Abstract
Keratins 6, 16, and 17 occupy unique positions within the keratin family. These proteins are not commonly found in the healthy, intact epidermis, but their expression increases in response to damage, inflammation, and hereditary skin conditions, as well as cancerous cell transformations and tumor growth. As a result, there is an active investigation into the potential use of these proteins as biomarkers for different pathologies. Recent studies have revealed the role of these keratins in regulating keratinocyte migration, proliferation, and growth, and more recently, their nuclear functions, including their role in maintaining nuclear structure and responding to DNA damage, have also been identified. This review aims to summarize the latest research on keratins 6, 16, and 17, their regulation in the epidermis, and their potential use as biomarkers in various skin conditions.
1. Introduction
Keratins are a superfamily of intermediate filamentous proteins that play important roles in the organization of the cytoskeleton. The cytoskeleton of mammalian epithelial cells is formed from pairs of type I and type II keratins. Type I keratins usually have a lower mass (40–56.5 kDa) and a more acidic total charge (pI 4.5–6.0), while type II keratins have a larger mass (50–70 kDa) and a basic neutral charge (pI 6.5–8.5) [1]. The heterodimers of keratin types I and II self-assemble into antiparallel, staggered tetramers, forming intermediate filaments through longitudinal and lateral interactions [2]. These keratin intermediate filaments (KIFs) have a diameter of approximately 10 nanometers and form a network within the cytoskeleton of cells, providing structural support and mechanical resilience.
The patterns of keratin expression in different tissues are not universal and mostly characterize specific types of epithelia. For example, keratins 8 and 18 are expressed in the simple epithelium, along with additional keratins 7, 19, 20, and 23. In contrast, stratified epithelium is characterized by keratins K4/K13 in mucous membranes and keratins K3/K12 in the cornea (reviewed by Kalabusheva et al. [3]). In the epidermis, actively proliferating basal keratinocytes express high levels of K5/K14 and low levels of K15. During epidermal differentiation, the proliferation of keratinocytes decreases, and keratins 5 and 14 are replaced by keratins 1 and 10 as the cells migrate to higher layers of the epidermis [4,5,6].
Moreover, the expression of certain keratins in the epidermis is highly restricted to specific anatomical sites. Keratin K6 (KRT6) and its polymerization partners K16 and K17 (KRT16 and KRT17, respectively) are only constitutively expressed in the palmoplantar epidermis as well as in skin appendages, including the nail plate, hair follicles, and sebaceous and sweat glands [2,7,8]. Keratins K6/16/17 are not detected in intact normal interfollicular epidermis; however, their expression significantly increases in wounds and is maintained at a high level throughout all stages of regeneration until the complete restoration of epithelial barrier function [2,9,10]. At the same time, the expression of K1/K10 rapidly decreases in keratinocytes at the wound’s edge, and K6/K16 induction and dimerization occur, accompanied by an increase in the level of cytoplasmic keratin K17 [10]. K6, K16, and K17 are known as damage-associated keratins, and they are often referred to as “stress keratins” because they play a central role in the process of damage regeneration.
High expression of these keratins has been detected in hyperproliferating keratinocytes in psoriasis and other inflammatory skin conditions [2,11,12]. In addition, in clinical settings, keratin K17 is well known as an oncogenic protein, the high expression of which is often linked to a poor prognosis. Additionally, an increasing number of recent studies have focused on the roles of keratins K16 and K6 in relation to carcinogenesis and tumor progression [13,14,15].
The study of molecular physiological processes regulated by keratin 6/16/17 has revealed many functions that are atypical for these proteins. These include the regulation of cell migration, growth, proliferation, apoptosis (programmed cell death), nuclear morphology, and responses to DNA damage. This review aimed to summarize the latest research data on the biological functions of these proteins, their encoded genes, and their roles under normal and abnormal conditions. Their potential as clinical markers and targets for therapy are also discussed.
2. Structure and Localization of K6/K16/K17
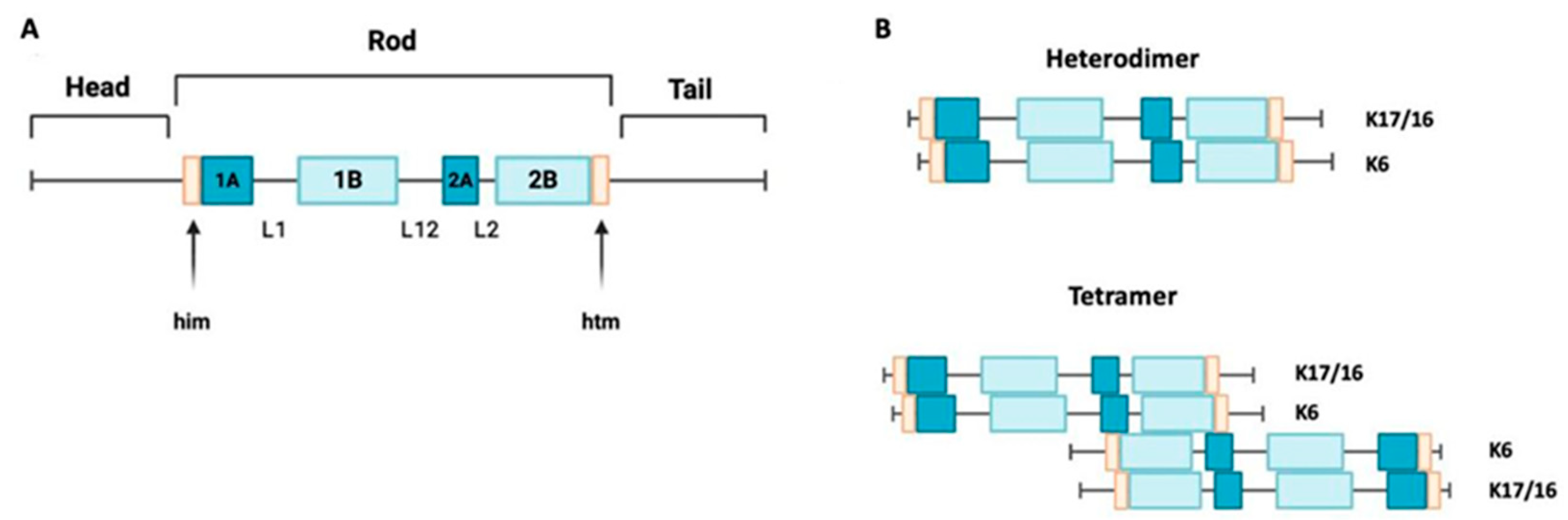
Keratin 17 is a type I keratin with a molecular weight of 48 kDa. It has a triplet structure consisting of 432 amino acids. Its structure (Figure 1A) includes a nonhelical head region (1–83), an alpha-helical rod region (84–392), and a nonhelical tail region (393–432) [16,17]. Within the core domain, there are segments containing heptadic repeats (coils 1A, 1B, and 2), which are interrupted by linker sequences at two conserved sites. Keratin 16 is also a type I keratin with a molecular weight of 50,924.66 Da. It is structurally similar to keratin 17, but it is slightly larger, consisting of 473 amino acids [1]. A mini family of three genes (KRT6A, KRT6B, and KRT6C) encodes homologous intermediate filaments of type II. The protein encoded by the KRT6A gene is dominant in this family. All genes in this mini family encode proteins with 564 amino acids, but the calculated molecular weights of these proteins vary slightly [9].
