Effects of Ulmus macrocarpa Extract and Catechin 7-O-β-D-apiofuranoside on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extract Materials
2.2. Pilot-Scale Extraction and Solvent Fractionation of Bark of Ulmus macrocarpa
2.3. Separation and Purification of Catechin Glycoside
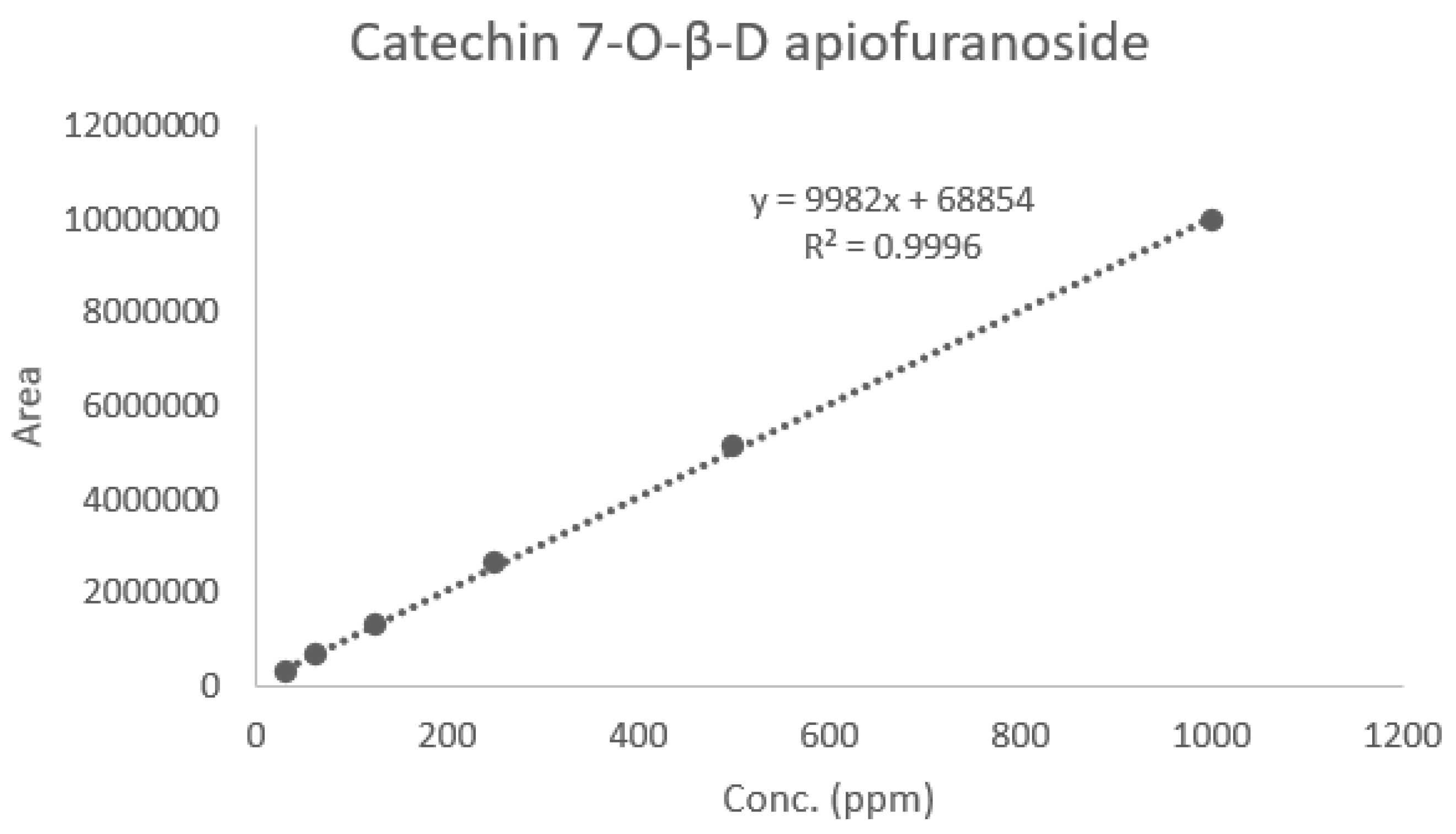
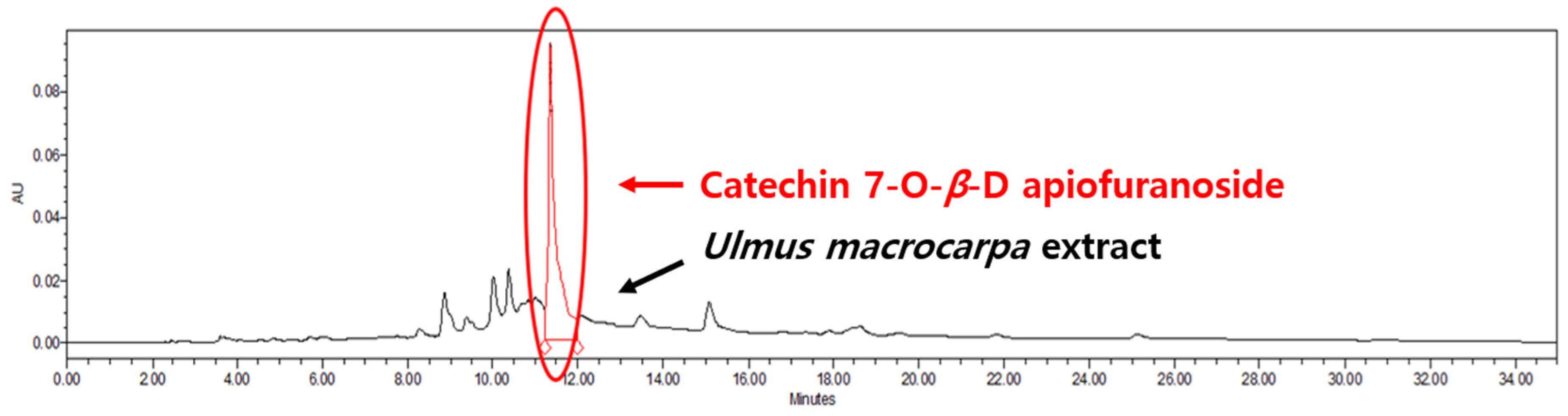
2.4. Quantitative Chromatographic Analysis of Ulmus macrocarpa Extract Powder (UME)
2.5. Cell Culture and Treatments
2.5.1. Apoptosis Induction and Treatment
2.5.2. Differentiation Induction and Treatment
2.6. Effects of Ulmus Macrocarpa Extract Powder (UME) and Catechin 7-O-β-D-apiofuranoside (CAG) on Muscle Apoptosis Biomarkers
2.6.1. Evaluation of Apoptosis in H2O2-Induced Myoblasts
2.6.2. Western Blot Analysis
2.7. Effects of Ulmus Macrocarpa Extract (UME) and Catechin 7-O-β-D apiofuranoside (CAG) on Muscle-Synthesis- and Muscle-Degradation-Related Proteins and Gene Expression
2.7.1. Measurement of Myotube Diameter
2.7.2. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.7.3. Western Blot Analysis
2.8. Statistical Analysis
3. Results
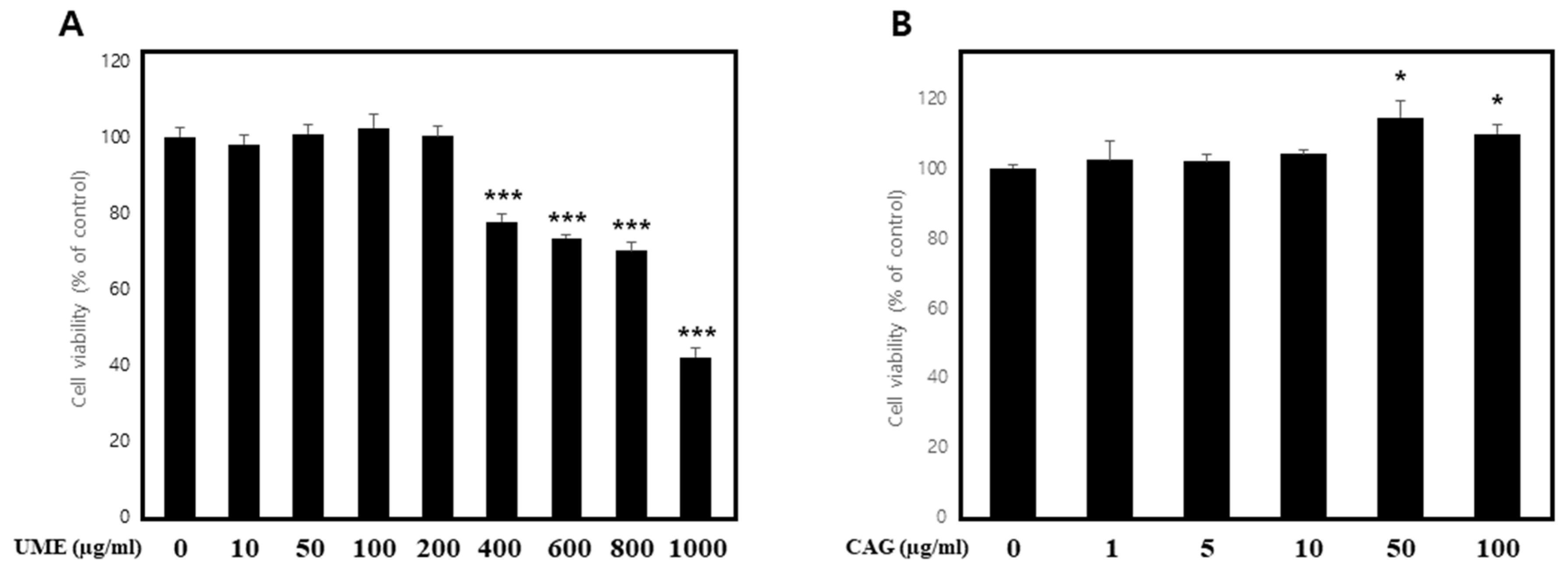
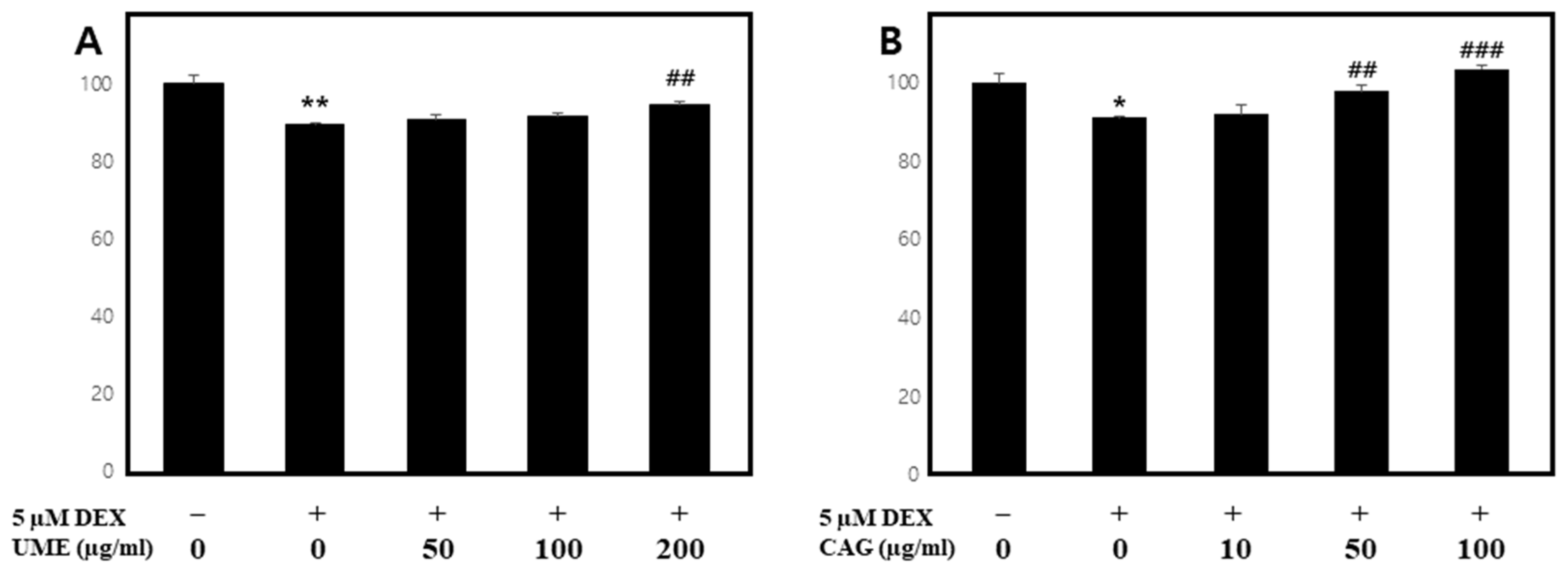
3.1. The Impact of UME and CAG on the Viability of C2C12 Cells
Measurement of Cell Viability under Normal Conditions
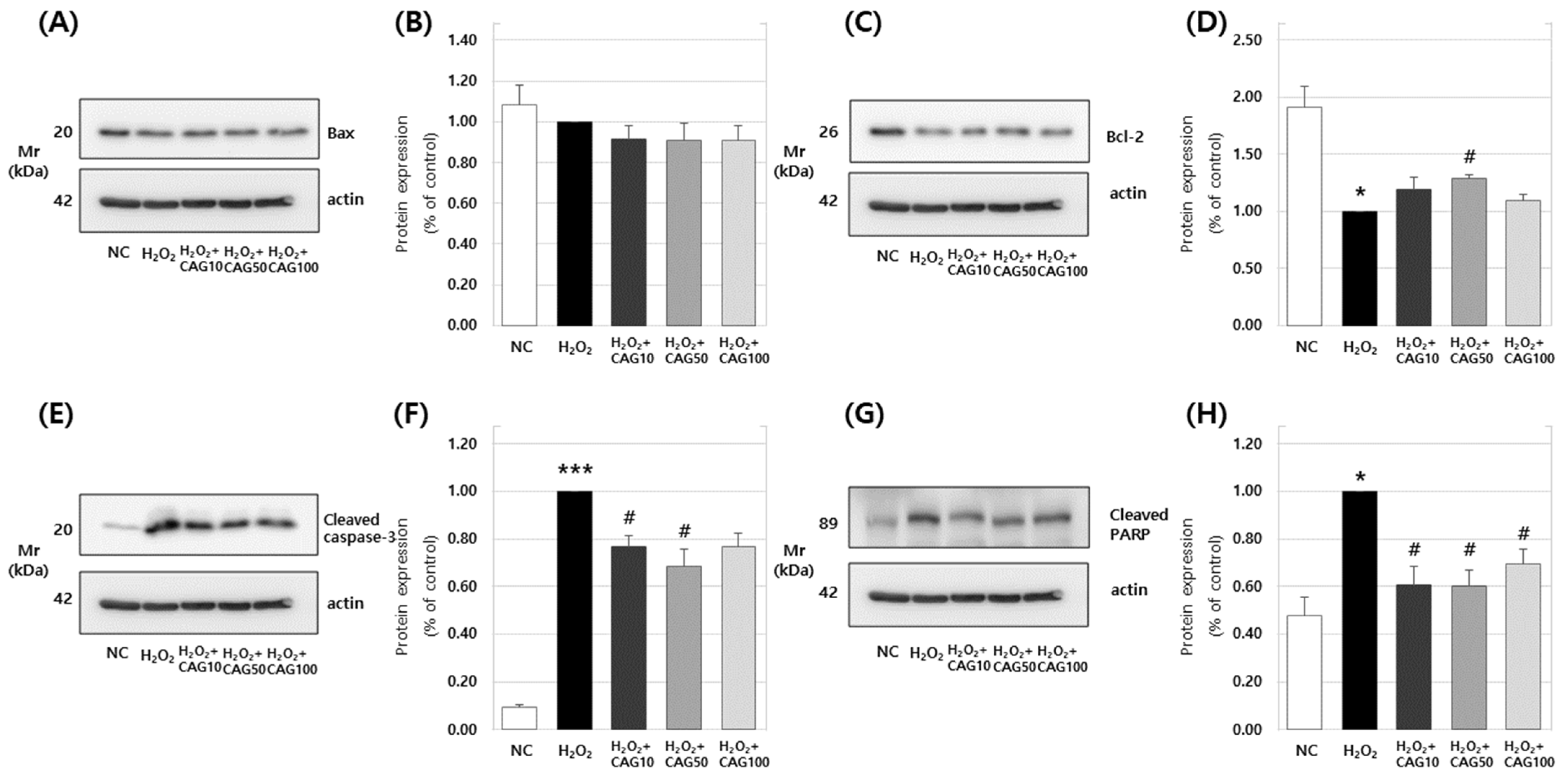
3.2. Effects of Ulmus Macrocarpa Extract (UME) and Catechin 7-O-β-D Apiofuranoside (CAG) on Muscle Apoptosis Biomarkers
3.2.1. Effects on H2O2-Induced Apoptosis in Myoblasts
3.2.2. Effects of Catechin 7-O-β-D apiofuranoside on Bax, Bcl-2, Cleaved Caspase-3, and Cleaved PARP Protein Expression
3.3. Effects of UME and CAG on Muscle-Synthesis- and Muscle-Degradation-Related Proteins and Gene Expression
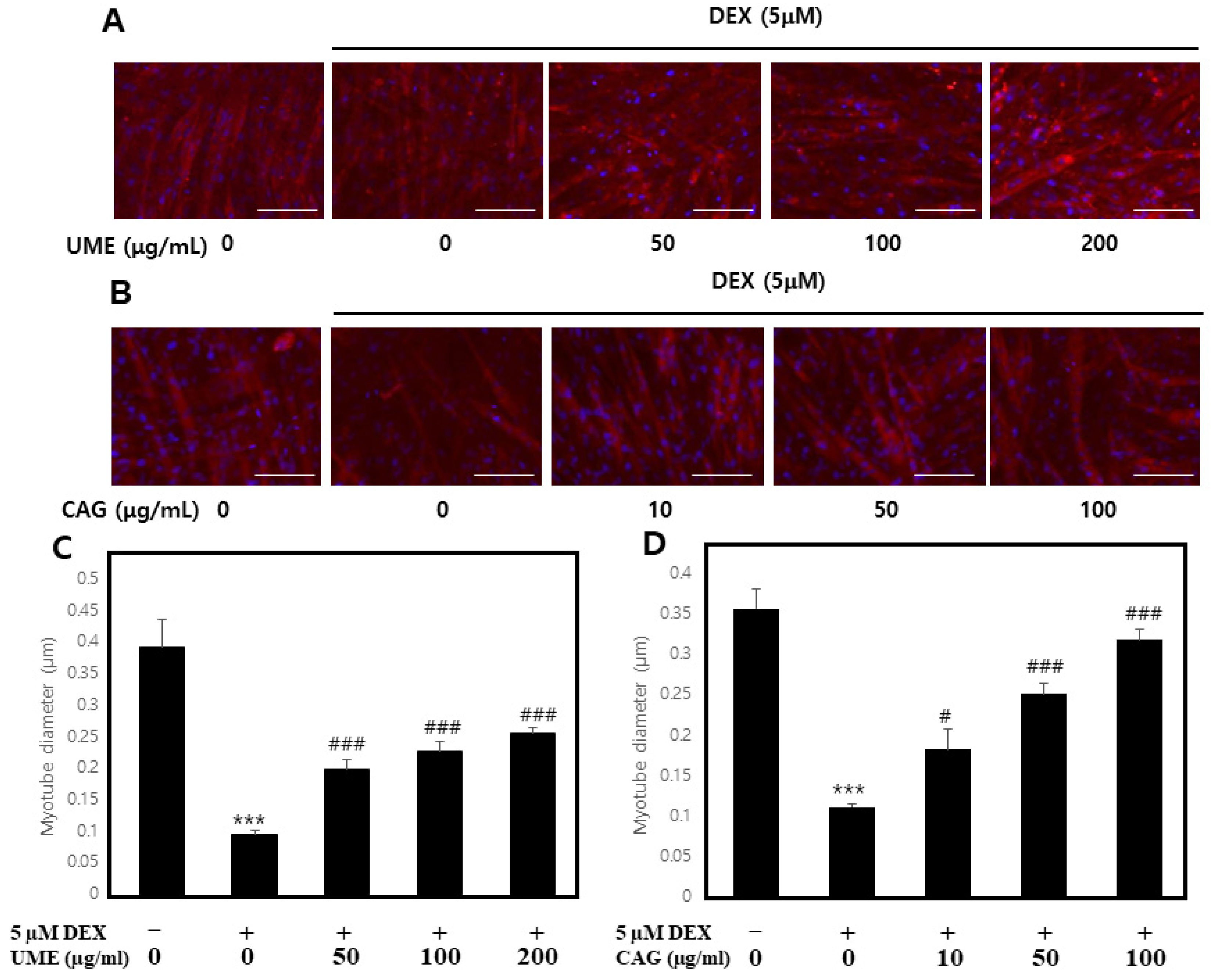
3.3.1. Impact of Dexamethasone-Induced Myotube Damage
3.3.2. Effect of UME and CAG on Dexamethasone-Induced Myotube Atrophy
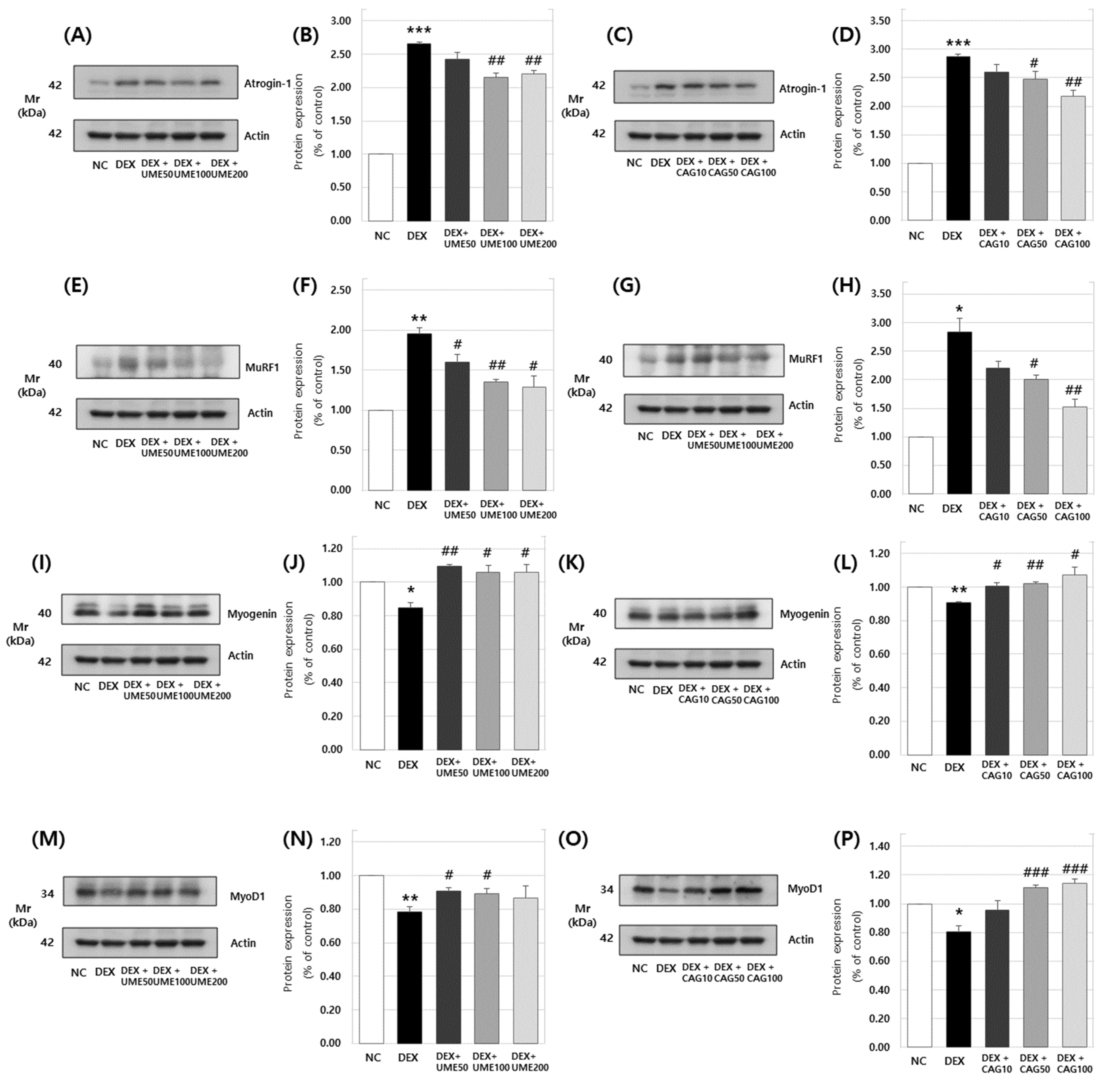
