Diagnostic and Prognostic Role of Circulating microRNAs in Patients with Coronary Artery Disease—Impact on Left Ventricle and Arterial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and RNA Extraction
2.3. Echocardiography
2.4. Arterial Function
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Echocardiographic and Arterial Stiffness Parameters
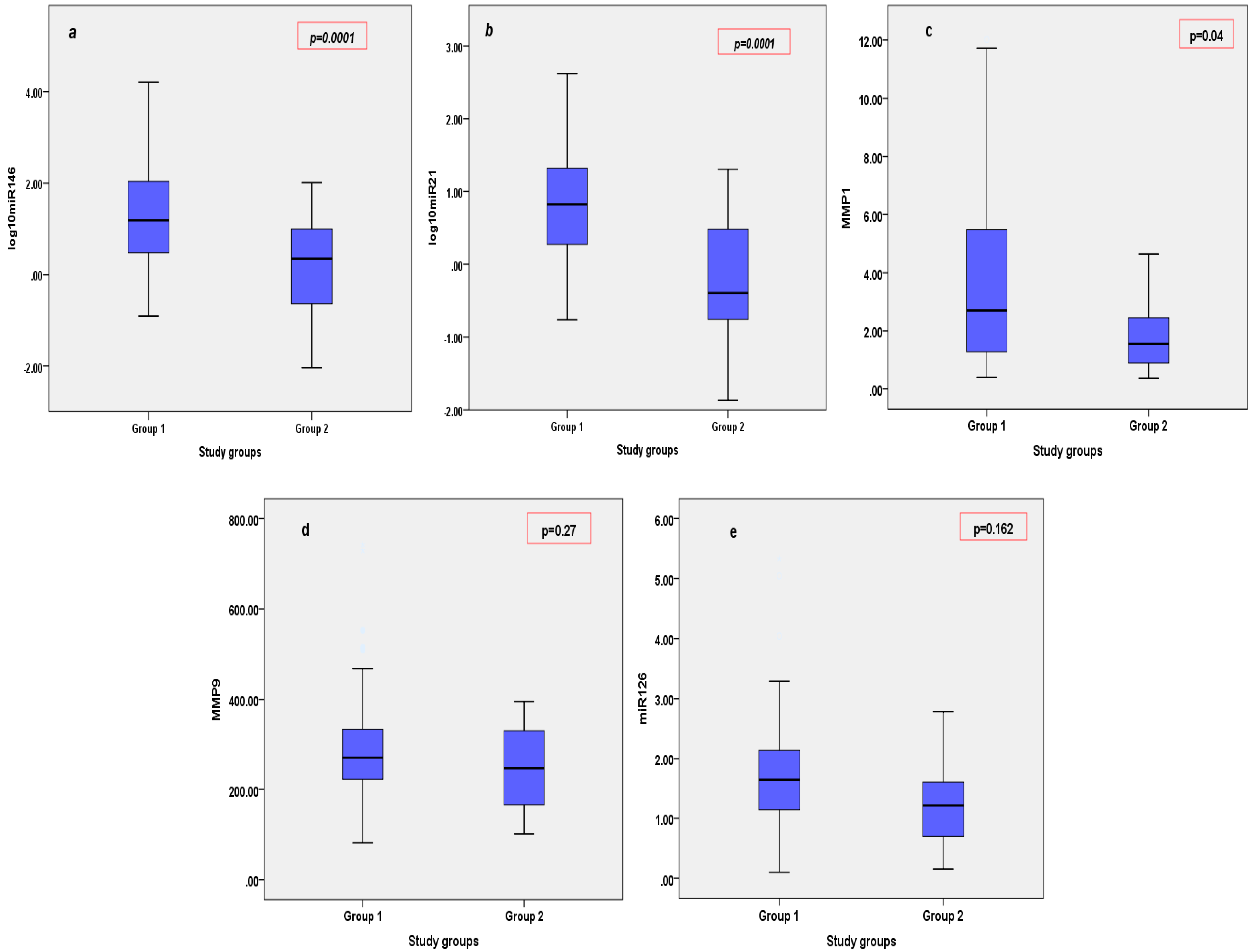
3.3. Circulating MicroRNAs and MMPs
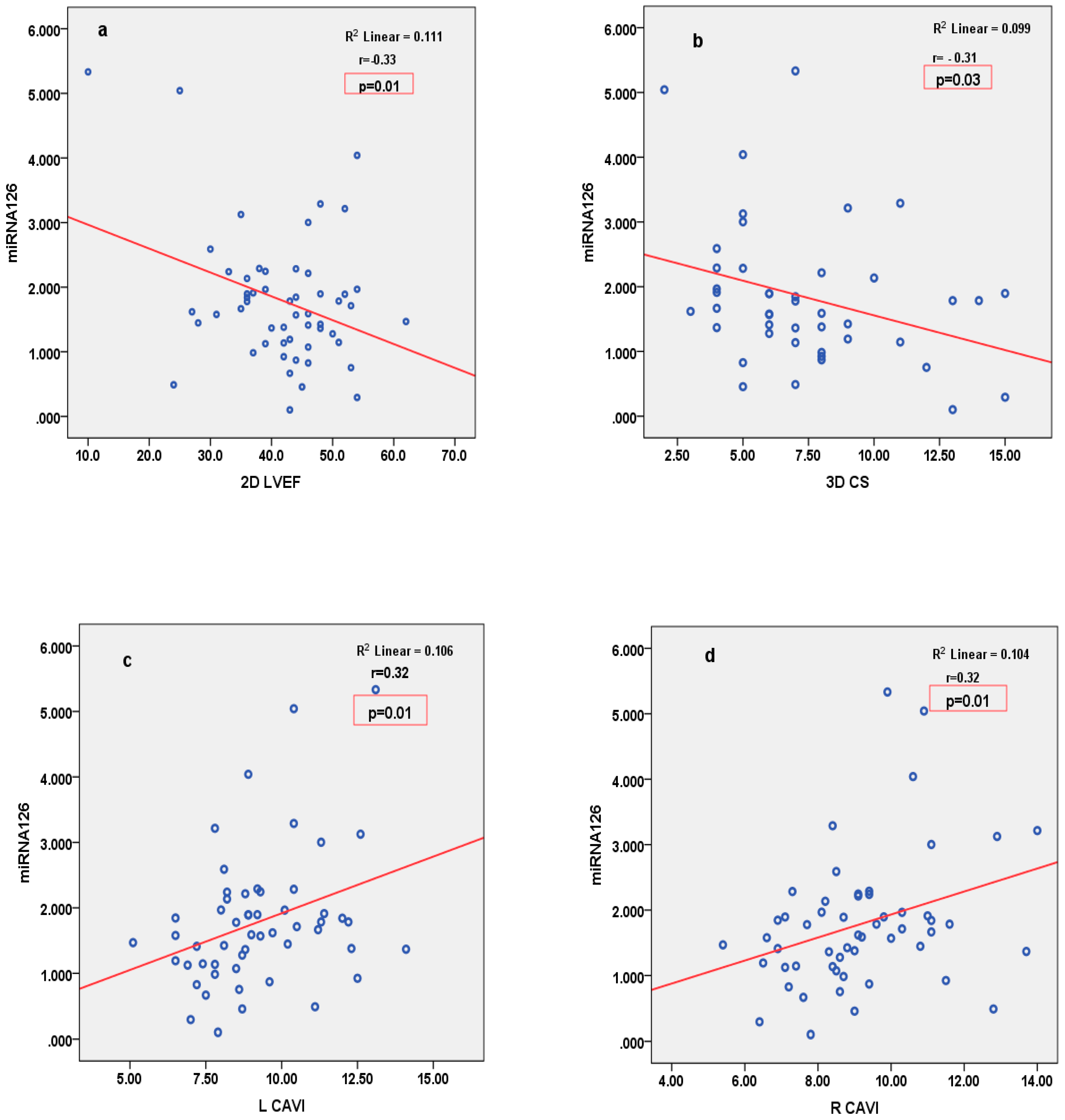
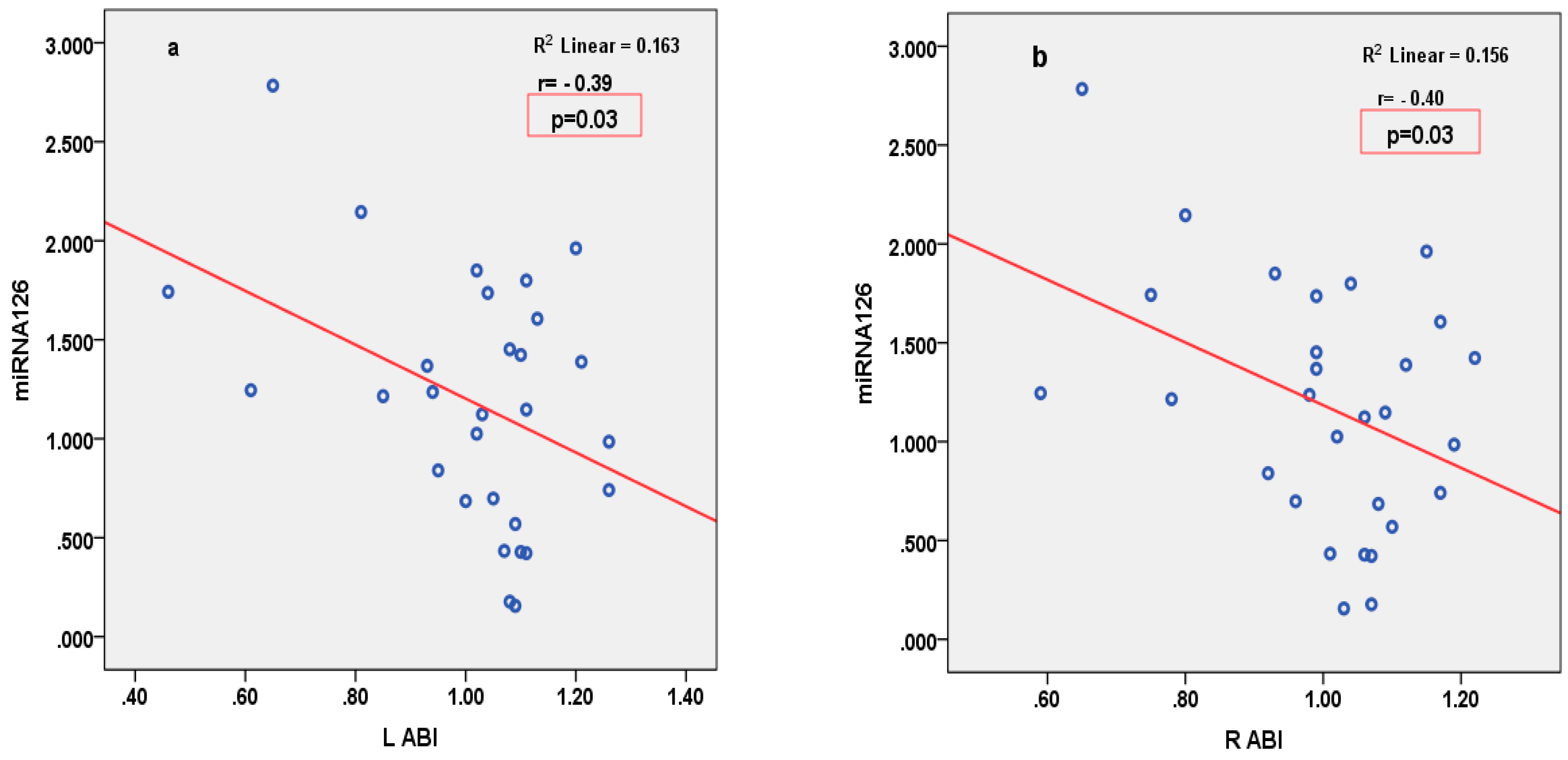
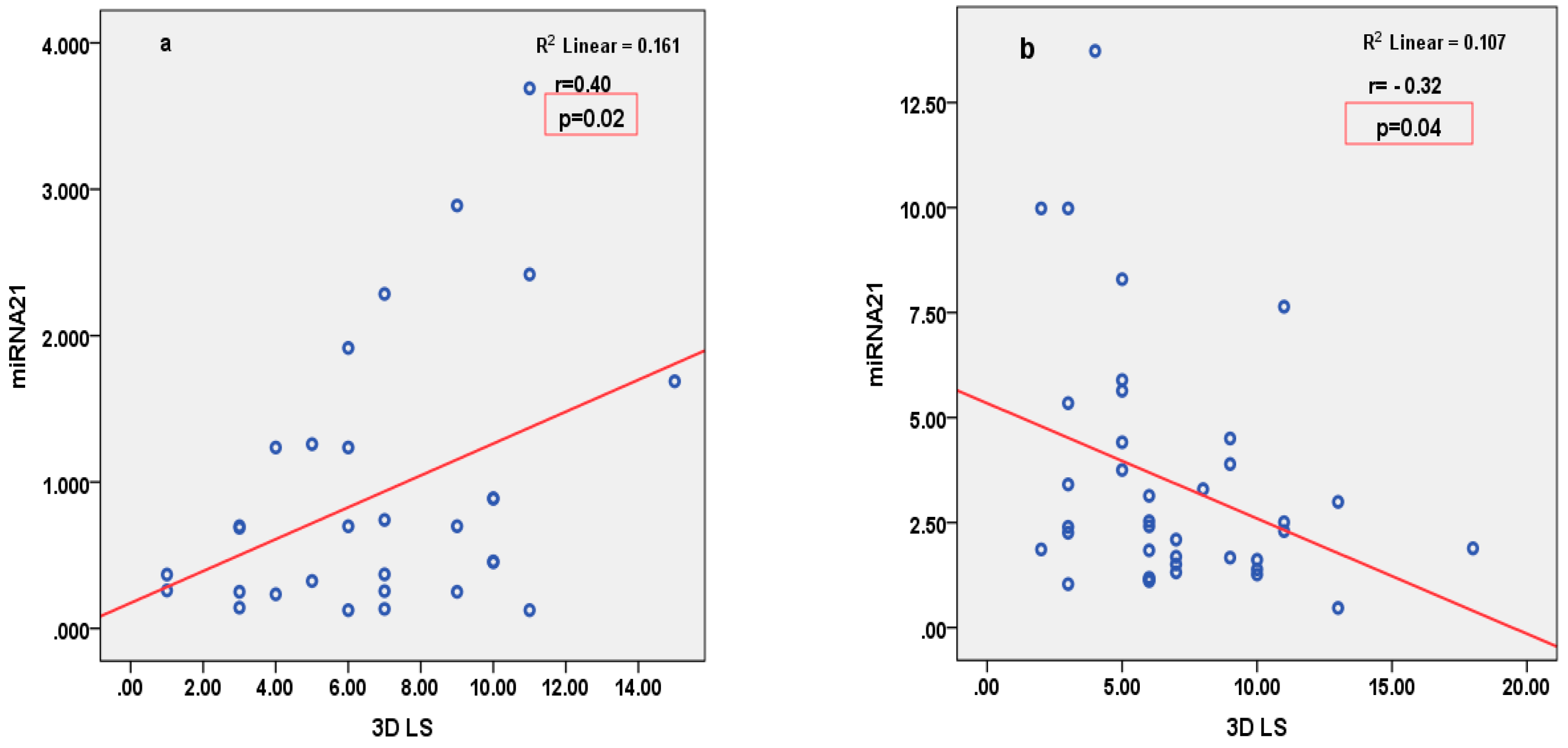
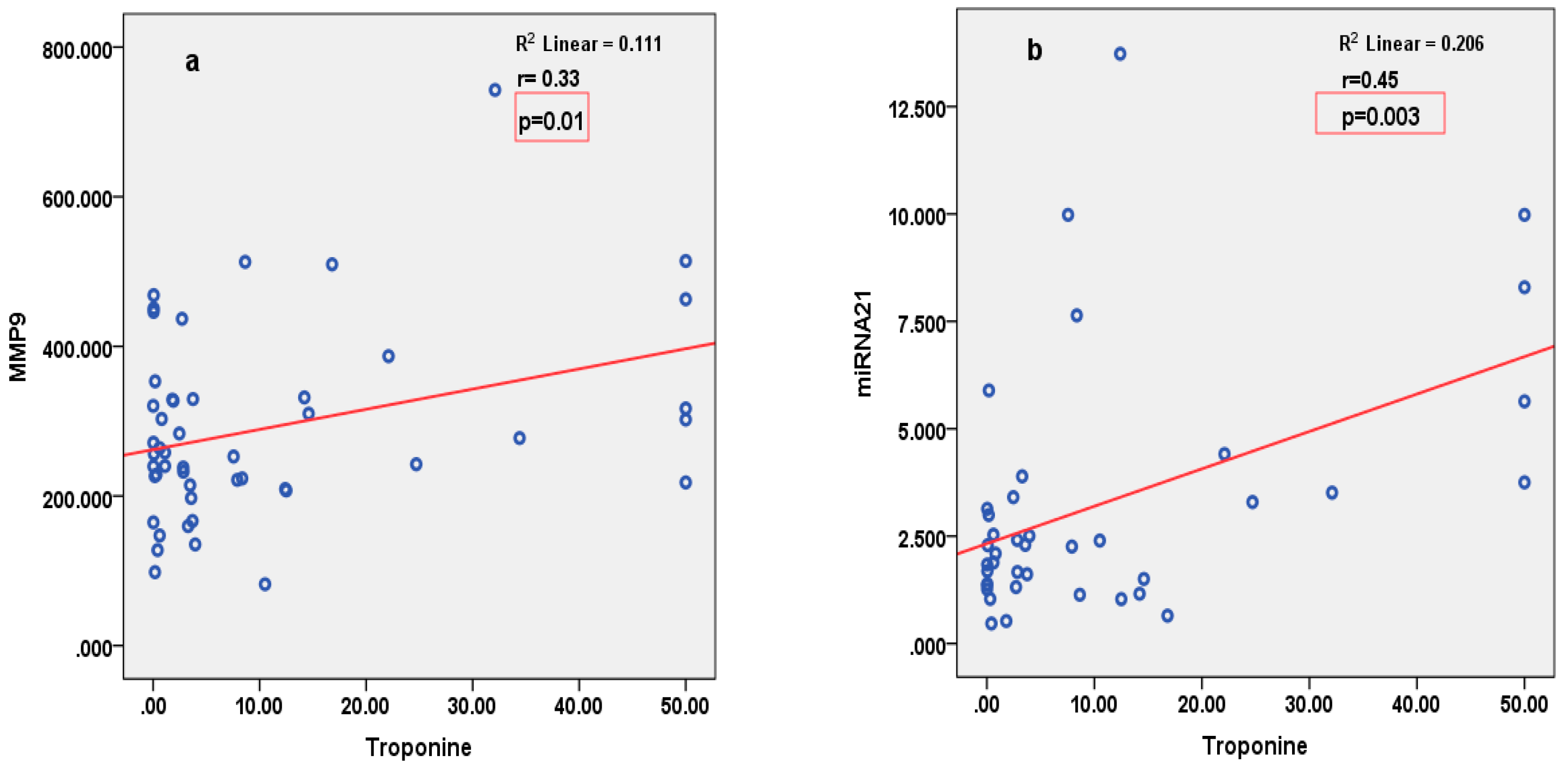
3.4. Correlations
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Li, Q.; Hosen, M.R.; Zietzer, A.; Flender, A.; Levermann, P.; Schmitz, T.; Frühwald, D.; Goody, P.; Nickenig, G.; et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ. Res. 2019, 124, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Nouraee, N.; Mowla, S.J. miRNA therapeutics in cardiovascular diseases: Promises and problems. Front. Genet. 2015, 6, 232. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, E.; Olson, E.N. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat. Rev. Drug Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Li, Q.; Miao, Y.; Zhang, Y.; Yuan, W.; Fan, L.; Liu, G.; Mi, Q.; Yang, J. The Emerging Role of MicroRNA-155 in Cardiovascular Diseases. BioMed Res. Int. 2016, 2016, 9869208. [Google Scholar] [CrossRef] [PubMed]
- Duggal, B.; Gupta, M.K.; Prasad, S.V.N. Potential Role of microRNAs in Cardiovascular Disease: Are They up to Their Hype? Curr. Cardiol. Rev. 2016, 12, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Darabi, F.; Aghaei, M.; Movahedian, A.; Pourmoghadas, A.; Sarrafzadegan, N. The role of serum levels of microRNA-21 and matrix metalloproteinase-9 in patients with acute coronary syndrome. Mol. Cell. Biochem. 2016, 422, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zhu, W.; Liu, D.; Su, Z.; Zhang, L.; Chang, Q.; Li, P. Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol. Med. 2019, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, M.; Sader, S. Matrix metalloproteinases in cardiovascular disease. Can. J. Cardiol. 2006, 22, 25B–30B. