, A.; Neagu, M. Catecholamines increase in vitro proliferation of murine B16F10 melanoma cells. Acta Endocrinol. 2014, 10, 545–558. [Google Scholar] [CrossRef]
- Scheau, C.; Draghici, C.; Ilie, M.A.; Lupu, M.; Solomon, I.; Tampa, M.; Georgescu, S.R.; Caruntu, A.; Constantin, C.; Neagu, M.; et al. Neuroendocrine Factors in Melanoma Pathogenesis. Cancers 2021, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Venier, N.A.; Colquhoun, A.J.; Sasaki, H.; Kiss, A.; Sugar, L.; Adomat, H.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate 2015, 75, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Han, K.Y.; Kim, E.C.; Kim, Y.M.; Lee, S.W.; Kim, O.H.; Kim, K.W.; Gho, Y.S.; Kwon, Y.G. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res. 2004, 64, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Vendrely, V.; Peuchant, E.; Buscail, E.; Moranvillier, I.; Rousseau, B.; Bedel, A.; Brillac, A.; de Verneuil, H.; Moreau-Gaudry, F.; Dabernat, S. Resveratrol and capsaicin used together as food complements reduce tumor growth and rescue full efficiency of low dose gemcitabine in a pancreatic cancer model. Cancer Lett. 2017, 390, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Perry, H.E.; Brown, K.C.; Gao, Y.; Lin, J.; Stevenson, C.D.; Hurley, J.D.; Nolan, N.A.; Akers, A.T.; Chen, Y.C.; et al. Capsaicin synergizes with camptothecin to induce increased apoptosis in human small cell lung cancers via the calpain pathway. Biochem. Pharmacol. 2017, 129, 54–66. [Google Scholar] [CrossRef]
- Dai, N.; Ye, R.; He, Q.; Guo, P.; Chen, H.; Zhang, Q. Capsaicin and sorafenib combination treatment exerts synergistic anti-hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. Oncol. Rep. 2018, 40, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Perry, H.E.; Brown, K.C.; Akers, A.T.; Nolan, N.A.; Stevenson, C.D.; Hurley, J.D.; Miles, S.L.; et al. Capsaicinoids enhance chemosensitivity to chemotherapeutic drugs. Adv. Cancer Res. 2019, 144, 263–298. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Scheau, C.; Constantin, C.; Neagu, M. Recent Advances in Signaling Pathways Comprehension as Carcinogenesis Triggers in Basal Cell Carcinoma. J. Clin. Med. 2020, 9, 3010. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Barthó, L. Capsaicin-sensitive afferents and their role in gastroprotection: An update. J. Physiol. Paris. 2001, 95, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Helyes, Z.; Oroszi, G.; Németh, J.; Pintér, E. Release of somatostatin and its role in the mediation of the anti-inflammatory effect induced by antidromic stimulation of sensory fibres of rat sciatic nerve. Br. J. Pharmacol. 1998, 123, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Helyes, Z.; Thán, M.; Oroszi, G.; Pintér, E.; Németh, J.; Kéri, G.; Szolcsányi, J. Anti-nociceptive effect induced by somatostatin released from sensory nerve terminals and by synthetic somatostatin analogues in the rat. Neurosci. Lett. 2000, 278, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Pintér, E.; Helyes, Z.; Szolcsányi, J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol. Ther. 2006, 112, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.M.; Parekh, D.; Townsend, C.M., Jr.; Thompson, J.C. Somatostatin and analogues in the treatment of cancer. A review. Ann. Surg. 1991, 213, 190–198. [Google Scholar] [CrossRef]
- Weckbecker, G.; Raulf, F.; Stolz, B.; Bruns, C. Somatostatin analogs for diagnosis and treatment of cancer. Pharmacol. Ther. 1993, 60, 245–264. [Google Scholar] [CrossRef]
- Scarpignato, C.; Pelosini, I. Somatostatin Analogs for Cancer Treatment and Diagnosis: An Overview. Chemotherapy 2001, 47 (Suppl. 2), 1–29. [Google Scholar] [CrossRef]
- Lamberts, S.W.J.; de Herder, W.W.; Hofland, L.J. Somatostatin analogs in the diagnosis and treatment of cancer. Trends Endocrinol. Metab. 2002, 13, 451–457. [Google Scholar] [CrossRef]
- Froidevaux, S.; Eberle, A.N. Somatostatin analogs and radiopeptides in cancer therapy. Pept. Sci. 2002, 66, 161–183. [Google Scholar] [CrossRef]
- Klironomos, S.; Notas, G.; Sfakianaki, O.; Kiagiadaki, F.; Xidakis, C.; Kouroumalis, E. Octreotide modulates the effects on fibrosis of TNF-α, TGF-β and PDGF in activated rat hepatic stellate cells. Regul. Pept. 2014, 188, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ayiomamitis, G.D.; Notas, G.; Zaravinos, A.; Drygiannakis, I.; Georgiadou, M.; Sfakianaki, O.; Mastrodimou, N.; Thermos, K.; Kouroumalis, E. Effects of octreotide and insulin on colon cancer cellular proliferation and correlation with hTERT activity. Oncoscience 2014, 1, 457. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Samonakis, D.; Notas, G. Somatostatin in hepatocellular carcinoma: Experimental and therapeutic implications. Hepatoma Res. 2018, 4, 34. [Google Scholar] [CrossRef]
- Periferakis, A.; Tsigas, G.; Periferakis, A.-T.; Badarau, I.A.; Scheau, A.-E.; Tampa, M.; Georgescu, S.R.; Didilescu, A.C.; Scheau, C.; Caruntu, C. Antitumoral and Anti-inflammatory Roles of Somatostatin and Its Analogs in Hepatocellular Carcinoma. Anal. Cell. Pathol. 2021, 2021, 1840069. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. Is There a Place for Somatostatin Analogues for the Systemic Treatment of Hepatocellular Carcinoma in the Immunotherapy Era? Livers 2022, 2, 315–335. [Google Scholar] [CrossRef]
- Qin, J.C.; Yu, W.T.; Li, H.X.; Liang, Y.Q.; Nong, F.F.; Wen, B. Cold exposure and capsaicin promote 1,2-dimethylhyrazine-induced colon carcinogenesis in rats correlates with extracellular matrix remodeling. World J. Gastroenterol. 2021, 27, 6615–6630. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.J.; Kim, G.E.; Cho, M.H.; Yoon, S.Y.; Davies, A.J.; Oh, S.B.; Lee, H.; Cho, Y.K.; Joo, C.H.; et al. Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis 2014, 35, 1652–1660. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, P.; Tao, Y.; Shen, C.; Wang, S.; Zhao, L.; Wu, H.; Fan, F.; Lin, C.; Chen, C.; et al. Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem. Toxicol. 2015, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wu, J.; Zong, G.; Wang, F.; Deng, R.; Tao, R.; Qian, C.; Shan, Y.; Wang, A.; Zhao, Y.; et al. Capsaicin shapes gut microbiota and pre-metastatic niche to facilitate cancer metastasis to liver. Pharmacol. Res. 2023, 188, 106643. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J. Peppers: The Domesticated Capsicums; University of Texas Press: Austin, TX, USA, 1995. [Google Scholar]