3.3.3. Expression of Atrogin-1, MuRF1, Myogenin, and MyoD1 Protein in DEX-Treated C2C12 Myotubes
3.3.4. Effects on Muscle-Degradation- and Muscle-Synthesis-Related Gene Expression
3.3.5. Akt and mTOR Signaling Pathway-Related Muscle Protein Expression Investigation
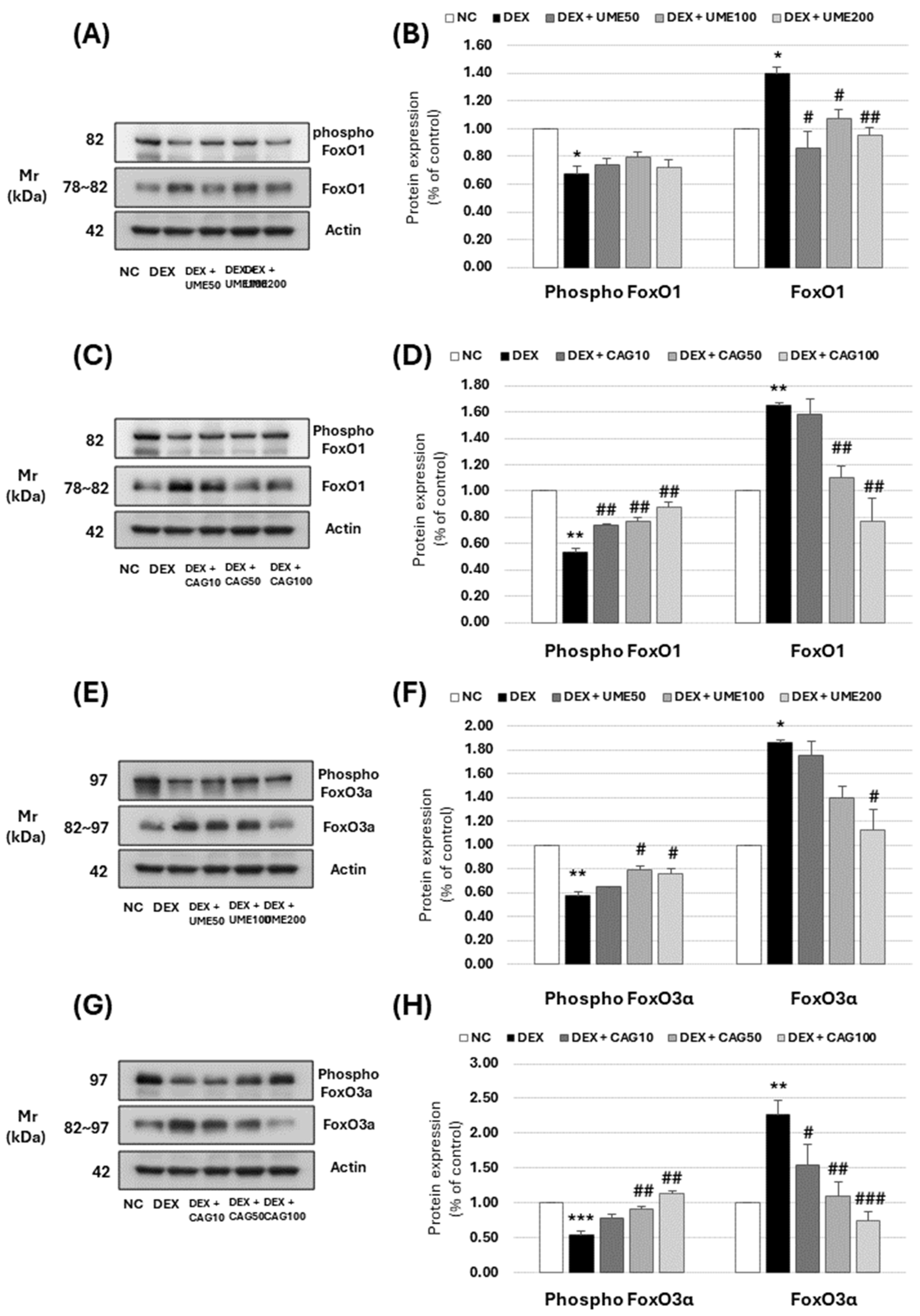
3.3.6. FoxO Signaling Pathway-Related Muscle Protein Expression Investigation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| Bax | bcl-2-associated X protein |
| Bcl-2 | B-cell leukemia/lymphoma 2 protein |
| CAG | catechin 7-O-β-D apiofuranoside |
| DAPI | 4′, 6-diamidino-2-phenylindole |
| DEX | dexamethasone |
| FoxO | forkhead box O |
| HPLC | high-performance liquid chromatography |
| mTOR | mammalian target of rapamycin |
| MuRF1 | muscle RING-finger protein-1 |
| MyoD | myoblast determination |
| PARP | Poly (ADP-ribose) polymerases |
| RT-PCR | real-time polymerase chain reaction |
| UME | Ulmus macrocarpa extract |
References
- Hong, S.; Choi, W. Recent perspectives on sarcopenia: Muscle wasting. Korean J. Intern. Med. 2012, 83, 444–454. [Google Scholar]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.; Kim, M. Physiological activities of Yu-geun-pi extract. J. Korean Soc. Food Preserv. Distrib. 2012, 19, 104–109. [Google Scholar] [CrossRef][Green Version]
- Lee, I.; Kwon, D.H.; Lee, S.H.; Lee, S.D.; Kim, D.W.; Lee, J.H.; Hyun, S.K.; Kang, K.-H.; Kim, C.; Kim, B.W.; et al. Immune-modulation effect of Ulmus macrocarpa Hance water extract on balb/c mice. J. Life Sci. 2014, 24, 1151–1156. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, S.; Choi, W.H.; Lee, Y.; Jo, Y.O.; Ha, T.Y. Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica. J. Med. Food 2010, 13, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, Y.J.; Shin, J.C.; Choi, S.E. Chemotaxonomic significance of catechin 7-O-beta-D-apiofuranoside in Ulmus species. J. Korean Wood Sci. Technol. 2020, 48, 888–895. [Google Scholar] [CrossRef]