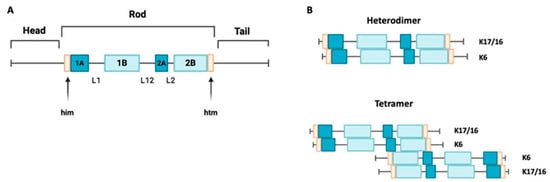
Figure 1.
Keratin 17 structure and assembly. (A) A schematic structure of keratin 17 showing non-helical domains at the N-terminus and C-terminus ends, with a central helical rod domain. K17’s structure consists of four alpha-helical domains (1A, 1B, 2A, and 2B), separated by three non-helical linkers (L1, L12, and L2). K17 also has two motifs, the helix initiation (him) and termination (hit) motifs, located at the ends of domains 1A and 2B, respectively. (B) A depiction of the alignment of the acidic type I keratins K17 and K16 along with the basic keratin K6, arranged in parallel to create a heterodimer. These heterodimers align in an antiparallel and staggered fashion to form a tetramer.
Keratins 16 and 17 can form dimers with keratin 6 (Figure 1B), so the expression of these intermediate filaments is often colocalized [18]. To investigate this further, data from the Genotype-Tissue Expression (GTEX) database were analyzed in a recent study. The goal was to reconstruct the tissue-specific expression patterns of keratins in the human body. This study confirmed the co-expression of several pairs of keratin genes, including KRT6 and KRT16, as well as KRT6 and KRT17 [19].
These data are in line with the fact that keratins K6, K16 and K17 are normally co-expressed in the palmar and plantar epidermis, as well as in epithelial appendages such as hair follicles. They are also found in epidermal lesions and in various pathological skin conditions [2,20,21]. In hair follicles, K6/K16/K17 are localized in the cells of the outer root sheath and in the cells of the companion layer and medulla [3,22,23,24].
In addition to the skin and its derivatives, K17 is expressed in normal basal cells of the respiratory epithelium, urothelial cells, squamous epithelial cells of the mucosal membrane, and basal cells of the oral cavity epithelium [10,25,26,27,28]. Keratin K16 has been detected at the protein level in various epithelial tissues and in nonkeratinizing stratified squamous epithelia [25,29,30]. K6 and K16 are also expressed in the duct cells of the mammary glands and sweat glands [31]. It has been reported that keratin 6 is expressed in the multilayered epithelium of the mucous membranes of the oral cavity, esophagus, and glandular epithelium [3,23,25]. However, given its colocalization with keratins K16 and K17, it is reasonable to assume that K6 is expressed in all types of epithelial cells expressing K16 and K17.
3. K6, K16, and K17 Are Essential for Regeneration
Proliferation is the final stage of skin wound healing, during which tissue integrity is restored due to cell division. At the same time, keratinocytes, which are the cells that make up the outermost layer of the skin, fill the damaged area by migrating and proliferating and participate in restoring the epidermal barrier through epidermal differentiation. During the healing process, keratinocytes near the wound temporarily suspend epidermal differentiation and undergo changes in protein translation, cell size, shape, and contact with other cells and the extracellular matrix, preparing for active migration and proliferation. Immediately after injury, keratinocytes at the edge of the wound significantly reduce the expression of keratin K1/K10 and induce the expression of keratins K6, K16, and K17 [2,32,33,34]. The high expression of keratins K6, K16, and K17 provides keratinocytes with the mechanical resilience required for migration and structural support [35]. However, the role of these keratins in regeneration extends beyond their structural function. The modulation of cell migration and adhesion to substrates is critical for successful regeneration and is an essential physiological function of keratins K6, K16, and K17. To close the defect in the epidermis, keratinocytes at the wound edge must first weaken their adhesion to each other and the basement membrane. This process is facilitated by alterations in the composition of the keratin cytoskeleton and desmosomes, which are considered the main factors determining adhesive strength. Wound healing is accompanied by a temporary reduction in desmosome adhesion, while keratin expression increases. Keratins 5/14 have been shown to contribute to the stabilization of desmosomes, while keratins K6/K17, on the contrary, lead to the destruction of desmosomes through the induction of protein kinase C and subsequent destabilization of epithelial layers [36]. Keratin 6 is able to interact directly with myosin II, which promotes cell adhesion and provides additional resistance to mechanical stress [37,38]. In addition, keratin 6 is expressed in cells at the edge of a wound, where it plays a role in regulating the collective migration of keratinocytes by inhibiting Src [39]. It also regulates cell–matrix interactions and intercellular adhesion [37]. Notably, keratinocytes isolated from KRT6A-/KRT6B-mice exhibit increased migration [38]. It has been shown in HaCaT keratinocytes that overexpression of keratin 16 leads to a decrease in migration activity. This decrease can be partially reversed by co-transfecting K6A [40]. In contrast, keratin 17 enhances the migratory activity of keratinocytes, as shown by data obtained from HaCaT cells in a scratch wound assay. The knockdown of KRT17 results in a decrease in the rate of diabetic wound healing in mouse skin [33]. Keratin 17 promotes damage repair by inducing cell proliferation through STAT3 [17] and modulates lipid metabolism in keratinocytes to restore epidermal barrier functions [41]. Generally, keratins K6/16 provide optimal intercellular adhesion and collective migration for damage repair, while keratin 17 induces cell migration and proliferation.
