Aquaporin Modulation by Cations, a Review
Abstract
:1. Introduction
2. Modulation by Alkaline Earth Metal Cations
2.1. Calcium Ions
2.2. Magnesium Ions
3. Transition Metals
3.1. Mercury Ions
3.2. Zinc Ions
3.3. Cadmium Ions
3.4. Gold Ion Compounds
3.5. Silver Ions
3.6. Copper Ions
3.7. Nickel Ions
3.8. Lead Ions
3.9. Manganese Ions
3.10. Iron Ions
4. Alkaline Metals
4.1. Lithium Ions
4.2. Sodium Ions
5. Discussion
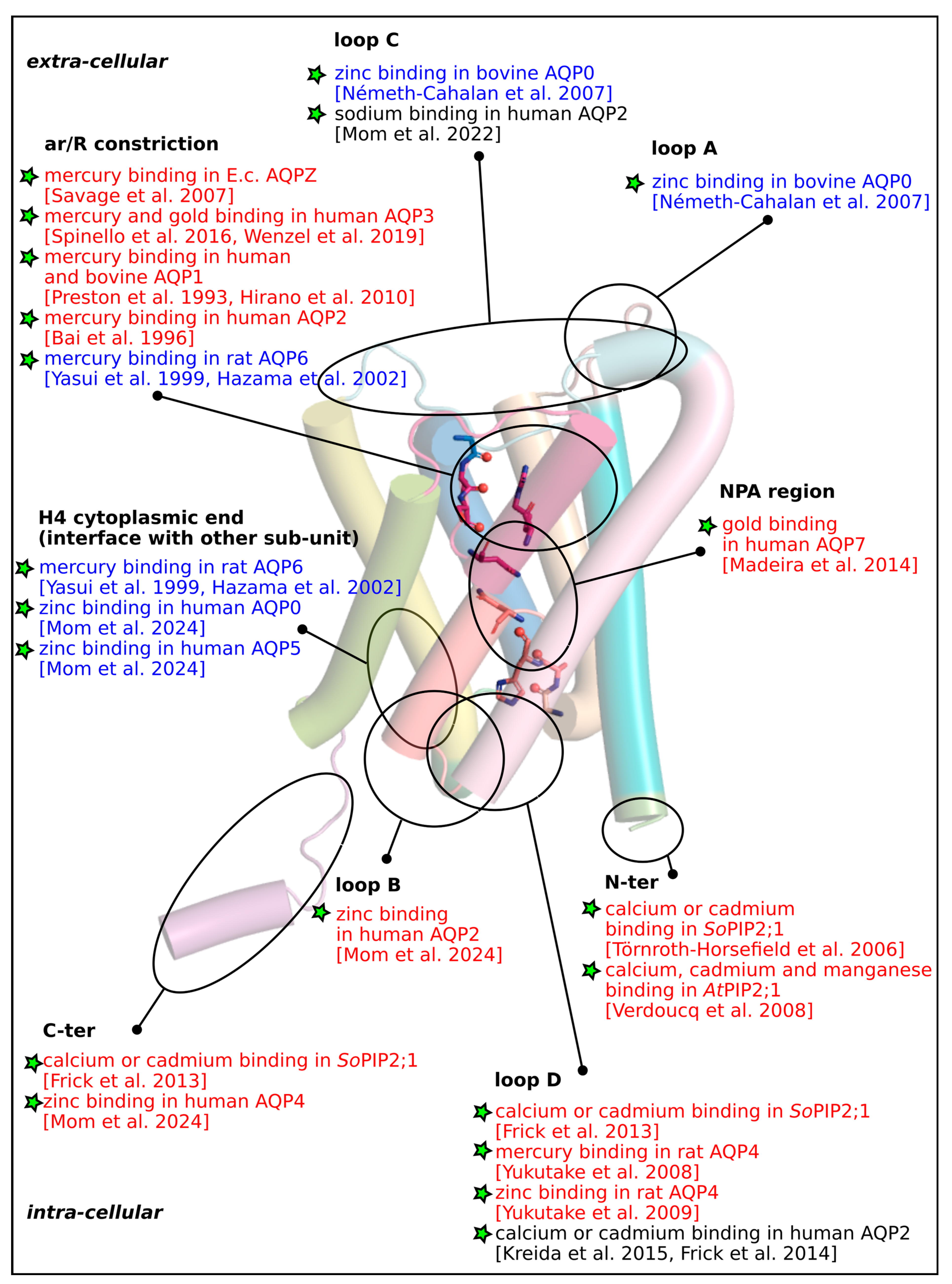
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and Evolution of Membrane Intrinsic Proteins. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A Family of Water Channel Proteins. Am. J. Physiol. 1993, 265, F461. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E. Aquaporins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-79885-9. [Google Scholar]
- Mukherjee, S.; Roy, S.; Corpas, F.J. Aquaporins: A Vital Nexus in H2O2-Gasotransmitter Signaling. Trends Plant Sci. 2024, 29, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes*. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-Facilitated Transmembrane Diffusion of Hydrogen Peroxide. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human Aquaporins: Regulators of Transcellular Water Flow. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Mola, M.G.; Sparaneo, A.; Gargano, C.D.; Spray, D.C.; Svelto, M.; Frigeri, A.; Scemes, E.; Nicchia, G.P. The Speed of Swelling Kinetics Modulates Cell Volume Regulation and Calcium Signaling in Astrocytes: A Different Point of View on the Role of Aquaporins. Glia 2016, 64, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Akai, M.; Onai, K.; Morishita, M.; Mino, H.; Shijuku, T.; Maruyama, H.; Arai, F.; Itoh, S.; Hazama, A.; Checchetto, V.; et al. Aquaporin AqpZ Is Involved in Cell Volume Regulation and Sensitivity to Osmotic Stress in Synechocystis Sp. Strain PCC 6803. J. Bacteriol. 2012, 194, 6828–6836. [Google Scholar] [CrossRef]
- Kida, H.; Miyoshi, T.; Manabe, K.; Takahashi, N.; Konno, T.; Ueda, S.; Chiba, T.; Shimizu, T.; Okada, Y.; Morishima, S. Roles of Aquaporin-3 Water Channels in Volume-Regulatory Water Flow in a Human Epithelial Cell Line. J. Membr. Biol. 2005, 208, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Duan, E. Aquaporins in Sperm Osmoadaptation: An Emerging Role for Volume Regulation. Acta Pharmacol. Sin. 2011, 32, 721–724. [Google Scholar] [CrossRef]
- Di Giusto, G.; Pizzoni, A.; Rivarola, V.; Beltramone, N.; White, A.; Ford, P.; Capurro, C. Aquaporin-2 and Na+/H+ Exchanger Isoform 1 Modulate the Efficiency of Renal Cell Migration. J. Cell. Physiol. 2020, 235, 4443–4454. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and Cell Migration. Pflug. Arch—Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.-H.; Nielsen, J.; Møller, H.B.; Fenton, R.A.; Nielsen, S.; Frøkiaer, J. Aquaporins in the Kidney. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 95–132. [Google Scholar] [CrossRef]
- Takeda, T.; Taguchi, D. Aquaporins as Potential Drug Targets for Meniere’s Disease and Its Related Diseases. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 171–184. [Google Scholar] [CrossRef]
- Delporte, C. Aquaporins and Gland Secretion. In Aquaporins; Yang, B., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 63–79. ISBN 978-94-024-1057-0. [Google Scholar]
- Kitchen, P.; Day, R.E.; Salman, M.M.; Conner, M.T.; Bill, R.M.; Conner, A.C. Beyond Water Homeostasis: Diverse Functional Roles of Mammalian Aquaporins. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2015, 1850, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Nagasawa, A.; Funahashi, T. Aquaporins and Glycerol Metabolism. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2006, 1758, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than Just Water Channels: Unexpected Cellular Roles of Aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.R.; Maistriaux, L.C.; Chaumont, F. Toward Understanding of the High Number of Plant Aquaporin Isoforms and Multiple Regulation Mechanisms. Plant Sci. 2017, 264, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Badaut, J.; Fukuda, A.M.; Jullienne, A.; Petry, K.G. Aquaporin and Brain Diseases. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Lodder, E.M.; Wilders, R. Aquaporin Channels in the Heart—Physiology and Pathophysiology. Int. J. Mol. Sci. 2019, 20, 2039. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G.; Annese, T.; Nico, B. Aquaporins in Cancer. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New Players in Cancer Biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Jana, A.; Bhattacharjee, S.; Mitra, S.; De, S.; Alghamdi, B.S.; Alam, M.Z.; Mahmoud, A.B.; Al Shareef, Z.; Abdel-Rahman, W.M.; et al. The Role of Aquaporins in Tumorigenesis: Implications for Therapeutic Development. Cell Commun. Signal. 2024, 22, 106. [Google Scholar] [CrossRef] [PubMed]
- Walz, T.; Fujiyoshi, Y.; Engel, A. The AQP Structure and Functional Implications. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–56. [Google Scholar] [CrossRef]
- de Groot, B.L.; Grubmüller, H. The Dynamics and Energetics of Water Permeation and Proton Exclusion in Aquaporins. Curr. Opin. Struct. Biol. 2005, 15, 176–183. [Google Scholar] [CrossRef]
- Beitz, E.; Wu, B.; Holm, L.M.; Schultz, J.E.; Zeuthen, T. Point Mutations in the Aromatic/Arginine Region in Aquaporin 1 Allow Passage of Urea, Glycerol, Ammonia, and Protons. Proc. Natl. Acad. Sci. USA 2006, 103, 269–274. [Google Scholar] [CrossRef]