- Nikawa, T.; Ulla, A.; Sakakibara, I. Polyphenols and their effects on muscle atrophy and muscle health. Molecules 2021, 26, 4887. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kwon, Y.E.; Kim, S.S.; Kim, H.J.; Kim, H.K.; Kim, J.-K.; Cheong, E.J.; Choi, S.E. Chemotaxonomic significance of catechin 7-O-beta-D-apiofuranoside in Ulmus species native to Asia. For. Sci. Technol. 2024, 1–9. [Google Scholar] [CrossRef]
- Lee, C.H.; Kwon, Y.; Park, S.; Kim, T.; Kim, M.S.; Kim, E.J.; Jung, J.I.; Min, S.; Park, K.-H.; Jeong, J.H.; et al. The Impact of Ulmus macrocarpa extracts on a model of sarcopenia-induced C57BL/6 mice. Int. J. Mol. Sci. 2024, 25, 6197. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Yeom, S.H.; Kim, M.K.; Lee, J.H.; Lee, M.W. Phenolic compounds from barks of Ulmus macrocarpa and their antioxidative activities. Korean J. Pharmacogn. 2002, 2, 376. [Google Scholar]
- Lee, S.J.; Lim, K.T. Glycoprotein isolated from Ulmus davidiana Nakai regulates expression of iNOS and COX-2 in vivo and in vitro. Food Chem. Toxicol. 2007, 45, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.E.; Choi, S.E.; Park, K.H. Regulation of Cytokines and dihydrotestosterone production in human hair follicle papilla cells by supercritical extraction-residues extract of Ulmus davidiana. Molecules 2022, 27, 1419. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jung, J.I.; Jeon, Y.E.; Kim, S.M.; Oh, T.K.; Lee, J.; Moon, J.M.; Kim, T.Y.; Kim, E.J. Gynostemma pentaphyllum extract and gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes. Nutr. Res. Pract. 2022, 16, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jung, J.I.; Jeon, Y.E.; Lee, H.S. Aqueous extract of Petasites Japonicus leaves promotes osteoblast differentiation via up-regulation of Runx2 and Osterix in MC3T3-E1 Cells. Nutr. Res. Pract. 2021, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Meguid, M.M.; Preziosa, I.; Fanelli, F.R. Oxidative stress and wasting in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.S.; Ashikhmin, Y.I.; Brown, L.A.; Guidot, D.M. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res. Ther. 2008, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Talarmin, H.; Derbré, F.; Lefeuvre-Orfila, L.; Léon, K.; Droguet, M.; Pennec, J.P.; Giroux-Metgès, M.A. The diaphragm is better protected from oxidative stress than hindlimb skeletal muscle during CLP-induced sepsis. Redox Rep. 2017, 22, 218–226. [Google Scholar] [CrossRef]
- Mastrocola, R.; Reffo, P.; Penna, F.; Tomasinelli, C.E.; Boccuzzi, G.; Baccino, F.M.; Aragno, M. and Costelli, P. Muscle wasting in diabetic and in tumor-bearing rats: Role of oxidative stress. Free Radic. Biol. Med. 2008, 44, 584–593. [Google Scholar] [CrossRef]
- Li, Y.P.; Chen, Y.; Li, A.S.; Reid, M.B. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am. J. Physiol.-Cell Physiol. 2003, 285, C806–C812. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free. Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Marcondes, M.C.C.; Tisdale, M.J. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. 2002, 180, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Jang, M.H.; Kim, E.K.; Han, S.W.; Cho, S.Y.; Kim, C.J. Nitric oxide induces apoptosis in mouse C2C12 myoblast cells. J. Pharmacol. Sci. 2005, 97, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Kim, D.J.; Kim, G.Y.; Cha, H.J.; Kim, S.; Kim, H.S.; Park, C.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; et al. Ethanol extract of Prunus mume fruit attenuates hydrogen peroxide-induced oxidative stress and apoptosis involving Nrf2/HO-1 activation in C2C12 myoblasts. Rev. Bras. Farmacogn. 2016, 26, 184–190. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.; Kim, Y.; Jeon, Y. Study of apoptosis-related genes following muscle atrophy induction and exercise. J. Korean Soc. Living Environ. Syst. 2009, 16, 246–253. [Google Scholar]
- Kim, K.B.; Kim, Y.A.; Park, J.J. Effects of 8-week exercise on Bcl-2, Bax, Caspase-8, Caspase-3 and HSP70 in mouse gastrocnemius muscle. J. Life Sci. 2010, 20, 1409–1414. [Google Scholar] [CrossRef]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef]
