Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review
Abstract
1. Introduction
2. Cytokine Dysregulations in MPNs
3. Relation between Inflammation and Thrombosis in MPNs
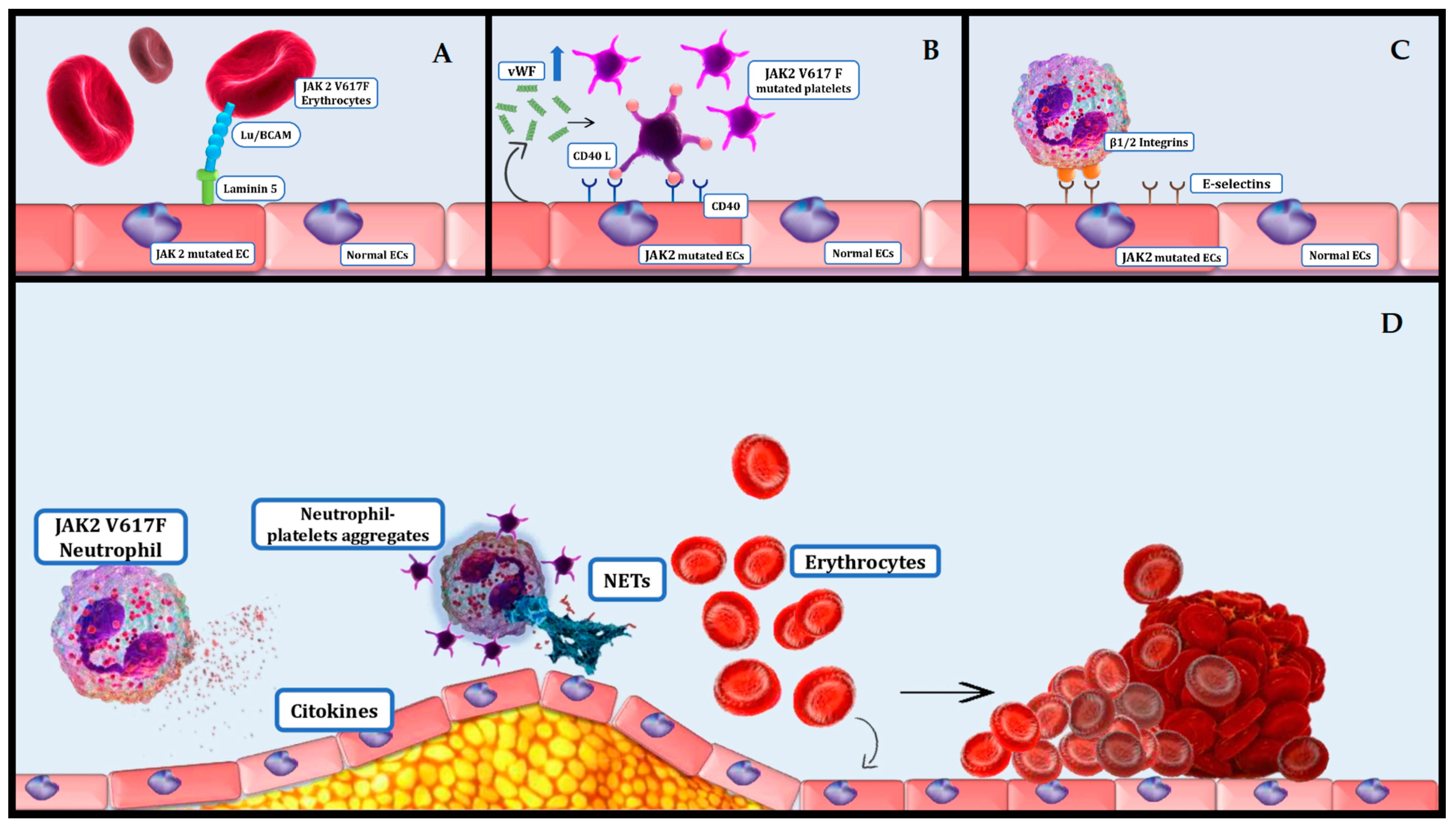
4. Platelets and Erythrocytes Effect on Thrombosis in MPNs
5. Complementary Role of JAK 2 Variants and Clonal Hematopoiesis of Indeterminate Potential (CHIP) in Cardiovascular Risk
6. Impact of MPNs on Atherosclerosis
7. Additional Factors Contributing to Cardiovascular Risk in MPNs
8. Other Biological Markers Associated with Cardiovascular Disease in MPNs
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tefferi, A.; Guglielmelli, P.; Larson, D.R.; Finke, C.; Wassie, E.A.; Pieri, L.; Gangat, N.; Fjerza, R.; Belachew, A.A.; Lasho, T.L.; et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014, 124, 2507–2513. [Google Scholar] [CrossRef]
- Gianelli, U.; Thiele, J.; Orazi, A.; Gangat, N.; Vannucchi, A.M.; Tefferi, A.; Kvasnicka, H.M. International Consensus Classification of myeloid and lymphoid neoplasms: Myeloproliferative neoplasms. Virchows Arch. 2023, 482, 53–68. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Ravn Landtblom, A.; Andréasson, B.; Samuelsson, J.; Dickman, P.W.; Kristinsson, S.Y.; Björkholm, M.; Andersson, T.M.-L. Incidence of myeloproliferative neoplasms—Trends by subgroup and age in a population-based study in Sweden. J. Intern. Med. 2020, 287, 448–454. [Google Scholar] [CrossRef]
- Passamonti, F.; Rumi, E.; Pietra, D.; Elena, C.; Boveri, E.; Arcaini, L.; Roncoroni, E.; Astori, C.; Merli, M.; Boggi, S.; et al. A prospective study of 338 patients with polycythemia vera: The impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 2010, 24, 1574–1579. [Google Scholar] [CrossRef]
- Rumi, E.; Boveri, E.; Bellini, M.; Pietra, D.; Ferretti, V.V.; Sant’Antonio, E.; Cavalloni, C.; Casetti, I.C.; Roncoroni, E.; Ciboddo, M.; et al. Clinical course and outcome of essential thrombocythemia and prefibrotic myelofibrosis according to the revised WHO 2016 diagnostic criteria. Oncotarget 2017, 8, 101735–101744. [Google Scholar] [CrossRef]
- Jeryczynski, G.; Thiele, J.; Gisslinger, B.; Wölfler, A.; Schalling, M.; Gleiß, A.; Burgstaller, S.; Buxhofer-Ausch, V.; Sliwa, T.; Schlögl, E.; et al. Pre-fibrotic/early primary myelofibrosis vs. WHO-defined essential thrombocythemia: The impact of minor clinical diagnostic criteria on the outcome of the disease. Am. J. Hematol. 2017, 92, 885–891. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Pacilli, A.; Rotunno, G.; Rumi, E.; Rosti, V.; Delaini, F.; Maffioli, M.; Fanelli, T.; Pancrazzi, A.; Pietra, D.; et al. Presentation and outcome of patients with 2016 WHO diagnosis of prefibrotic and overt primary myelofibrosis. Blood 2017, 129, 3227–3236. [Google Scholar] [CrossRef]
- Beer, P.A.; Campbell, P.J.; Scott, L.M.; Bench, A.J.; Erber, W.N.; Bareford, D.; Wilkins, B.S.; Reilly, J.T.; Hasselbalch, H.C.; Bowman, R.; et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood 2008, 112, 141–149. [Google Scholar] [CrossRef]
- Pardanani, A.D.; Levine, R.L.; Lasho, T.; Pikman, Y.; Mesa, R.A.; Wadleigh, M.; Steensma, D.P.; Elliott, M.A.; Wolanskyj, A.P.; Hogan, W.J.; et al. MPL515 mutations in myeloproliferative and other myeloid disorders: A study of 1182 patients. Blood 2006, 108, 3472–3476. [Google Scholar] [CrossRef]
- Klampfl, T.; Gisslinger, H.; Harutyunyan, A.S.; Nivarthi, H.; Rumi, E.; Milosevic, J.D.; Them, N.C.C.; Berg, T.; Gisslinger, B.; Pietra, D.; et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med. 2013, 369, 2379–2390. [Google Scholar] [CrossRef]
- Nangalia, J.; Massie, C.E.; Baxter, E.J.; Nice, F.L.; Gundem, G.; Wedge, D.C.; Avezov, E.; Li, J.; Kollmann, K.; Kent, D.G.; et al. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N. Engl. J. Med. 2013, 369, 2391–2405. [Google Scholar] [CrossRef]
- Iurlo, A.; Cattaneo, D.; Gianelli, U. Blast Transformation in Myeloproliferative Neoplasms: Risk Factors, Biological Findings, and Targeted Therapeutic Options. Int. J. Mol. Sci. 2019, 20, 1839. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef]
- Falchi, L.; Kantarjian, H.M.; Verstovsek, S. Assessing the thrombotic risk of patients with essential thrombocythemia in the genomic era. Leukemia 2017, 31, 1845–1854. [Google Scholar] [CrossRef]
- Masarova, L.; Bose, P.; Pemmaraju, N.; Daver, N.G.; Sasaki, K.; Chifotides, H.T.; Zhou, L.; Kantarjian, H.M.; Estrov, Z.; Verstovsek, S. Improved survival of patients with myelofibrosis in the last decade: Single-center experience. Cancer 2022, 128, 1658–1665. [Google Scholar] [CrossRef]
- Kroll, M.H.; Michaelis, L.C.; Verstovsek, S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev. 2015, 29, 215–221. [Google Scholar] [CrossRef]