4. K6, K16, and K17 Are Involved in the Pathogenesis of Various Skin Diseases
4.1. Psoriasis
The involvement of K17 in the development of psoriasis was first identified in 1990 [42]. In 1995, Leigh and his colleagues discovered that K17 can be found in the upper layers of psoriatic skin both in vivo and in vitro [11]. This discovery formed the basis for further research in this area. Since then, the understanding of K17 as a marker for psoriasis has increased, and studies have shown that its level correlates with the severity of the condition [43]. The role of keratin 17 in the pathogenesis of this disease is primarily realized by inducing keratinocyte proliferation through the STAT3 and 14-3-3/mTOR pathways and modulating the immune response [44,45]. Keratin 17 is an autoantigen that stimulates the production of proinflammatory cytokines by T cells [44]. These cytokines, in turn, induce the expression of stress keratins. Interestingly, the a/a sequence (amino acids 102–116 and 188–196) of keratin 17 closely resembles that of the streptococcal M6 protein, a superantigen in psoriasis [44,46]. Keratins 6 and 16 are highly expressed in psoriatic plaques and are characteristic of psoriatic keratinocytes [47]. However, their role in the pathogenesis of psoriasis has been less well studied than that of keratin 17. Keratin 16 has also been shown to contribute to the hyperproliferation of keratinocytes in psoriasis, and silencing keratin 16 leads to a reduction in VEGF secretion by psoriatic keratinocytes [12]. Keratin 16 is also involved in regulating inflammation by modulating damage-associated molecular patterns (DAMPs) and cytokines through the mitogen-activated protein kinase (MAPK) and/or epidermal growth factor receptor (EGFR) pathways [48].
4.2. Atopic Dermatitis
Atopic dermatitis (AD), also known as eczema, is a common chronic inflammatory autoimmune skin disorder. Increased expression of KRT6A has been observed in patients with this condition. In keratinocytes from patients with AD, KRT6A appears to inhibit inflammation-induced apoptosis and suppress IL-17A expression, making the K6A/IL-17 pathway a potential target for treatment [49]. In addition, a recent study revealed a correlation between variations in the KRT6A gene (single nucleotide polymorphisms) and disease severity [50]. Although there is less evidence to support the role of keratin 16 in AD, recent research has shown that increased expression of KRT16 may be a marker for hyperproliferative keratinocytes in AD, and KRT16’s expression is significantly reduced in patients treated with dupilumab for AD [51,52]. Although it is not yet clear whether keratin 17 plays a role in the development of AD, this condition is characterized by an increase in the expression of several cytokines that trigger the production of keratin 17 [53].
4.3. Pachyonychia Congenita
Pachyonychia congenita (PC) is a rare genetic disorder that is inherited in an autosomal dominant pattern. It is characterized by keratoderma in the palms and soles of the feet, nail dystrophy, cyst formation, hyperkeratosis of hair follicles, and leukokeratosis of mucous membranes. Hyperhidrosis is also a common symptom of this condition. Mutations in genes such as KRT6A, KRT16, and KRT17 have been found in patients with PC [54,55,56]. In particular, the Asn92Asp mutation in the KRT17 gene and the Leu130Pro mutation in KRT16 (both of which are located in the helix initiation motif) have been linked to this condition. Additionally, mutations in KRT6A and KRT6B have been identified in some patients with PC [57,58]. Mutations of p.Tyr465His in exon 7 of KRT6A and p.Glu413Gln in exon 6 of KRT16 have also been linked to PC [59].
To date, progress in PC treatment is still unsatisfactory, and many therapies are at different stages of clinical development. Naturally, keratin 6/16/17 have been identified as potential therapeutic targets for PC. Sirolimus (rapamycin), an mTOR inhibitor, has been shown to reduce the expression of keratin 6A in human keratinocytes and reduce the severity of PC symptoms, although it has some side effects [60,61]. Other approaches include the use of statins for Stat1 repression [62], botulinum toxin injections [63], and siRNA therapy [64,65]. These methods involve complex and painful medical procedures that are not perfect. Therefore, the development of new therapies for treating PC or reducing its symptoms is urgently needed. Since PC serves as a prototype for siRNA-based therapies, genome editing techniques such as CRISPR-based therapeutics could potentially be considered for future research.
4.4. Other Skin Pathologies
In addition to psoriasis and PC, keratin 17 is also involved in the development of other skin conditions, such as lichen planus (LP). The pathogenesis of these conditions shares some similarities, including increased expression of keratin 17. In lichen planus, this expression is triggered by the induction of interferon gamma (IFN-γ) [66]. Similarly, KRT6A, KRT6B, and KRT6C are increased in patients with LP [67]. Elevated levels of KRT17 have been noted in patients with prurigo nodularis (PN), a chronic inflammatory dermatosis characterized by the presence of multiple hyperkeratotic papules [68]. In addition, in PN, there is increased expression of K6 in all layers of the skin with lesions, particularly in the spinous layer. Conversely, K16 has been detected only in the basal and lower suprabasal layers [69]. In another study, using single-cell RNA profiling, increased expression of the KRT6A, KRT6B, KRT6C, and KRT16 genes significantly characterized PN keratinocytes [70]. Inflammatory skin diseases are likely to lead to the production of these keratins, as the expression of K6/K16/K17 is associated with skin damage and strongly upregulated by proinflammatory cytokines.
5. Cancer
For many types of cancer, keratin 17 can be used as a diagnostic, prognostic, and predictive marker. Its increased expression often correlates with tumor aggressiveness and has a negative prognosis [71,72,73,74,75]. According to the analysis of data from various types of cancer, based on The Gene Expression Omnibus (GEO), The Cancer Genome Atlas (TCGA), and The Human Protein Atlas (HPA), as well as TIMER2 and other sources, a high level of keratin 17 was noted in most malignant neoplasms compared to normal tissues [76,77]. In addition, high expression of keratin 17 has been correlated with tumor size, depth of invasion, and metastasis in gastric cancer [78]. It has also been found to determine the low level of response to therapy in patients with squamous cell carcinoma of the head and neck (HNSCC), especially in those treated with immune checkpoint blockades [79]. Keratin 17 has been reported to be a negative prognostic biomarker for several types of cancer, including squamous cell carcinoma of the lungs (LSCC) and pulmonary adenocarcinoma (LUAD) [80]. It has also been linked to pancreatic ductal adenocarcinoma (PDAC) [81] and colorectal cancer (CRC) [82]. Keratin 17 has been shown to induce and promote the growth of oral squamous cell carcinoma (OSCC) [83]. Although the expression of keratin 17 is generally associated with an unfavorable prognosis for cancer patients, this trend does not always hold true. For example, in invasive breast carcinoma (BRCA) and chromophobic kidney cancer (KICH), the levels of keratin 17 are lower than those in normal tissue. Additionally, there are no significant differences in the levels of keratin 17 between tumors and normal tissues in urothelial bladder carcinoma (BLCA), papillary carcinoma of the kidney (KIRP), or pancreatic adenocarcinoma (PAAD) [16].