- Dominov, J.A.; Dunn, J.J.; Miller, J.B. Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J. Cell Biol. 1998, 142, 537–544. [Google Scholar] [CrossRef]
- Reed, J.C. Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 1994, 124, 1–6. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H. Bax protein in cancer treatment. J. Korean Med. Assoc. 2007, 50, 1016–1022. [Google Scholar] [CrossRef]
- Kim, T.; Kim, D.; Moon, Y.; Lim, G.; Woo, W. Induction of apoptosis through the mitochondria/caspase pathway by mulberry extract in A549 cells. J. Korean Soc. Pathol. 2016, 30, 150–156. [Google Scholar]
- Snigdha, S.; Smith, E.D.; Prieto, G.A.; Cotman, C.W. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci. Bull. 2012, 28, 14–24. [Google Scholar] [CrossRef]
- Menconi, M.; Gonnella, P.; Petkova, V.; Lecker, S.; Hasselgren, P.O. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J. Cell Biochem. 2008, 105, 353–364. [Google Scholar] [CrossRef]
- Jeon, S.K.; Kim, O.H.; Park, S.M.; Lee, J.H.; Park, S.D. Effects of glucoraphanin in dexamethasone-induced skeletal muscle atrophy in vitro model. Herb. Formula Sci. 2020, 28, 29–39. [Google Scholar]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Desler, M.M.; Jones, S.J.; Smith, C.W.; Woods, T.L. Effects of dexamethasone and anabolic agents on proliferation and protein synthesis and degradation in C2C12 myogenic cells. J. Anim. Sci. 1996, 74, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Mitch, W.E.; Wang, X.; Price, S.R. Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by NF-κB. J. Biol. Chem. 2000, 275, 19661–19666. [Google Scholar] [CrossRef]