- Nabhan, G.P. Why Some Like It Hot: Food, Genes, and Cultural Diversity; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Kim, S.; Park, M.; Yeom, S.I.; Kim, Y.M.; Lee, J.M.; Lee, H.A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.K.; De, A.K. Capsicum: Historical and botanical perspectives. In Capsicum; CRC Press: Boca Raton, FL, USA, 2003; pp. 21–35. [Google Scholar]
- Eshbaugh, W.H. Genetic and biochemical systematic studies of chili peppers (Capsicum-Solanaceae). Bull. Torrey Bot. Club 1975, 102, 396–403. [Google Scholar] [CrossRef]
- Eshbaugh, W.H. The taxonomy of the genus Capsicum. In Peppers: Botany, Production and Uses; CAB International: Wallingford UK, 2012; pp. 14–28. [Google Scholar]
- Heiser, C.B., Jr.; Pickersgill, B. Names for the Cultivated Capsicum Species (Solanaceae). Taxon 1969, 18, 277–283. [Google Scholar] [CrossRef]
- Votava, E.J.; Baral, J.B.; Bosland, P.W. Genetic diversity of chile (Capsicum annuum var. annuum L.) landraces from northern New Mexico, Colorado, and Mexico. Econ. Bot. 2005, 59, 8–17. [Google Scholar] [CrossRef]
- Dewitt, D.; Bosland, P.W. The Complete Chile Pepper Book—A Gardener’s Guide to Choosing, Growing, Preserving and Cooking; Timber Press: London, UK, 2009. [Google Scholar]
- Carmack, R.M.; Gasco, J.L.; Gossen, G.H. The Legacy of Mesoamerica: History and Culture of a Native American Civilization; Routledge: Oxfordshire, UK, 2016. [Google Scholar]
- Pilcher, J.M. Tamales or timbales: Cuisine and the formation of Mexican national identity, 1821–1911. Americas 1996, 53, 193–216. [Google Scholar] [CrossRef]
- Haverluk, T.W. Chile peppers and identity construction in Pueblo, Colorado. J. Study Food Soc. 2002, 6, 45–59. [Google Scholar] [CrossRef]
- Meotti, F.C.; Lemos de Andrade, E.; Calixto, J.B. TRP modulation by natural compounds. Handb. Exp. Pharmacol. 2014, 223, 1177–1238. [Google Scholar] [CrossRef]
- Padilha, H.; Barbieri, R. Plant breeding of chili peppers (Capsicum, Solanaceae)-A review. Aust. J. Basic. Appl. Sci. 2016, 10, 148–154. [Google Scholar]
- Romero Cóndor, C.; Acurio, L.; Paucar-Ayala, S.; Herrera-Robalino, J.; Cabrera, H.; Albán-Villacreces, A.; Sangucho-Montenegro, C.; Veliz-Zambrano, M. Brief Historical review of the geological cartography in Ecuador. Cienc. Lat. Rev. Científica Multidiscip. 2023, 7, 2584–2621. [Google Scholar] [CrossRef]
- Grieder, T.; Bueno Mendoza, A.; Smith, C.E.; Malina, R.M. La Galgada in the World of Its Time. In La Galgada, Peru: A Preceramic Culture in Transition; University of Texas Press: Austin, TX, USA, 1988; pp. 192–203. [Google Scholar]
- Pearsall, D.M. Plant domestication and the shift to agriculture in the Andes. In The Handbook of South American Archaeology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 105–120. [Google Scholar]
- Lanning, E.P. Peru before the Incas; Prentice-Hall: Saddle River, NJ, USA, 1967. [Google Scholar]
- Sarmiento de Gamboa, P. The History of the Incas; University of Texas Press: Austin, TX, USA, 2007. [Google Scholar]
- Thompson, J.E.S. Maya History and Religion; University of Oklahoma Press: Norman, OK, USA, 1990; Volume 99. [Google Scholar]
- Smith, M.E. The Aztecs; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Periferakis, A. Geology and the Aztecs: How the Ore Deposits of Mesoamerica Influenced the Socioeconomic Development of an Empire, from its Emergence to its Downfall. In Proceedings of the 15th International Congress of the Geological Society of Greece, Athens, Greece, 22–24 May 2019; pp. 700–701. [Google Scholar]
- Periferakis, A. The Influence of Ore Deposits to the Development and Collapse of the Inca Civilisation between the 15th and the 16th Century. In Proceedings of the 15th International Congress of the Geological Society of Greece, Athens, Greece, 22–24 May 2019; pp. 702–703. [Google Scholar]
- De Feo, V. The ritual use of Brugmansia species in traditional Andean medicine in Northern Peru. Econ. Bot. 2004, 58, S221–S229. [Google Scholar] [CrossRef]
- Dafni, A.; Petanidou, T.; Vallianatou, I.; Kozhuharova, E.; Blanché, C.; Pacini, E.; Peyman, M.; Dajić Stevanovic, Z.; Franchi, G.G.; Benítez, G. Myrtle, Basil, Rosemary, and Three-Lobed Sage as Ritual Plants in the Monotheistic Religions: An Historical–Ethnobotanical Comparison. Econ. Bot. 2020, 74, 330–355. [Google Scholar] [CrossRef]
- Kohek, M.; Sánchez Avilés, C.; Romaní, O.; Bouso, J.C. Ancient psychoactive plants in a global village: The ritual use of cannabis in a self-managed community in Catalonia. Int. J. Drug Policy 2021, 98, 103390. [Google Scholar] [CrossRef] [PubMed]
- Yaldiz, G.; Ozguven, M.; Sekeroglu, N. Variation in capsaicin contents of different Capsicum species and lines by varying drying parameters. Ind. Crops Prod. 2010, 32, 434–438. [Google Scholar] [CrossRef]
- Cho, H.; Kwon, Y. Development of a database of capsaicinoid contents in foods commonly consumed in Korea. Food Sci. Nutr. 2020, 8, 4611–4624. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Praveen, P.K.; Para, P.A.; Sharma, V. Medicinal Properties of chilli pepper in human diet: An editorial. ARC J. Public. Health Community Med. 2017, 2, 6–7. [Google Scholar]
- Aguilar-Meléndez, A.; Vásquez-Dávila, M.A.; Manzanero-Medina, G.I.; Katz, E. Chile (Capsicum spp.) as Food-Medicine Continuum in Multiethnic Mexico. Foods 2021, 10, 2502. [Google Scholar] [CrossRef]
- Martínez-Aceviz, Y.; Sobrevilla-Navarro, A.A.; Ramos-Lopez, O. Dietary Intake of Capsaicin and Its Association with Markers of Body Adiposity and Fatty Liver in a Mexican Adult Population of Tijuana. Healthcare 2023, 11, 3001. [Google Scholar] [CrossRef] [PubMed]
- Meghvansi, M.K.; Siddiqui, S.; Khan, M.H.; Gupta, V.K.; Vairale, M.G.; Gogoi, H.K.; Singh, L. Naga chilli: A potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. J. Ethnopharmacol. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Saleh, B.K.; Omer, A.; Teweldemedhin, B. Medicinal uses and health benefits of chili pepper (Capsicum spp.): A review. MOJ Food Process Technol. 2018, 6, 325–328. [Google Scholar] [CrossRef]
- Montiel-Oseguera, A. La Persistencia de la Costumbre Pima. Interpretaciones Desde La Antropología Cognitiva; UAM, INAH y ENAH del Norte de México: Mexico City, Mexico, 2013. [Google Scholar]
- Long-Solís, J. Capsicum y Cultura: La Historia Del Chilli; Fondo De Cultura Economica: Mexico City, Mexico, 2013. [Google Scholar]