- Pei, Y.Q.; Wu, Y.; Wang, F.; Cui, W. Prognostic value of CALR vs. JAK2V617F mutations on splenomegaly, leukemic transformation, thrombosis, and overall survival in patients with primary fibrosis: A meta-analysis. Ann. Hematol. 2016, 95, 1391–1398. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, C.; Gao, Z.; Wang, L.; Chen, C.; Zheng, Y.; Meng, Y. PKM2 promotes angiotensin-II-induced cardiac remodelling by activating TGF-β/Smad2/3 and Jak2/Stat3 pathways through oxidative stress. J. Cell. Mol. Med. 2021, 25, 10711–10723. [Google Scholar] [CrossRef]
- Su, S.-A.; Yang, D.; Wu, Y.; Xie, Y.; Zhu, W.; Cai, Z.; Shen, J.; Fu, Z.; Wang, Y.; Jia, L.; et al. EphrinB2 Regulates Cardiac Fibrosis Through Modulating the Interaction of Stat3 and TGF-β/Smad3 Signaling. Circ. Res. 2017, 121, 617–627. [Google Scholar] [CrossRef]
- Leiva, O.; Ren, S.; Neuberg, D.; Bhatt, A.; Jenkins, A.; Rosovsky, R.; Karp Leaf, R.; Goodarzi, K.; Hobbs, G. Pulmonary hypertension is associated with poor cardiovascular and hematologic outcomes in patients with myeloproliferative neoplasms and cardiovascular disease. Int. J. Hematol. 2023, 117, 90–99. [Google Scholar] [CrossRef]
- Asosingh, K.; Aldred, M.A.; Vasanji, A.; Drazba, J.; Sharp, J.; Farver, C.; Comhair, S.A.A.; Xu, W.; Licina, L.; Huang, L.; et al. Circulating Angiogenic Precursors in Idiopathic Pulmonary Arterial Hypertension. Am. J. Pathol. 2008, 172, 615–627. [Google Scholar] [CrossRef]
- Singh, I.; Mikita, G.; Green, D.; Risquez, C.; Sanders, A. Pulmonary extra-medullary hematopoiesis and pulmonary hypertension from underlying polycythemia vera: A case series. Pulm. Circ. 2017, 7, 261–267. [Google Scholar] [CrossRef]
- Vrtovec, M.; Anzic, A.; Zupan, I.P.; Zaletel, K.; Blinc, A. Carotid artery stiffness, digital endothelial function, and coronary calcium in patients with essential thrombocytosis, free of overt atherosclerotic disease. Radiol. Oncol. 2017, 51, 203–210. [Google Scholar] [CrossRef][Green Version]
- Landolfi, R.; Di Gennaro, L. Pathophysiology of thrombosis in myeloproliferative neoplasms. Haematologica 2011, 96, 183–186. [Google Scholar] [CrossRef]
- Fleischman, A.G.; Aichberger, K.J.; Luty, S.B.; Bumm, T.G.; Petersen, C.L.; Doratotaj, S.; Vasudevan, K.B.; LaTocha, D.H.; Yang, F.; Press, R.D.; et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood 2011, 118, 6392–6398. [Google Scholar] [CrossRef]
- Auteri, G.; Sansone, V.; Bartoletti, D.; Di Pietro, C.; Sutto, E.; Mazzoni, C.; Vianelli, N.; Cavo, M.; Piscaglia, F.; Palandri, F. Spleen and Liver Fibrosis Is Associated to Treatment Response and Prognosis in Philadelphia-Negative Chronic Myeloproliferative Neoplasms. Blood 2021, 138 (Suppl. S1), 3626. [Google Scholar] [CrossRef]
- Pettersson, H.; Knutsen, H.; Holmberg, E.; Andréasson, B. Increased incidence of another cancer in myeloproliferative neoplasms patients at the time of diagnosis. Eur. J. Haematol. 2015, 94, 152–156. [Google Scholar] [CrossRef]
- Cerquozzi, S.; Tefferi, A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: A literature review of incidence and risk factors. Blood Cancer J. 2015, 5, e366. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Rai, S.; Zhang, Y.; Grockowiak, E.; Kimmerlin, Q.; Hansen, N.; Stoll, C.B.; Usart, M.; Luque Paz, D.; Hao-Shen, H.; Zhu, Y.; et al. IL-1β promotes MPN disease initiation by favoring early clonal expansion of JAK2 -mutant hematopoietic stem cells. Blood Adv. 2024, 8, 1234–1249. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef]
- Arranz, L.; Arriero, M.D.M.; Villatoro, A. Interleukin-1β as emerging therapeutic target in hematological malignancies and potentially in their complications. Blood Rev. 2017, 31, 306–317. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, S.; Liu, N.; He, N.; Zhang, A.; Meng, S.; Ji, C.; Ma, D.; Ye, J. Genetic polymorphisms and expression of NLRP3 inflammasome-related genes are associated with Philadelphia chromosome-negative myeloproliferative neoplasms. Hum. Immunol. 2020, 81, 606–613. [Google Scholar] [CrossRef]
- Bauvois, B.; Susin, S.A. Revisiting Neutrophil Gelatinase-Associated Lipocalin (NGAL) in Cancer: Saint or Sinner? Cancers 2018, 10, 336. [Google Scholar] [CrossRef]
- Kagoya, Y.; Yoshimi, A.; Tsuruta-Kishino, T.; Arai, S.; Satoh, T.; Akira, S.; Kurokawa, M. JAK2V617F+ myeloproliferative neoplasm clones evoke paracrine DNA damage to adjacent normal cells through secretion of lipocalin-2. Blood 2014, 124, 2996–3006. [Google Scholar] [CrossRef]
- Øbro, N.F.; Grinfeld, J.; Belmonte, M.; Irvine, M.; Shepherd, M.S.; Rao, T.N.; Karow, A.; Riedel, L.M.; Harris, O.B.; Baxter, E.J.; et al. Longitudinal Cytokine Profiling Identifies GRO-α and EGF as Potential Biomarkers of Disease Progression in Essential Thrombocythemia. HemaSphere 2020, 4, e371. [Google Scholar] [CrossRef]
- Pourcelot, E.; Trocme, C.; Mondet, J.; Bailly, S.; Toussaint, B.; Mossuz, P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: Clinical implications. Exp. Hematol. 2014, 42, 360–368. [Google Scholar] [CrossRef]
- Ho, C.L.; Lasho, T.L.; Butterfield, J.H.; Tefferi, A. Global cytokine analysis in myeloproliferative disorders. Leuk. Res. 2007, 31, 1389–1392. [Google Scholar] [CrossRef]