- Törnroth-Horsefield, S.; Hedfalk, K.; Fischer, G.; Lindkvist-Petersson, K.; Neutze, R. Structural Insights into Eukaryotic Aquaporin Regulation. FEBS Lett. 2010, 584, 2580–2588. [Google Scholar] [CrossRef]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural Mechanism of Plant Aquaporin Gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Németh-Cahalan, K.L.; Kalman, K.; Hall, J.E. Molecular Basis of pH and Ca2+ Regulation of Aquaporin Water Permeability. J. Gen. Physiol. 2004, 123, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.T.; Conner, A.C.; Bill, R.M. Inhibitors of Mammalian Aquaporin Water Channels. Int. J. Mol. Sci. 2019, 20, 1589. [Google Scholar] [CrossRef]
- Nguyen, A.T.P.; Weigle, A.T.; Shukla, D. Functional Regulation of Aquaporin Dynamics by Lipid Bilayer Composition. Nat. Commun. 2024, 15, 1848. [Google Scholar] [CrossRef]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Nuclear Calcium: A Key Regulator of Gene Expression. Biometals 1998, 11, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Greenberg, M.E. Calcium Signaling in Neurons: Molecular Mechanisms and Cellular Consequences. Science 1995, 268, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.P.; Bird, G.S.; Hajnóczky, G.; Robb-Gaspers, L.D.; Putney, J.W. Spatial and Temporal Aspects of Cellular Calcium Signaling. FASEB J. 1996, 10, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Means, A.R. Calmodulin: A Prototypical Calcium Sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Lewit-Bentley, A.; Réty, S. EF-Hand Calcium-Binding Proteins. Curr. Opin. Struct. Biol. 2000, 10, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Reichow, S.L.; Gonen, T. Noncanonical Binding of Calmodulin to Aquaporin-0: Implications for Channel Regulation. Structure 2008, 16, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Németh-Cahalan, K.L.; Hall, J.E. pH and Calcium Regulate the Water Permeability of Aquaporin 0 *. J. Biol. Chem. 2000, 275, 6777–6782. [Google Scholar] [CrossRef] [PubMed]
- Girsch, S.J.; Peracchia, C. Lens Cell-to-Cell Channel Protein: I. Self-Assembly into Liposomes and Permeability Regulation by Calmodulin. J. Membrain Biol. 1985, 83, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lindsey Rose, K.M.; Wang, Z.; Magrath, G.N.; Hazard, E.S.; Hildebrandt, J.D.; Schey, K.L. Aquaporin 0−Calmodulin Interaction and the Effect of Aquaporin 0 Phosphorylation. Biochemistry 2008, 47, 339–347. [Google Scholar] [CrossRef]
- Reichow, S.L.; Clemens, D.M.; Freites, J.A.; Németh-Cahalan, K.L.; Heyden, M.; Tobias, D.J.; Hall, J.E.; Gonen, T. Allosteric Mechanism of Water-Channel Gating by Ca2+–Calmodulin. Nat. Struct. Mol. Biol. 2013, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Roche, J.V.; Missel, J.W.; Al-Jubair, T.; Hagströmer, C.J.; Wittenbecher, V.; Linse, S.; Gourdon, P.; Törnroth-Horsefield, S. The Role of Phosphorylation in Calmodulin-Mediated Gating of Human AQP0. Biochem. J. 2024, 481, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Virginia Roche, J.; Olsson, C.; Linse, S.; Törnroth-Horsefield, S. Protein–Protein Interactions in AQP Regulation—Biophysical Characterization of AQP0–CaM and AQP2–LIP5 Complex Formation. Faraday Discuss. 2018, 209, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Rabaud, N.E.; Song, L.; Wang, Y.; Agre, P.; Yasui, M.; Carbrey, J.M. Aquaporin 6 Binds Calmodulin in a Calcium-Dependent Manner. Biochem. Biophys. Res. Commun. 2009, 383, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Vogel, H.J.; Conner, A.C.; Kitchen, P.; Bill, R.M.; MacDonald, J.A. Simultaneous Binding of the N- and C-Terminal Cytoplasmic Domains of Aquaporin 4 to Calmodulin. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2022, 1864, 183837. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Hasler, U.; Leroy, V.A.A.; de Seigneux, S.; Dimitrov, M.; Mordasini, D.; Rousselot, M.; Martin, P.-Y.; Fe[Combining Acute Accent]raille, E. Calcium-Sensing Receptor Attenuates AVP-Induced Aquaporin-2 Expression via a Calmodulin-Dependent Mechanism. J. Am. Soc. Nephrol. 2008, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-L.; Yip, K.-P.; Michea, L.; Kador, K.; Ferraris, J.D.; Wade, J.B.; Knepper, M.A. Regulation of Aquaporin-2 Trafficking by Vasopressin in the Renal Collecting Duct: Roles of Ryanodine-Sensitive Ca2+ Stores and Calmodulin *. J. Biol. Chem. 2000, 275, 36839–36846. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, L.; Sham, J.S.K.; Yip, K.-P. Calcium Signaling in Vasopressin-Induced Aquaporin-2 Trafficking. Pflug. Arch—Eur. J. Physiol. 2008, 456, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Procino, G.; Tamma, G.; Carmosino, M.; Svelto, M. Minireview: Aquaporin 2 Trafficking. Endocrinology 2005, 146, 5063–5070. [Google Scholar] [CrossRef] [PubMed]