- Rakhshandehroo, E.; Asadpour, M.; Jafari, A.; Malekpour, S.H. The effectiveness of Cinnamomum zeylanicum, Punica granatum flower and Capsicum annuum extracts against Parascaris equorum infective larvae. İstanbul Üniversitesi Vet. Fakültesi Derg. 2016, 42, 132–137. [Google Scholar] [CrossRef]
- Frei, B.; Baltisberger, M.; Sticher, O.; Heinrich, M. Medical ethnobotany of the Zapotecs of the Isthmus-Sierra (Oaxaca, Mexico): Documentation and assessment of indigenous uses. J. Ethnopharmacol. 1998, 62, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen-Rascón, F.; Paredes, A. Tarahumara Medicine: Ethnobotany and Healing among the Rarámuri of Mexico; University of Oklahoma Press: Norman, OK, USA, 2015. [Google Scholar]
- Alcorn, J.B. Huastec Mayan Ethnobotany; University of Texas Press: Austin, TX, USA, 1984. [Google Scholar]
- Martínez, T.H.L. Etnobotánica del Chile Quipín (Capsicum annuum var. glabriusculum) en la Sierra Gorda y Semidesierto de Querétaro. Master’s Thesis, Colegio de postgraduados, campus Montecillo Botánica, Montecillo, Mexico, 2007. [Google Scholar]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.R.; Silva, B. Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters Part I; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016. [Google Scholar]
- Roys, R.L. The Ethno-Botany of the Maya; Tulane University: New Orleans, LA, USA, 1931. [Google Scholar]
- López Austin, A. Textos acerca de las partes del cuerpo humano y de las enfermedades y medicinas en los primeros memoriales de Sahagún. Estud. Cult. Nahuatl 1972, 10, 129–153. [Google Scholar] [PubMed]
- Madero, R.A. Recetario Totonaco de la Costa de Veracruz; Dirección General de Culturas Populares de Conaculta: Mexico City, Mexico, 2000. [Google Scholar]
- Bañuelos, N.; Salido, P.L.; Gardea, A. Etnobotánica del chiltepín: Pequeño gran señor en la cultura de los sonorenses. Estud. Soc. 2008, 16, 177–205. [Google Scholar]
- Magaña Alejandro, M.A.; Gama Campillo, L.M.; Mariaca Méndez, R. El uso de las plantas medicinales en las comunidades Maya-Chontales de Nacajuca, Tabasco, México. Polibotánica 2010, 29, 213–262. [Google Scholar]
- Cook, S. The Forest of the Lacandon Maya: An Ethnobotanical Guide; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Aguilar-Meléndez, A.; Vásquez, M.A.; Katz, E.; Colorado, M.R.H. Los Chiles Que le Dan Sabor al Mundo: Contribuciones MULTIDISCIPLINARIAS; IRD Éditions: Paris, France, 2018. [Google Scholar]
- Breedlove, D.E.; Laughlin, R.M. Flowering of Man: A Tzotzil Botany of Zinacantán; Smithsonian Institution Press: Washington, DC, USA, 1993; Volume 2. [Google Scholar]
- Chemin Bassler, H. Recetario Pame de San Luis Potosí y Querétaro; Dirección General de Culturas Populares, Consejo Nacional para la Cultura y las Artes: Coyoacán, México, 2000. [Google Scholar]
- Laughlin, R.M. The Huastec. In Handbook of Middle American Indians; University of Texas Press: Austin, TX, USA, 1969; Volume 7, pp. 298–311. [Google Scholar]
- Fernández, D.L. Medicina indígena y males infantiles entre los nahuas de Texcoco: Pérdida de la guía, caída de mollera, tiricia y mal de ojo. Proc. An. De Antropol. 2015, 49, 101–148. [Google Scholar] [CrossRef]
- Geck, M.S.; Cabras, S.; Casu, L.; Reyes García, A.J.; Leonti, M. The taste of heat: How humoral qualities act as a cultural filter for chemosensory properties guiding herbal medicine. J. Ethnopharmacol. 2017, 198, 499–515. [Google Scholar] [CrossRef]
- Alayza Escardó, F. Historia de la Cirugía en el Perú. In Historia de la Cirugia en el Peru; Editorial Monterrico, S.A.: Lima, Peru, 1992. [Google Scholar]
- Froeschner, E.H. Two examples of ancient skull surgery. J. Neurosurg. 1992, 76, 550–552. [Google Scholar] [CrossRef]
- Periferakis, A. A review of obsidian source exploitation in pre-columbian south America. Bull. Geol. Soc. Greece 2019, 55, 65–108. [Google Scholar] [CrossRef]
- Schmiedel, U.; Araya, Y.; Bortolotto, M.I.; Boeckenhoff, L.; Hallwachs, W.; Janzen, D.; Kolipaka, S.S.; Novotny, V.; Palm, M.; Parfondry, M.; et al. Contributions of paraecologists and parataxonomists to research, conservation, and social development. Conserv. Biol. 2016, 30, 506–519. [Google Scholar] [CrossRef]
- Nabhan, G.P. Ethnobiology for the Future: Linking Cultural and Ecological Diversity; University of Arizona Press: Tucson, AZ, USA, 2016. [Google Scholar]
- Fan, Y.; Kim, D.H.; Gwak, Y.S.; Ahn, D.; Ryu, Y.; Chang, S.; Lee, B.H.; Bills, K.B.; Steffensen, S.C.; Yang, C.H.; et al. The role of substance P in acupuncture signal transduction and effects. Brain Behav. Immun. 2021, 91, 683–694. [Google Scholar] [CrossRef]
- Du, G.-h.; Yuan, T.-y.; Du, L.-d.; Zhang, Y.-x. Chapter Twelve—The Potential of Traditional Chinese Medicine in the Treatment and Modulation of Pain. In Advances in Pharmacology; Barrett, J.E., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 75, pp. 325–361. [Google Scholar]
- Li, S.-H.; Li, L.; Yang, R.-N.; Liang, S.-D. Compounds of traditional Chinese medicine and neuropathic pain. Chin. J. Nat. Med. 2020, 18, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ghiţă, M.A.; Căruntu, C.; Rosca, A.E.; Căruntu, A.; Moraru, L.; Constantin, C.; Neagu, M.; Boda, D. Real-Time Investigation of Skin Blood Flow Changes Induced by Topical Capsaicin. Acta Dermatovenerol. Croat. 2017, 25, 223–227. [Google Scholar]
- Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int. J. Toxicol. 2007, 26 (Suppl. 1), 3–106. [CrossRef] [PubMed]
- Surh, Y.-J.; Sup Lee, S. Capsaicin, a double-edged sword: Toxicity, metabolism, and chemopreventive potential. Life Sci. 1995, 56, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.B. Hunan hand. N. Engl. J. Med. 1981, 305, 1020. [Google Scholar]
- Williams, S.R.; Clark, R.F.; Dunford, J.V. Contact Dermatitis Associated with Capsaicin: Hunan Hand Syndrome. Ann. Emerg. Med. 1995, 25, 713–715. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E.; Such, G.; Joó, F.; Nagy, A. Neurotoxic effect of capsaicin in mammals. Acta Physiol. Hung. 1987, 69, 295–313. [Google Scholar]
- Ritter, S.; Dinh, T.T. Capsaicin-induced neuronal degeneration in the brain and retina of preweanling rats. J. Comp. Neurol. 1990, 296, 447–461. [Google Scholar] [CrossRef]