The involvement of K6 and K16 in carcinogenesis and tumor progression has been studied to a lesser extent. However, the number of studies characterizing these oncoproteins has increased in recent years. Keratin 16, in particular, has been identified as a marker for circulating tumor cells. Its high expression in breast cancer samples has been correlated with an intermediate mesenchymal phenotype [14]. Huang and colleagues demonstrated that high levels of keratin 16 are associated with a poorer prognosis, pathological differentiation, and lymph node involvement in patients with oral squamous cell carcinoma (OSCC) [13]. Increased expression of keratin 6A and the involvement of keratin 16 in the development of this type of tumor have also been reported in LUAD [84]. For keratin 6, a correlation with squamous cell differentiation in As-transformed UROtsa cells has been shown [85]. In addition, increased expression of KRT6A/B/C has also been observed in primary melanoma cells, and high levels of these keratins have been associated with a poor prognosis for patients with skin cutaneous melanoma (SKCM) [20]. High levels of keratin 6A are associated with a poorer prognosis and increased invasiveness in colorectal cancer (CRC) [86]. Data on the diagnostic significance of K6, K16, and K17 proteins are summarized in Table 1.

Table 1.
Expression of K6/16/17 in various types of cancer.
6. The Role of K6, K16, and K17 in Carcinogenesis
Keratin 17 plays a role in the development of cancer through several key processes: (I) promoting cell growth and proliferation; (II) supporting cell survival; and (III) modulating the immune system’s response. Keratin 17 has been shown to be involved in regulating the proliferation, migration, and invasion of tumor cells. For example, it has been found to be important in the development of gastric cancer [78] and osteosarcoma [107], among other types of cancer. Thus, keratin 17 promotes cell proliferation and migration by activating Akt/mTOR [83,108], and its inhibition can lead to a reduction in the activity of ERK1/2, Bad, p38 MAPK, and SAPK/JNK [12]. Keratin 17 also contributes to the uncontrolled progression of the cell cycle by promoting the degradation of p27KIP1; therefore, K17 may be considered a marker for uncontrolled proliferation [109]. The discovery of the role of K17 in regulating the DDR response and cell survival suggests that one possible mechanism of carcinogenesis is the maintenance of cell viability with DNA damage. This, in turn, could contribute to the accumulation of mutations and genomic instability [110]. Suppression of keratin 17 expression can lead to the induction of tumor cell apoptosis through changes in the levels of Bcl-2 family proteins and enhanced regulation of caspase-3 cleavage. This contributes to stopping the cell cycle at the G1/S phase by reducing the expression of cyclin E1 and cyclin D [78]. Another important aspect of tumor progression is the role of keratin 17 in regulating glycolysis through its co-expression with HIF1α. When HIF1α is translocated to the nucleus, it binds to the promoters of specific target genes, such as GLUT1 [107]. In addition, keratin 17 covalently binds to α-enolase (ENO1), which supports its phosphorylation by K17-Ser44. This phosphorylation enhances the nuclear translocation of keratin 17 and the transcriptional activation of LDHA, leading to increased glycolysis and cell proliferation [8].
In addition, K17 plays a role in the process of tumor cells evading immune surveillance. CD8+ T cells have been shown to play a role in the recognition and induction of apoptosis in tumor cells, while K17 can block the infiltration of these cells into tumor tissues in some cancers. This has been demonstrated, in particular, in pancreatic cancer and proliferative processes associated with the papilloma virus [111]. At the same time, KRT17 knockdown slows down HNSCC growth and increases the infiltration of CD8+ T cells [103]. In addition, K17 suppresses macrophage-mediated CXCL9/CXCL10 chemokine signaling [112,113]. K17 also plays an oncogenic role by inducing the expression of IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin 10 (IL-10). These cytokines lead to the activation of different types of macrophages [114,115].
An increasing number of studies have investigated the functions of keratins 6 and 16 in cancer development and progression. One study revealed that silencing KRT6A led to a reduction in the expression of TIM-2 and MMP-9, two proteins involved in tumor invasion and metastasis in nasopharyngeal carcinoma [116]. A recent study revealed that KRT6A contributes to the progression of bladder cancer by enhancing cell viability, proliferation, and adhesion while also inhibiting cancer cell apoptosis [93]. In addition, activation of Notch1 in kidney cancer cells may lead to increased expression of KRT6 [117]. KRT6A overexpression in LUAD promotes tumor cell proliferation, epithelial-to-mesenchymal transition (EMT), and metastasis [15]. Keratin 16 has also been reported to promote tumor progression and metastasis by inducing epithelial-to-mesenchymal transition (EMT), as has been demonstrated in LUAD and MCF-7 cells [14,84]. In addition, keratin 16 inhibits the degradation of vimentin, which can also promote metastasis [118]. A recent study showed that iRhom2 acts as a positive regulator of keratin 16. GOF mutations in iRhom2, which are found in tumors with esophageal cancer (TOC), support tumor hyperproliferation by modulating the expression of K16 [97].
7. Cell Functions of K6, K16, and K17
The most studied function of keratin 17 is its positive regulation of cell growth and proliferation. KRT17-deficient keratinocytes are smaller and exhibit reduced translation and suppressed expression of the mTOR/AKT signaling pathway, which is essential for regulating cell growth and synthesis [119]. In addition, keratin 17 promotes the phosphorylation of STAT3 and its nuclear transport, leading to the induction of cyclin D1 and the hyperproliferation of keratinocytes [17]. Phosphorylated keratin 17 regulates cell growth by binding to the adapter protein 14-3-3σ. In contrast, hypophosphorylation of keratin 17 leads to the sequestration of 14-3-3σ in the nucleus, inhibiting protein synthesis and cell growth [119]. Recent studies have revealed the functions of keratin 17 in the nuclear pool. This protein regulates the morphology of the nucleus, the structure of chromatin, RNA processing, and gene expression. It is also involved in regulating the DNA damage response by interacting with various proteins that are involved in DDRs, including Aire [120], hnRNPK [114], H2AX [121], DNA-PKcs [121], 53BP1 [121], and HMGN2 [110]. In addition, recent research has revealed that keratin 17 is essential for the overall regulation of gene expression. However, most of the mechanisms involved are still unknown [122].
The functions of keratin 16 have not been studied in detail, but it is known to play a role in regulating inflammation and innate immunity after damage to the skin barrier. Keratin 16 may influence the MAPK and EGFR signaling pathways, thereby regulating the overall levels of DAMPs [48]. K16 is also thought to be involved in protecting against oxidative stress through its interaction with the Nrf2 pathway. A recent finding was that keratin 16 helps maintain the normal redox balance in cells by activating Nrf2 and stimulating glutathione synthesis [123]. In addition, keratin 16 and its partner, keratin 6, are involved in regulating mitochondrial morphology, which could explain the signs of oxidative stress observed in PC patients [123].