- Evenson, A.R.; Fareed, M.U.; Menconi, M.J.; Mitchell, J.C.; Hasselgren, P.O. GSK-3β inhibitors reduce protein degradation in muscles from septic rats and in dexamethasone-treated myotubes. Int. J. Biochem. Cell Biol. 2005, 37, 2226–2238. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D. and Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Mikura, M.; Hirasaka, K.; Okumura, Y.; Nikawa, T.; Kawano, Y.; Nakayama, M.; Ikeda, M. Effects of dimethyl sulphoxide and dexamethasone on mRNA expression of myogenesis-and muscle proteolytic system-related genes in mouse myoblastic C2C12 cells. J. Biochem. 2008, 144, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.P.K.; Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. Nerve activity-independent regulation of skeletal muscle atrophy: Role of MyoD and myogenin in satellite cells and myonuclei. Am. J. Physiol.-Cell Physiol. 2003, 285, C1161–C1173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tintignac, L.A.; Lagirand, J.; Batonnet, S.; Sirri, V.; Leibovitch, M.P.; Leibovitch, S.A. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J. Biol. Chem. 2005, 280, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.M.; Leal, J.F.M.; Seger, R.; Taya, Y.; Oren, M. Cross-talk between Akt, p53 and Mdm2: Possible implications for the regulation of apoptosis. Oncogene 2002, 21, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, I.S.; Park, S.Y.; Park, O.J.; Kim, Y.M. Quercetin induces apoptosis via regulation of mTOR-VASP signaling pathway in HT-29 colon cancer cells. J. Cancer Prev. 2011, 16, 340–347. [Google Scholar]
- Eo, H.; Reed, C.H.; Valentine, R.J. Imoxin prevents dexamethasone-induced promotion of muscle-specific E3 ubiquitin ligases and stimulates anabolic signaling in C2C12 myotubes. Biomed. Pharmacother. 2020, 128, 110238. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.Y.; Kim, H.K.; Park, Y.; Whang, K.Y. Cortisone and dexamethasone inhibit myogenesis by modulating the AKT/mTOR signaling pathway in C2C12. Biosci. Biotechnol. Biochem. 2016, 80, 2093–2099. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.O.; Park, K.H.; Wie, M.B.; Choi, S.E.; Yun, J.H. A 60% edible ethanolic extract of ulmus davidiana inhibits vascular endothelial growth factor-induced angiogenesis. Molecules 2021, 26, 781. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, H.S.; Opitz, J.; Kabayama, K.; Kim, T.J. Effect of Cymbidium root extracts on oxidative stress-induced myoblasts damage. J. Life Sci. 2014, 24, 1019–1024. [Google Scholar] [CrossRef][Green Version]
- Ishibashi, J.; Perry, R.L.; Asakura, A.; Rudnicki, M.A. MyoD induces myogenic differentiation through cooperation of its NH2-and COOH-terminal regions. J. Cell Biol. 2005, 171, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef] [PubMed]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflügers Arch.-Eur. J. Physiol. 2011, 461, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 2014, 147, 73–80. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-Response 2017, 15, 1559325817691158. [Google Scholar] [CrossRef]
- Jung, U.J. Sarcopenic obesity: Involvement of oxidative stress and beneficial role of antioxidant flavonoids. Antioxidants 2023, 12, 1063. [Google Scholar] [CrossRef]




| mRNA | Primer Sequences | |
|---|---|---|
| Atrogin-1 | Forward | 5′-GCCCTCCACACTAGTTGACC-3′ |
| Reverse | 5′-GACGGATTGACAGCCAGGAA-3′ | |
| MuRf-1 | Forward | 5′-GAGGGCCATTGACTTTGGGA-3′ |
| Reverse | 5′-TTTACCCTCTGTGGTCACGC-3′ | |
| MyoD1 | Forward | 5′-GCACTACAGTGGCGACTCAGAT-3′ |
| Reverse | 5′-TAGTAGGCGGTGTCGTAGCCAT-3′ | |
| Myogenin | Forward | 5′-CCATCCAGTACATTGAGCGCCT-3′ |
| Reverse | 5′-CTGTGGGAGTTGCATTCACTGG-3′ | |
| GAPDH | Forward | 5′-TGGGTGTGAACCATGAGAAG-3′ |
| Reverse | 5′-GCTAAGCAGTTGGTGGTGC-3′ | |
| DEX (5 μM) | UME (μg/mL) | mRNA | |||
|---|---|---|---|---|---|
| Atrogin-1 | MuRF-1 | Myo D | Myogenin | ||
| − | − | 0.07 ± 0.02 | 0.10 ± 0.18 | 3.24 ± 0.27 | 2.47 ± 0.24 |
| + | − | 1.00 ± 0.22 ** | 1.00 ± 0.10 *** | 1.00 ± 0.11 *** | 1.00 ± 0.07 *** |
| + | 50 | 1.03 ± 0.12 | 0.90 ± 0.06 | 1.47 ± 0.21 | 1.27 ± 0.09 # |
| + | 100 | 0.60 ± 0.04 | 0.56 ± 0.04 ## | 2.02 ± 0.26 ## | 1.51 ± 0.10 ## |
| + | 200 | 0.40 ± 0.04 # | 0.31 ± 0.06 ### | 2.26 ± 0.29 ## | 1.76 ±0.17 ## |