- Freites, J.A.; Németh-Cahalan, K.L.; Hall, J.E.; Tobias, D.J. Cooperativity and Allostery in Aquaporin 0 Regulation by Ca2+. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2019, 1861, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.B.; Németh-Cahalan, K.L.; Freites, J.A.; Vorontsova, I.; Hall, J.E.; Tobias, D.J. Calmodulin Gates Aquaporin 0 Permeability through a Positively Charged Cytoplasmic Loop *. J. Biol. Chem. 2017, 292, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kreida, S.; Törnroth-Horsefield, S. Structural Insights into Aquaporin Selectivity and Regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, H.; Jensen, M.Ø.; Mouritsen, O.G. To Gate or Not to Gate: Using Molecular Dynamics Simulations to Morph Gated Plant Aquaporins into Constitutively Open Conformations. J. Phys. Chem. B 2009, 113, 5239–5244. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, M.; Frick, A.; Wang, Y.; Ekvall, M.; Hallgren, K.; Hedfalk, K.; Neutze, R.; Tajkhorshid, E.; Törnroth-Horsefield, S. Structural and Functional Analysis of SoPIP2;1 Mutants Adds Insight into Plant Aquaporin Gating. J. Mol. Biol. 2009, 387, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Mom, R.; Réty, S.; Mocquet, V.; Auguin, D. Plant Aquaporin Gating Is Reversed by Phosphorylation on Intracellular Loop D—Evidence from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2023, 24, 13798. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Järvå, M.; Ekvall, M.; Uzdavinys, P.; Nyblom, M.; Törnroth-Horsefield, S. Mercury Increases Water Permeability of a Plant Aquaporin through a Non-Cysteine-Related Mechanism. Biochem. J. 2013, 454, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Larsson, C.; Ek, B.; Kjellbom, P. The Major Integral Proteins of Spinach Leaf Plasma Membranes Are Putative Aquaporins and Are Phosphorylated in Response to Ca2+ and Apoplastic Water Potential. Plant Cell 1996, 8, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Grondin, A.; Maurel, C. Structure–Function Analysis of Plant Aquaporin AtPIP2;1 Gating by Divalent Cations and Protons. Biochem. J. 2008, 415, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Eriksson, U.K.; Mattia, F.d.; Öberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.T.; Törnroth-Horsefield, S. X-Ray Structure of Human Aquaporin 2 and Its Implications for Nephrogenic Diabetes Insipidus and Trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.M.; Savelkoul, P.J.M.; Markovich, D.; Hofman, E.; Nielsen, S.; van der Sluijs, P.; Deen, P.M.T. The Role of Putative Phosphorylation Sites in the Targeting and Shuttling of the Aquaporin-2 Water Channel*. J. Biol. Chem. 2002, 277, 41473–41479. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.B.; Rittig, S.; Fenton, R.A. Nephrogenic Diabetes Insipidus: Essential Insights into the Molecular Background and Potential Therapies for Treatment. Endocr. Rev. 2013, 34, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Park, T.J. Magnesium Metabolism. Electrolytes Blood Press. E BP 2008, 6, 86–95. [Google Scholar] [CrossRef]
- Okahira, M.; Kubota, M.; Iguchi, K.; Usui, S.; Hirano, K. Regulation of Aquaporin 3 Expression by Magnesium Ion. Eur. J. Pharmacol. 2008, 588, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Mochiduki, T.; Takasaki, A.; Ushiki, T.; Baba, K.; Ishii, M.; Kudo, T.; Ito, K.; Toda, T.; Ochiai, W.; et al. A Mechanism by Which the Osmotic Laxative Magnesium Sulphate Increases the Intestinal Aquaporin 3 Expression in HT-29 Cells. Life Sci. 2011, 88, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Kawai, H.; Shinozaki, Y.; Tabata, K.; Yoshida, R.; Nishinaka, Y.; Kon, R.; Sakai, H.; Hosoe, T. Magnesium Compounds Increase Aquaporin-3 in Human Epidermal Keratinocyte HaCaT Cells. Dermatol. Ther. 2023, 2023, 8896599. [Google Scholar] [CrossRef]
- Ghabriel, M.N.; Thomas, A.; Vink, R. Magnesium Restores Altered Aquaporin-4 Immunoreactivity Following Traumatic Brain Injury to a Pre-Injury State. In Proceedings of the Brain Edema XIII, Ann Arbor, MI, USA, 1–3 June 2005; Hoff, J.T., Keep, R.F., Xi, G., Hua, Y., Eds.; Springer: Vienna, Austria, 2006; pp. 402–406. [Google Scholar]
- Li, X.; Liu, H.; Yang, Y. Magnesium Sulfate Attenuates Brain Edema by Lowering AQP4 Expression and Inhibits Glia-Mediated Neuroinflammation in a Rodent Model of Eclampsia. Behav. Brain Res. 2019, 364, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yin, H.; Sun, H.; Qian, T.; Zhu, J.; Qi, G.; Wang, Y.; Qi, B. The Clinical Value of Aquaporin-4 in Children with Hand, Foot, and Mouth Disease and the Effect of Magnesium Sulfate on Its Expression: A Prospective Randomized Clinical Trial. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1343–1349. [Google Scholar] [CrossRef]
- Euser, A.G.; Bullinger, L.; Cipolla, M.J. Magnesium Sulphate Treatment Decreases Blood–Brain Barrier Permeability during Acute Hypertension in Pregnant Rats. Exp. Physiol. 2008, 93, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xiao, M.; Li, S.; Yang, B. Aquaporins in Nervous System. In Aquaporins; Yang, B., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 81–103. ISBN 978-94-024-1057-0. [Google Scholar]
- Carbrey, J.M.; Agre, P. Discovery of the Aquaporins and Development of the Field. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–28. [Google Scholar] [CrossRef]
- Macey, R.I. Transport of Water and Urea in Red Blood Cells. Am. J. Physiol. -Cell Physiol. 1984, 246, C195–C203. [Google Scholar] [CrossRef]
- Zeidel, M.L.; Ambudkar, S.V.; Smith, B.L.; Agre, P. Reconstitution of Functional Water Channels in Liposomes Containing Purified Red Cell CHIP28 Protein. Biochemistry 1992, 31, 7436–7440. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, K.; Uchida, S.; Harat, Y.; Hirata, Y.; Marumo, F.; Sasaki, S. Cloning and Expression of Apical Membrane Water Channel of Rat Kidney Collecting Tubule. Nature 1993, 361, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Maurel, C. The Role of Aquaporins in Root Water Uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The Mercury-Sensitive Residue at Cysteine 189 in the CHIP28 Water Channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kuang, K.; Haller, J.F.; Shi, G.; Kang, F.; Cheung, M.; Iserovich, P.; Fischbarg, J. Mercurial Sensitivity of Aquaporin 1 Endofacial Loop B Residues. Protein Sci. 2001, 10, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; Stroud, R.M. Structural Basis of Aquaporin Inhibition by Mercury. J. Mol. Biol. 2007, 368, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Yukutake, Y.; Tsuji, S.; Hirano, Y.; Adachi, T.; Takahashi, T.; Fujihara, K.; Agre, P.; Yasui, M.; Suematsu, M. Mercury Chloride Decreases the Water Permeability of Aquaporin-4-Reconstituted Proteoliposomes. Biol. Cell 2008, 100, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Gu, Y.; Ishibashi, K.; Marumo, F.; Sasaki, S. Mercury-Sensitive Residues and Pore Site in AQP3 Water Channel. Biochemistry 1997, 36, 13973–13978. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Hazama, A.; Kwon, T.-H.; Nielsen, S.; Guggino, W.B.; Agre, P. Rapid Gating and Anion Permeability of an Intracellular Aquaporin. Nature 1999, 402, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Spinello, A.; de Almeida, A.; Casini, A.; Barone, G. The Inhibition of Glycerol Permeation through Aquaglyceroporin-3 Induced by Mercury(II): A Molecular Dynamics Study. J. Inorg. Biochem. 2016, 160, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Fushimi, K.; Sasaki, S.; Marumo, F. Structure of Aquaporin-2 Vasopressin Water Channel (∗). J. Biol. Chem. 1996, 271, 5171–5176. [Google Scholar] [CrossRef]
- Hirano, Y.; Okimoto, N.; Kadohira, I.; Suematsu, M.; Yasuoka, K.; Yasui, M. Molecular Mechanisms of How Mercury Inhibits Water Permeation through Aquaporin-1: Understanding by Molecular Dynamics Simulation. Biophys. J. 2010, 98, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Y.; Chen, L.Y. Mercury Inhibits the L170C Mutant of Aquaporin Z by Making Waters Clog the Water Channel. Biophys. Chem. 2012, 160, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hazama, A.; Kozono, D.; Guggino, W.B.; Agre, P.; Yasui, M. Ion Permeation of AQP6 Water Channel Protein. J. Biol. Chem. 2002, 277, 29224–29230. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, H.; Yu, K.; Yang, J. Mechanism of Unusual AQP6 Activation by Mercury Binding to a Pore-External Residue C155. Biochem. Biophys. Res. Commun. 2022, 618, 1–7. [Google Scholar] [CrossRef]
- Xie, H.; Ma, S.; Zhao, Y.; Zhou, H.; Tong, Q.; Chen, Y.; Zhang, Z.; Yu, K.; Lin, Q.; Kai, L.; et al. Molecular Mechanisms of Mercury-Sensitive Aquaporins. J. Am. Chem. Soc. 2022, 144, 22229–22241. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.J.; Chaumont, F.; Mirkov, T.E.; Chrispeels, M.J. Characterization of a New Vacuolar Membrane Aquaporin Sensitive to Mercury at a Unique Site. Plant Cell 1996, 8, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Secchi, F.; Maciver, B.; Zeidel, M.L.; Zwieniecki, M.A. Functional Analysis of Putative Genes Encoding the PIP2 Water Channel Subfamily in Populus Trichocarpa. Tree Physiol. 2009, 29, 1467–1477. [Google Scholar] [CrossRef]
- Bottino, C.; Vázquez, M.; Devesa, V.; Laforenza, U. Impaired Aquaporins Expression in the Gastrointestinal Tract of Rat after Mercury Exposure. J. Appl. Toxicol. 2016, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C. Zinc. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-Finger Proteins in Health and Disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and Mechanism of Zinc Metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, B.L. Role of Zinc in Plasma Membrane Function. J. Nutr. 2000, 130, 1432S–1436S. [Google Scholar] [CrossRef] [PubMed]
- Klug, A.; Schwabe, J.W.R. Zinc Fingers. FASEB J. 1995, 9, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Németh-Cahalan, K.L.; Kalman, K.; Froger, A.; Hall, J.E. Zinc Modulation of Water Permeability Reveals That Aquaporin 0 Functions as a Cooperative Tetramer. J. Gen. Physiol. 2007, 130, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Mom, R.; Réty, S.; Mocquet, V.; Auguin, D. Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach. Int. J. Mol. Sci. 2024, 25, 2267. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective Secretion of Saliva in Transgenic Mice Lacking Aquaporin-5 Water Channels *. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Jo, Y.; Lee, Y.-H.; Park, K.; Park, H.-K.; Choi, S.-Y. Zn2+ Stimulates Salivary Secretions via Metabotropic Zinc Receptor ZnR/GPR39 in Human Salivary Gland Cells. Sci. Rep. 2019, 9, 17648. [Google Scholar] [CrossRef]
- Yukutake, Y.; Hirano, Y.; Suematsu, M.; Yasui, M. Rapid and Reversible Inhibition of Aquaporin-4 by Zinc. Biochemistry 2009, 48, 12059–12061. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.J.; Sinclair, T.R.; Taliercio, E. Silver and Zinc Inhibitors Influence Transpiration Rate and Aquaporin Transcript Abundance in Intact Soybean Plants. Environ. Exp. Bot. 2016, 122, 168–175. [Google Scholar] [CrossRef]
- Gitto, A.; Fricke, W. Zinc Treatment of Hydroponically Grown Barley Plants Causes a Reduction in Root and Cell Hydraulic Conductivity and Isoform-Dependent Decrease in Aquaporin Gene Expression. Physiol. Plant. 2018, 164, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, H.; Zaghdoud, C.; Nortes, P.A.; Carvajal, M.; Martínez-Ballesta, M.d.C. Differential Aquaporin Response to Distinct Effects of Two Zn Concentrations after Foliar Application in Pak Choi (Brassica rapa L.) Plants. Agronomy 2020, 10, 450. [Google Scholar] [CrossRef]