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, D.; Florescu, M.; Bruja, R.; Patrascu, N.; Vladareanu, A.-M.; Vinereanu, D. 3D echocardiography, arterial stiffness, and biomarkers in early diagnosis and prediction of CHOP-induced cardiotoxicity in non-Hodgkin’s lymphoma. Sci. Rep. 2020, 10, 18473. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; Pérez De Isla, L.; et al. EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 3–46. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Kogame, N.; Iijima, R.; Nakamura, M.; Sugi, K. Carotid artery intima-media thickness and plaque score can predict the SYNTAX score. Eur. Heart J. 2012, 33, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Niki, K.; Furuhata, H.; Ohnishi, S.; Suzuki, S. Relationship between the pressure and diameter of the carotid artery in humans. Heart Vessel. 2000, 15, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, D.J.; Florescu, M.; Suran, B.M.C.; Enescu, O.A.; Mincu, R.I.; Magda, S.; Patrascu, N.; Vinereanu, D. Comparison of pulse wave velocity assessed by three different techniques: Arteriograph, Complior, and Echo-tracking. Heart Vessel. 2016, 31, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A Novel Blood Pressure-independent Arterial Wall Stiffness Parameter; Cardio-Ankle Vascular Index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Okada, T.; Niki, K.; Chang, D.; Sugawara, M. On-line noninvasive one-point measurements of pulse wave velocity. Heart Vessel. 2002, 17, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Iino, H.; Okano, T.; Daimon, M.; Sasaki, K.; Chigira, M.; Nakao, T.; Mizuno, Y.; Yamazaki, T.; Kurano, M.; Yatomi, Y.; et al. Usefulness of Carotid Arterial Strain Values for Evaluating the Arteriosclerosis. J. Atheroscler. Thromb. 2019, 26, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Muroya, T.; Kawano, H.; Koga, S.; Ikeda, S.; Yamamoto, F.; Maemura, K. Aortic Stiffness Is Associated with Coronary Microvascular Dysfunction in Patients with Non-obstructive Coronary Artery Disease. Intern. Med. 2020, 59, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Kirigaya, J.; Iwahashi, N.; Tahakashi, H.; Minamimoto, Y.; Gohbara, M.; Abe, T.; Akiyama, E.; Okada, K.; Matsuzawa, Y.; Maejima, N.; et al. Impact of Cardio-Ankle Vascular Index on