- Lechner, A.; Alderson, T.; Gautam, S.; Flaker, G. Ventricular fibrillation due to coronary spasm after pepper spray. Pacing Clin. Electrophysiol. 2021, 44, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gu, Q.; Qu, C. Capsaicin pretreatment reversed pulmonary arterial hypertension by alleviating inflammation via p38MAPK pathway. Exp. Lung Res. 2017, 43, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Baz, R.A.; Scheau, C.; Niscoveanu, C.; Bordei, P. Morphometry of the Entire Internal Carotid Artery on CT Angiography. Medicina 2021, 57, 832. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef]
- MacAlpin, R.N. Contribution of dynamic vascular wall thickening to luminal narrowing during coronary arterial constriction. Circulation 1980, 61, 296–301. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Song, Y.S.; Sorenson, C.M.; Sheibani, N. Thrombospondin-1 in vascular development, vascular function, and vascular disease. Semin. Cell Dev. Biol. 2024, 155, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Bergren, D.R. Capsaicin challenge, reflex bronchoconstriction, and local action of substance P. Am. J. Physiol. 1988, 254, R845–R852. [Google Scholar] [CrossRef]
- Thomas, K.C.; Ethirajan, M.; Shahrokh, K.; Sun, H.; Lee, J.; Cheatham, T.E., 3rd; Yost, G.S.; Reilly, C.A. Structure-activity relationship of capsaicin analogs and transient receptor potential vanilloid 1-mediated human lung epithelial cell toxicity. J. Pharmacol. Exp. Ther. 2011, 337, 400–410. [Google Scholar] [CrossRef]
- Trevisan, G.; Rossato, M.F.; Hoffmeister, C.; Oliveira, S.M.; Silva, C.R.; Matheus, F.C.; Mello, G.C.; Antunes, E.; Prediger, R.D.; Ferreira, J. Mechanisms involved in abdominal nociception induced by either TRPV1 or TRPA1 stimulation of rat peritoneum. Eur. J. Pharmacol. 2013, 714, 332–344. [Google Scholar] [CrossRef]
- Lee, K.J.; Vos, R.; Tack, J. Effects of capsaicin on the sensorimotor function of the proximal stomach in humans. Aliment. Pharmacol. Ther. 2004, 19, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Estimation of Dietary Capsaicinoid Exposure in Korea and Assessment of Its Health Effects. Nutrients 2021, 13, 2461. [Google Scholar] [CrossRef] [PubMed]
- Lassen, C.L.; Meyer, K.; Bredthauer, A.; Klier, T.W. Facial and Oral Cross-Contamination of a 3-Year-Old Child with High Concentration Capsaicin: A Case Report. A A Pract. 2020, 14, e01258. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.B.; Holloway, C. Characteristics of pepper spray-related injuries reported to the National Electronic Injury Surveillance System during 2000–2020. Clin. Toxicol. 2022, 60, 348–355. [Google Scholar] [CrossRef]
- Dragosloveanu, C.D.M.; Celea, C.G.; Dragosloveanu, S. Comparison of 360 degrees circumferential trabeculotomy and conventional trabeculotomy in primary pediatric glaucoma surgery: Complications, reinterventions and preoperative predictive risk factors. Int. Ophthalmol. 2020, 40, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Yenigun, O.M.; Thanassi, M. Capsaicin: An Uncommon Exposure and Unusual Treatment. Clin. Pract. Cases Emerg. Med. 2019, 3, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Kim-Katz, S.Y.; Anderson, I.B.; Kearney, T.E.; MacDougall, C.; Hudmon, K.S.; Blanc, P.D. Topical antacid therapy for capsaicin-induced dermal pain: A poison center telephone-directed study. Am. J. Emerg. Med. 2010, 28, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.F.; Tang, W.Y. Clinicopathological effects of pepper (oleoresin capsicum) spray. Hong Kong Med. J. 2015, 21, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Çirakli, S.; Doganay, S.; Kaya Ozcora, G.D.; Canpolat, M.; Kumandas, S. Biber gazı maruziyeti sonucu gelişen Guillain-Barre sendromunu taklit eden polinöropati. Türk. Pediatri. Arşivi. 2019, 54, 53–56. [Google Scholar] [CrossRef]
- Snyman, T.; Stewart, M.J.; Steenkamp, V. A fatal case of pepper poisoning. Forensic Sci. Int. 2001, 124, 43–46. [Google Scholar] [CrossRef]
- Akçay, A.B.; Ozcan, T.; Seyis, S.; Acele, A. Coronary vasospasm and acute myocardial infarction induced by a topical capsaicin patch. Turk. Kardiyol. Dern. Ars. 2009, 37, 497–500. [Google Scholar] [PubMed]
- Xie, M.; Wu, H.; Bian, J.; Huang, S.; Xia, Y.; Qin, Y.; Yan, Z. Synthesis and biological evaluation of capsaicin analogues as antioxidant and neuroprotective agents. RSC Adv. 2023, 13, 32150–32159. [Google Scholar] [CrossRef] [PubMed]
- Dou, F.; Wu, B.; Chen, J.; Liu, T.; Yu, Z.; Chen, C. Capsaicin inhibits A7r5 cell senescence via the mitochondrial carrier protein Slc25a12. Exp. Cell Res. 2023, 433, 113856. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hao, K.; Xiong, Y.; Xu, R.; Huang, H.; Wang, H. Capsaicin alleviates neuronal apoptosis and schizophrenia-like behavioral abnormalities induced by early life stress. Schizophrenia 2023, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Li, S.; Zha, Z.; Chen, Y.; Wang, Q.; Zhou, S.; Huang, X.; Xu, M. Capsaicin alleviates doxorubicin-induced acute myocardial injury by regulating iron homeostasis and PI3K-Akt signaling pathway. Aging 2023, 15, 11845–11859. [Google Scholar] [CrossRef] [PubMed]
- Dragoș, D.; Petran, M.; Gradinaru, T.C.; Gilca, M. Phytochemicals and Inflammation: Is Bitter Better? Plants 2022, 11, 2991. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, O.; Drago, F. Pharmacological significance of extra-oral taste receptors. Eur. J. Pharmacol. 2021, 910, 174480. [Google Scholar] [CrossRef]
- Töle, J.C.; Behrens, M.; Meyerhof, W. Taste receptor function. Handb. Clin. Neurol. 2019, 164, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Lang, T. Extra-Oral Taste Receptors-Function, Disease, and Perspectives. Front. Nutr. 2022, 9, 881177. [Google Scholar] [CrossRef]
- Nevo, S.; Kadouri, N.; Abramson, J. Tuft cells: From the mucosa to the thymus. Immunol. Lett. 2019, 210, 1–9. [Google Scholar] [CrossRef]
- O’Leary, C.E.; Schneider, C.; Locksley, R.M. Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu. Rev. Immunol. 2019, 37, 47–72. [Google Scholar] [CrossRef]
- Howitt, M.R.; Cao, Y.G.; Gologorsky, M.B.; Li, J.A.; Haber, A.L.; Biton, M.; Lang, J.; Michaud, M.; Regev, A.; Garrett, W.S. The Taste Receptor TAS1R3 Regulates Small Intestinal Tuft Cell Homeostasis. Immunohorizons 2020, 4, 23–32. [Google Scholar] [CrossRef]
- Ki, S.Y.; Jeong, Y.T. Taste Receptors beyond Taste Buds. Int. J. Mol. Sci. 2022, 23, 9677. [Google Scholar] [CrossRef]
- Hendel, S.K.; Kellermann, L.; Hausmann, A.; Bindslev, N.; Jensen, K.B.; Nielsen, O.H. Tuft Cells and Their Role in Intestinal Diseases. Front. Immunol. 2022, 13, 822867. [Google Scholar] [CrossRef]