- Cacemiro, M.D.C.; Cominal, J.G.; Tognon, R.; Nunes, N.D.S.; Simões, B.P.; Figueiredo-Pontes, L.L.D.; Catto, L.F.B.; Traina, F.; Souto, E.X.; Zambuzi, F.A.; et al. Philadelphia-negative myeloproliferative neoplasms as disorders marked by cytokine modulation. Hematol. Transfus. Cell Ther. 2018, 40, 120–131. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Pace, E.; Alonci, A.; Ferraro, M.; Petrungaro, A.; Saitta, S.; Gerace, D.; Russo, S.; Penna, G.; et al. Evaluation of interleukin-23 plasma levels in patients with polycythemia vera and essential thrombocythemia. Cell. Immunol. 2012, 278, 91–94. [Google Scholar] [CrossRef]
- Boissinot, M.; Cleyrat, C.; Vilaine, M.; Jacques, Y.; Corre, I.; Hermouet, S. Anti-inflammatory cytokines hepatocyte growth factor and interleukin-11 are over-expressed in Polycythemia vera and contribute to the growth of clonal erythroblasts independently of JAK2V617F. Oncogene 2011, 30, 990–1001. [Google Scholar] [CrossRef]
- Tefferi, A.; Vaidya, R.; Caramazza, D.; Finke, C.; Lasho, T.; Pardanani, A. Circulating Interleukin (IL)-8, IL-2R, IL-12, and IL-15 Levels Are Independently Prognostic in Primary Myelofibrosis: A Comprehensive Cytokine Profiling Study. J. Clin. Oncol. 2011, 29, 1356–1363. [Google Scholar] [CrossRef]
- Vaidya, R.; Gangat, N.; Jimma, T.; Finke, C.M.; Lasho, T.L.; Pardanani, A.; Tefferi, A. Plasma cytokines in polycythemia vera: Phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am. J. Hematol. 2012, 87, 1003–1005. [Google Scholar] [CrossRef]
- White, T.A.; Johnson, T.; Zarzhevsky, N.; Tom, C.; Delacroix, S.; Holroyd, E.W.; Maroney, S.A.; Singh, R.; Pan, S.; Fay, W.P.; et al. Endothelial-derived tissue factor pathway inhibitor regulates arterial thrombosis but is not required for development or hemostasis. Blood 2010, 116, 1787–1794. [Google Scholar] [CrossRef][Green Version]
- Kristinsson, S.Y.; Landgren, O.; Samuelsson, J.; Bjorkholm, M.; Goldin, L.R. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica 2010, 95, 1216–1220. [Google Scholar] [CrossRef]
- Guy, A.; Gourdou-Latyszenok, V.; Le Lay, N.; Peghaire, C.; Kilani, B.; Dias, J.V.; Duplaa, C.; Renault, M.-A.; Denis, C.; Villeval, J.L.; et al. Vascular endothelial cell expression of JAK2 V617F is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica 2019, 104, 70–81. [Google Scholar] [CrossRef]
- Guadall, A.; Lesteven, E.; Letort, G.; Awan Toor, S.; Delord, M.; Pognant, D.; Brusson, M.; Verger, E.; Maslah, N.; Giraudier, S.; et al. Endothelial Cells Harbouring the JAK2V617F Mutation Display Pro-Adherent and Pro-Thrombotic Features. Thromb. Haemost. 2018, 118, 1586–1599. [Google Scholar] [CrossRef]
- Najem, M.Y.; Couturaud, F.; Lemarié, C.A. Cytokine and chemokine regulation of venous thromboembolism. J. Thromb. Haemost. 2020, 18, 1009–1019. [Google Scholar] [CrossRef]
- Bjørn, M.E.; Hasselbalch, H.C. The Role of Reactive Oxygen Species in Myelofibrosis and Related Neoplasms. Mediat. Inflamm. 2015, 2015, 648090. [Google Scholar] [CrossRef]
- Moisa, C.; Gaman, M.A.; Diaconu, C.C.; Gaman, A.M. Oxidative Stress Levels, JAK2V617F Mutational Status and Thrombotic Complications in Patients with Essential Thrombocythemia. Rev. Chim. 2019, 70, 2822–2825. [Google Scholar] [CrossRef]
- Landolfi, R.; Marchioli, R.; Kutti, J.; Gisslinger, H.; Tognoni, G.; Patrono, C.; Barbui, T. Efficacy and Safety of Low-Dose Aspirin in Polycythemia Vera. N. Engl. J. Med. 2004, 350, 114–124. [Google Scholar] [CrossRef]
- Tefferi, A.; Elliott, M. Thrombosis in Myeloproliferative Disorders: Prevalence, Prognostic Factors, and the Role of Leukocytes and JAK2 V617F. Semin. Thromb. Hemost. 2007, 33, 313–320. [Google Scholar] [CrossRef]
- Kaifie, A.; Kirschner, M.; Wolf, D.; Maintz, C.; Hänel, M.; Gattermann, N.; Gökkurt, E.; Platzbecker, U.; Hollburg, W.; Göthert, J.R.; et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): Analysis from the German SAL-MPN-registry. J. Hematol. Oncol. 2016, 9, 18. [Google Scholar] [CrossRef]
- Swystun, L.L.; Liaw, P.C. The role of leukocytes in thrombosis. Blood 2016, 128, 753–762. [Google Scholar] [CrossRef]
- Edelmann, B.; Gupta, N.; Schnoeder, T.M.; Oelschlegel, A.M.; Shahzad, K.; Goldschmidt, J.; Philipsen, L.; Weinert, S.; Ghosh, A.; Saalfeld, F.C.; et al. JAK2-V617F promotes venous thrombosis through β1/β2 integrin activation. J. Clin. Investig. 2018, 128, 4359–4371. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Kumar, A.; Dubey, M.; Singh, A.K.; Pathak, P.; Chandra, T.; Barthwal, M.K.; Dikshit, M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic. Biol. Med. 2016, 93, 190–203. [Google Scholar] [CrossRef]
- Delgado-Rizo, V.; Martínez-Guzmán, M.A.; Iñiguez-Gutierrez, L.; García-Orozco, A.; Alvarado-Navarro, A.; Fafutis-Morris, M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front. Immunol. 2017, 8, 81. [Google Scholar] [CrossRef]
- Elaskalani, O.; Abdol Razak, N.B.; Metharom, P. Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. Cell Commun. Signal. 2018, 16, 24. [Google Scholar] [CrossRef]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307. [Google Scholar] [CrossRef]