Long-Term Outcome in Patients with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2020, 27, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Li, M.; Wu, Y. Association between Atherosclerosis and Diabetic Retinopathy in Chinese Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Takahashi, K.; Dannoura, M.; Oomori, K.; Miyoshi, A.; Inada, T.; Miyoshi, H. The Association of Cardio-Ankle Vascular Index and Ankle-Brachial Index with Macroangiopathy in Patients with Type 2 Diabetes Mellitus. J. Atheroscler. Thromb. 2019, 26, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. MicroRNAs: Role in cardiovascular biology and disease. Clin. Sci. 2008, 114, 699–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Tian, L.; Sun, Q. Diagnostic and prognostic value of circulating miRNA-499 and miRNA-22 in acute myocardial infarction. J. Clin. Lab. Anal. 2020, 34, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Wang, F.; Nie, X.; Du, H.; Zhao, Y.; Yin, Z.; Li, H.; Fan, J.; Wen, Z.; Wang, D.W.; et al. The Cell Type–Specific Functions of miR-21 in Cardiovascular Diseases. Front. Genet. 2020, 11, 563166. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Gupta, S.K.; Thum, T. MicroRNA-Based Therapeutic Approaches in the Cardiovascular System. Cardiovasc. Ther. 2010, 30, e9–e15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, Y.; Chen, S.; Zhang, G.; Zhang, M.; Gong, Y.; Li, X. Circulating MicroRNA-146a and MicroRNA-21 Predict Left Ventricular Remodeling after ST-Elevation Myocardial Infarction. Cardiology 2015, 132, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.M.; Brecht, W.J.; Xu, Q.; Mahley, R.W.; Huang, Y. Increased tau Phosphorylation in Apolipoprotein E4 Transgenic Mice Is Associated with Activation of Extracellular Signal-regulated Kinase. J. Biol. Chem. 2004, 279, 44795–44801. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Olson, E.N. AngiomiRs—Key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009, 19, 205–211. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Davis, S. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006, 34, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Iacobescu, L.; Ciobanu, A.-O.; Corlatescu, A.