Advances in Microflow Cytometry-Based Molecular Detection Methods for Improved Future MDS Cancer Diagnosis
Abstract
1. Introduction
- focusing the particles to be analyzed in the microfluidic channel,
- miniaturization of the fluid-handling components,
- miniaturization of the optics, and
- integration and applications development.
2. Recent Progress on Cancer Diagnosis Using Flow Cytometry
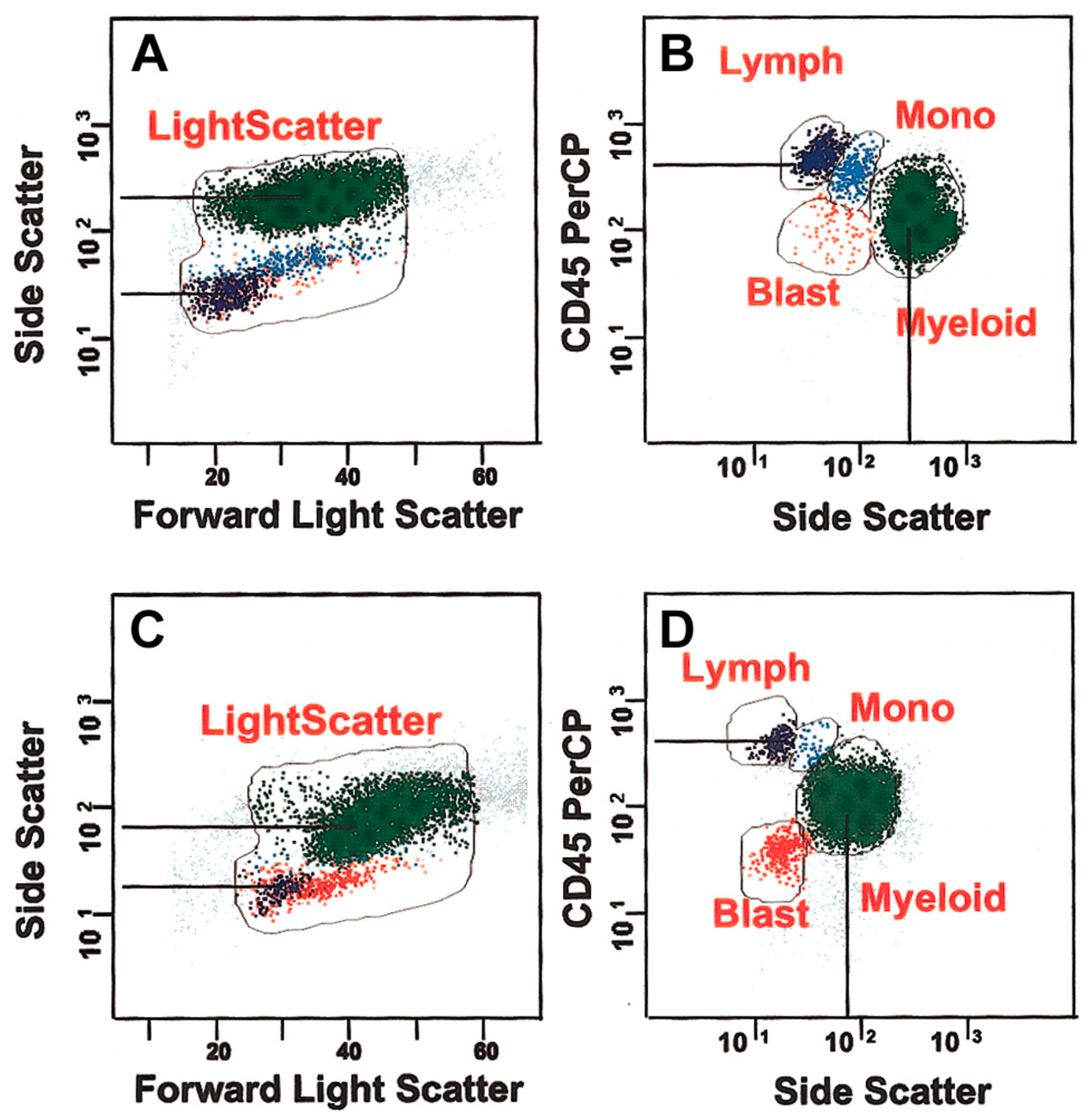
2.1. MDS Diagnosis
2.2. Leukemia Diagnosis
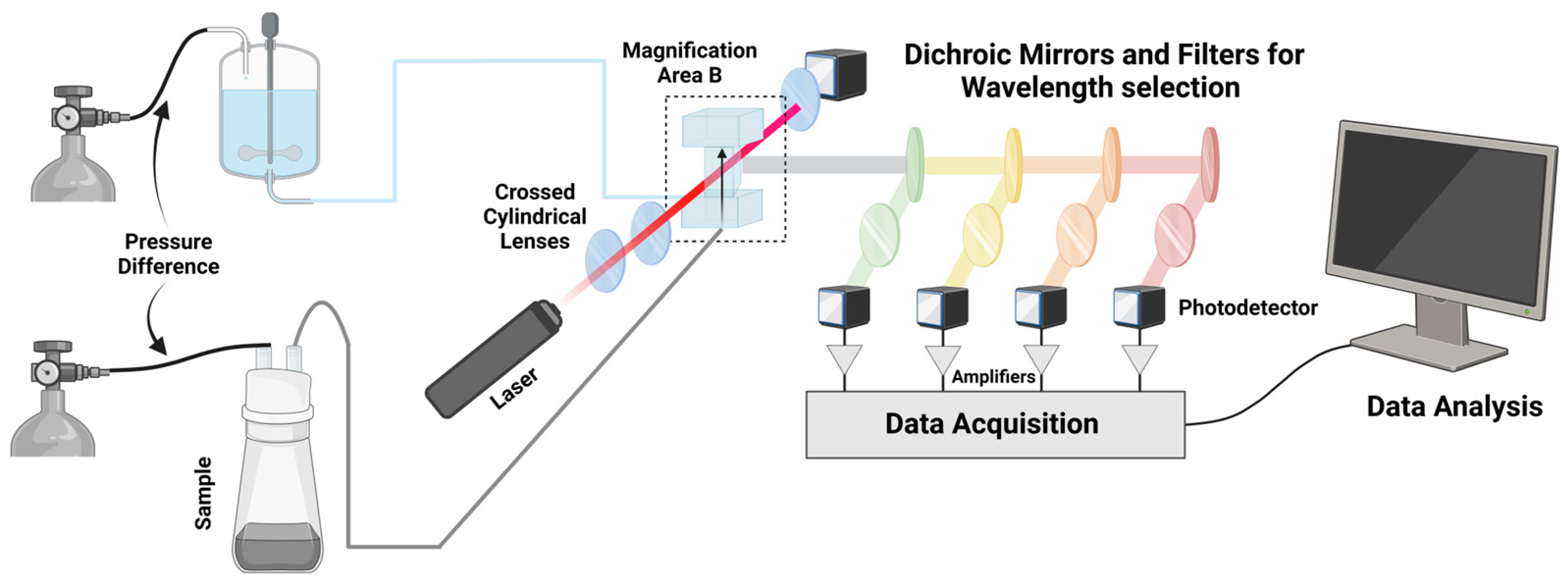
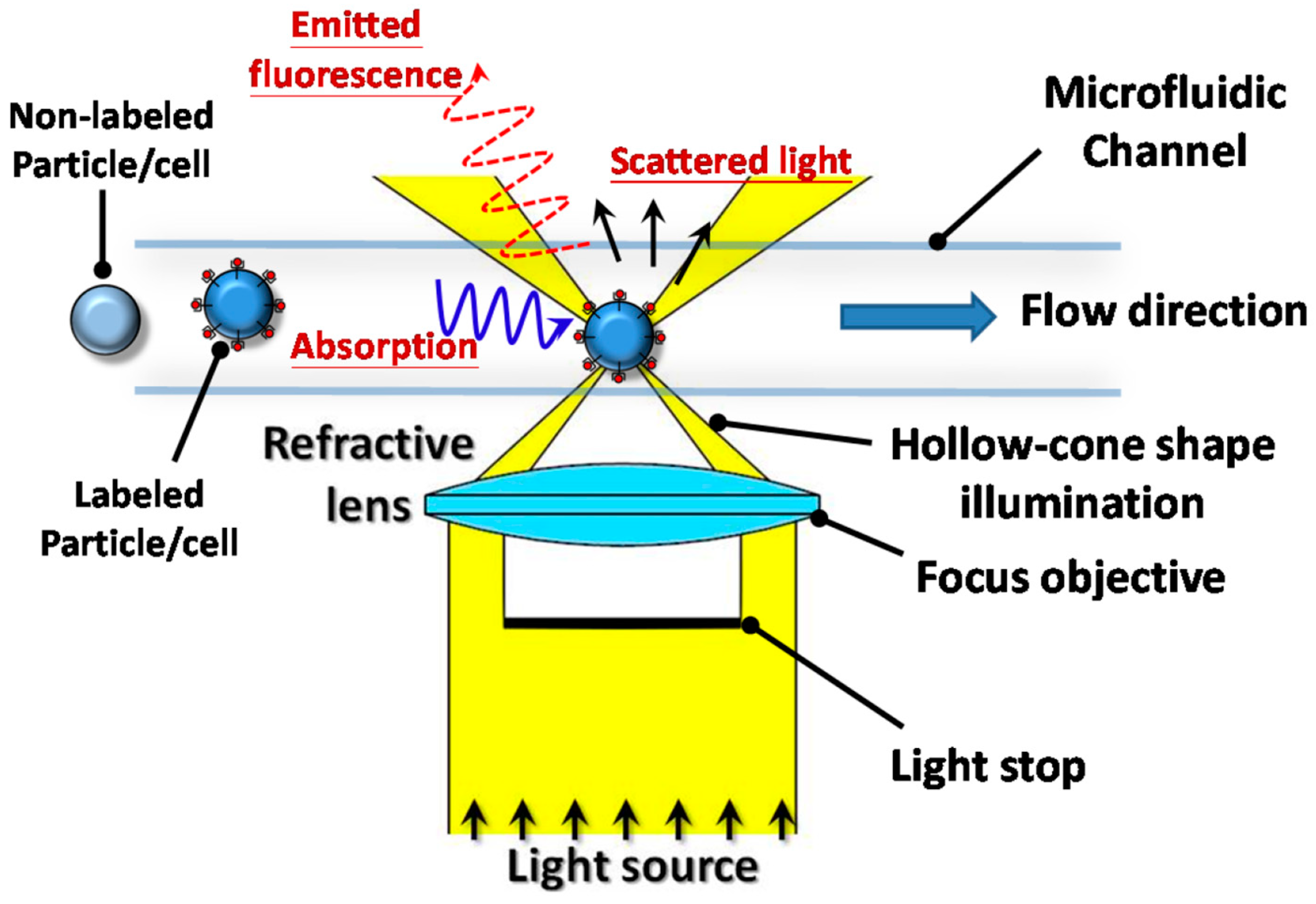
3. Microflow Cytometry as a Novel Integrated Platform for Diagnostics
3.1. Microflow Cytometry as a Diagnostic Tool
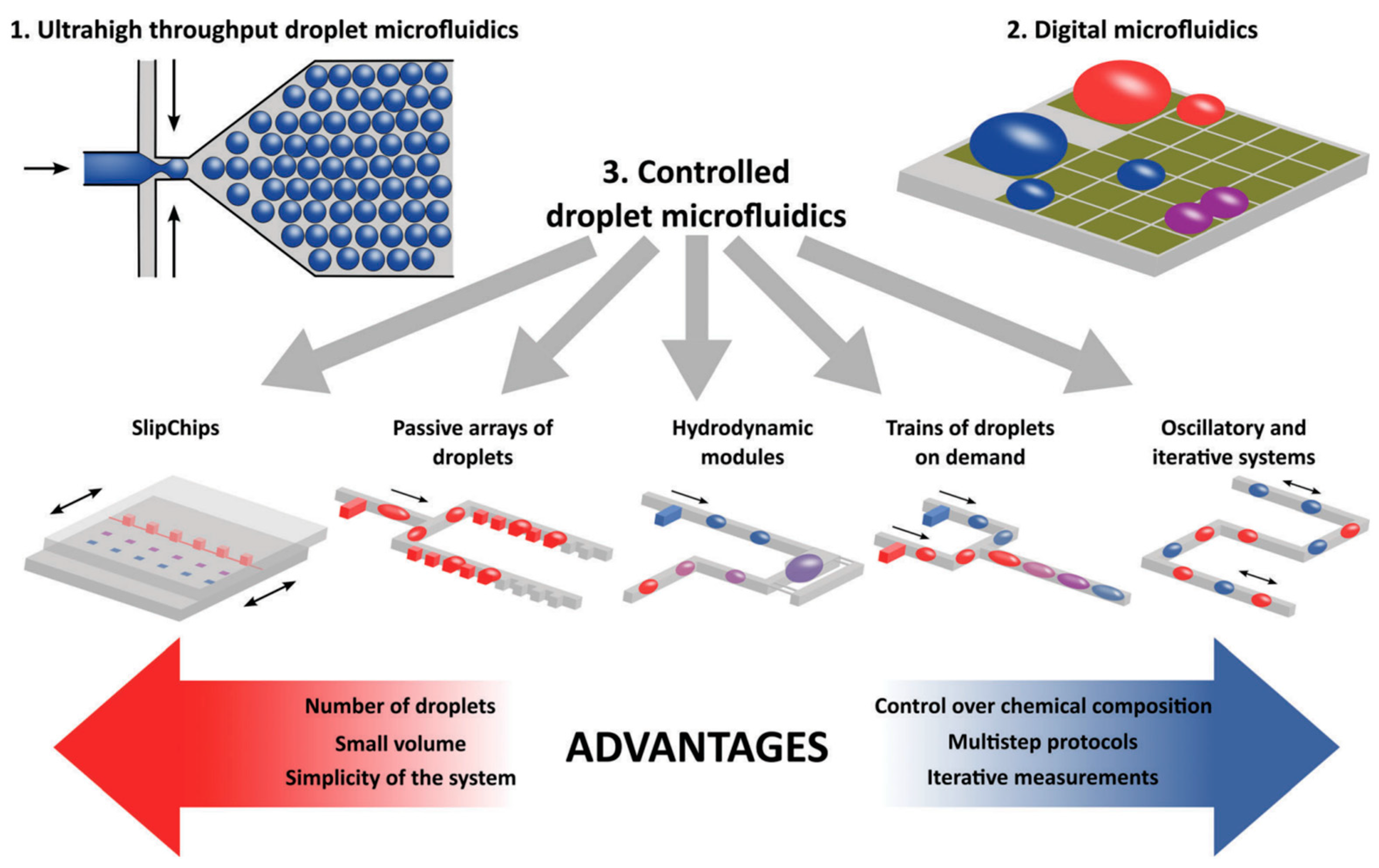
3.2. Microfluidic Subtypes and Classifications