The currently known functions of keratin 6 mainly involve regulating keratinocyte migration through enhancing cellular adhesion and attachment to the substrate. This is achieved through the interactions between keratin 6a/6b and myosin II and desmoplakin [37]. However, there is still potential for discovering new and unexpected roles for keratin 6. Studies have shown that the addition of recombinant keratin 6A to cell culture media can slow proliferation and stabilize stem cells while also inducing the expression of stem cell-related genes such as CD133, OCT4, KLF4, SOX2, NANOG, and ANAX2 [124]. The cell functions of K6, K16, and K17 are illustrated in Figure 2.
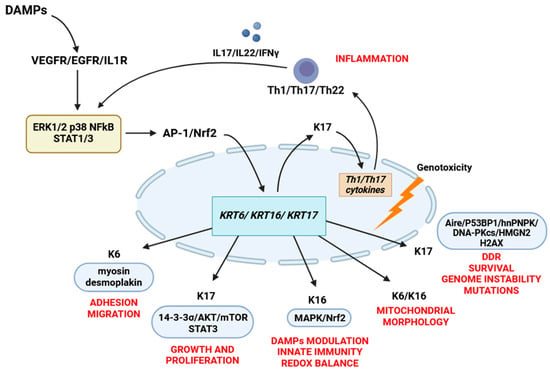
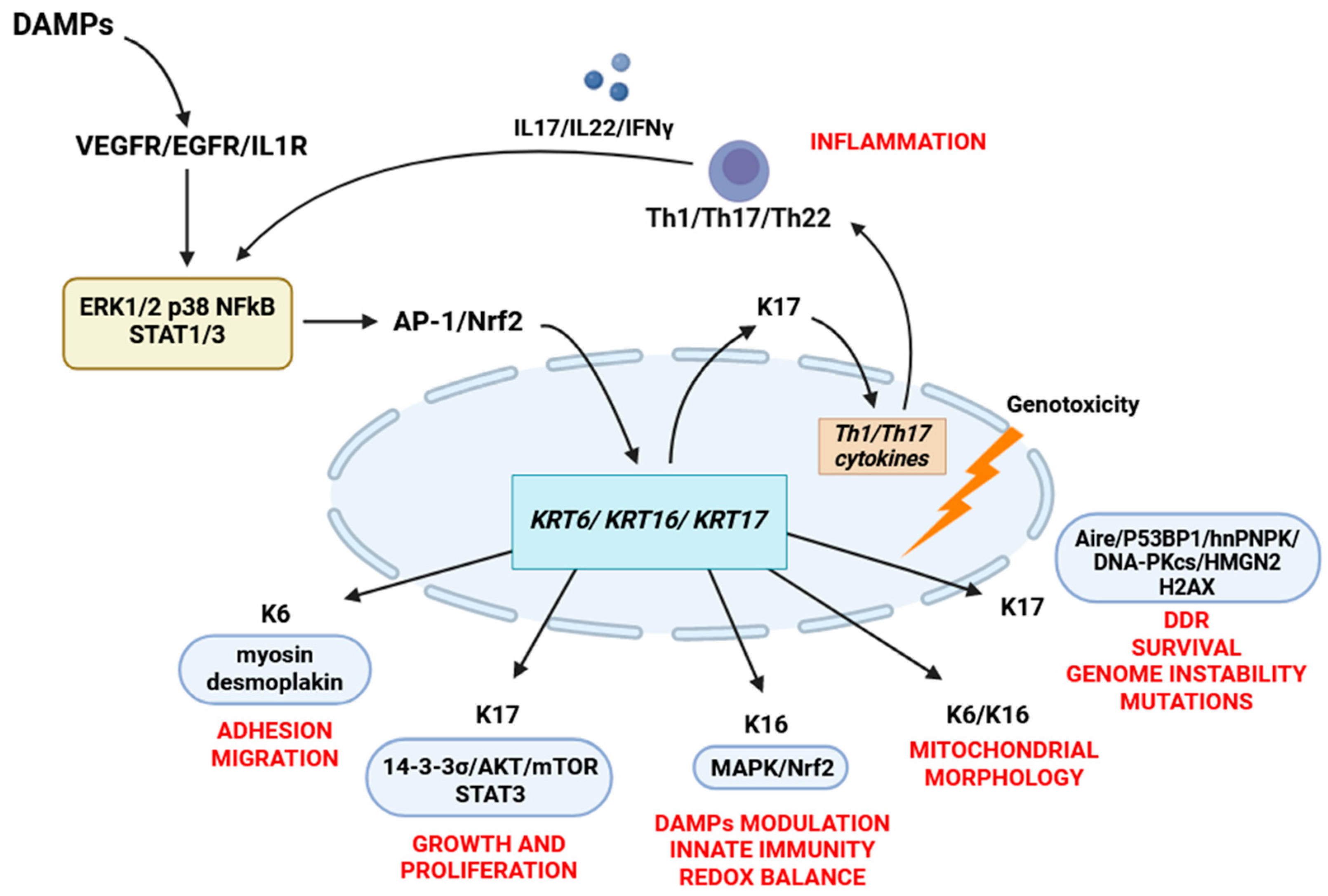
Figure 2.
The cell functions of K6, K16, and K17. Damaged keratinocytes release various DAMPs, which induce the expression of K6/16/17 by activating the AP-1, NFkB, Nrf2, MAPK (ERK1/2 and p38) pathways. Altered expression of K17 promotes the secretion of Th1 cytokines (IFNγ, IL17A, and IL22) that maintain K6/16/17 expression through an autoimmune feedback loop. Keratin 17 regulates cell growth and proliferation by binding to the 14-3-3σ/AKT/mTOR pathway and STAT3 phosphorylation. When located in the nucleus, keratin 17 is involved in the DNA damage response by interacting with various DNA damage response proteins, including Aire, hnRNPK, H2AX, DNA-PKcs, 53BP1, and HMGN2. Keratin 16 modulates the secretion of DAMPs through the MAPK and EGFR signaling pathways and plays a role in maintaining the redox balance by interacting with the Nrf2 signaling pathway. Keratins K6 and K16 also play a role in regulating the morphology and functions of mitochondria. Keratin 6 is involved in regulating cell adhesion by directly interacting with myosin IIA and desmosomal proteins, which provide the mechanical properties necessary for wound healing.