-D.; Simionescu, M.; Iacobescu, G.L.; Dragomir, E.; Vinereanu, D. The Role of Circulating MicroRNAs in Cardiovascular Diseases: A Novel Biomarker for Diagnosis and Potential Therapeutic Targets? Cureus 2024, 16, e64100. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Ferreira, V.V.; Pereira-Da-Silva, T.; Ferreira, R.C. The role of miRNAs in the diagnosis of stable atherosclerosis of different arterial territories: A critical review. Front. Cardiovasc. Med. 2022, 9, 1040971. [Google Scholar] [CrossRef] [PubMed]
- Barwari, T.; Rienks, M.; Mayr, M. MicroRNA-21 and the Vulnerability of Atherosclerotic Plaques. Mol. Ther. 2018, 26, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Funayama, H.; Yoshioka, T.; Ishikawa, S.-E.; Momomura, S.-I.; Kario, K. Close Association of Matrix Metalloproteinase-9 Levels with the Presence of Thin-Cap Fibroatheroma in Acute Coronary Syndrome Patients: Assessment by Optical Coherence Tomography and Intravascular Ultrasonography. Cardiovasc. Revascularization Med. 2021, 32, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; George, M.; Jha, D.; Goenka, L. Long Term Prognostic Utility of Matrix Metalloproteinase-9 in Patients with Acute Coronary Syndrome—A Systematic Review. CPB 2021, 22, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Somuncu, M.U.; Pusuroglu, H.; Karakurt, H.; Bolat, I.; Karakurt, S.T.; Demir, A.R.; Isıksacan, N.; Akgul, O.; Surgit, O. The prognostic value of elevated matrix metalloproteinase-9 in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: A two-year prospective study. Rev. Port. Cardiol. 2020, 39, 267–276. [Google Scholar] [CrossRef] [PubMed]

| General Characteristics | STEMI (n = 60) | SIHD (n = 30) | p-Value |
|---|---|---|---|
| Age (years) | 57.3 ± 13.4 | 61.1 ± 10.6 | 0.19 |
| Male/female (n/n) | 43/17 | 18/12 | 0.26 |
| BMI (kg/m2) | 28.0 ± 4.1 | 28.5 ± 3.5 | 0.54 |
| SBP (mmHg) | 129.8 ± 22.9 | 134.3 ± 16.9 | 0.35 |
| DBP (mmHg) | 76.6 ± 9.4 | 80.2 ± 9.8 | 0.09 |
| Heart rate (beats/min) | 75.9 ± 12.1 | 67.4 ± 11.5 | 0.02 |
| Cardiovascular risk factors | |||
| Current smoker (%) | 50.0 | 10.0 | 0.001 |
| Hypertension (%) | 73.3 | 96.7 | 0.008 |
| Type 2 diabetes (%) | 50.0 | 26.7 | 0.035 |
| Dyslipidaemia (%) | 88.3 | 100 | 0.05 |
| Parameter | STEMI (n = 60) | SIHD (n = 30) | p-Value |