- Kwon, S.S.; Yoon, S.Y.; Jeong, S.Y.; Lee, M.Y.; Kim, K.H.; Lee, N.; Won, J.-H. Neutrophil–lymphocyte ratio and carotid plaque burden in patients with essential thrombocythemia and polycythemia vera. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1913–1916. [Google Scholar] [CrossRef]
- Holstein, K.; Matysiak, A.; Witt, L.; Sievers, B.; Beckmann, L.; Haddad, M.; Renné, T.; Voigtlaender, M.; Langer, F. LPS-induced expression and release of monocyte tissue factor in patients with haemophilia. Ann. Hematol. 2020, 99, 1531–1542. [Google Scholar] [CrossRef]
- Falanga, A.; Vignoli, A.; Marchetti, M.; Russo, L.; Panova-Noeva, M.; Balducci, D.; Salmoiraghi, S.; Barbui, T.; Cate, H.T.; Rambaldi, A. Impact of V617F JAK2 Mutation on Monocyte Tissue Factor and Procoagulant Activity in Patients with Essential Thrombocythemia(ET) or Polycythemia VERA (PV). Blood 2008, 112, 3736. [Google Scholar] [CrossRef]
- Butenas, S.; Orfeo, T.; Mann, K.G. Tissue Factor in Coagulation: Which? Where? When? Arterioscler.Thromb. Vasc. Biol. 2009, 29, 1989–1996. [Google Scholar] [CrossRef]
- Lucchesi, A.; Napolitano, R.; Bochicchio, M.T.; Giordano, G.; Napolitano, M. Platelets Contribution to Thrombin Generation in Philadelphia-Negative Myeloproliferative Neoplasms: The “Circulating Wound” Model. Int. J. Mol. Sci. 2021, 22, 11343. [Google Scholar] [CrossRef]
- Moore, S.F.; Hunter, R.W.; Harper, M.T.; Savage, J.S.; Siddiq, S.; Westbury, S.K.; Poole, A.W.; Mumford, A.D.; Hers, I. Dysfunction of the PI3 kinase/Rap1/integrin αIIbβ3 pathway underlies ex vivo platelet hypoactivity in essential thrombocythemia. Blood 2013, 121, 1209–1219. [Google Scholar] [CrossRef]
- Hauschner, H.; Bokstad Horev, M.; Misgav, M.; Nagar, M.; Seligsohn, U.; Rosenberg, N.; Koren-Michowitz, M. Platelets from Calreticulin mutated essential thrombocythemia patients are less reactive than JAK2 V617F mutated platelets. Am. J. Hematol. 2020, 95, 379–386. [Google Scholar] [CrossRef]
- Campbell, P.J.; MacLean, C.; Beer, P.A.; Buck, G.; Wheatley, K.; Kiladjian, J.J.; Forsyth, C.; Harrison, C.N.; Green, A.R. Correlation of blood counts with vascular complications in essential thrombocythemia: Analysis of the prospective PT1 cohort. Blood 2012, 120, 1409–1411. [Google Scholar] [CrossRef]
- Carobbio, A.; Finazzi, G.; Antonioli, E.; Guglielmelli, P.; Vannucchi, A.M.; Delaini, F.; Guerini, V.; Ruggeri, M.; Rodeghiero, F.; Rambaldi, A.; et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood 2008, 112, 3135–3137. [Google Scholar] [CrossRef]
- Mela Osorio, M.J.; Ferrari, L.; Goette, N.P.; Gutierrez, M.I.; Glembotsky, A.C.; Maldonado, A.C.; Lev, P.R.; Alvarez, C.; Korin, L.; Marta, R.F.; et al. Long-term follow-up of essential thrombocythemia patients treated with anagrelide: Subgroup analysis according toJAK2/CALR/MPLmutational status. Eur. J. Haematol. 2016, 96, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Gowin, K.; Thapaliya, P.; Samuelson, J.; Harrison, C.; Radia, D.; Andreasson, B.; Mascarenhas, J.; Rambaldi, A.; Barbui, T.; Rea, C.J.; et al. Experience with pegylated interferon -2a in advanced myeloproliferative neoplasms in an international cohort of 118 patients. Haematologica 2012, 97, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G.; Mesa, R.; Finazzi, G.; Harrison, C.; Kiladjian, J.J.; Lengfelder, E.; McMullin, M.F.; Passamonti, F.; Vannucchi, A.M.; Besses, C.; et al. Revised response criteria for polycythemia vera and essential thrombocythemia: An ELN and IWG-MRT consensus project. Blood 2013, 121, 4778–4781. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Rodrigo, E.; Alvarez-Larrán, A.; Reverter, J.C.; Villamor, N.; Colomer, D.; Cervantes, F. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica 2006, 91, 169–175. [Google Scholar] [PubMed]
- Falanga, A.; Marchetti, M.; Vignoli, A.; Balducci, D.; Barbui, T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp. Hematol. 2005, 33, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Larrán, A.; Arellano-Rodrigo, E.; Reverter, J.C.; Domingo, A.; Villamor, N.; Colomer, D.; Cervantes, F. Increased platelet, leukocyte, and coagulation activation in primary myelofibrosis. Ann. Hematol. 2008, 87, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Evangelista, V.; Vignoli, A.; Licini, M.; Balicco, M.; Manarini, S.; Finazzi, G.; Cerletti, C.; Barbui, T. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood 2000, 96, 4261–4266. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Rodrigo, E.; Alvarez-Larrán, A.; Reverter, J.; Colomer, D.; Villamor, N.; Bellosillo, B.; Cervantes, F. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: Relationship with thrombosis occurrence and JAK 2 V617F allele burden. Am. J. Hematol. 2009, 84, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Rosti, V.; Villani, L.; Riboni, R.; Poletto, V.; Bonetti, E.; Tozzi, L.; Bergamaschi, G.; Catarsi, P.; Dallera, E.; Novara, F.; et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013, 121, 360–368. [Google Scholar] [CrossRef]