8. Regulation of K6, K16, and K17
One of the common properties of all cytokeratins is the ability of their promoters to bind various transcription factors. Among these transcription factors, those involved in the regulation of KRT6/16/17 include proteins from the AP-1 and NFκB signaling pathways. Specifically, in cooperation with Sp1, AP-1 can activate the promoters of KRT6 and KRT16 [125,126]. Considering that the AP-1 and NFκB response elements can be physically separated, it is assumed that the interaction between these signaling pathways regulates the expression of epidermal genes. Activation of these factors leads to the differential expression of cytokeratins during epidermal differentiation, wound healing, and skin diseases.
Another transcription factor that regulates KRT6/KRT16/KRT17 expression is Nrf2 [47,127]. Nrf2 regulates the expression of keratins 6, 16, and 17 by binding to specific regions of their promoters. These regions, known as ARE (antioxidant response element) domains, are located within the DNA sequences encoding these keratins [47]. Nrf2 is highly expressed in various types of skin cancer and other diseases. Its increased regulation can lead to the hyperproliferation of keratinocytes by inducing the expression of keratins 16 and 17 [47,127]. All three keratins are transcriptionally activated by the Hedgehog-regulated factors GLI1 and GLI2, suggesting a connection between these keratin proteins and the Hedgehog signaling pathway [128,129,130].
Some members of the p53 family of transcription factors are also involved in the regulation of K6/K16/K17. P53 is essential for the normal differentiation of epithelial cells [131]. The mechanisms by which p53 affects keratin are complex and involve both the activation and suppression of genes. p53 can indirectly suppress the expression of Krt14 in basal keratinocytes, contributing to their differentiation [132]. However, a recent study showed that removing p53 promotes differentiation by partially regulating the expression of KRT6A [133]. Therefore, the effect of p53 is complex and multifaceted. It directly binds to the promoter region of the KRT17 gene and negatively regulates its expression. However, the induction of KRT17 expression does not depend on the presence of p53 [134]. Additionally, the promoter sequence of KRT17 contains two p53 response elements [134].
Other important factors involved in the regulation of K6/16/17 include cytokines and growth factors. Moreover, the initial activation of these proteins is carried out autonomously by keratinocytes. Damaged keratinocytes secrete a variety of DAMPs, such as dsRNA, DNA, high mobility group box 1 (HMGB1), uric acid, and heat shock proteins (HSPs) [135,136,137]. These molecules are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs). This leads to the activation of AP-1 and NFkB, which, in turn, activate KRT6/16/17. The expression of these keratins triggers the secretion of cytokines (IFN, IL17A, and IL22), which, in turn, support the expression of K6, K16, and K17 [2,37,43,128,134,138]. Epidermal growth factor (EGF) induces the expression of keratin on 6/16/17 through the NFκB and/or MAPK (ERK1/2) pathways [139,140,141]. TGF-β also activates the expression of keratin 17 in normal keratinocytes [17].
The expression of the K6, K16, and K17 genes can also be regulated in response to various external stimuli such as mechanical stimuli (stretching and scratches). These stimuli lead to the phosphorylation of the EGFR and ERK1/2 proteins, which, in turn, activate the expression of keratin 6 and repress the expression of K10 [142]. The expression of keratin 17 is induced by ionizing radiation (IR), and high levels of keratin 17 are characteristic of IR-induced dermatitis [134]. Keratin 17 is also induced by UV radiation from both UVA and UVB sources [143,144]. Our group reported that the expression of keratin 17 significantly increases in primary human keratinocytes exposed to cadmium in vitro [145]. Notably, a similar response was observed in HaCaT cells with knocked-out mutant p53, but not in wild-type HaCaT cells [146].
Funding
This work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021–2030) (no. 122022800481-0).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, M.; Zhang, L. Keratin 6, 16 and 17—Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Kalabusheva, E.P.; Shtompel, A.S.; Rippa, A.L.; Ulianov, S.V.; Razin, S.V.; Vorotelyak, E.A. A Kaleidoscope of Keratin Gene Expression and the Mosaic of Its Regulatory Mechanisms. Int. J. Mol. Sci. 2023, 24, 5603. [Google Scholar] [CrossRef] [PubMed]
- Rosdy, M.; Clauss, L.C. Terminal epidermal differentiation of human keratinocytes grown in chemically defined medium on inert filter substrates at the air-liquid interface. J. Investig. Dermatol. 1990, 95, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Gómez, N.; Freije, A.; Gandarillas, A. Keratinocyte Differentiation by Flow Cytometry. Methods Mol. Biol. 2020, 2109, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L.; Tomić, L.; Parać, E.; Pedić, L.; Lazić-Mosler, E. Key Factors in the Complex and Coordinated Network of Skin Keratinization: Their Significance and Involvement in Common Skin Conditions. Int. J. Mol. Sci. 2024, 25, 236. [Google Scholar] [CrossRef]
- Gunnarsson, M.; Larsson, S.; Malak, M.; Ericson, M.B.; Topgaard, D.; Sparr, E. Molecular Mobility in Keratin-Rich Materials Monitored by Nuclear Magnetic Resonance: A Tool for the Evaluation of Structure-Giving Properties. Biomacromolecules 2023, 24, 2661–2673. [Google Scholar] [CrossRef]
- Luo, Y.; Pang, B.; Hao, J.; Li, Q.; Qiao, P.; Zhang, C.; Bai, Y.; Xiao, C.; Chen, J.; Zhi, D.; et al. Keratin 17 covalently binds to alpha-enolase and exacerbates proliferation of keratinocytes in psoriasis. Int. J. Biol. Sci. 2023, 19, 3395–3411. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Paladini, R.D.; Coulombe, P.A. Cloning and characterization of multiple human genes and cDNAs encoding highly related type II keratin 6 isoforms. J. Biol. Chem. 1995, 270, 18581–18592. [Google Scholar] [CrossRef] [PubMed]
- McGowan, K.M.; Coulombe, P.A. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J. Cell Biol. 1998, 143, 469–486. [Google Scholar] [CrossRef]