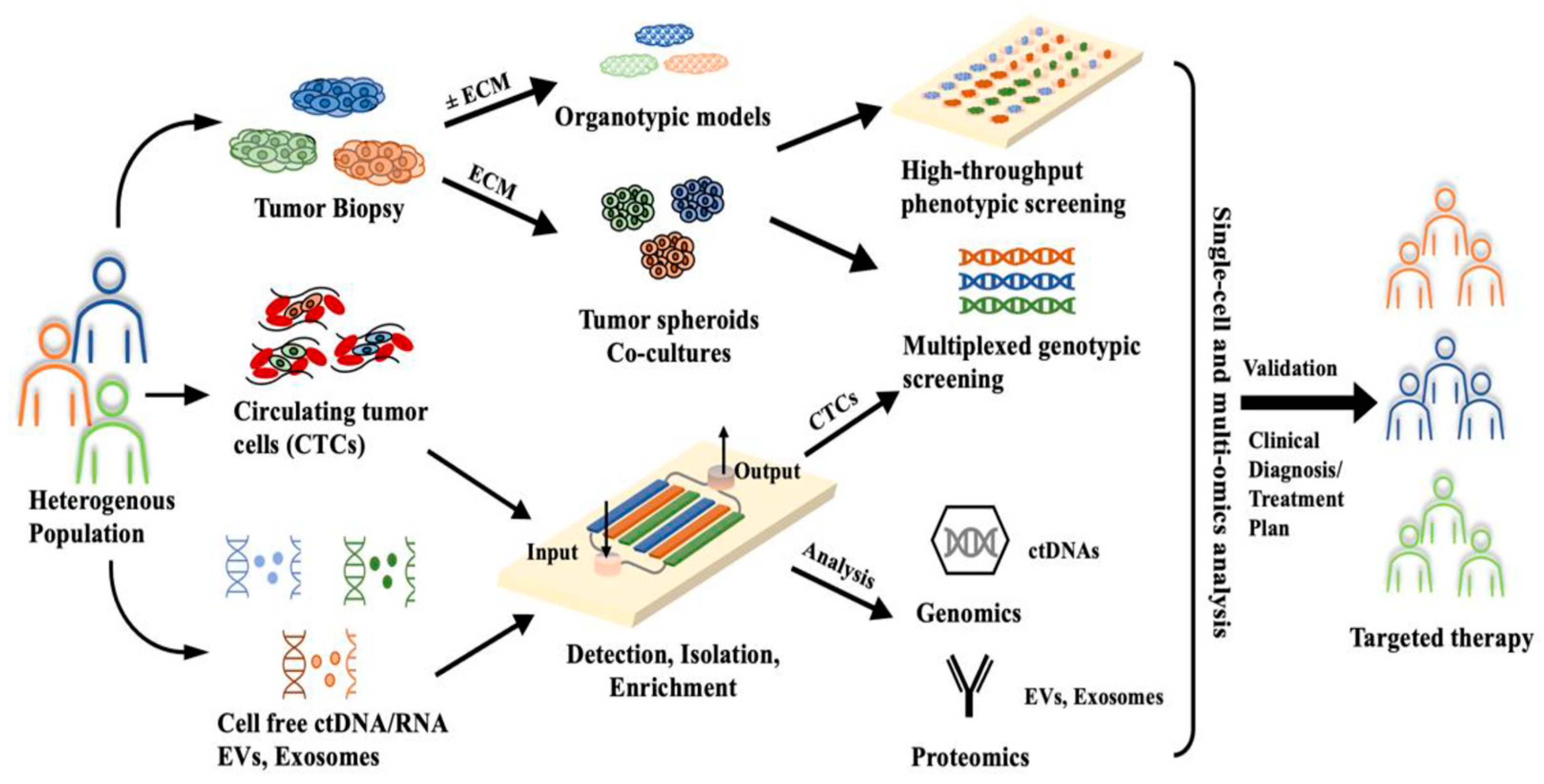
4. Next Steps: MDS Diagnosis with Microflow Cytometry
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Burden Growing, amidst Mounting Need for Services. Available online: https://www.iarc.who.int/news-events/global-cancer-burden-growing-amidst-mounting-need-for-services/ (accessed on 18 April 2024).