- Sell, E.A.; Ortiz-Carpena, J.F.; Herbert, D.R.; Cohen, N.A. Tuft cells in the pathogenesis of chronic rhinosinusitis with nasal polyps and asthma. Ann. Allergy Asthma Immunol. 2021, 126, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhu, X.; Geng, J.; Xu, Y.; Wu, R.; Li, C.; Fan, D.; Qin, X.; Du, Y.; Tian, Y.; et al. Intestinal Tuft-2 cells exert antimicrobial immunity via sensing bacterial metabolite N-undecanoylglycine. Immunity 2022, 55, 686–700.e7. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.E.; Sbierski-Kind, J.; Kotas, M.E.; Wagner, J.C.; Liang, H.E.; Schroeder, A.W.; de Tenorio, J.C.; von Moltke, J.; Ricardo-Gonzalez, R.R.; Eckalbar, W.L.; et al. Bile acid-sensitive tuft cells regulate biliary neutrophil influx. Sci. Immunol. 2022, 7, eabj1080. [Google Scholar] [CrossRef]
- Merritt, J.C.; Richbart, S.D.; Moles, E.G.; Cox, A.J.; Brown, K.C.; Miles, S.L.; Finch, P.T.; Hess, J.A.; Tirona, M.T.; Valentovic, M.A.; et al. Anti-cancer activity of sustained release capsaicin formulations. Pharmacol. Ther. 2022, 238, 108177. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef]
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. [Google Scholar] [CrossRef]
- Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A.; et al. Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Xie, C.; Jiang, Y.; Ai, X.; Xing, B.; Pu, K. Semiconducting Photothermal Nanoagonist for Remote-Controlled Specific Cancer Therapy. Nano Lett. 2018, 18, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, D.A.; Swami, P.A.; Nadaf, S.J.; Choudhari, P.B.; Kumbar, V.M.; More, H.N.; Killedar, S.G.; Kawtikwar, P.S. Capsaicin Loaded Solid SNEDDS for Enhanced Bioavailability and Anticancer Activity: In-Vitro, In-Silico, and In-Vivo Characterization. J. Pharm. Sci. 2021, 110, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, J.; Mu, Y.; Foda, M.F.; Han, H. Activation of TRPV1 by capsaicin-loaded CaCO3 nanoparticle for tumor-specific therapy. Biomaterials 2022, 284, 121520. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.-A.; Georgatos-Garcia, S.; Scheau, A.-E.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; et al. Use of Biomaterials in 3D Printing as a Solution to Microbial Infections in Arthroplasty and Osseous Reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2024, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Tong, L.; Ao, Y.; Zhang, G.; Liu, Y.; Zhang, H. Novel design of NIR-triggered plasmonic nanodots capped mesoporous silica nanoparticles loaded with natural capsaicin to inhibition of metastasis of human papillary thyroid carcinoma B-CPAP cells in thyroid cancer chemo-photothermal therapy. J. Photochem. Photobiol. B 2019, 197, 111534. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, S.Y.; Kim, G.G.; Hur, M.G.; Yang, S.D.; Park, J.H.; Kim, S.W. Development of (68)Ga-SCN-DOTA-Capsaicin as an Imaging Agent Targeting Apoptosis and Cell Cycle Arrest in Breast Cancer. Cancer Biother. Radiopharm. 2017, 32, 169–175. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem. Biophys. Res. Commun. 2019, 516, 365–372. [Google Scholar] [CrossRef]
- McNamara, F.N.; Randall, A.; Gunthorpe, M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br. J. Pharmacol. 2005, 144, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. Eur. J. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef] [PubMed]
- Kolonko, A.K.; Efing, J.; González-Espinosa, Y.; Bangel-Ruland, N.; van Driessche, W.; Goycoolea, F.M.; Weber, W.M. Capsaicin-Loaded Chitosan Nanocapsules for wtCFTR-mRNA Delivery to a Cystic Fibrosis Cell Line. Biomedicines 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Manzo, E.; Schiano Moriello, A.; Tinto, F.; Verde, R.; Allarà, M.; De Petrocellis, L.; Pagano, E.; Izzo, A.A.; Di Marzo, V.; Petrosino, S. A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator. Cells 2021, 10, 450. [Google Scholar] [CrossRef]
- Scheau, C.; Mihai, L.; Badarau, I.A.; Caruntu, C. Emerging applications of some important natural compounds in the field of oncology. Farmacia 2020, 68, 984–991. [Google Scholar] [CrossRef]
- Rajput, H.; Nangare, S.; Khan, Z.; Patil, A.; Bari, S.; Patil, P. Design of lactoferrin functionalized carboxymethyl dextran coated egg albumin nanoconjugate for targeted delivery of capsaicin: Spectroscopic and cytotoxicity studies. Int. J. Biol. Macromol. 2023, 256, 128392. [Google Scholar] [CrossRef] [PubMed]
- Leibing, E.; Leonhardt, U.; Köster, G.; Goerlitz, A.; Rosenfeldt, J.-A.; Hilgers, R.; Ramadori, G. Acupuncture treatment of chronic low-back pain—A randomized, blinded, placebo-controlled trial with 9-month follow-up. Pain 2002, 96, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, C.I.; Argyra, E.; Vadalouka, A.; Vasilakos, D.G. Acupuncture as an adjunctive therapy to pharmacological treatment in patients with chronic pain due to osteoarthritis of the knee: A 3-armed, randomized, placebo-controlled trial. Pain 2012, 153, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Schmucker, C.; Naumann, J.; Schlueter, N.; Huber, R.; Lederer, A.K. Acupuncture in management of acute dental pain—A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2023, 59, 114–128. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Q.; Huo, M.; Song, H.; Chang, H.; Cao, J.; Fang, Y.; Zhang, D. The mechanistic basis for the effects of electroacupuncture on neuropathic pain within the central nervous system. Biomed. Pharmacother. 2023, 161, 114516. [Google Scholar] [CrossRef]
- Lu, W.W.; Zhang, J.M.; Lv, Z.T.; Chen, A.M. Update on the Clinical Effect of Acupuncture Therapy in Patients with Gouty Arthritis: Systematic Review and Meta-Analysis. Evid. Based Complement. Altern. Med. 2016, 2016, 9451670. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Qian, H.; Li, B.; Su, X. Acupuncture alleviates acute peritonitis: A case report. Heliyon 2023, 9, e15290. [Google Scholar] [CrossRef]
- Periferakis, K.; Periferakis, A. Treating gout caused by renal insufficiency with acupuncture and moxibustion: A case report. Rom. J. Clin. Res. 2023, 6, 21–28. [Google Scholar]
- Chen, B.; Liu, D.; Li, T.; Zheng, L.; Lan, L.; Yang, N.; Huang, Y. Research Hotspots and Trends on Acupuncture for Anti-Inflammation: A Bibliometric Analysis from 2011 to 2021. J. Pain Res. 2023, 16, 1197–1217. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and Anti-Virulence Activity of Capsaicin Against Erythromycin-Resistant, Cell-Invasive Group A Streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef]