|---|---|---|---|
| 2D LVEDV (mL) | 124.4 ± 34.5 | 120.4 ± 34.1 | 0.60 |
| 2D LVESV (mL) | 71.9 ± 27.6 | 61.5 ± 23.8 | 0.08 |
| 2D LVEF (%) | 42.0 ± 8.9 | 49.6 ± 7.6 | 0.0001 |
| MAPSE (mm) | 12.5 ± 6 | 13.6 ± 2.1 | 0.32 |
| TDI Smi_m (cm/s) | 6.8 ± 1.5 | 6.7 ± 1.2 | 0.88 |
| TDI Smi_l (cm/s) | 7.4 ± 2.3 | 7.9 ± 2.0 | 0.28 |
| 2D RS (%) | 16.1 ± 8.4 | 21.3 ± 9.0 | 0.011 |
| 2D CS (%) | 12.7 ± 3.7 | 14.3 ± 3.8 | 0.06 |
| 2D LS (%) | 10.0 ± 3.8 | 14.1 ± 3.1 | 0.0001 |
| 3D LVEDV (mL) | 111.3 ± 27.7 | 103.7 ± 28.6 | 0.25 |
| 3D LVESV (mL) | 62.9 ± 21.5 | 52.2 ± 20.0 | 0.31 |
| 3D LVEF (%) | 44.3 ± 7.4 | 50.6 ± 7.2 | 0.0001 |
| 3D RS (%) | 16.6 ± 8.9 | 18.4 ± 8.7 | 0.37 |
| 3D CS (%) | 7.4 ± 3.3 | 8.4 ± 3.6 | 0.21 |
| 3D LS (%) | 6.5 ± 3.4 | 6.8 ± 3.3 | 0.72 |
| IMT (mm) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.52 |
| Ep (kPa) | 131.7 ± 56.4 | 142.7 ± 61.2 | 0.42 |
| β index | 10.0 ± 3.9 | 11.3 ± 4.8 | 0.20 |
| AC (mm2/kPa) | 0.7 ± 0.3 | 0.7 ± 0.4 | 0.99 |
| AIX (%) | 4.4 ± 24.3 | 12.3 ± 14.3 | 0.12 |
| RCAVI | 9.3 ± 1.9 | 8.9 ± 1.7 | 0.36 |
| LCAVI | 9.3 ± 1.9 | 8.9 ± 1.5 | 0.32 |
| RABI | 0.9 ± 0.17 | 0.9 ± 0.1 | 0.92 |
| LABI | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.98 |
| Parameter | miR126 | miR146 | miR21 | MMP1 | MMP9 | |
|---|---|---|---|---|---|---|
| Pearson correlation (R) and p-value | STEMI | |||||
| 2D LVEDV (mL) | R = −0.03, p = 0.81 | R = 0.05, p = 0.71 | R = 0.13, p = 0.36 | R = 0.03, p = 0.82 | R = 0.08, p = 0.53 | |
| 2D LVESV (mL) | R = 0.01, p = 0.94 | R = −0.02, p = 0.86 | R = 0.09, p = 0.51 | R = 0.11, p = 0.38 | R = 0.11, p = 0.40 | |
| 2D LVEF (%) | R = −0.33, p = 0.01 | R = 0.15, p = 0.26 | R = −0.07, p = 0.63 | R = −0.27, p = 0.04 | R = −0.40, p = 0.001 | |
| MAPSE (mm) | R = 0.35, p = 0.009 | R = −0.02, p = 0.86 | R = 0.10, p = 0.49 | R = 0.09, p = 0.46 | R = 0.33, p = 0.009 | |
| TDI Smi_m (cm/s) | R = −0.23, p = 0.08 | R = 0.17, p = 0.23 | R = −0.02, p = 0.86 | R = −0.23, p = 0.08 | R = −0.14, p = 0.29 | |
| TDI Smi_l (cm/s) | R = 0.04, p = 0.74 | R = 0.25, p = 0.06 | R = 0.26, p = 0.08 | R = −0.18, p = 0.16 | R = −0.03, p = 0.79 | |
| 2D RS (%) | R = −0.20, p = 0.13 | R = 0.07, p = 0.59 | R = 0.02, p = 0.89 | R = −0.18, p = 0.18 | R = −0.26, p = 0.05 | |