- Almeida, A.d.; Soveral, G.; Casini, A. Gold Compounds as Aquaporin Inhibitors: New Opportunities for Therapy and Imaging. Med. Chem. Commun. 2014, 5, 1444–1453. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but Elusive Drug Targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Graziani, V.; Marrone, A.; Re, N.; Coletti, C.; Platts, J.A.; Casini, A. A Multi-Level Theoretical Study to Disclose the Binding Mechanisms of Gold(III)–Bipyridyl Compounds as Selective Aquaglyceroporin Inhibitors. Chem. —A Eur. J. 2017, 23, 13802–13813. [Google Scholar] [CrossRef] [PubMed]
- Serna, A.; Galán-Cobo, A.; Rodrigues, C.; Sánchez-Gomar, I.; Toledo-Aral, J.J.; Moura, T.F.; Casini, A.; Soveral, G.; Echevarría, M. Functional Inhibition of Aquaporin-3 With a Gold-Based Compound Induces Blockage of Cell Proliferation. J. Cell. Physiol. 2014, 229, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.d.; Mósca, A.F.; Wragg, D.; Wenzel, M.; Kavanagh, P.; Barone, G.; Leoni, S.; Soveral, G.; Casini, A. The Mechanism of Aquaporin Inhibition by Gold Compounds Elucidated by Biophysical and Computational Methods. Chem. Commun. 2017, 53, 3830–3833. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.N.; Mósca, A.F.; Graziani, V.; Aikman, B.; Thomas, S.R.; de Almeida, A.; Platts, J.A.; Re, N.; Coletti, C.; Marrone, A.; et al. Insights into the Mechanisms of Aquaporin-3 Inhibition by Gold(III) Complexes: The Importance of Non-Coordinative Adduct Formation. Inorg. Chem. 2019, 58, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Cobo, A.G.; Echevarría, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting Aquaporin Function: Potent Inhibition of Aquaglyceroporin-3 by a Gold-Based Compound. PLoS ONE 2012, 7, e37435. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Ciancetta, A.; de Almeida, A.; Marrone, A.; Re, N.; Soveral, G.; Casini, A. Aquaporin Inhibition by Gold(III) Compounds: New Insights. ChemMedChem 2013, 8, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; de Almeida, A.; de Graaf, C.; Camps, M.; Zorzano, A.; Moura, T.F.; Casini, A.; Soveral, G. A Gold Coordination Compound as a Chemical Probe to Unravel Aquaporin-7 Function. ChemBioChem 2014, 15, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Liao, P.-L.; Tsai, C.-H.; Chan, Y.-J.; Cheng, Y.-W.; Hwang, L.-L.; Lin, K.-H.; Yen, T.-L.; Li, C.-H. Inhaled Gold Nanoparticles Cause Cerebral Edema and Upregulate Endothelial Aquaporin 1 Expression, Involving Caveolin 1 Dependent Repression of Extracellular Regulated Protein Kinase Activity. Part. Fibre Toxicol. 2019, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, C.M.; Tyerman, S.D. New Potent Inhibitors of Aquaporins: Silver and Gold Compounds Inhibit Aquaporins of Plant and Human Origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Frare, R.; Ayub, N.; Alleva, K.; Soto, G. The Ammonium Channel NOD26 Is the Evolutionary Innovation That Drives the Emergence, Consolidation, and Dissemination of Nitrogen-Fixing Symbiosis in Angiosperms. J. Mol. Evol. 2018, 86, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Harris, Z.L. Genetic Defects in Copper Metabolism. J. Nutr. 2003, 133, 1527S–1531S. [Google Scholar] [CrossRef] [PubMed]
- Zelenina, M.; Tritto, S.; Bondar, A.A.; Zelenin, S.; Aperia, A. Copper Inhibits the Water and Glycerol Permeability of Aquaporin-3 *. J. Biol. Chem. 2004, 279, 51939–51943. [Google Scholar] [CrossRef] [PubMed]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a Potent Copper-Based Aquaporin Inhibitor With Cytotoxic Effect Against Cancer Cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Zelenina, M.; Bondar, A.A.; Zelenin, S.; Aperia, A. Nickel and Extracellular Acidification Inhibit the Water Permeability of Human Aquaporin-3 in Lung Epithelial Cells *. J. Biol. Chem. 2003, 278, 30037–30043. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Selvín-Testa, A.; Capani, F.; Loidl, C.F.; López, E.M.; Pecci-Saavedra, J. Prenatal and Postnatal Lead Exposure Induces 70 kDa Heat Shock Protein in Young Rat Brain Prior to Changes in Astrocyte Cytoskeleton. Neurotoxicology 1997, 18, 805–817. [Google Scholar]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin Water Channels in the Nervous System. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef]
- Gunnarson, E.; Axehult, G.; Baturina, G.; Zelenin, S.; Zelenina, M.; Aperia, A. Lead Induces Increased Water Permeability in Astrocytes Expressing Aquaporin 4. Neuroscience 2005, 136, 105–114. [Google Scholar] [CrossRef]
- Bosma, E.F.; Rau, M.H.; van Gijtenbeek, L.A.; Siedler, S. Regulation and Distinct Physiological Roles of Manganese in Bacteria. FEMS Microbiol. Rev. 2021, 45, fuab028. [Google Scholar] [CrossRef]
- Pankau, C.; Cooper, R.L. Molecular Physiology of Manganese in Insects. Curr. Opin. Insect Sci. 2022, 51, 100886. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, L.W.; Elvehjem, C.A.; Hart, E.B. Studies on the Physiology of Manganese in the Rat. Am. J. Physiol. -Leg. Content 1943, 140, 72–82. [Google Scholar] [CrossRef]
- Campbell, L.C.; Nable, R.O. Physiological Functions of Manganese in Plants. In Manganese in Soils and Plants: Proceedings of the International Symposium on ‘Manganese in Soils and Plants’ Held at the Waite Agricultural Research Institute, The University of Adelaide, Glen Osmond, Australia, 22–26 August 1988 as an Australian Bicentennial Event; Graham, R.D., Hannam, R.J., Uren, N.C., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 139–154. ISBN 978-94-009-2817-6. [Google Scholar]
- Lei, L.; Huang, M.; Su, L.; Xie, D.; Mamuya, F.A.; Ham, O.; Tsuji, K.; Păunescu, T.G.; Yang, B.; Lu, H.A.J. Manganese Promotes Intracellular Accumulation of AQP2 via Modulating F-Actin Polymerization and Reduces Urinary Concentration in Mice. Am. J. Physiol. -Ren. Physiol. 2018, 314, F306–F316. [Google Scholar] [CrossRef] [PubMed]