- Sozer, S.; Fiel, M.I.; Schiano, T.; Xu, M.; Mascarenhas, J.; Hoffman, R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009, 113, 5246–5249. [Google Scholar] [CrossRef]
- Trappenburg, M.C.; Van Schilfgaarde, M.; Marchetti, M.; Spronk, H.M.; Cate, H.T.; Leyte, A.; Terpstra, W.E.; Falanga, A. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica 2009, 94, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M. Insights into the pathogenesis and management of thrombosis in polycythemia vera and essential thrombocythemia. Intern. Emerg. Med. 2010, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Ovesná, P.; Černá, O.; Kissová, J.; Soukupová, J.M.; Brychtová, Y.; Doubek, M.; Červinek, L.; Cmunt, E.; Dulíček, P.; et al. Thrombosis in thrombocythemic Ph- myeloproliferations is associated with higher platelet count prior to the event: Results of analyses of prothrombotic risk factors from a registry of patients treated with anagrelide. Eur. J. Haematol. 2016, 96, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Accurso, V.; Santoro, M.; Mancuso, S.; Siragusa, S. Cardiovascular risk factor in MPN patients. J. Thromb. Thrombolysis 2020, 50, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Găman, M.A.; Kipkorir, V.; Srichawla, B.S.; Dhali, A.; Găman, A.M.; Diaconu, C.C. Primary Arterial Hypertension and Drug-Induced Hypertension in Philadelphia-Negative Classical Myeloproliferative Neoplasms: A Systematic Review. Biomedicines 2023, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Baker, R.; Lopez, J.A.; Spencer, S. Myeloproliferative Disorders and the Hyperviscosity Syndrome. Hematol. Oncol. Clin. North Am. 2010, 24, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.C.; Wetherley-Mein, G. Vascular occlusive episodes and venous hæmatocrit in primary proliferative polycythæmlx. Lancet 1978, 312, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Presti, R.L.; Caracciolo, C.; Montana, M.; Barone, R.; Catania, A.; Caimi, G. Erythrocyte deformability evaluated by laser diffractometry in polycythemia vera. Clin. Hemorheol. Microcirc. 2012, 50, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, Z.; Dybowicz, A.J.; Marchewka, A.; Teległów, A.; Skotnicki, A.; Zduńczyk, A.; Aleksander, P.; Filar-Mierzwa, K. Elongation index of erythrocytes, study of activity of chosen erythrocyte enzymes, and the levels of glutathione, malonyldialdehyde in polycythemia vera (PV). Clin. Hemorheol. Microcirc. 2011, 47, 169–176. [Google Scholar] [CrossRef]
- De Grandis, M.; Cambot, M.; Wautier, M.P.; Cassinat, B.; Chomienne, C.; Colin, Y.; Wautier, J.-L.; Le Van Kim, C.; El Nemer, W. JAK2V617F activates Lu/BCAM-mediated red cell adhesion in polycythemia vera through an EpoR-independent Rap1/Akt pathway. Blood 2013, 121, 658–665. [Google Scholar] [CrossRef]
- Lucero, H.A.; Kagan, H.M. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cell Mol. Life Sci. 2006, 63, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, T.; Bejar, J.; Attias, D.; Mischenko, E.; Sabo, E.; Neufeld, G.; Vadasz, Z. The expression of lysyl-oxidase gene family members in myeloproliferative neoplasms. Am. J. Hematol. 2013, 88, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Leiva, O.; Ng, S.K.; Chitalia, S.; Balduini, A.; Matsuura, S.; Ravid, K. The role of the extracellular matrix in primary myelofibrosis. Blood Cancer J. 2017, 7, e525. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Mi, R.; Koupenova, M.; Eliades, A.; Patterson, S.; Toselli, P.; Thon, J.; Italiano, J.E.; Trackman, P.C.; Papadantonakis, N.; et al. Lysyl oxidase is associated with increased thrombosis and platelet reactivity. Blood 2016, 127, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Eliades, A.; Papadantonakis, N.; Bhupatiraju, A.; Burridge, K.A.; Johnston-Cox, H.A.; Migliaccio, A.R.; Crispino, J.D.; Lucero, H.A.; Trackman, P.C.; Ravid, K. Control of Megakaryocyte Expansion and Bone Marrow Fibrosis by Lysyl Oxidase. J. Biol. Chem. 2011, 286, 27630–27638. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.C.; Touw, I.; Yoshimura, A. The Jak-Stat pathway in normal and perturbed hematopoiesis. Blood 2000, 95, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Penta, K.; Sawyer, S.T. Erythropoietin Induces the Tyrosine Phosphorylation, Nuclear Translocation, and DNA Binding of STAT1 and STAT5 in Erythroid Cells. J. Biol. Chem. 1995, 270, 31282–31287. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.G.; Reddy, E.P. Janus kinases: Components of multiple signaling pathways. Oncogene 2000, 19, 5662–5679. [Google Scholar] [CrossRef] [PubMed]