- Lemieux, M.E.; Reveles, X.T.; Rebeles, J.; Bederka, L.H.; Araujo, P.R.; Sanchez, J.R.; Grayson, M.; Lai, S.C.; DePalo, L.R.; Habib, S.A.; et al. Detection of early-stage lung cancer in sputum using automated flow cytometry and machine learning. Respir. Res. 2023, 24, 23. [Google Scholar] [CrossRef]
- Andreou, M.; Vartholomatos, E.; Harissis, H.; Markopoulos, G.S.; Alexiou, G.A. Past, Present and Future of Flow Cytometry in Breast Cancer—A Systematic Review. EJIFCC 2019, 30, 423–437. [Google Scholar]
- Austin Suthanthiraraj, P.P.; Graves, S.W. Fluidics. Curr. Protoc. Cytom. 2013, 65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shapiro, H.M. Practical Flow Cytometry; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Zhang, Y.; Watts, B.R.; Guo, T.; Zhang, Z.; Xu, C.; Fang, Q. Optofluidic Device Based Microflow Cytometers for Particle/Cell Detection: A Review. Micromachines 2016, 7, 70. [Google Scholar] [CrossRef]
- Perfetto, S.P.; Chattopadhyay, P.K.; Roederer, M. Seventeen-colour flow cytometry: Unravelling the immune system. Nat. Rev. Immunol. 2004, 4, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.N.; Bejar, R. MDS overlap disorders and diagnostic boundaries. Blood 2019, 133, 1086–1095. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Taylor, J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review. JAMA 2022, 328, 872–880. [Google Scholar] [CrossRef]
- Hasserjian, R.P.; Germing, U.; Malcovati, L. Diagnosis and classification of myelodysplastic syndromes. Blood 2023, 142, 2247–2257. [Google Scholar] [CrossRef]
- van de Loosdrecht, A.A.; Alhan, C.; Bene, M.C.; Della Porta, M.G.; Drager, A.M.; Feuillard, J.; Font, P.; Germing, U.; Haase, D.; Homburg, C.H.; et al. Standardization of flow cytometry in myelodysplastic syndromes: Report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica 2009, 94, 1124–1134. [Google Scholar] [CrossRef]
- Bento, L.C.; Correia, R.P.; Pitangueiras Mangueira, C.L.; De Souza Barroso, R.; Rocha, F.A.; Bacal, N.S.; Marti, L.C. The Use of Flow Cytometry in Myelodysplastic Syndromes: A Review. Front. Oncol. 2017, 7, 270. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Picone, C.; Pascutto, C.; Malcovati, L.; Tamura, H.; Handa, H.; Czader, M.; Freeman, S.; Vyas, P.; Porwit, A.; et al. Multicenter validation of a reproducible flow cytometric score for the diagnosis of low-grade myelodysplastic syndromes: Results of a European LeukemiaNET study. Haematologica 2012, 97, 1209–1217. [Google Scholar] [CrossRef]
- Grille Montauban, S.; Hernandez-Perez, C.R.; Velloso, E.D.R.P.; Novoa, V.; Lorand-Metze, I.; Gonzalez, J.; Solari, L.; Cismondi, V.; Serrano, J.C.; Burgnini, A.; et al. Flow cytometry “Ogata score” for the diagnosis of myelodysplastic syndromes in a real-life setting. A Latin American experience. Int. J. Lab. Hematol. 2019, 41, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A. The Myelodysplastic Syndromes. In Current Clinical Medicine, 2nd ed.; Cleveland, C., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2010; pp. 606–609. [Google Scholar]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Toprak, S.K. Past, present and future in low-risk myelodysplastic syndrome. Front. Med. 2022, 9, 967900. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.J.; Ebert, B.L.; Steensma, D.P. Chapter 60—Myelodysplastic Syndromes. In Hematology, 7th ed.; Hoffman, R., Benz, E.J., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 944–969.e913. [Google Scholar]
- Bernard, E.; Tuechler, H.; Greenberg Peter, L.; Hasserjian Robert, P.; Arango Ossa Juan, E.; Nannya, Y.; Devlin Sean, M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef] [PubMed]
- Ateya, D.A.; Erickson, J.S.; Howell, P.B., Jr.; Hilliard, L.R.; Golden, J.P.; Ligler, F.S. The good, the bad, and the tiny: A review of microflow cytometry. Anal. Bioanal. Chem. 2008, 391, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, R.S.; Sen, A.K. The Microflow Cytometer. In Environmental, Chemical and Medical Sensors; Bhattacharya, S., Agarwal, A.K., Chanda, N., Pandey, A., Sen, A.K., Eds.; Springer: Singapore, 2018; pp. 371–387. [Google Scholar]
- Lin, S.-W.; Lin, C.-H. Chip-Based Cytometry Illuminated by a Blade-Shape Continuous Light for Multispectral Detection. Appl. Sci. 2016, 6, 229. [Google Scholar] [CrossRef]
- Pillai, S.; Kwan, J.C.; Yaziji, F.; Yu, H.; Tran, S.D. Mapping the Potential of Microfluidics in Early Diagnosis and Personalized Treatment of Head and Neck Cancers. Cancers 2023, 15, 3894. [Google Scholar] [CrossRef]
- Emde, B.; Kreher, H.; Bäumer, N.; Bäumer, S.; Bouwes, D.; Tickenbrock, L. Microfluidic-Based Detection of AML-Specific Biomarkers Using the Example of Promyelocyte Leukemia. Int. J. Mol. Sci. 2020, 21, 8942. [Google Scholar] [CrossRef]
- Gonidec, M.; Puigmartí-Luis, J. Continuous- versus Segmented-Flow Microfluidic Synthesis in Materials Science. Crystals 2019, 9, 12. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Garstecki, P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem. Soc. Rev. 2017, 46, 6210–6226. [Google Scholar] [CrossRef] [PubMed]
- Elshoeibi, A.M.; Badr, A.; Elsayed, B.; Metwally, O.; Elshoeibi, R.; Elhadary, M.R.; Elshoeibi, A.; Attya, M.A.; Khadadah, F.; Alshurafa, A.; et al. Integrating AI and ML in Myelodysplastic Syndrome Diagnosis: State-of-the-Art and Future Prospects. Cancers 2024, 16, 65. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.; Zhou, L.; Pierce, S.; Kantarjian, H.; Estrov, Z.; Verstovsek, S. Clinical Significance of Bone Marrow Blast Percentage in Patients with Myelofibrosis and the Effect of Ruxolitinib Therapy. Clin. Lymphoma Myeloma Leuk. 2021, 21, 318–327 e316. [Google Scholar] [CrossRef]
- Hodes, A.; Calvo, K.R.; Dulau, A.; Maric, I.; Sun, J.; Braylan, R. The challenging task of enumerating blasts in the bone marrow. Semin. Hematol. 2019, 56, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Flow Cytometry in the Diagnosis of Leukemias. In Leukemia; Li, W., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Wells, D.A.; Benesch, M.; Loken, M.R.; Vallejo, C.; Myerson, D.; Leisenring, W.M.; Deeg, H.J. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood 2003, 102, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Dietrich Werner, D.N.; George, D. What Are Other Cellular Populations That May Fall in the SCC vs. CD45 Dim Area but Are Not Blasts CD34 Positive. Available online: https://www.cytometry.org/web/q_view.php?id=355&filter=Interpretation%20and%20Clinical%20Application (accessed on 20 April 2024).