- Leigh, I.M.; Navsaria, H.; Purkis, P.E.; McKay, I.A.; Bowden, P.E.; Riddle, P.N. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br. J. Dermatol. 1995, 133, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-G.; Fan, H.-Y.; Wang, T.; Lin, L.-Y.; Cai, T.-G. Silencing KRT16 inhibits keratinocyte proliferation and VEGF secretion in psoriasis via inhibition of ERK signaling pathway. Kaohsiung J. Med. Sci. 2019, 35, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Jang, T.-H.; Tung, S.-L.; Yen, T.-C.; Chan, S.-H.; Wang, L.-H. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 89. [Google Scholar] [CrossRef] [PubMed]
- Elazezy, M.; Schwentesius, S.; Stegat, L.; Wikman, H.; Werner, S.; Mansour, W.Y.; Failla, A.V.; Peine, S.; Müller, V.; Thiery, J.P.; et al. Emerging Insights into Keratin 16 Expression during Metastatic Progression of Breast Cancer. Cancers 2021, 13, 3869. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, W.; Zhang, M.; Wang, X.; Peng, S.; Zhang, R. KRT6A Promotes EMT and Cancer Stem Cell Transformation in Lung Adenocarcinoma. Technol. Cancer Res. Treat. 2020, 19, 1533033820921248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Xia, T.; Lu, L.; Luo, M.; Chen, Y.; Liu, Y.; Li, Y. The Role of Keratin17 in Human Tumours. Front. Cell Dev. Biol. 2022, 10, 818416. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jin, L.; Ke, Y.; Fan, X.; Zhang, T.; Zhang, C.; Bian, H.; Wang, G. E3 Ligase Trim21 Ubiquitylates and Stabilizes Keratin 17 to Induce STAT3 Activation in Psoriasis. J. Investig. Dermatol. 2018, 138, 2568–2577. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.M.; Lee, C.-H.; Coulombe, P.A. A small surface hydrophobic stripe in the coiled-coil domain of type I keratins mediates tetramer stability. J. Cell Biol. 2005, 168, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Thompson, B.; Fisk, J.N.; Nebert, D.W.; Bruford, E.A.; Vasiliou, V.; Bunick, C.G. Update of the keratin gene family: Evolution, tissue-specific expression patterns, and relevance to clinical disorders. Hum. Genom. 2022, 16, 1. [Google Scholar] [CrossRef]
- Han, W.; Hu, C.; Fan, Z.-J.; Shen, G.-L. Transcript levels of keratin 1/5/6/14/15/16/17 as potential prognostic indicators in melanoma patients. Sci. Rep. 2021, 11, 1023. [Google Scholar] [CrossRef]
- Cohen, E.; Johnson, C.N.; Wasikowski, R.; Billi, A.C.; Tsoi, L.C.; Kahlenberg, J.M.; Gudjonsson, J.E.; Coulombe, P.A. Significance of stress keratin expression in normal and diseased epithelia. iScience 2024, 27, 108805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lyle, S.; Yang, Z.; Cotsarelis, G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Investig. Dermatol. 2003, 121, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Langbein, L.; Schweizer, J. Keratins of the human hair follicle. Int. Rev. Cytol. 2005, 243, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Bulyk, M.L.; Jambhekar, A.; Lahav, G. The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 2019, 20, 199–210. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Fleckman, P.; Jaeger, K.; Silva, K.A.; Sundberg, J.P. Comparative Anatomy of Mouse and Human Nail Units. Anat. Rec. 2013, 296, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Troyanovsky, S.M.; Leube, R.E.; Franke, W.W. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur. J. Cell Biol. 1992, 59, 127–137. [Google Scholar] [PubMed]
- Troyanovsky, S.M.; Guelstein, V.I.; Tchipysheva, T.A.; Krutovskikh, V.A.; Bannikov, G.A. Patterns of expression of keratin 17 in human epithelia: Dependency on cell position. J. Cell Sci. 1989, 93 Pt 3, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Warland, G.; Nakagawa, H.; Opitz, O.G.; Rustgi, A.K. Cellular characterization and successful transfection of serially subcultured normal human esophageal keratinocytes. J. Cell. Physiol. 1998, 177, 274–281. [Google Scholar] [CrossRef]
- Bernot, K.M.; Coulombe, P.A.; McGowan, K.M. Keratin 16 expression defines a subset of epithelial cells during skin morphogenesis and the hair cycle. J. Investig. Dermatol. 2002, 119, 1137–1149. [Google Scholar] [CrossRef]
- Karantza, V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011, 30, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Mazzalupo, S.; Wong, P.; Martin, P.; Coulombe, P.A. Role for keratins 6 and 17 during wound closure in embryonic mouse skin. Dev. Dyn. 2003, 226, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Feng, H.; Qin, W.; Li, Q. KRT17 from skin cells with high glucose stimulation promotes keratinocytes proliferation and migration. Front. Endocrinol. 2023, 14, 1237048. [Google Scholar] [CrossRef] [PubMed]
- McGowan, K.; Coulombe, P.A. The wound repair-associated keratins 6, 16, and 17. Insights into the role of intermediate filaments in specifying keratinocyte cytoarchitecture. Subcell. Biochem. 1998, 31, 173–204. [Google Scholar] [PubMed]
- Tombultürk, F.K. Effects of keratin6/16 heterodimer on diabetic wound healing treatment with topical metformin. Front. Life Sci. Relat. Technol. 2024, 5, 65–73. [Google Scholar] [CrossRef]
- Loschke, F.; Homberg, M.; Magin, T.M. Keratin Isotypes Control Desmosome Stability and Dynamics through PKCα. J. Investig. Dermatol. 2016, 136, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, S.; Liu, H.B.; Parent, C.A.; Coulombe, P.A. Keratin 6 regulates collective keratinocyte migration by altering cell–cell and cell–matrix adhesion. J. Cell Biol. 2018, 217, 4314–4330. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Coulombe, P.A. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol. 2003, 163, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Rotty, J.D.; Coulombe, P.A. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J. Cell Biol. 2012, 197, 381–389. [Google Scholar] [CrossRef]