- Leiva, O.; Hobbs, G.; Ravid, K.; Libby, P. Cardiovascular disease in myeloproliferative neoplasms: JACC: Cardiooncology state-of-the-art review. CardioOncology 2022, 4, 166–182. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Bayes-Genis, A.; Díez-Díez, M.; Hernández-Vicente, Á.; Vázquez-Andrés, D.; De La Barrera, J.; Vazquez, E.; Quintas, A.; Zuriaga, M.A.; Asensio-López, M.C.; et al. Clonal Hematopoiesis and Risk of Progression of Heart Failure with Reduced Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, R.; Sellar, R.S.; Vromman, A.; Sausen, G.; Folco, E.; Sukhova, G.K.; McConke, M.E.; Corbo, C.; Ebert, B.L.; Libby, P. The clonal hematopoiesis mutation Jak2 aggravates endothelial injury and thrombosis in arteries with erosion-like intimas. Int. J. Cardiol. 2024, 409, 132184. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.K.; Izukawa, T.; Young, S.; Rosen, G.; Jamali, M.; Zhang, L.; Johnson, D.; Bain, E.; Hilland, J.; Ferrone, C.K.; et al. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv. 2019, 3, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Valanti, E.K.; Dalakoura-Karagkouni, K.; Sanoudou, D. Current and Emerging Reconstituted HDL-apoA-I and HDL-apoE Approaches to Treat Atherosclerosis. J. Pers. Med. 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Feig, J.E.; Rong, J.X.; Shamir, R.; Sanson, M.; Vengrenyuk, Y.; Liu, J.; Rayner, K.; Moore, K.; Garabedian, M.; Fisher, E.A. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7166–7171. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, W.; Fidler, T.; Wang, Y.; Tang, Y.; Woods, B.; Welch, C.; Cai, B.; Silvestre-Roig, C.; Ai, D.; et al. Macrophage Inflammation, Erythrophagocytosis, and Accelerated Atherosclerosis in Jak2 V617F Mice. Circ. Res. 2018, 123, e35–e47. [Google Scholar] [CrossRef] [PubMed]
- Fidler, T.P.; Xue, C.; Yalcinkaya, M.; Hardaway, B.; Abramowicz, S.; Xiao, T.; Liu, W.; Thomas, D.G.; Hajebrahimi, M.A.; Pircher, J.; et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 2021, 592, 296–301. [Google Scholar] [CrossRef]
- Libby, P.; Molinaro, R.; Sellar, R.S.; Ebert, B.L. Jak-ing Up the Plaque’s Lipid Core…and Even More. Circ. Res. 2018, 123, 1180–1182. [Google Scholar] [CrossRef]
- Michel, J.B.; Libby, P.; Franck, G. Internal Bleeding. JACC Basic Transl. Sci. 2018, 3, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I.; Yang, J.; Ikeda, J.; Roth, Z.; Pollock-Tahiri, E.; Desai, H.; Sivasubramaniyam, T.; Rehal, S.; Rapps, J.; Li, Y.Z.; et al. Macrophage Jak2 deficiency accelerates atherosclerosis through defects in cholesterol efflux. Commun. Biol. 2022, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Anžič Drofenik, A.; Vrtovec, M.; Božič Mijovski, M.; Sever, M.; Preložnik Zupan, I.; Kejžar, N.; Blinc, A. Progression of coronary calcium burden and carotid stiffness in patients with essential thrombocythemia associated with JAK2 V617F mutation. Atherosclerosis 2020, 296, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Koh, J.S.; Kang, S.; Ryu, H.; Song, I.C.; Lee, H.J.; Yun, H.-J.; Kim, S.Y.; Kim, S.S.; Jo, D.-Y. Abdominal aortic calcification in patients newly diagnosed with essential thrombocythemia. Blood Res. 2023, 58, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Fernex De Mongex, A.; Delrue, M.; Ghaffari, P.; Jaillette, C.; Yannoutsos, A.; Emmerich, J.; Priollet, P. Foot ischemia related to essential thrombocytemia and atherosclerosis. JMV J. Méd. Vasc. 2021, 46, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, P.; Pasca, S.; Jurj, A.; Gafencu, G.; Joelsson, J.; Selicean, S.; Moldovan, C.; Munteanu, R.; Onaciu, A.; Tigu, A.; et al. Transforming growth factor β-mediated micromechanics modulates disease progression in primary myelofibrosis. J. Cell. Mol. Med. 2020, 24, 11100–11110. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Qin, L.; Baeyens, N.; Li, G.; Afolabi, T.; Budatha, M.; Tellides, G.; Schwartz, M.A.; Simons, M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Investig. 2015, 125, 4514–4528. [Google Scholar] [CrossRef]
- Janda, K.; Krzanowski, M.; Dumnicka, P.; Kusnierz-Cabala, B.; Krasniak, A.; Sulowicz, W. Transforming Growth Factor Beta 1 as a Risk Factor for Cardiovascular Diseases in End-Stage Renal Disease Patients Treated with Peritoneal Dialysis. Clin. Lab. 2014, 60, 1163–1168. [Google Scholar] [CrossRef]
- Wesseling, M.; Sakkers, T.R.; De Jager, S.C.A.; Pasterkamp, G.; Goumans, M.J. The morphological and molecular mechanisms of epithelial/endothelial-to-mesenchymal transition and its involvement in atherosclerosis. Vasc. Pharmacol. 2018, 106, 1–8. [Google Scholar] [CrossRef]
- Misaka, T.; Kimishima, Y.; Yokokawa, T.; Ikeda, K.; Takeishi, Y. Clonal hematopoiesis and cardiovascular diseases: Role of JAK2V617F. J. Cardiol. 2023, 81, 3–9. [Google Scholar] [CrossRef]
- Mehta, J.K.; Kaur, G.; Buttar, H.S.; Bagabir, H.A.; Bagabir, R.A.; Bagabir, S.A.; Haque, S.; Tuli, H.S.; Telessy, I.G. Role of the renin-angiotensin system in the pathophysiology of coronary heart disease and heart failure: Diagnostic biomarkers and therapy with drugs and natural products. Front Physiol. 2023, 14, 1034170. [Google Scholar] [CrossRef]