- Alhan, C.; Westers, T.M.; Cremers, E.M.P.; Cali, C.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Application of flow cytometry for myelodysplastic syndromes: Pitfalls and technical considerations. Cytom. Part B Clin. Cytom. 2016, 90, 358–367. [Google Scholar] [CrossRef]
- van de Loosdrecht, A.A.; Kern, W.; Porwit, A.; Valent, P.; Kordasti, S.; Cremers, E.; Alhan, C.; Duetz, C.; Dunlop, A.; Hobo, W.; et al. Clinical application of flow cytometry in patients with unexplained cytopenia and suspected myelodysplastic syndrome: A report of the European LeukemiaNet International MDS-Flow Cytometry Working Group. Cytom. B Clin. Cytom. 2023, 104, 77–86. [Google Scholar] [CrossRef]
- Liu, F.; Cao, Q. Transformation of myelodysplastic syndrome to acute myeloid leukemia: A case with whole-body 2-[F18] fluoro-2-deoxy-D-glucose positron emission tomography. Indian J. Nucl. Med. 2011, 26, 104–106. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.; Abdul-Hay, M. Acute lymphoblastic leukemia: A comprehensive review and 2017 update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef] [PubMed]
- Fayed, D.; Donia, T.; El-Shanshory, M.; Ali, E.M.M.; Mohamed, T.M. Evaluation of MicroRNA92, MicroRNA638 in Acute Lymphoblastic Leukemia of Egyptian Children. Asian Pac. J. Cancer Prev. 2021, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Shehata, H.H.; Moussa, M.; Ibrahim, T.M. Prognostic significance of survivin and tumor necrosis factor-alpha in adult acute lymphoblastic leukemia. Clin. Biochem. 2012, 45, 112–116. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M. Clinical significance of serum p53 and epidermal growth factor receptor in patients with acute leukemia. Asian Pac. J. Cancer Prev. 2013, 14, 4295–4299. [Google Scholar] [CrossRef][Green Version]
- Liang, T.; Wang, N.; Li, W.; Li, A.; Wang, J.; Cui, J.; Liu, N.; Li, Y.; Li, L.; Yang, G.; et al. Identification of complement C3f-desArg and its derivative for acute leukemia diagnosis and minimal residual disease assessment. Proteomics 2010, 10, 90–98. [Google Scholar] [CrossRef]
- Pane, F.; Savoia, M.; Fortunato, G.; Camera, A.; Rotoli, B.; Salvatore, F.; Sacchetti, L. Serum pseudouridine in the diagnosis of acute leukaemias and as a novel prognostic indicator in acute lymphoblastic leukaemia. Clin. Biochem. 1993, 26, 513–520. [Google Scholar] [CrossRef]
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef]
- Wierda, W.G.; O’Brien, S.; Wang, X.; Faderl, S.; Ferrajoli, A.; Do, K.-A.; Cortes, J.; Thomas, D.; Garcia-Manero, G.; Koller, C.; et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood 2007, 109, 4679–4685. [Google Scholar] [CrossRef]
- Hallek, M.; Wanders, L.; Ostwald, M.; Busch, R.; Senekowitsch, R.; Stern, S.; Schick, H.-D.; Kuhn-Hallek, I.; Emmerich, B. Serum β2-Microglobulin and Serum Thymidine Kinase are Independent Predictors of Progression-Free Survival in Chronic Lymphocytic Leukemia and Immunocytoma. Leuk. Lymphoma 1996, 22, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Pratt, G.; Thomas, P.; Marden, N.; Alexander, D.; Davis, Z.; Hussey, D.; Parry, H.; Harding, S.; Catovsky, D.; Begley, J.; et al. Evaluation of serum markers in the LRF CLL4 trial: β2-microglobulin but not serum free light chains, is an independent marker of overall survival. Leuk. Lymphoma 2016, 57, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Damle, R.N.; Wasil, T.; Fais, F.; Ghiotto, F.; Valetto, A.; Allen, S.L.; Buchbinder, A.; Budman, D.; Dittmar, K.; Kolitz, J.; et al. Ig V Gene Mutation Status and CD38 Expression As Novel Prognostic Indicators in Chronic Lymphocytic Leukemia: Presented in part at the 40th Annual Meeting of the American Society of Hematology, Miami Beach, FL, December 4–8 1998. Blood 1999, 94, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Falay, M.; Ceran, F.; Gunes, A.K.; Dagdas, S.; Ayli, M.; Ozet, G. CD38 Expression and Variation as a Prognostic Factor Chronic Lymphocytic Leukemia. Clin. Lab. 2016, 62, 1287–1293. [Google Scholar] [CrossRef]
- Wiestner, A. Flow cytometry for ZAP-70: New colors for chronic lymphocytic leukemia. Cytom. Part B Clin. Cytom. 2006, 70B, 201–203. [Google Scholar] [CrossRef]
- Rozovski, U.; Keating, M.J.; Estrov, Z. Why Is the Immunoglobulin Heavy Chain Gene Mutation Status a Prognostic Indicator in Chronic Lymphocytic Leukemia? Acta Haematol. 2018, 140, 51–54. [Google Scholar] [CrossRef]
- Hu, B.; Patel, K.P.; Chen, H.-C.; Wang, X.; Luthra, R.; Routbort, M.J.; Kanagal-Shamanna, R.; Medeiros, L.J.; Yin, C.C.; Zuo, Z.; et al. Association of gene mutations with time-to-first treatment in 384 treatment-naive chronic lymphocytic leukaemia patients. Br. J. Haematol. 2019, 187, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Farahat, N.M.G.; Elkaffash, D.M.N.E.D.; Alghandour, A.H.; Swelem, R.S.; Abo El-Wafa, R.A.H. Study of microRNA Profile as a Molecular Biomarker in Egyptian Chronic Lymphocytic Leukemia. Indian J. Hematol. Blood Transfus. 2019, 35, 89–99. [Google Scholar] [CrossRef]
- Balatti, V.; Pekarky, Y.; Croce, C.M. Role of microRNA in chronic lymphocytic leukemia onset and progression. J. Hematol. Oncol. 2015, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Calin George, A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik Sylwia, E.; Iorio Marilena, V.; Visone, R.; Sever Nurettin, I.; Fabbri, M.; et al. A MicroRNA Signature Associated with Prognosis and Progression in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2005, 353, 1793–1801. [Google Scholar] [CrossRef]