- Das, J.; Deka, M.; Gogoi, K. Antimicrobial activity of chilli extracts (Capsicum chinense) against food borne pathogens Escherichia coli and Staphylococcus aureus. Int. J. Res. Anal. Rev. (IJRAR) 2018, 5, 717–720. [Google Scholar]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Buitimea-Cantúa, N.E.; Rocha-Pizaña, M.d.R.; Hernández-Morales, A.; Magaña-Barajas, E.; Molina-Torres, J. Inhibitory effect of Capsicum chinense and Piper nigrum fruits, capsaicin and piperine on aflatoxins production in Aspergillus parasiticus by downregulating the expression of afl D, afl M, afl R, and afl S genes of aflatoxins biosynthetic pathway. J. Environ. Sci. Health Part B 2020, 55, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans. Int. J. Mol. Sci. 2023, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Mann, T.S.; McFawn, P.K.; Han, L.; Dong, X.; Henry, P.J. Investigating the role of MRGPRC11 and capsaicin-sensitive afferent nerves in the anti-influenza effects exerted by SLIGRL-amide in murine airways. Respir. Res. 2016, 17, 1–14. [Google Scholar] [CrossRef]
- Menezes, R.P.; Bessa, M.A.S.; Siqueira, C.P.; Teixeira, S.C.; Ferro, E.A.V.; Martins, M.M.; Cunha, L.C.S.; Martins, C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 63, 9580–9604. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Scheau, A.-E.; Savulescu-Fiedler, I.; Caruntu, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. Int. J. Mol. Sci. 2023, 24, 16299. [Google Scholar] [CrossRef] [PubMed]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-Staphylococcus aureus activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Shu, Y.; Liu, Y.; Li, L.; Feng, J.; Lou, B.; Zhou, X.; Wu, H. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar]
- Jaisinghani, R.N. Antibacterial properties of quercetin. Microbiol. Res. 2017, 8, 6877. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Ali, S.A. Synergistic antibacterial activity of Curcumin with antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar]
- Yun, D.G.; Lee, D.G. Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Yao, Y.; Yu, Y.; Zeng, Y. Enhanced antibacterial activity of curcumin by combination with metal ions. Colloid. Interface Sci. Commun. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Ojala, T.; Remes, S.; Haansuu, P.; Vuorela, H.; Hiltunen, R.; Haahtela, K.; Vuorela, P. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 2000, 73, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected naturally occurring and synthetic coumarins. Int. J. Antimicrob. Agents 2009, 33, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, A.a.A.; Ayoub, M.T.; Shakya, A.K.; Abu Safieh, K.A.; Mubarak, M.S. Synthesis, characterization, and antimicrobial activity of some new coumarin derivatives. Med. Chem. Res. 2012, 21, 468–476. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.; Chin, V.K.; Wong, E.H.; Madhavan, P.; Tay, S.T.; Yong, P.V.C.; Chong, P.P. Review: Antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020, 65, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.I.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides (Review). Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Neu, H.C. The Crisis in Antibiotic Resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef]
- Miyakis, S.; Pefanis, A.; Tsakris, A. The Challenges of Antimicrobial Drug Resistance in Greece. Clin. Infect. Dis. 2011, 53, 177–184. [Google Scholar] [CrossRef]
- Rafila, A.; Talapan, D.; Dorobăţ, O.M.; Popescu, G.A.; Piţigoi, D.; Florea, D.; Buicu, F.C. Emergence of Carbapenemase-producing Enterobacteriaceae, a Public Health Threat: A Romanian Infectious Disease Hospital Based Study/Emergenţa Enterobacteriaceaelor producătoare de carbapenemaze, o ameninţare pentru sănătatea publică: Un studiu realizat într-un spital romanesc de boli infectioase. Rev. Romana De Med. De Lab. 2015, 23, 295–301. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public. Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Medicine 2017, 45, 622–628. [Google Scholar] [CrossRef]
- Popa, L.I.; Gheorghe, I.; Barbu, I.C.; Surleac, M.; Paraschiv, S.; Măruţescu, L.; Popa, M.; Pîrcălăbioru, G.G.; Talapan, D.; Niţă, M.; et al. Multidrug Resistant Klebsiella pneumoniae ST101 Clone Survival Chain from Inpatients to Hospital Effluent after Chlorine Treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef] [PubMed]
- Tălăpan, D.; Rafila, A. Five-Year Survey of Asymptomatic Colonization with Multidrug-Resistant Organisms in a Romanian Tertiary Care Hospital. Infect. Drug Resist. 2022, 15, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, O.; Abu-Abaa, M.; Huang, K.; Hasan, S. The Rare Adverse Effect of Cefepime-Induced Neutropenia. Cureus 2023, 15, e38274. [Google Scholar] [CrossRef] [PubMed]
- Massoth, C.; Saadat-Gilani, K.; Wenk, M. [allergy to penicillin—No beta-lactam antibiotics?]. Anasthesiol. Intensivmed. Notfallmed Schmerzther. 2023, 58, 264–266. [Google Scholar] [CrossRef]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public Health 2022, 19, 4669. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef]
- Rosca, A.E.; Iesanu, M.I.; Zahiu, C.D.M.; Voiculescu, S.E.; Paslaru, A.C.; Zagrean, A.M. Capsaicin and Gut Microbiota in Health and Disease. Molecules 2020, 25, 5681. [Google Scholar] [CrossRef]
- Weng, G.; Duan, Y.; Zhong, Y.; Song, B.; Zheng, J.; Zhang, S.; Yin, Y.; Deng, J. Plant Extracts in Obesity: A Role of Gut Microbiota. Front. Nutr. 2021, 8, 727951. [Google Scholar] [CrossRef] [PubMed]
- Dumitrache, M.D.; Jieanu, A.S.; Scheau, C.; Badarau, I.A.; Popescu, G.D.A.; Caruntu, A.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review). Exp. Ther. Med. 2021, 22, 917. [Google Scholar] [CrossRef] [PubMed]