- Campbell, P.J.; Scott, L.M.; Buck, G.; Wheatley, K.; East, C.L.; Marsden, J.T.; Duffy, A.; Boyd, E.M.; Bench, A.J.; Scott, M.A.; et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: A prospective study. Lancet 2005, 366, 1945–1953. [Google Scholar] [CrossRef]
- Vrsalovic, M.M.; Pejsa, V.; Veic, T.S.; Kolonic, S.O.; Ajdukovic, R.; Haris, V.; Jaksic, O.; Kusec, R. Bone marrow renin-angiotensin system expression in polycythemia vera and essential thrombocythemia depends on JAK2 mutational status. Cancer Biol. Ther. 2007, 6, 1430–1432. [Google Scholar] [CrossRef][Green Version]
- Barbui, T.; Masciulli, A.; Ghirardi, A.; Carobbio, A. ACE inhibitors and cytoreductive therapy in polycythemia vera. Blood 2017, 129, 1226–1227. [Google Scholar] [CrossRef] [PubMed]
- Corey, S.J.; Jha, J.; McCart, E.A.; Rittase, W.B.; George, J.; Mattapallil, J.J.; Mehta, H.; Ognoon, M.; Bylicky, M.A.; Summers, T.A.; et al. Captopril mitigates splenomegaly and myelofibrosis in the Gata1low murine model of myelofibrosis. J. Cell. Mol. Med. 2018, 22, 4274–4282. [Google Scholar] [CrossRef] [PubMed]
- Garypidou, V.; Vakalopoulou, S.; Dimitriadis, D.; Tziomalos, K.; Sfikas, G.; Perifanis, V. Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. Haematologica 2004, 89, 245–246. [Google Scholar]
- Kim, J.; Krichevsky, S.; Xie, L.; Palumbo, M.C.; Rodriguez-Diego, S.; Yum, B.; Brouwer, L.; Silver, R.T.; Schafer, A.I.; Ritchie, E.K.; et al. Incremental Utility of Right Ventricular Dysfunction in Patients with Myeloproliferative Neoplasm–Associated Pulmonary Hypertension. J. Am. Soc. Echocardiogr. 2019, 32, 1574–1585. [Google Scholar] [CrossRef]
- Roach, E.C.; Park, M.M.; Wilson Tang, W.H.; Thomas, J.D.; Asosingh, K.; Kalaycio, M.; Erzurum, S.C.; Farha, S. Impaired right ventricular-pulmonary vascular function in myeloproliferative neoplasms. J. Heart Lung Transplant. 2015, 34, 390–394. [Google Scholar] [CrossRef]
- Yoder, M.C. Defining human endothelial progenitor cells. J. Thromb. Haemost. 2009, 7, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C.; Ingram, D.A. Endothelial progenitor cell: Ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Cur. Opin. Hematol. 2009, 16, 269–273. [Google Scholar] [CrossRef]
- Lyden, D.; Hattori, K.; Dias, S.; Costa, C.; Blaikie, P.; Butros, L.; Chadburn, A.; Heissig, B.; Marks, W.; Witte, L.; et al. Impaired recruitment of bone-marrow–derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001, 7, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Farha, S.; Asosingh, K.; Xu, W.; Sharp, J.; George, D.; Comhair, S.; Park, M.; Tang, W.H.W.; Loyd, J.E.; Theil, K.; et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: A link to the intrinsic myeloid abnormalities. Blood 2011, 117, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, C.A.; Verweyen, J.; Halank, M.; Benjamin, N.; Fischer, C.; Mayer, E.; Guth, S.; Wiedenroth, C.B.; Egenlauf, B.; Harutyunova, S.; et al. Myeloproliferative Diseases as Possible Risk Factor for Development of Chronic Thromboembolic Pulmonary Hypertension—A Genetic Study. Int. J. Mol. Sci. 2020, 21, 3339. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Li, Q.; Miao, Y.; Zhang, Y.; Yuan, W.; Fan, L.; Liu, G.; Mi, Q.; Yang, J. The Emerging Role of MicroRNA-155 in Cardiovascular Diseases. BioMed Res. Int. 2016, 2016, 9869208. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.Q.D.; Pedersen, O.H.; Larsen, M.L.; Grove, E.L.; Kristensen, S.D.; Hvas, A.M.; Nissen, P.H. Platelet microRNA expression and association with platelet maturity and function in patients with essential thrombocythemia. Platelets 2020, 31, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.S.; McCoy, C.E.; Lloyd, A.T.; O’Farrelly, C.; Stevenson, N.J. miR-19a: An Effective Regulator of SOCS3 and Enhancer of JAK-STAT Signalling. Jin, D.Y.; editor. PLoS ONE 2013, 8, e69090. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Pairet, S.; Álvarez-Larrán, A.; Pons, A.; Ferrer, G.; Longarón, R.; Fernández-Rodríguez, C.; Camacho, L.; Monzó, M.; Besses, C.; et al. miR-203 and miR-221 regulate SOCS1 and SOCS3 in essential thrombocythemia. Blood Cancer J. 2016, 6, e406. [Google Scholar] [CrossRef]
- Girardot, M.; Pecquet, C.; Boukour, S.; Knoops, L.; Ferrant, A.; Vainchenker, W.; Giraudier, S.; Constantinescu, S.N. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood 2010, 116, 437–445. [Google Scholar] [CrossRef]
- Zhan, H.; Cardozo, C.; Yu, W.; Wang, A.; Moliterno, A.R.; Dang, C.V.; Spivak, J.L. MicroRNA deregulation in polycythemia vera and essential thrombocythemia patients. Blood CellsMol. Dis. 2013, 50, 190–195. [Google Scholar] [CrossRef][Green Version]
- Volck, B.; Price, P.A.; Johansen, J.S.; Sørensen, O.; Benfield, T.L.; Nielsen, H.J.; Calafat, J.; Borregaard, N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Physicians 1998, 110, 351–360. [Google Scholar]
- Biggar, R.J.; Johansen, J.S.; Smedby, K.E.; Rostgaard, K.; Chang, E.T.; Adami, H.O.; Glimelius, B.; Molin, D.; Hamilton-Dutoit, S.; Melbye, M.; et al. Serum YKL-40 and Interleukin 6 Levels in Hodgkin Lymphoma. Clin. Cancer Res. 2008, 14, 6974–6978. [Google Scholar] [CrossRef] [PubMed]