- Rama Rao, K.V.; Jayakumar, A.R.; Reddy, P.V.B.; Tong, X.; Curtis, K.M.; Norenberg, M.D. Aquaporin-4 in Manganese-Treated Cultured Astrocytes. Glia 2010, 58, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; McLaren, G.D. (Eds.) Iron Physiology and Pathophysiology in Humans; Humana Press: Totowa, NJ, USA, 2012; ISBN 978-1-60327-484-5. [Google Scholar]
- Belaidi, A.A.; Bush, A.I. Iron Neurochemistry in Alzheimer’s Disease and Parkinson’s Disease: Targets for Therapeutics. J. Neurochem. 2016, 139, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Ximenes-da-Silva, A. Metal Ion Toxins and Brain Aquaporin-4 Expression: An Overview. Front. Neurosci. 2016, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cui, Z.; Zhong, Z.; Sun, Y.; Sun, Q.; Yang, G.; Bian, L. Curcumin Attenuates Brain Edema in Mice with Intracerebral Hemorrhage through Inhibition of AQP4 and AQP9 Expression. Acta Pharmacol. Sin. 2015, 36, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Sun, Y.; Wang, B.; Sun, Q.; Yang, G.; Bian, L. Involvement of Mitogen-Activated Protein Kinase Pathways in Ferrous Iron-Induced Aquaporin-4 Expression in Cultured Astrocytes. NeuroToxicology 2019, 73, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Qing, W.G.; Dong, Y.Q.; Ping, T.Q.; Lai, L.G.; Fang, L.D.; Min, H.W.; Xia, L.; Heng, P.Y. Brain Edema after Intracerebral Hemorrhage in Rats: The Role of Iron Overload and Aquaporin 4: Laboratory Investigation. J. Neurosurg. 2009, 110, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nan, D.; Liu, R.; Li, J.; Zhang, Z.; Deng, J.; Zhang, Y.; Yan, Z.; Hou, C.; Yao, E.; et al. Aquaporin 4 Mediates the Effect of Iron Overload on Hydrocephalus After Intraventricular Hemorrhage. Neurocrit Care 2024, 40, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Freeman, S.A. Lithium: Clinical Considerations in Internal Medicine. Am. J. Med. 2006, 119, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Timmer, R.T.; Sands, J.M. Lithium Intoxication. J. Am. Soc. Nephrol. 1999, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.-H.; Laursen, U.H.; Marples, D.; Maunsbach, A.B.; Knepper, M.A.; Frøkiær, J.; Nielsen, S. Altered Expression of Renal AQPs and Na+transporters in Rats with Lithium-Induced NDI. Am. J. Physiol. -Ren. Physiol. 2000, 279, F552–F564. [Google Scholar] [CrossRef] [PubMed]
- Marples, D.; Christensen, S.; Christensen, E.I.; Ottosen, P.D.; Nielsen, S. Lithium-Induced Downregulation of Aquaporin-2 Water Channel Expression in Rat Kidney Medulla. J. Clin. Investig. 1995, 95, 1838–1845. [Google Scholar] [CrossRef]
- Walker, R.J.; Weggery, S.; Bedford, J.J.; Mcdonald, F.J.; Ellis, G.; Leader, J.P. Lithium-Induced Reduction in Urinary Concentrating Ability and Urinary Aquaporin 2 (AQP2) Excretion in Healthy Volunteers. Kidney Int. 2005, 67, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Kortenoeven, M.L.A.; Schweer, H.; Cox, R.; Wetzels, J.F.M.; Deen, P.M.T. Lithium Reduces Aquaporin-2 Transcription Independent of Prostaglandins. Am. J. Physiol. -Cell Physiol. 2012, 302, C131–C140. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Hoffert, J.D.; Knepper, M.A.; Agre, P.; Nielsen, S.; Fenton, R.A. Proteomic Analysis of Lithium-Induced Nephrogenic Diabetes Insipidus: Mechanisms for Aquaporin 2 down-Regulation and Cellular Proliferation. Proc. Natl. Acad. Sci. USA 2008, 105, 3634–3639. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Edemir, B. Lithium Chloride and GSK3 Inhibition Reduce Aquaporin-2 Expression in Primary Cultured Inner Medullary Collecting Duct Cells Due to Independent Mechanisms. Cells 2020, 9, 1060. [Google Scholar] [CrossRef] [PubMed]
- Kortenoeven, M.L.A.; Li, Y.; Shaw, S.; Gaeggeler, H.-P.; Rossier, B.C.; Wetzels, J.F.M.; Deen, P.M.T. Amiloride Blocks Lithium Entry through the Sodium Channel Thereby Attenuating the Resultant Nephrogenic Diabetes Insipidus. Kidney Int. 2009, 76, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Roxas, B.; Farjah, M.; Danziger, R.S. Aquaporin-2 Transcript Is Differentially Regulated by Dietary Salt in Sprague–Dawley and Dahl SS/Jr Rats. Biochem. Biophys. Res. Commun. 2002, 296, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Sutters, M.; Duncan, R.; Peart, W.S. Effect of Dietary Salt Restriction on Renal Sensitivity to Vasopressin in Man. Clin. Sci. 1995, 89, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Buemi, M.; Bolignano, D.; Coppolino, G.; Di Pasquale, G.; Cosentini, V.; Campo, S.; Barillà, A.; Aloisi, C. Aquaporin-2 (AQP2) Urinary Excretion and Assumption of Water with Different Mineral Content in Healthy Subjects. Ren. Fail. 2007, 29, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, X.; Shi, M.; Knepper, M.A.; Ecelbarger, C.A. Effects of Dietary Fat, NaCl, and Fructose on Renal Sodium and Water Transporter Abundances and Systemic Blood Pressure. Am. J. Physiol. Ren. Physiol. 2004, 287, F1204–F1212. [Google Scholar] [CrossRef] [PubMed]
- Graffe, C.C.; Bech, J.N.; Pedersen, E.B. Effect of High and Low Sodium Intake on Urinary Aquaporin-2 Excretion in Healthy Humans. Am. J. Physiol. -Ren. Physiol. 2012, 302, F264–F275. [Google Scholar] [CrossRef] [PubMed]
- Storm, R.; Klussmann, E.; Geelhaar, A.; Rosenthal, W.; Maric, K. Osmolality and Solute Composition Are Strong Regulators of AQP2 Expression in Renal Principal Cells. Am. J. Physiol. -Ren. Physiol. 2003, 284, F189–F198. [Google Scholar] [CrossRef] [PubMed]