| 2D CS (%) | R = −0.04, p = 0.73 | R = 0.03, p = 0.79 | R = 0.04, p = 0.76 | R = −0.02, p = 0.83 | R = −0.09, p = 0.47 | |
| 2D LS (%) | R = −0.13, p = 0.33 | R = 0.02, p = 0.85 | R = −0.08, p = 0.56 | R = −0.27, p = 0.03 | R = −0.26, p = 0.04 | |
| 3D LVEDV (mL) | R = −0.02, p = 0.83 | R = 0.00, p = 0.99 | R = 0.02, p = 0.86 | R = 0.04, p = 0.79 | R = 0.08, p = 0.58 | |
| 3D LVESV (mL) | R = 0.03, p = 0.80 | R = 0.00, p = 0.97 | R = 0.07, p = 0.63 | R = 0.05, p = 0.71 | R = 0.09, p = 0.52 | |
| 3D LVEF (%) | R = −0.11, p = 0.43 | R = −0.02, p = 0.86 | R = −0.18, p = 0.26 | R = −0.07, p = 0.60 | R = −0.14, p = 0.33 | |
| 3D RS (%) | R = −0.25, p = 0.08 | R = −0.10, p = 0.49 | R = −0.28, p = 0.08 | R = −0.21, p = 0.16 | R = −0.30, p = 0.03 | |
| 3D CS (%) | R = −0.31, p = 0.03 | R = −0.04, p = 0.77 | R = −0.25, p = 0.11 | R = −0.19, p = 0.19 | R = −0.31, p = 0.03 | |
| 3D LS (%) | R = −0.16, p = 0.27 | R = −0.18, p = 0.23 | R = −0.32, p = 0.04 | R = −0.19, p = 0.20 | R = −0.22, p = 0.12 | |
| IMT (mm) | R = 0.21, p = 0.13 | R = 0.02, p = 0.85 | R = −0.17, p = 0.29 | R = 0.22, p = 0.12 | R = 0.17, p = 0.24 | |
| Ep (kPa) | R = 0.05, p = 0.72 | R = 0.09, p = 0.53 | R = 0.15, p = 0.32 | R = 0.10, p = 0.47 | R = 0.11, p = 0.40 | |
| β index | R = 0.03, p = 0.78 | R = 0.02, p = 0.84 | R = −0.33, p = 0.01 | R = 0.06, p = 0.65 | R = 0.12, p = 0.36 | |
| AC (mm2/kPa) | R = −0.09, p = 0.52 | R = −0.07, p = 0.63 | R = 0.13, p = 0.40 | R = −0.06, p = 0.66 | R = 0.00, p = 0.94 | |
| AIX (%) | R = 0.07, p = 0.71 | R = 0.01, p = 0.94 | R = −0.21, p = 0.17 | R = 0.05, p = 0.72 | R = −0.01, p = 0.92 | |
| RCAVI | R = 0.32, p = 0.01 | R = 0.07, p = 0.58 | R = 0.07, p = 0.62 | R = 0.16, p = 0.22 | R = 0.18, p = 0.15 | |
| LCAVI | R = 0.32, p = 0.01 | R = 0.08, p = 0.53 | R = 0.15, p = 0.29 | R = 0.11, p = 0.40 | R = 0.14, p = 0.27 | |
| RABI | R = −0.02, p = 0.84 | R = 0.17, p = 0.23 | R = 0.14, p = 0.32 | R = −0.07, p = 0.57 | R = 0.01, p = 0.93 | |
| LABI | R = −0.02, p = 0.83 | R = 0.13, p = 0.38 | R = 0.09, p = 0.52 | R = −0.11, p = 0.41 | R = −14, p = 0.27 | |
| Pearson correlation (R) and p-value | SIHD | |||||
| 2D LVEDV (mL) | R = 0.07, p = 0.78 | R = 0.06, p = 0.75 | R = −0.15, p = 0.41 | R = −10, p = 0.60 | R = 0.15, p = 0.41 | |