- Vakiti, A.; Mewawalla, P. Acute Myeloid Leukemia. In StatPearls; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Boissel, N.; Leroy, H.; Brethon, B.; Philippe, N.; de Botton, S.; Auvrignon, A.; Raffoux, E.; Leblanc, T.; Thomas, X.; Hermine, O.; et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML). Leukemia 2006, 20, 965–970. [Google Scholar] [CrossRef]
- Boissel, N.; Renneville, A.; Biggio, V.; Philippe, N.; Thomas, X.; Cayuela, J.-M.; Terre, C.; Tigaud, I.; Castaigne, S.; Raffoux, E.; et al. Prevalence, clinical profile, and prognosis of NPM mutations in AML with normal karyotype. Blood 2005, 106, 3618–3620. [Google Scholar] [CrossRef]
- Fröhling, S.; Schlenk, R.F.; Stolze, I.; Bihlmayr, J.; Benner, A.; Kreitmeier, S.; Tobis, K.; Döhner, H.; Döhner, K. CEBPA Mutations in Younger Adults With Acute Myeloid Leukemia and Normal Cytogenetics: Prognostic Relevance and Analysis of Cooperating Mutations. J. Clin. Oncol. 2004, 22, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Marková, J.; Michková, P.; Burčková, K.; Březinová, J.; Michalová, K.; Dohnalová, A.; Maaloufová, J.S.; Soukup, P.; Vítek, A.; Cetkovský, P.; et al. Prognostic impact of DNMT3A mutations in patients with intermediate cytogenetic risk profile acute myeloid leukemia. Eur. J. Haematol. 2012, 88, 128–135. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Ma, L.; Merker, J.D.; Gotlib, J.R.; Schrijver, I.; Zehnder, J.L.; Arber, D.A. Next-generation sequencing of acute myeloid leukemia identifies the significance of TP53, U2AF1, ASXL1, and TET2 mutations. Mod. Pathol. 2015, 28, 706–714. [Google Scholar] [CrossRef]
- Abbas, S.; Lugthart, S.; Kavelaars, F.G.; Schelen, A.; Koenders, J.E.; Zeilemaker, A.; van Putten, W.J.L.; Rijneveld, A.W.; Löwenberg, B.; Valk, P.J.M. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: Prevalence and prognostic value. Blood 2010, 116, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, E.; Ramus, C.; Berthier, S.; Arlotto, M.; Bouamrani, A.; Lefebvre, C.; Morel, F.; Garin, J.; Ifrah, N.; Berger, F.; et al. Expression of S100A8 in leukemic cells predicts poor survival in de novo AML patients. Leukemia 2011, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; He, A.; Zhang, W.; Huang, C.; Yang, J.; Yang, Y.; Wang, J.; Zhang, Y. Potential biomarkers for adult acute myeloid leukemia minimal residual disease assessment searched by serum peptidome profiling. Proteome Sci. 2013, 11, 39. [Google Scholar] [CrossRef]
- Cho, J.W.; Kim, J.J.; Park, S.G.; Lee, D.H.; Lee, S.C.; Kim, H.J.; Park, B.C.; Cho, S. Identification of B-cell translocation gene 1 as a biomarker for monitoring the remission of acute myeloid leukemia. Proteomics 2004, 4, 3456–3463. [Google Scholar] [CrossRef]
- Kaźmierczak, M.; Luczak, M.; Lewandowski, K.; Handschuh, L.; Czyż, A.; Jarmuż, M.; Gniot, M.; Michalak, M.; Figlerowicz, M.; Komarnicki, M. Esterase D and gamma 1 actin level might predict results of induction therapy in patients with acute myeloid leukemia without and with maturation. Med. Oncol. 2013, 30, 725. [Google Scholar] [CrossRef]
- Sampaio, M.M.; Santos, M.L.C.; Marques, H.S.; Goncalves, V.L.S.; Araujo, G.R.L.; Lopes, L.W.; Apolonio, J.S.; Silva, C.S.; Santos, L.K.S.; Cuzzuol, B.R.; et al. Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review. World J. Clin. Oncol. 2021, 12, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Zhang, J.; Chen, X.; Xu, H.; Heng, G.; Chen, J.; Zhao, Y.; Li, J.; Ni, Y.; et al. CD9, a potential leukemia stem cell marker, regulates drug resistance and leukemia development in acute myeloid leukemia. Stem Cell Res. Ther. 2021, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, M.; Hu, Y.; Xing, H.; Chen, X.; Zhang, Y.; Zhu, P. Significance of CD71 expression by flow cytometry in diagnosis of acute leukemia. Leuk. Lymphoma 2014, 55, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.W.; Nathwani, B.N.; Rappaport, H. Flow cytometry in the diagnosis and classification of malignant lymphoma and leukemia. Cancer 1982, 50, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, F.; Durrieu, F.; Briais, A.; Dumain, P.; Belloc, F.; Bascans, E.; Reiffers, J.; Boisseau, M.R.; Bernard, P. Flow cytometry CD45 gating for immunophenotyping of acute myeloid leukemia. Leukemia 1997, 11, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Al-Mawali, A.; Gillis, D.; Hissaria, P.; Lewis, I. Incidence, sensitivity, and specificity of leukemia-associated phenotypes in acute myeloid leukemia using specific five-color multiparameter flow cytometry. Am. J. Clin. Pathol. 2008, 129, 934–945. [Google Scholar] [CrossRef]
- Virk, H.; Sachdeva, M.U.S. Flow Cytometric MRD Assessment in Acute Lymphoblastic Leukemias. Indian J. Med. Paediatr. Oncol. 2023, 44, 494–504. [Google Scholar] [CrossRef]
- Burnusuzov, H.A.; Spasova, M.I.; Murdjeva, M.A.; Stoyanova, A.A.; Mumdziev, I.N.; Kaleva, V.I.; Belcheva, M.I.; Bosheva, M.N. Immunophenotypic Modulation of the Blast Cells in Childhood Acute Lymphoblastic Leukemia Minimal Residual Disease Detection. Folia Med. 2016, 58, 28–35. [Google Scholar] [CrossRef]
- Tembhare, P.; Badrinath, Y.; Ghogale, S.; Subramanian, P.G. Method for DNA Ploidy Analysis along with Immunophenotyping for Rare Populations in a Sample using FxCycle Violet. Curr. Protoc. Cytom. 2017, 80, 6.38.31–36.38.15. [Google Scholar] [CrossRef]
- Dong, X.; Liu, L.; Tu, Y.; Zhang, J.; Miao, G.; Zhang, L.; Ge, S.; Xia, N.; Yu, D.; Qiu, X. Rapid PCR powered by microfluidics: A quick review under the background of COVID-19 pandemic. TrAC Trends Anal. Chem. 2021, 143, 116377. [Google Scholar] [CrossRef]