- Mom, R.; Robert-Paganin, J.; Mom, T.; Chabbert, C.; Réty, S.; Auguin, D. A Perspective for Ménière’s Disease: In Silico Investigations of Dexamethasone as a Direct Modulator of AQP2. Biomolecules 2022, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Mom, R.; Réty, S.; Auguin, D. Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids. Int. J. Mol. Sci. 2023, 24, 1499. [Google Scholar] [CrossRef] [PubMed]
- Vaziriyeganeh, M.; Carvajal, M.; Du, N.; Zwiazek, J.J. Salinity Tolerance of Halophytic Grass Puccinellia Nuttalliana Is Associated with Enhancement of Aquaporin-Mediated Water Transport by Sodium. Int. J. Mol. Sci. 2022, 23, 5732. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Beitz, E. Aquaporins with Selectivity for Unconventional Permeants. Cell. Mol. Life Sci. 2007, 64, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Rambow, J.; Wu, B.; Rönfeldt, D.; Beitz, E. Aquaporins with Anion/Monocarboxylate Permeability: Mechanisms, Relevance for Pathogen–Host Interactions. Front. Pharmacol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Yoshinari, A.; Luu, D.-T. Plant Aquaporin Trafficking. In Plant Aquaporins: From Transport to Signaling; Chaumont, F., Tyerman, S.D., Eds.; Signaling and Communication in Plants; Springer International Publishing: Cham, Switzerland, 2017; pp. 47–81. ISBN 978-3-319-49395-4. [Google Scholar]
- Yaneff, A.; Sigaut, L.; Marquez, M.; Alleva, K.; Pietrasanta, L.I.; Amodeo, G. Heteromerization of PIP Aquaporins Affects Their Intrinsic Permeability. Proc. Natl. Acad. Sci. USA 2014, 111, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Hedfalk, K.; Törnroth-Horsefield, S.; Nyblom, M.; Johanson, U.; Kjellbom, P.; Neutze, R. Aquaporin Gating. Curr. Opin. Struct. Biol. 2006, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Z.; McDill, B.W.; Kovach, P.A.; Ding, L.; Go, W.Y.; Ho, S.N.; Chen, F. Calcineurin-NFATc Signaling Pathway Regulates AQP2 Expression in Response to Calcium Signals and Osmotic Stress. Am. J. Physiol. -Cell Physiol. 2007, 292, C1606–C1616. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.T.; Mason, P.E.; Anderson, J.L.R.; Dempsey, C.E. Arginine Side Chain Interactions and the Role of Arginine as a Gating Charge Carrier in Voltage Sensitive Ion Channels. Sci. Rep. 2016, 6, 21759. [Google Scholar] [CrossRef] [PubMed]
- Ruta, V.; Chen, J.; MacKinnon, R. Calibrated Measurement of Gating-Charge Arginine Displacement in the KvAP Voltage-Dependent K+ Channel. Cell 2005, 123, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Marracino, P.; Ghaani, M.R.; Liberti, M.; Del Signore, F.; Burnham, C.J.; Gárate, J.-A.; Apollonio, F.; English, N.J. Human Aquaporin 4 Gating Dynamics under Axially Oriented Electric-Field Impulses: A Non-Equilibrium Molecular-Dynamics Study. J. Chem. Phys. 2018, 149, 245102. [Google Scholar] [CrossRef]
- Burnham, C.J.; English, N.J. Electropumping of Water Through Human Aquaporin 4 by Circularly Polarized Electric Fields: Dramatic Enhancement and Control Revealed by Non-Equilibrium Molecular Dynamics. J. Phys. Chem. Lett. 2017, 8, 4646–4651. [Google Scholar] [CrossRef] [PubMed]
- Marracino, P.; Liberti, M.; Trapani, E.; Burnham, C.J.; Avena, M.; Garate, J.-A.; Apollonio, F.; English, N.J. Human Aquaporin 4 Gating Dynamics under Perpendicularly-Oriented Electric-Field Impulses: A Molecular Dynamics Study. Int. J. Mol. Sci. 2016, 17, 1133. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, H.; Kamali, R. Non-Equilibrium Molecular Dynamics Study of Human Aquaporin-2 in the Static External Electric Fields. J. Biomol. Struct. Dyn. 2022, 40, 10793–10801. [Google Scholar] [CrossRef]
- Reale, R.; English, N.J.; Garate, J.-A.; Marracino, P.; Liberti, M.; Apollonio, F. Human Aquaporin 4 Gating Dynamics under and after Nanosecond-Scale Static and Alternating Electric-Field Impulses: A Molecular Dynamics Study of Field Effects and Relaxation. J. Chem. Phys. 2013, 139, 205101. [Google Scholar] [CrossRef] [PubMed]
- Garate, J.-A.; English, N.J.; MacElroy, J.M.D. Human Aquaporin 4 Gating Dynamics in Dc and Ac Electric Fields: A Molecular Dynamics Study. J. Chem. Phys. 2011, 134, 055110. [Google Scholar] [CrossRef] [PubMed]
- English, N.J.; Garate, J.-A. Near-Microsecond Human Aquaporin 4 Gating Dynamics in Static and Alternating External Electric Fields: Non-Equilibrium Molecular Dynamics. J. Chem. Phys. 2016, 145, 085102. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.S.; Aponte-Santamaría, C.; Grubmüller, H.; de Groot, B.L. Voltage-Regulated Water Flux through Aquaporin Channels In Silico. Biophys. J. 2010, 99, L97–L99. [Google Scholar] [CrossRef]
- Mom, R.; Muries, B.; Benoit, P.; Robert-Paganin, J.; Réty, S.; Venisse, J.-S.; Padua, A.; Label, P.; Auguin, D. Voltage-Gating of Aquaporins, a Putative Conserved Safety Mechanism during Ionic Stresses. FEBS Lett. 2021, 595, 41–57. [Google Scholar] [CrossRef] [PubMed]


| Ion Name | Name of the Regulated AQP | Direct Interaction with AQP | Water Channel Function | Residues Involved in Binding | Residues Involved in Molecular Mechanism | Transcripts and/or Protein Abundance |
|---|---|---|---|---|---|---|
| Calcium | SoPIP2;1 | yes | inhibition [31] | D28 and E31 [31] T183 and A267 [60] | D28, E31, R190, R191 and H193 [31,57] | Unknown |
| Calcium | SoPIP2;1 | No | Activation | None | S115, S188 and S274 [31,58,59] | unknown |
| Calcium | AtPIP2;1 | Yes | Inhibition | E31, H199 [62] | E31, H199 and R124 [62] | unknown |
| Calcium | Human AQP2 | yes | Unknown | Q57, E155 [56,63] | Unknown | increase [50,165] |