| DEX (5 μM) | CAG (μg/mL) | mRNA | |||
|---|---|---|---|---|---|
| Atrogin-1 | MuRF-1 | Myo D | Myogenin | ||
| − | − | 0.06 ± 0.01 | 0.11 ± 0.02 | 2.98 ± 0.29 | 3.34 ± 0.55 |
| + | − | 1.00 ± 0.16 ** | 1.00 ± 0.08 *** | 1.00 ± 0.09 *** | 1.00 ± 0.10 ** |
| + | 10 | 0.75 ± 0.05 | 0.75 ± 0.06 # | 1.16 ± 0.12 | 1.44 ± 0.14 # |
| + | 50 | 0.39 ± 0.04 ## | 0.45 ± 0.05 ### | 1.56 ± 0.08 ## | 2.10 ± 0.17 ## |
| + | 100 | 0.16 ± 0.02 ### | 0.20 ± 0.03 ### | 2.12 ± 0.13 ### | 2.26 ±0.16 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.S.; Park, S.; Kwon, Y.; Kim, T.; Lee, C.H.; Jang, H.; Kim, E.J.; Jung, J.I.; Min, S.; Park, K.-H.; et al. Effects of Ulmus macrocarpa Extract and Catechin 7-O-β-D-apiofuranoside on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells. Curr. Issues Mol. Biol. 2024, 46, 8320-8339. https://doi.org/10.3390/cimb46080491
Kim MS, Park S, Kwon Y, Kim T, Lee CH, Jang H, Kim EJ, Jung JI, Min S, Park K-H, et al. Effects of Ulmus macrocarpa Extract and Catechin 7-O-β-D-apiofuranoside on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells. Current Issues in Molecular Biology. 2024; 46(8):8320-8339. https://doi.org/10.3390/cimb46080491
Chicago/Turabian StyleKim, Min Seok, Sunmin Park, Yeeun Kwon, TaeHee Kim, Chan Ho Lee, HyeonDu Jang, Eun Ji Kim, Jae In Jung, Sangil Min, Kwang-Hyun Park, and et al. 2024. "Effects of Ulmus macrocarpa Extract and Catechin 7-O-β-D-apiofuranoside on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells" Current Issues in Molecular Biology 46, no. 8: 8320-8339. https://doi.org/10.3390/cimb46080491
APA StyleKim, M. S., Park, S., Kwon, Y., Kim, T., Lee, C. H., Jang, H., Kim, E. J., Jung, J. I., Min, S., Park, K.-H., & Choi, S. E. (2024). Effects of Ulmus macrocarpa Extract and Catechin 7-O-β-D-apiofuranoside on Muscle Loss and Muscle Atrophy in C2C12 Murine Skeletal Muscle Cells. Current Issues in Molecular Biology, 46(8), 8320-8339. https://doi.org/10.3390/cimb46080491