| 2D LVESV (mL) | R = −0.04, p = 0.98 | R = −0.01, p = 0.93 | R = −0.11, p = 0.55 | R = 0.05, p = 0.80 | R = 0.19, p = 0.32 | |
| 2D LVEF (%) | R = 0.06, p = 0.73 | R = 0.07, p = 0.70 | R = 0.04, p = 0.98 | R = −0.29, p = 0.15 | R = −0.08, p = 0.68 | |
| MAPSE (mm) | R = 0.41, p = 0.02 | R = 0.23, p = 0.24 | R = −0.37, p = 0.04 | R = −0.29, p = 0.14 | R = −0.04, p = 0.82 | |
| TDI Smi_m (cm/s) | R = 0.08, p = 0.64 | R = 0.24, p = 0.22 | R = 0.06, p = 0.73 | R = −0.25, p = 0.20 | R = 0.03, p = 0.87 | |
| TDI Smi_l (cm/s) | R = 0.00, p = 0.99 | R = 0.00, p = 0.98 | R = 0.−29, p = 0.11 | R = −0.09, p = 0.65 | R = 0.08, p = 0.68 | |
| 2D RS (%) | R = −0.14, p = 0.47 | R = 0.11, p = 0.56 | R = −0.01, p = 0.94 | R = −0.18, p = 0.37 | R = −0.21, p = 0.29 | |
| 2D CS (%) | R = −0.004, p = 0.98 | R = 0.05, p = 0.77 | R = −0.13, p = 0.47 | R = −0.26, p = 0.20 | R = 0.14, p = 0.48 | |
| 2D LS (%) | R = 0.23, p = 0.21 | R = 0.27, p = 0.16 | R = −0.19, p = 0.30 | R = −0.11, p = 0.56 | R = −0.02, p = 0.88 | |
| 3D LVEDV (mL) | R = −0.03, p = 0.86 | R = −0.07, p = 0.73 | R = −0.30, p = 0.10 | R = −0.14, p = 0.48 | R = 0.17, p = 0.37 | |
| 3D LVESV (mL) | R = −0.07, p = 0.71 | R = −0.14, p = 0.47 | R = −0.26, p = 0.16 | R = 0.02, p = 0.91 | R = 0.23, p = 0.23 | |
| 3D LVEF (%) | R = 0.07, p = 0.71 | R = 0.18, p = 0.35 | R = 0.13, p = 0.48 | R = −0.28, p = 0.16 | R = −0.25, p = 0.19 | |
| 3D RS (%) | R = −0.13, p = 0.47 | R = 0.24, p = 0.21 | R = 0.50, p = 0.005 | R = 0.02, p = 0.91 | R = −0.06, p = 0.76 | |
| 3D CS (%) | R = −0.16, p = 0.40 | R = 0.26, p = 0.18 | R = 0.46, p = 0.009 | R = 0.02, p = 0.91 | R = −0.05, p = 0.77 | |
| 3D LS (%) | R = −0.05, p = 0.78 | R = −25, p = 0.19 | R = 0.40, p = 0.02 | R = −0.003, p = 0.99 | R = −0.37, p = 0.85 | |
| IMT (mm) | R = 0.24, p = 0.23 | R = 0.15, p = 0.48 | R = −0.02, p = 0.88 | R = 0.18, p = 0.40 | R = −0.08, p = 0.70 | |
| Ep (kPa) | R = −0.21, p = 0.29 | R = −0.13, p = 0.52 | R = −0.09, p = 0.63 | R = 0.17, p = 0.42 | R = −0.02, p = 0.89 | |
| β index | R = −0.18, p = 0.37 | R = −0.04, p = 0.85 | R = −0.13, p = 0.51 | R = 0.08, p = 0.69 | R = −0.08, p = 0.70 | |
| AC (mm2/kPa) | R = 0.27, p = 0.16 | R = 0.18, p = 0.37 | R = 0.09, p = 0.63 | R = −0.17, p = 0.43 | R = −0.13, p = 0.51 | |