| Calcium | Ovine AQP0 Human AQP0 | No | Inhibition | None | L227, L234, A240 and R156 (CaM binding) [41,55] S229, S231 and S235 (phosphorylation sites) [46] R187, Y149, Y23 (water permeability modulation) [45,54] | unknown |
| Calcium | Human AQP4 | No | Unknown | None | Residues 6-31 in AQP4-M1 residues 256-280 in AQP4-M1 (CaM binding) [49] | unknown |
| Calcium | Mouse AQP6 rat AQP6 human AQP6 | No | Unknown | None | L15, L19, A22 and F28 in human AQP6 [48] | unknown |
| Magnesium | Human AQP3 | Unknown | Unknown | Unknown | Unknown | increase [67,68,69] |
| Magnesium | Rat AQP4 human AQP4 | Unknown | Unknown | Unknown | Unknown | No effect or decrease [70,71,72,73] |
| Mercury | E. coli AQPZ | yes | Inhibition | C20 [82] | C20 [82] R189 [92] | unknown |
| Mercury | Human AQP3 | yes | Inhibition | C40 | F63, R218 [86] | Decrease (rat AQP3) [95] |
| Mercury | Human AQP1 bovine AQP1 | yes | Inhibition | Human: C189 [80] bovine: C191 [88] | For bovine AQP1: R197, H182, F58, I193, G192, C191, G190, E144 [88] | Unknown |
| Mercury | Human AQP2 | yes | Inhibition | C181 [87] | Unknown | unknown |
| Mercury | rat AQP4 | yes | Inhibition | C178 [83] | Unknown | Decrease [95] |
| Mercury | Rat AQP6 | yes | Activation | C155 and C190 [85,90] | R196 [92] and M160 [91] | No effect |
| Mercury | AtTIPs | Yes | Inhibition | C116 or C118 [93] | C116 or C118 [93] | unknown |
| Mercury | Rat AQP7 | Unknown | Unknown | Unknown | Unknown | decrease [95] |
| Zinc | bovine AQP0 human AQP0 | yes | Activation | H40 and H122 [101] C144, T54, Q140 [102] | R187 [102] | unknown |
| Zinc | Human AQP5 | yes | Activation | C145, S149, S164, S168, T55, Q58 [102] | Human: R187 [102] | increase [104] |
| Zinc | Rat AQP4 Human AQP4 | yes | Inhibition | Rat: C178 [105] human: C253, D179, H90 [102] | Human: R216 [102] | unknown |
| Zinc | Human AQP2 | yes | Inhibition | C75 [102] | R216 [102] | unknown |
| Zinc | Soybean AQPs | Unknown | Unknown | Unknown | Unknown | No effect or increase [106] |
| Zinc | Barley AQPs (HvPIP1;3, HvPIP2;4 and HvPIP2;5) | Unknown | Unknown | Unknown | Unknown | decrease [107] |
| Zinc | Pak choi AQPs (PIP1 isoforms) | unknown | Unknown | Unknown | Unknown | increase [108] |
| Zinc | Human AQP3 | No | No effect [124] | None | None | unknown |
| Cadmium | Human AQP2 | Yes | Unknown | Q57, E155 [63] | Unknown | unknown |
| Cadmium | SoPIP2;1 | Yes | Inhibition | D28 and E31 [31] T183 and A267 [60] | D28, E31, R190, R191 and H193 [31,57] | unknown |
| Cadmium | AtPIP2;1 | Yes | Inhibition | E31 and H199 [62] | E31, H199 and R124 [62] | unknown |
| Cadmium | Human AQP3 | No | No effect [124] | None | None | Unknown |
| Gold | Human AQP3 | yes | Inhibition | C40 [114] | R218 [114] | unknown |
| Gold | Human AQP7 | yes | Inhibition | Met47 [117] | unknown | Unknown |
| Gold | mouse AQP1 | Unknown | Unknown | Unknown | Unknown | increase [118] |
| Silver | Soybean NOD26 | Unknown | inhibition [119] | Unknown | Unknown | unknown |
| Silver | Beet root PIPs | Unknown | inhibition [119] | Unknown | Unknown | unknown |
| Silver | Human AQP1 and human AQP3 | Unknown | Inhibition [119] | Unknown | Unknown | unknown |
| Silver | Soybean AQPs | Unknown | Unknown | Unknown | Unknown | No effect or increase [106] |
| Copper | Human AQP3 | Unknown | inhibition | unknown | W128, S152, H241 [122] | Unknown |
| Copper | Human AQP4 | no | No effect [122] | None | none | unknown |
| Copper | Mouse AQP7 | no | No effect [122] | None | none | Unknown |
| Nickel | Human AQP3 | Unknown | Inhibition | unknown | W128, S152, H241 [124] | Unknown |
| Nickel | Human AQP4 | no | No effect [124] | none | none | unknown |
| Nickel | Human AQP5 | no | No effect [124] | None | None | unknown |
| Nickel | AtPIP2;1 | Yes | Inhibition [62] | Unknown | unknown | unknown |
| Lead | Mouse AQP4 | unknown | Activation | Unknown | S111 [128] | No effect [128] |
| Lead | Human AQP3 | No | No effect [128] | None | None | unknown |
| Manganese | AtPIP2;1 | Yes | Inhibition | E31 and H199 [62] | E31, H199 and R124 [62] | unknown |
| Manganese | Rat AQP2 | Unknown | Inhibition [133] | Unknown | Unknown | unknown |
| Manganese | Rat AQP4 | Unknown | Activation [134] | Unknown | Unknown | No effect |
| Iron | Mouse AQP4 rat AQP4 | Unknown | Unknown | Unknown | Unknown | increase [138,139,140,141] |
| Iron | Mouse AQP9 | Unknown | Unknown | Unknown | Unknown | increase [138] |
| Lithium | Human AQP2 [146] rat AQP2 [144,145,148,149] mouse AQP2 [147,150] | Unknown | Unknown | Unknown | Unknown | decrease [144,145,146,147,148,149,150] |
| Lithium | Rat AQP3 | Unknown | Unknown | Unknown | Unknown | decrease [144] |
| Lithium | Rat AQP1 | Unknown | Unknown | Unknown | Unknown | No effect [144] |
| Sodium | Rat AQP2 [151,154,156] human AQP2 [152,153,155] | yes [157] | Unknown | E106, D111, D115, D199 and D200 in human AQP2 [157] | Unknown | Conflicting results: decrease [151] increase [152,153,154,155,156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mom, R.; Mocquet, V.; Auguin, D.; Réty, S. Aquaporin Modulation by Cations, a Review. Curr. Issues Mol. Biol. 2024, 46, 7955-7975. https://doi.org/10.3390/cimb46080470
Mom R, Mocquet V, Auguin D, Réty S. Aquaporin Modulation by Cations, a Review. Current Issues in Molecular Biology. 2024; 46(8):7955-7975. https://doi.org/10.3390/cimb46080470
Chicago/Turabian StyleMom, Robin, Vincent Mocquet, Daniel Auguin, and Stéphane Réty. 2024. "Aquaporin Modulation by Cations, a Review" Current Issues in Molecular Biology 46, no. 8: 7955-7975. https://doi.org/10.3390/cimb46080470