- Mylin, A.K.; Andersen, N.F.; Johansen, J.S.; Abildgaard, N.; Heickendorff, L.; Standal, T.; Gimsing, P.; Knudsen, L.M. Serum YKL-40 and bone marrow angiogenesis in multiple myeloma. Int. J Cancer 2009, 124, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.S.; Schultz, N.A.; Jensen, B.V. Plasma YKL-40: A Potential New Cancer Biomarker? Future Oncol. 2009, 5, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Renkema, G.H.; Boot, R.G.; Au, F.L.; Donker-Koopman, W.E.; Strijland, A.; Muijsers, A.O.; Hrebicek, M.; Aerts, J.M.F.G. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J Biochem. 1998, 251, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.G.; Van Achterberg, T.A.E.; Van Aken, B.E.; Renkema, G.H.; Jacobs, M.J.H.M.; Aerts, J.M.F.G.; De Vries, C.J.M. Strong Induction of Members of the Chitinase Family of Proteins in Atherosclerosis: Chitotriosidase and Human Cartilage gp-39 Expressed in Lesion Macrophages. Arter. Thromb. Vasc. Biol. 1999, 19, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Krečak, I.; Gverić-Krečak, V.; Lapić, I.; Rončević, P.; Gulin, J.; Fumić, K.; Krečak, F.; Holik, H.; Duraković, N. Circulating YKL-40 in Philadelphia-negative myeloproliferative neoplasms. Acta Clin. Belg. 2021, 76, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Vannucchi, A.M.; Carobbio, A.; Rumi, E.; Finazzi, G.; Gisslinger, H.; Ruggeri, M.; Randi, M.L.; Cazzola, M.; Rambaldi, A.; et al. The effect of arterial hypertension on thrombosis in low-risk polycythemia vera. Am. J. Hematol. 2017, 92, E5–E6. [Google Scholar] [CrossRef] [PubMed]
- Horvat, I.; Boban, A.; Zadro, R.; Antolic, M.R.; Serventi-Seiwerth, R.; Roncevic, P.; Radman, I.; Sertic, D.; Vodanovic, M.; Pulanic, D.; et al. Influence of Blood Count, Cardiovascular Risks, Inherited Thrombophilia, and JAK2 V617F Burden Allele on Type of Thrombosis in Patients with Philadelphia Chromosome Negative Myeloproliferative Neoplasms. Clin. Lymphoma Myeloma Leuk. 2019, 19, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Landolfi, R.; Di Gennaro, L.; Barbui, T.; De Stefano, V.; Finazzi, G.; Marfisi, R.; Tognoni, G.; Marchioli, R.; for the European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP). Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 2007, 109, 2446–2452. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Thiele, J.; Kvasnicka, H.M.; Orazi, A.; Gianelli, U.; Gangat, N.; Vannucchi, A.M.; Barbui, T.; Arber, D.A.; Tefferi, A. The international consensus classification of myeloid neoplasms and acute Leukemias: Myeloproliferative neoplasms. Am. J. Hematol. 2023, 98, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Andriani, A.; Latagliata, R.; Anaclerico, B.; Spadea, A.; Rago, A.; Di Veroli, A.; Spirito, F.; Porrini, R.; De Muro, M.; Crescenzi Leonetti, S.; et al. Spleen enlargement is a risk factor for thrombosis in essential thrombocythemia: Evaluation on 1,297 patients. Am. J. Hematol. 2016, 91, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Accurso, V.; Santoro, M.; Raso, S.; Contrino, A.D.; Perez, A.; Di Piazza, F.; Florena, A.M.; Russo, A.; Siragusa, S. The Essential Thrombocythemia, Thrombotic Risk Stratification, and Cardiovascular Risk Factors. Adv. Hematol. 2020, 2020, 9124821. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Carobbio, A.; Cervantes, F.; Vannucchi, A.M.; Guglielmelli, P.; Antonioli, E.; Alvarez-Larrán, A.; Rambaldi, A.; Finazzi, G.; Barosi, G. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood 2010, 115, 778–782. [Google Scholar] [CrossRef] [PubMed]
| MPNs | Major Criteria | Minor Criteria | Cardiovascular Risk Factors and Complications | References |
|---|---|---|---|---|
| PV |
|
| Hypertension (40–70%) [147] Diabetes melitius (7–16%) [148] Obesity (7.5%) and smoking (5–10%) [38] | [38,147,148,149,150,151] |
| ET |
|
| Thrombotic risk before diagnostic 18% [152] Each cardiovascular risk increases the thrombotic risk [153]:
| [150,151,152,153] |
| PMF |
|
| Thrombotic events [154]:
| [150,151,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todor, S.B.; Ichim, C.; Boicean, A.; Mihaila, R.G. Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 8407-8423. https://doi.org/10.3390/cimb46080496
Todor SB, Ichim C, Boicean A, Mihaila RG. Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review. Current Issues in Molecular Biology. 2024; 46(8):8407-8423. https://doi.org/10.3390/cimb46080496
Chicago/Turabian StyleTodor, Samuel Bogdan, Cristian Ichim, Adrian Boicean, and Romeo Gabriel Mihaila. 2024. "Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review" Current Issues in Molecular Biology 46, no. 8: 8407-8423. https://doi.org/10.3390/cimb46080496
APA StyleTodor, S. B., Ichim, C., Boicean, A., & Mihaila, R. G. (2024). Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review. Current Issues in Molecular Biology, 46(8), 8407-8423. https://doi.org/10.3390/cimb46080496