- Popescu, G.D.A.; Scheau, C.; Badarau, I.A.; Dumitrache, M.D.; Caruntu, A.; Scheau, A.E.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules 2020, 26, 94. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Li, Y.; Ding, G. Interstitial fluid flow: The mechanical environment of cells and foundation of meridians. Evid. Based Complement. Altern. Med. 2012, 2012, 853516. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yang, J.F.; Chen, M.; Xu, L.; Wang, W.C.; Wang, F.; Tong, J.B.; Wang, C.Y. Visualized regional hypodermic migration channels of interstitial fluid in human beings: Are these ancient meridians? J. Altern. Complement. Med. 2008, 14, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tong, H.; Xu, W.; Hu, J.; Liu, N.; Li, H.; Cao, L. Perivascular space: Possible anatomical substrate for the meridian. J. Altern. Complement. Med. 2003, 9, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Zhao, Y.; Kjell, F. Understanding propagated sensation along meridians by volume transmission in peripheral tissue. Chin. J. Integr. Med. 2013, 19, 330–339. [Google Scholar] [CrossRef]
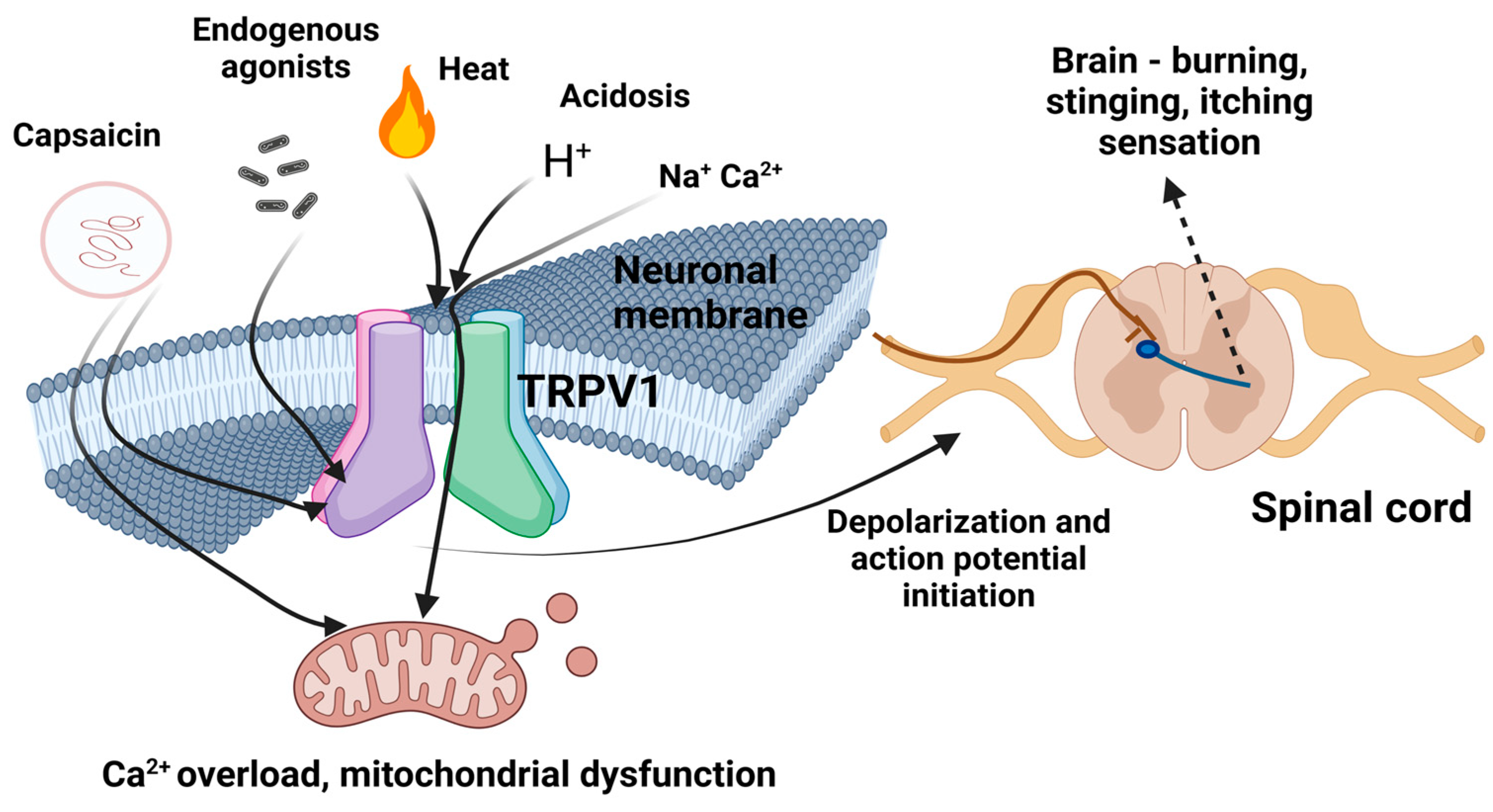
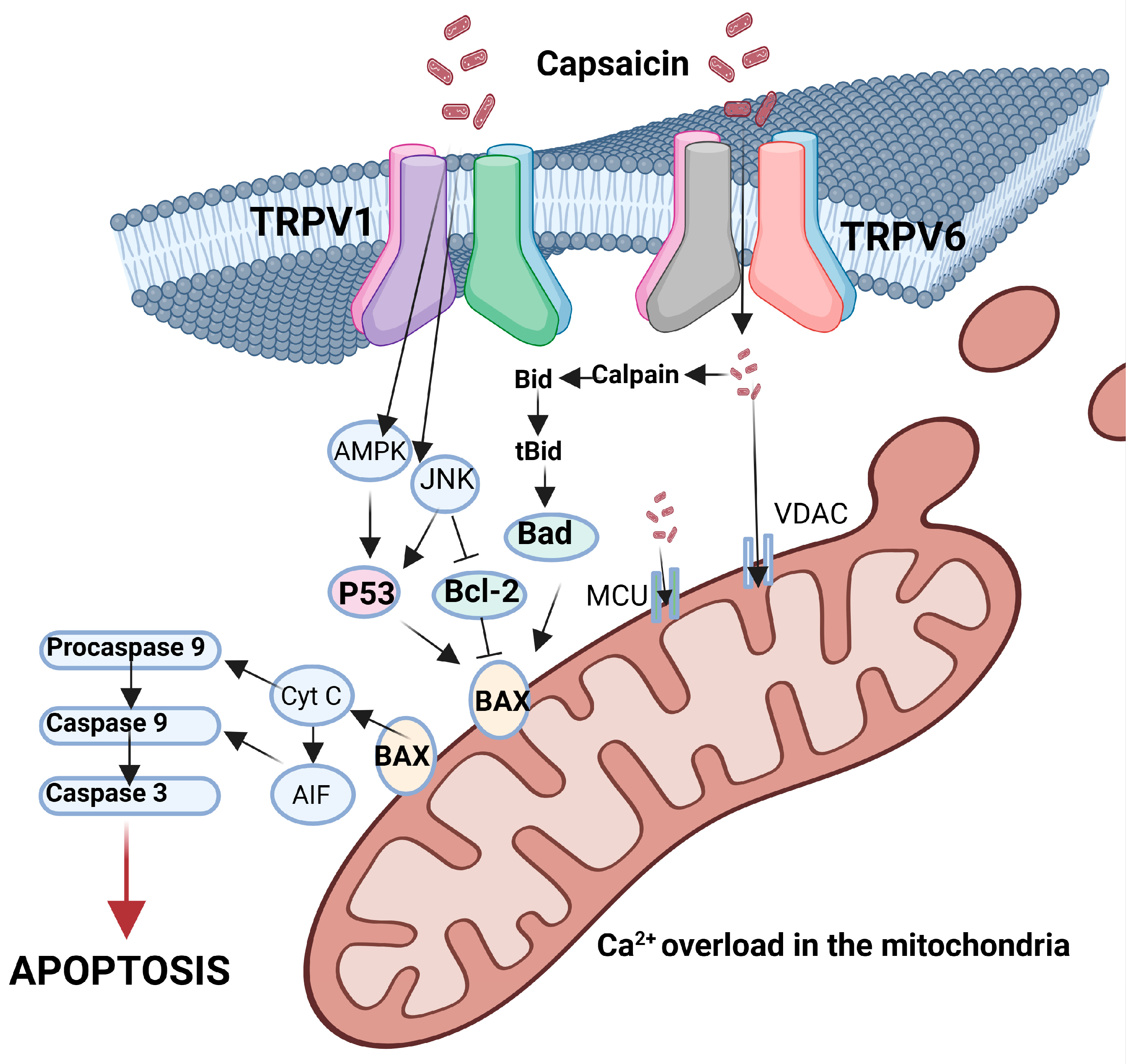
| Use of Capsaicin and Its Derivatives | References | |
|---|---|---|
| Animal repellents | [43,44,45,46] | |
| Food industry—fragrance ingredient | [47,48,49] | |
| Pesticides | [50,51,52,53] | |
| Veterinary medicine (various uses) | [54,55,56] | |
| Medical Uses | Chronic pain—cream application (local adm.) | [57,58,59,60,61] |
| Gastroprotection in cases of drug administration | [62] | |
| Post-operative nausea and vomiting | [63,64,65] | |
| Post-operative sore throat | [66] | |
| Pruritus | [67,68,69,70,71] | |
| Urinary bladder hyperactivity | [72,73] | |
| Skin conditioning creams | [74,75] | |
| Indication | Formulation | Effect | Action Mechanism | Type of Study | Year | References |
|---|---|---|---|---|---|---|
| Hepatocellular carcinoma | 200 mM capsaicin or Met-capsaicin | Pro-apoptotic, potential chemopreventive | DNA fragmentation/nuclear condensation/activation of caspase-3 | In vitro—SK-Hep-1 hepatocellular carcinoma cells | 2001 | [131] |
| Colon cancer | 200–300 mM | Proapoptotic, chemopreventive | PPARγ pathway activation (non-TRPV1 related) | In vitro—HT-29 human colon cancer cells | 2004 | [133] |
| Gastric adenocarcinoma | 1 mmol/L | Proapoptotic | Reduction of Bcl-2 and antiapoptotic protein/DNA fragmentation | In vitro AGS cells | 2005 | [134] |
| Prostate cancer | Different doses | Profound antiproliferative effect | Prevention of NF-kappaB activation | In vitro—prostate cancer cell lines/In vivo—prostate cancer cell xenografts on mice | 2006 | [135] |
| Gastric cancer | 50 μM | Proapoptotic | TRPV6-mediated capsaicin-induced apoptosis/stabilization of p53 through JNK-regulated p53 phosphorylation/increase of Bax and p53 protein without increasing transcription | In vitro—human gastric cancer AGS cells/GES-1 cells | 2007 | [293] |
| Melanoma | 50–200 μM | Proapoptotic | Down-regulation of Bcl-2 expression, nuclear condensation, internucleosomal DNA fragmentation | In vitro—B16-F10 melanoma cells | 2007 | [294] |
| Benzo(a)pyrene induced experimental Lung cancer | 10 mg/kg | Chemoprotective | Decreased lung mitochondrial lipid peroxidation | In vivo—animal | 2008 | [295] |
| Pancreatic cancer | 2.5 mg capsaicin/kg body weight 5 times/week, 5 mg capsaicin/kg 3 times/week by oral gavage (in vivo part) | Proapoptotic | Increased expression of Bax, down-regulation of Bcl-2, significant release of cytochrome c and AIF in the cytosol | In vivo—athymic nude mice; in vitro—AsPC-1 and BxPC-3 cells | 2008 | [296] |
| Small cell lung cancer | Oral administration of 10 mg capsaicin/kg of mice; 50 Μm capsaicin for cell cultures | Antiproliferative | Decreased expression of E2F-responsive proliferative genes cyclin E, thymidylate synthase, cdc25A and cdc6at | In vivo–animal, in vitro-human SCLC cell lines NCI-H69, NCI-H82, DMS53 and DMS114 | 2010 | [297] |
| Chronic Pancreatitis and pancreatic intraepithelial neoplasia | Oral administration of 10 ppm. capsaicin or 20 ppm depending on research group | Chemopreventive | Reduction of cell proliferation and suppressed phosphorylation of ERK and c-Jun, blocked Hedgehog/GLI pathway activation | In vivo—animal | 2011 | [298] |
| Breast cancer | 200 μM | Proapoptotic | Decreased mitochondria membrane potential, induced cleavage of PARP-1, decreased procaspase-7 expression | In vitro—MCF-7 and BT-20 human breast cancer cell lines | 2011 | [299] |
| Tongue cancer | 50, 100 and 150 µM | Proapoptotic, prooxidant | Activation of caspase 3, DNA fragmentation, induction of G0/G1 phase arrest, activation of ROS | In vitro—SCC-4 human tongue cancer cells | 2012 | [300] |
| Colorectal cancer | 50 and 100 μM | Anti-proliferative, proapoptotic | Suppression of TCF-4 expression and disruption of TCF-4 and β-catenin interaction | In vitro—SW480, LoVo, and HCT-116 colorectal cancer cells | 2012 | [301] |
| Bladder cancer | 50, 100, 150, 200 µM | Anti-proliferative, proapoptotic | Inhibition of CDK2, CDK4 and CDK6; cell death induction by ROS increase and decreased mitochondrial membrane potential | In vitro—5637 human urinary bladder cancer cell line | 2012 | [302] |
| Human KB cancer cells | 1, 50, 100, 150, 200 and 250 μM | Anti-proliferative, proapoptotic | Mitochondrial membrane permeabilization and caspase activation | In vitro—human KB cancer cells | 2013 | [303] |
| Gastric cancer | 10–300 μM | Anti-proliferative (probably), proapoptotic | Decreased expression of phosphorylated ERK 1/2, p38 MAPK or JNK | In vitro—human gastric cancer cells (AGS cells) | 2014 | [304] |
| Cholangiocarcinoma | 150–200 µM | Impaired cell proliferation, migration, invasion, epithelial to mesenchymal transition growth inhibition in soft agar colonies | Inhibition of Hedgehog signalling pathway | In vitro—human CC cell lines (SZ-1 and TFK-1) | 2014 | [305] |
| Pancreatic neuroendocrine tumours | 10–200 μM | Cytotoxic | Disruption of mitochondrial membrane potential and inhibition of ATP synthesis | In vitro—BON and QGP-1 cells | 2014 | [306] |
| Bladder cancer | 10–250 μM | Mediation of cancer cell apoptosis | Activation of dendritic cells via CD91 | In vitro—T24 and SD48 human urinary bladder cancercell lines | 2015 | [307] |
| Prostate cancer | 50, 100, 150 and 200 μM | Antiproliferative | Restoration of miR-449a profiling in cancer cells leading to negative modulation of the androgen receptor | In vitro—human C4-2 and LNCaP cells | 2015 | [308] |
| Bladder cancer | 100 and 200 μM | Antiproliferative, anti-migration, cell cycle prolongation | Inhibition of tNOX and sirtuin 1 (SIRT1) | In vitro—TSGH8301 and T24 urinary bladder cancer cells | 2016 | [309] |
| Gastric cancer | 0–16 μg/mL | Chemopreventive, antiprofirative, proapoptotic | Reduced hMOF activity | In vitro—colon cancer SW-480, gastric cancer MGC-803 and gastric mucosal GES-1 cells | 2016 | [310] |