| AIX (%) | R = −0.001, p = 0.99 | R = −0.07, p = 0.73 | R = −0.04, p = 0.84 | R = −0.26, p = 0.21 | R = −0.16, p = 0.42 | |
| RCAVI | R = −0.25, p = 0.18 | R = −0.34, p = 0.07 | R = −0.11, p = 0.55 | R = 0.16, p = 0.41 | R = 0.32, p = 0.04 | |
| LCAVI | R = −0.27, p = 0.15 | R = −0.28, p = 0.15 | R = −0.05, p = 0.76 | R = 0.09, p = 0.63 | R = 0.40, p = 0.03 | |
| RABI | R = −0.40, p = 0.03 | R = 0.16, p = 0.41 | R = 0.07, p = 0.70 | R = 0.08, p = 0.66 | R = −0.14, p = 0.45 | |
| LABI | R = −0.39, p = 0.03 | R = 0.17, p = 0.37 | R = 0.16, p = 0.38 | R = 0.19, p = 0.35 | R = −0.06, p = 0.76 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobescu, L.; Ciobanu, A.O.; Macarie, R.; Vadana, M.; Ciortan, L.; Tucureanu, M.M.; Butoi, E.; Simionescu, M.; Vinereanu, D. Diagnostic and Prognostic Role of Circulating microRNAs in Patients with Coronary Artery Disease—Impact on Left Ventricle and Arterial Function. Curr. Issues Mol. Biol. 2024, 46, 8499-8511. https://doi.org/10.3390/cimb46080500
Iacobescu L, Ciobanu AO, Macarie R, Vadana M, Ciortan L, Tucureanu MM, Butoi E, Simionescu M, Vinereanu D. Diagnostic and Prognostic Role of Circulating microRNAs in Patients with Coronary Artery Disease—Impact on Left Ventricle and Arterial Function. Current Issues in Molecular Biology. 2024; 46(8):8499-8511. https://doi.org/10.3390/cimb46080500
Chicago/Turabian StyleIacobescu, Loredana, Andrea Olivia Ciobanu, Razvan Macarie, Mihaela Vadana, Letitia Ciortan, Monica Madalina Tucureanu, Elena Butoi, Maya Simionescu, and Dragos Vinereanu. 2024. "Diagnostic and Prognostic Role of Circulating microRNAs in Patients with Coronary Artery Disease—Impact on Left Ventricle and Arterial Function" Current Issues in Molecular Biology 46, no. 8: 8499-8511. https://doi.org/10.3390/cimb46080500
APA StyleIacobescu, L., Ciobanu, A. O., Macarie, R., Vadana, M., Ciortan, L., Tucureanu, M. M., Butoi, E., Simionescu, M., & Vinereanu, D. (2024). Diagnostic and Prognostic Role of Circulating microRNAs in Patients with Coronary Artery Disease—Impact on Left Ventricle and Arterial Function. Current Issues in Molecular Biology, 46(8), 8499-8511. https://doi.org/10.3390/cimb46080500