- Moragues, T.; Arguijo, D.; Beneyton, T.; Modavi, C.; Simutis, K.; Abate, A.R.; Baret, J.-C.; deMello, A.J.; Densmore, D.; Griffiths, A.D. Droplet-based microfluidics. Nat. Rev. Methods Primers 2023, 3, 32. [Google Scholar] [CrossRef]
- Piyasena, M.E.; Graves, S.W. The intersection of flow cytometry with microfluidics and microfabrication. Lab Chip 2014, 14, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Thakur, M.; Singh, S.; Tripathi, A. Microfluidic Devices as a Tool for Drug Delivery and Diagnosis: A Review. Int. J. Appl. Pharm. 2021, 13, 95–102. [Google Scholar] [CrossRef]
- Kopparthy, V.L.; Crews, N.D. A versatile oscillating-flow microfluidic PCR system utilizing a thermal gradient for nucleic acid analysis. Biotechnol. Bioeng. 2020, 117, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Monat, C.; Domachuk, P.; Eggleton, B.J. Integrated optofluidics: A new river of light. Nat. Photonics 2007, 1, 106–114. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Guan, X.; Yang, Y.; Tang, B.; Guo, W.; Sun, C.; Duan, X. A Microflow Cytometer Enabled by Monolithic Integration of a Microreflector with an Acoustic Resonator. ACS Sens. 2024, 9, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- van de Loosdrecht, A.A.; Westers, T.M.; Westra, A.H.; Dräger, A.M.; van der Velden, V.H.J.; Ossenkoppele, G.J. Identification of distinct prognostic subgroups in low- and intermediate-1–risk myelodysplastic syndromes by flow cytometry. Blood 2008, 111, 1067–1077. [Google Scholar] [CrossRef]
- Malcovati, L.; Della Porta, M.G.; Lunghi, M.; Pascutto, C.; Vanelli, L.; Travaglino, E.; Maffioli, M.; Bernasconi, P.; Lazzarino, M.; Invernizzi, R.; et al. Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia 2005, 19, 776–783. [Google Scholar] [CrossRef][Green Version]
- Dhingra, G.; Dass, J.; Arya, V.; Gupta, N.; Saraf, A.; Langer, S.; Aggarwal, S.; Kotwal, J.; Bhargava, M. Evaluation of multiparametric flow cytometry in diagnosis & prognosis of myelodysplastic syndrome in India. Indian J. Med. Res. 2020, 152, 254–262. [Google Scholar] [CrossRef]
- Mathis, S.; Chapuis, N.; Debord, C.; Rouquette, A.; Radford-Weiss, I.; Park, S.; Dreyfus, F.; Lacombe, C.; Béné, M.C.; Kosmider, O.; et al. Flow cytometric detection of dyserythropoiesis: A sensitive and powerful diagnostic tool for myelodysplastic syndromes. Leukemia 2013, 27, 1981–1987. [Google Scholar] [CrossRef]
- Westers, T.M.; Cremers, E.M.; Oelschlaegel, U.; Johansson, U.; Bettelheim, P.; Matarraz, S.; Orfao, A.; Moshaver, B.; Brodersen, L.E.; Loken, M.R.; et al. Immunophenotypic analysis of erythroid dysplasia in myelodysplastic syndromes. A report from the IMDSFlow working group. Haematologica 2017, 102, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, S.; Faridi, A.; Ramachandraiah, H.; Kumar, T.; Margulis, W.; Laurell, F.; Russom, A. High performance micro-flow cytometer based on optical fibres. Sci. Rep. 2017, 7, 5628. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xue, C.; Hu, G. Sheathless Separation of Particles and Cells by Viscoelastic Effects in Straight Rectangular Microchannels. Procedia Eng. 2015, 126, 721–724. [Google Scholar] [CrossRef]
- Leshansky, A.M.; Bransky, A.; Korin, N.; Dinnar, U. Tunable Nonlinear Viscoelastic “Focusing’’ in a Microfluidic Device. Phys. Rev. Lett. 2007, 98, 234501. [Google Scholar] [CrossRef] [PubMed]
- D’Avino, G.; Romeo, G.; Villone, M.M.; Greco, F.; Netti, P.A.; Maffettone, P.L. Single line particle focusing induced by viscoelasticity of the suspending liquid: Theory, experiments and simulations to design a micropipe flow-focuser. Lab Chip 2012, 12, 1638–1645. [Google Scholar] [CrossRef]
- Holzner, G.; Mateescu, B.; van Leeuwen, D.; Cereghetti, G.; Dechant, R.; Stavrakis, S.; deMello, A. High-throughput multiparametric imaging flow cytometry: Toward diffraction-limited sub-cellular detection and monitoring of sub-cellular processes. Cell Rep. 2021, 34, 108824. [Google Scholar] [CrossRef]
- Dieujuste, D.; Qiang, Y.; Du, E. A portable impedance microflow cytometer for measuring cellular response to hypoxia. Biotechnol. Bioeng. 2021, 118, 4041–4051. [Google Scholar] [CrossRef]
- Frankowski, M.; Bock, N.; Kummrow, A.; Schädel-Ebner, S.; Schmidt, M.; Tuchscheerer, A.; Neukammer, J. A microflow cytometer exploited for the immunological differentiation of leukocytes. Cytom. Part A 2011, 79A, 613–624. [Google Scholar] [CrossRef]
- Khalilian, P.; Eskandari, N.; Sharifi, M.J.; Soltani, M.; Nematollahi, P. Toll-Like Receptor 4, 2, and Interleukin 1 Receptor Associated Kinase4: Possible Diagnostic Biomarkers in Myelodysplastic Syndrome Patients. Adv. Biomed. Res. 2024, 13, 17. [Google Scholar] [CrossRef]

| Scoring Technique | Criteria | Effectiveness |
|---|---|---|
| Ogata Score (diagnostic tool) | Score of 0 to 4, +1 for each of the following:
|
|
| IPSS (prognosis tool) | Adds a variable number of points to score (pts) depending on the range for various variables [16]
| - |
| IPSS-R (prognosis tool) | Adds a variable number of points to score (pts) depending on the range for various variables [17]:
| Concordance of 0.74 for overall patient survival, concordance of 0.89 [18] for leukemia-free survival [19] |
| IPSS-M (prognosis tool) | Each of these factors is weighted differently based on statistical analysis. The model outputs a single number that ranks individuals in six categories, from very low to very high [20]:
| Concordance of 0.81 for overall patient survival concordance of 0.89 for leukemia free survival [19] |
| Biomarker | Blood Sample | Area under the ROC Curve | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| miR-92a [41] | Plasma | 0.755 | 41.5 | 100 | 100 | 36.7 |
| miR-638 [41] | Plasma | 0.86 | 54.7 | 100 | 100 | 42.9 |
| TNF-α [42] | Serum | 0.94 | 91.7 | 100 | - | - |
| Survivin [42] | Serum | 0.98 | 90 | 80 | - | - |
| p53 [43] | Serum | 0.8 | 52 | 100 | - | - |
| EGFR [43] | Serum | 0.93 | 73.9 | 95.8 | - | - |
| C3f [44] | Serum | 0.99 | 97 | 100 | - | - |
| Pseudouridine [45] | Serum | - | 90 | 97.5 | - | - |
| Category | Prognostic Biomarkers | Prognostic Value |
|---|---|---|
| Serum Markers | LDT | LDT ≤ 12 months predicts a poor prognosis, LDT > 12 months correlates with a long treatment-free period and survival [47] |
| s-β2M | Elevated levels predict poor outcomes and are used in risk stratification [48] | |
| s-TK | Elevated levels predict disease progression and are associated with shorter LDT and unmutated IGHV status [48,49] | |
| LDH | Indicator of time to first treatment (TTFT), associated with shorter PFS and OS [48] | |
| Immunophenotyping | CD38 | Predicts TTFT, resistance to treatment, hepatomegaly, and shorter survival [50,51] |
| ZAP70 | Predicts disease progression, Richter’s syndrome, and correlates with IGHV mutation status [52] | |
| IGHV Mutation Status | Mutated-CLL and Unmutated-CLL | Unmutated status is associated with an aggressive course and predicts shorter TTFT, CD38 positivity, and resistance to treatment [53,54] |
| MicroRNAs | MiR-15a, MiR-16-1, MiR-34a, and MiR-155 | Various impacts on prognosis in CLL, association with disease aggressiveness, and therapy response [55,56,57] |
| Category | Prognostic Biomarkers | Prognostic Value |
|---|---|---|
| Genetics | FLT3 (FLT3-ITD) | FLT3 mutations in AML, particularly FLT3-ITD, are associated with poor prognosis, a higher risk of relapse, and lower overall survival rates. Screening for FLT3 mutations can help identify patients who may benefit from intensified treatment protocols or FLT3 inhibitors [59]. |
| NPM1 | Nucleophosmin (NPM) mutations are found in 47% of AML cases with a normal karyotype, associated with a high white blood cell count and monocytic lineage involvement, but do not significantly impact complete remission or long-term outcomes, requiring further study for definitive prognostic value clarification [60]. | |
| CEBPA | Mutations in the CEBPA gene indicate a favorable prognosis and could enhance risk assessment for AML patients with normal cytogenetics [61]. | |
| DNMT3A | DNMT3A mutations in AML are associated with a higher risk of relapse and inferior overall survival, particularly in patients achieving complete remission. “Double-mutated” patients, with both DNMT3A and another mutation, have particularly poor outcomes [62]. | |
| TP53 | TP53 mutations in AML are independently associated with worse overall survival (OS) and disease-free survival (DFS). These mutations also correlate with specific clinicopathologic features, AML subtypes, and morphologic dysplasia [63]. | |
| IDH 1/2 | Mutations in IDH1 are associated with poor survival outcomes in a genetic subgroup that lacks FLT3 (ITD) and NPM1 (mutant). Therefore, IDH1 and IDH2 mutations are frequent in AML, and IDH1 mutations could be prognostically significant in specific AML subtypes [64]. | |
| Protomics | Calgranulin A | Analysis confirmed the expression of Calgranulin A mainly in AML patients with the worst prognosis, indicating a selective deregulation associated with poor outcomes. This suggests that the expression of Calgranulin A in leukemic cells is a predictor of low survival [65]. |
| UBA1, FIBA, and PF4 | The peptides could serve as potential markers for monitoring minimal residual disease, assessing clinical outcomes, and predicting poor prognosis and relapse [66]. | |
| BTG1 | BTG1 might be involved in myeloid cell differentiation, suggesting its potential use as a biomarker for monitoring remission status in AML-M2 and M3 patients undergoing treatment and as a predictor of a good prognosis [67]. | |
| Gamma 1 actin | Gamma 1 actin in AML predicts resistance to standard induction therapy, guiding the use of alternative treatments for better outcomes [68]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonsalves, M.; Escobar, A.; Altarabishi, A.D.; Xu, C.-Q. Advances in Microflow Cytometry-Based Molecular Detection Methods for Improved Future MDS Cancer Diagnosis. Curr. Issues Mol. Biol. 2024, 46, 8053-8070. https://doi.org/10.3390/cimb46080476
Gonsalves M, Escobar A, Altarabishi AD, Xu C-Q. Advances in Microflow Cytometry-Based Molecular Detection Methods for Improved Future MDS Cancer Diagnosis. Current Issues in Molecular Biology. 2024; 46(8):8053-8070. https://doi.org/10.3390/cimb46080476
Chicago/Turabian StyleGonsalves, Marc, Andres Escobar, Ahmad Diaa Altarabishi, and Chang-Qing Xu. 2024. "Advances in Microflow Cytometry-Based Molecular Detection Methods for Improved Future MDS Cancer Diagnosis" Current Issues in Molecular Biology 46, no. 8: 8053-8070. https://doi.org/10.3390/cimb46080476
APA StyleGonsalves, M., Escobar, A., Altarabishi, A. D., & Xu, C.-Q. (2024). Advances in Microflow Cytometry-Based Molecular Detection Methods for Improved Future MDS Cancer Diagnosis. Current Issues in Molecular Biology, 46(8), 8053-8070. https://doi.org/10.3390/cimb46080476