| Prostate cancer | 20, 80 μM | Antiproliferative, induced autophagy | Activation of ROS generation, increased levels of LC3-II, accumulation of p62 | In vitro—prostate cancer (LNCaP and PC-3) cells | 2016 | [311] |
| Renal cell carcinoma | 0–400 μM | Proapoptotic | Up-regulation of pro-apoptotic genes (c-myc, FADD, Bax andcleaved-caspase-3,-8, and-9), down-regulation of Bcl2, activated p38 and JNK MAPK pathways | In vitro—human renal cell carcinoma 786-O, ACHN, Caki-1 cells | 2016 | [312] |
| Ovarian cancers | 0.1–50 μg/mL | Proapoptotic | Cell cycle arrest | In vitro—SKOV-3 ovarian cancer cells/in vivo-male SD rats | 2017 | [313] |
| Nasopharyngeal carcinoma | 100, 150, 200 and 300 μM/L | Antiproliferative, proapoptotic, induced autophagy | Increased G1 phase cell cycle arrest, increased LC3-II and Atg5 levels, decreased p62 and Fap-1 expression, increased caspase-3 activity | In vitro—NPC-TW01 cells | 2017 | [314] |
| Oesophageal squamous cell carcinoma | 120 µM | Antiproliferative, propapoptotic | Inhibition of glycolysis, decreased HK-2 expression | In vitro—Het-1A cell | 2018 | [315] |
| Oral squamous cancer | 50–350 µM | Antiproliferative, proapoptotic | Disruption of the mitochondrial-membrane potential, activation of caspase-3, -7 and -9, DNA fragmentation | In vitro—ORL-48 cells | 2019 | [316] |
| Osteosarcoma | 20 µM | Proapoptotic | Mitochondrial dysfunction, overproduction of ROS and JNK, activation of AMPK-p53 pathway | In vitro—MG63 cells | 2019 | [317] |
| Breast cancer | 0, 10, 50, 100 or 200 µM | Proapoptotic | Induced G2/M cell cycle arrest, reduced CDK8 expression levels, decreased phosphorylation of PI3K and Akt, downregulation of Wnt and β-catenin | In vitro—MDA MB 231 breast cancer cells | 2020 | [318] |
| Prostate cancer | 1, 5, 10 µM | Proapoptotic | Decreased expression of Wnt-2, p-GSK3β, β-catenin, c-myc and cyclin D1 | In vitro—PC-3 and DU145 prostate cancer stem cells | 2020 | [319] |
| Gastric cancer | IC50 of 0.6 ± 0.0421 μM | Antiproliferative | Inhibition of histone methylation KDM1A | In vitro—gastric cancer cell line BGC-823 | 2020 | [320] |
| Glioblastoma | (IC50) values of capsaicin were 325.7 ± 12.4 μM at 24 h and 265.7 ± 10.2 μM at 48 h | Proapoptotic | Upregulation of peroxisome proliferator-activated receptor gamma | In vitro—human glioblastoma LN-18 cell line | 2020 | [321] |
| Breast cancer | In vitro: 150 μΜ/L for 72 h; in vivo: 10 mg/kg 1 time per 3 days for 21 days | Proapoptotic, antiproliferative | Down-regulation of FBI-1-mediated NF-κB pathway | In vivo—female BALB/c athymic nude mice; in vitro—human breast cancer cell lines (MCF-7 and MDA-MB-23) | 2021 | [322] |
| Lung cancer cells | 0–200 µM | Antiproliferative | Reduced accumulation of HIF-1α protein inhibition of mitochondrial respiration | In vitro—A549, H1299, H2009, and H23 cell lines | 2022 | [323] |
| Renal cancer | 0, 5, 10, 25, 50, 100 and 200 μM | Inhibition of cell migration, invasion and epithelial-mesenchymal transition Induced autophagy | AMPK/mTOR pathway | In vitro—renal cell carcinoma (RCC) 786-O and CAKI-1 cell lines | 2022 | [324] |
| Epithelial lung cancer NSCLC | 100, 200 and 300 μM/L | Inhibition of proliferation and promotion of ferroptosis | Increase of total iron levels and ferrous ion levels by regulating the SLC7A11/GPX4 axis | In vitro—NSCLC cells (A549 and NCI-H23) | 2022 | [325] |
| Hepatocarcinogenesis | 100, 200 and 300 μM/L | Significant inhibition of hepatocarcinogenesis | Inhibition of SIRT1/SOX2 signalling; SIRT1 downregulation | In vitro—HepG2 and WB-F344 cells | 2022 | [326] |
| Anaplastic thyroid cancer | 50, 100 and 200 μM | Stemness-inhibitory effect | Calcium-dependent autophagic degradation of OCT4A, following TRPV1 activation | In vitro—8505C and FRO cells | 2022 | [327] |
| Use | Tribe(s)/Country | Capsicum Species Used | References |
|---|---|---|---|
| Antibacterial/antimicrobial | Pimas/Mexico | Capsicum sp. | [391] |
| Nematicide | Pimas/Mexico | Capsicum sp. | [392,393] |
| Fever alleviation | Zapotecs and Raramuris/Mexico | Capsicum sp. | [394,395] |
| Mental, behavioural and neurological disorders | Raramuris and Mestizos/Mexico | C. annuum var. glabriusculum | [395] |
| Ocular pathologies treatment | Mestizos and Zapotecs/Mexico | C. annuum var. glabriusculum | [394,396,397,398,399] |
| Auricular pathologies treatment | Nahua/Mexico | C. annuum var. glabriusculum | [394,398,399] |
| Respiratory pathologies treatment | Nahua and Mestizos/Mexico | Capsicum sp. | [77,397,400,401,402,403] |
| Pathologies of the gastrointestinal tract | Mestizos, Nahua and Lacandon Maya/Mexico | Capsicum sp. | [77,397,400,401,404,405] |
| Skin pathologies | Mestizos, Zapotecs and Tzotzil/Mexico | Capsicum sp. | [77,396,397,403,406,407] |
| Disease of the musculoskeletal system and associated tissues | Ramamuris/Mexico | Capsicum sp. | [395,408] |
| Pathologies of the genito-urinary system | Local tribes/Mexico | Capsicum sp. | [77,400,401] |
| Labor pain mitigation/delivery promotion | Local tribes/Mexico | Capsicum sp. | [400] |
| Poisoning treatment (for spider bites) | Mestizos/Mexico | C. annuum var. glabriusculum | [400,403] |
| General health benefits | Local people/Eritrea | Capsicum sp. | [390] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petran, E.M.; Periferakis, A.; Troumpata, L.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Periferakis, K.; Caruntu, A.; Savulescu-Fiedler, I.; Sima, R.-M.; et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Curr. Issues Mol. Biol. 2024, 46, 7895-7943. https://doi.org/10.3390/cimb46080468
Petran EM, Periferakis A, Troumpata L, Periferakis A-T, Scheau A-E, Badarau IA, Periferakis K, Caruntu A, Savulescu-Fiedler I, Sima R-M, et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Current Issues in Molecular Biology. 2024; 46(8):7895-7943. https://doi.org/10.3390/cimb46080468
Chicago/Turabian StylePetran, Elena Madalina, Argyrios Periferakis, Lamprini Troumpata, Aristodemos-Theodoros Periferakis, Andreea-Elena Scheau, Ioana Anca Badarau, Konstantinos Periferakis, Ana Caruntu, Ilinca Savulescu-Fiedler, Romina-Marina Sima, and et al. 2024. "Capsaicin: Emerging Pharmacological and Therapeutic Insights" Current Issues in Molecular Biology 46, no. 8: 7895-7943. https://doi.org/10.3390/cimb46080468