- Trost, A.; Desch, P.; Wally, V.; Haim, M.; Maier, R.H.; Reitsamer, H.A.; Hintner, H.; Bauer, J.W.; Önder, K. Aberrant heterodimerization of keratin 16 with keratin 6A in HaCaT keratinocytes results in diminished cellular migration. Mech. Ageing Dev. 2010, 131, 346–353. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Z.; Xiao, C.; Luo, Y.; Fang, H.; Bai, Y.; Sun, Z.; Ma, J.; Dang, E.; Wang, G. Keratin 17 Is Required for Lipid Metabolism in Keratinocytes and Benefits Epidermal Permeability Barrier Homeostasis. Front. Cell Dev. Biol. 2022, 9, 779257. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Dean, D.; Lane, E.B.; Dawber, R.P.; Leigh, I.M. Keratinocyte differentiation in psoriatic scalp: Morphology and expression of epithelial keratins. Br. J. Dermatol. 1994, 131, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Jin, L.; Dang, E.; Chang, T.; Feng, Z.; Liu, Y.; Wang, G. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J. Investig. Dermatol. 2011, 131, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, G. Keratin 17: A critical player in the pathogenesis of psoriasis. Med. Res. Rev. 2014, 34, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, W.; Li, B.; Wang, G. Keratin 17 in psoriasis: Current understanding and future perspectives. Semin. Cell Dev. Biol. 2022, 128, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, G.; Fan, J.-Y.; Li, W.; Liu, Y.-F. HLA DR B1*04, *07-restricted epitopes on Keratin 17 for autoreactive T cells in psoriasis. J. Dermatol. Sci. 2005, 38, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, X.; Cui, T.; Dang, E.; Wang, G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Investig. Dermatol. 2017, 137, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.C.; Piña-Paz, S.; Rotty, J.D.; Hickerson, R.P.; Kaspar, R.L.; Balmain, A.; Coulombe, P.A. Keratin 16 regulates innate immunity in response to epidermal barrier breach. Proc. Natl. Acad. Sci. USA 2013, 110, 19537–19542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q. KRT6A Inhibits IL-1β-Mediated Pyroptosis of Keratinocytes via Blocking IL-17 Signaling. Crit. Rev. Eukaryot. Gene Expr. 2024, 34, 1–11. [Google Scholar] [CrossRef]
- Zhu, A.Y.; Mitra, N.; Margolis, D.J. Longitudinal association of atopic dermatitis progression and keratin 6A. Sci. Rep. 2022, 12, 13629. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Calatroni, A.; Zaramela, L.S.; LeBeau, P.K.; Dyjack, N.; Brar, K.; David, G.; Johnson, K.; Leung, S.; Ramirez-Gama, M.; et al. The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci. Transl. Med. 2019, 11, eaav2685. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.D.; Suárez-Fariñas, M.; Dhingra, N.; Cardinale, I.; Li, X.; Kostic, A.; Ming, J.E.; Radin, A.R.; Krueger, J.G.; Graham, N.; et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Kumari, L.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Janmeda, P.; Chuturgoon, S.; Chuturgoon, A. Unraveling Atopic Dermatitis: Insights into Pathophysiology, Therapeutic Advances, and Future Perspectives. Cells 2024, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- McLean, W.H.; Rugg, E.L.; Lunny, D.P.; Morley, S.M.; Lane, E.B.; Swensson, O.; Dopping-Hepenstal, P.J.; Griffiths, W.A.; Eady, R.A.; Higgins, C. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat. Genet. 1995, 9, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Leachman, S.A.; Kaspar, R.L.; Fleckman, P.; Florell, S.R.; Smith, F.J.D.; McLean, W.H.I.; Lunny, D.P.; Milstone, L.M.; van Steensel, M.A.M.; Munro, C.S.; et al. Clinical and Pathological Features of Pachyonychia Congenita. J. Investig. Dermatol. Symp. Proc. 2005, 10, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.J.D.; Liao, H.; Cassidy, A.J.; Stewart, A.; Hamill, K.J.; Wood, P.; Joval, I.; van Steensel, M.A.M.; Björck, E.; Callif-Daley, F.; et al. The genetic basis of pachyonychia congenita. J. Investig. Dermatol. Symp. Proc. 2005, 10, 21–30. [Google Scholar] [CrossRef]
- McLean, W.H.I.; Smith, F.J.D.; Cassidy, A.J. Insights into genotype-phenotype correlation in pachyonychia congenita from the human intermediate filament mutation database. J. Investig. Dermatol. Symp. Proc. 2005, 10, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bowden, P.E.; Haley, J.L.; Kansky, A.; Rothnagel, J.A.; Jones, D.O.; Turner, R.J. Mutation of a type II keratin gene (K6a) in pachyonychia congenita. Nat. Genet. 1995, 10, 363–365. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S.; Wang, D.; Wang, T.; Ren, X.; Yuan, Y.; Cui, H. A KRT6A and a Novel KRT16 Gene Mutations in Chinese Patients with Pachyonychia Congenita. Int. J. Gen. Med. 2021, 14, 903–907. [Google Scholar] [CrossRef]
- Teng, J.M.C.; Bartholomew, F.B.; Patel, V.; Sun, G. Novel treatment of painful plantar keratoderma in pachyonychia congenita using topical sirolimus. Clin. Exp. Dermatol. 2018, 43, 968–971. [Google Scholar] [CrossRef]
- Hickerson, R.P.; Leake, D.; Pho, L.N.; Leachman, S.A.; Kaspar, R.L. Rapamycin selectively inhibits expression of an inducible keratin (K6a) in human keratinocytes and improves symptoms in pachyonychia congenita patients. J. Dermatol. Sci. 2009, 56, 82–88. [Google Scholar] [CrossRef]
- Zhao, Y.; Gartner, U.; Smith, F.J.D.; McLean, W.H.I. Statins downregulate K6a promoter activity: A possible therapeutic avenue for pachyonychia congenita. J. Investig. Dermatol. 2011, 131, 1045–1052. [Google Scholar] [CrossRef]
- Koren, A.; Sprecher, E.; Reider, E.; Artzi, O. A treatment protocol for botulinum toxin injections in the treatment of pachyonychia congenita-associated keratoderma. Br. J. Dermatol. 2020, 182, 671–677. [Google Scholar] [CrossRef]
- Leachman, S.A.; Hickerson, R.P.; Hull, P.R.; Smith, F.J.D.; Milstone, L.M.; Lane, E.B.; Bale, S.J.; Roop, D.R.; McLean, W.H.I.; Kaspar, R.L. Therapeutic siRNAs for dominant genetic skin diseases including pachyonychia congenita. J. Dermatol. Sci. 2008, 51, 151–157. [Google Scholar] [CrossRef]
- Leachman, S.A.; Hickerson, R.P.; Schwartz, M.E.; Bullough, E.E.; Hutcherson, S.L.; Boucher, K.M.; Hansen, C.D.; Eliason, M.J.; Srivatsa, G.S.; Kornbrust, D.J.; et al. First-in-human Mutation-targeted siRNA Phase Ib Trial of an Inherited Skin Disorder. Mol. Ther. 2010, 18, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhu, Z.; Feng, Y.; Zhang, Y. Keratin 17 is Not Always a Marker of Proliferation of Keratinocytes in Skin Diseases. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Dickman, J.; Howell, M.; Hoopes, R.; Wang, Y.; Dickerson, T.J.; Bottomley, M.; Shamma, H.N.; Rapp, C.M.; Turner, M.J.; Rohan, C.A.; et al. Insights into Lichen Planus Pigmentosus Inversus using Minimally Invasive Dermal Patch and Whole Transcriptome Analysis. J. Clin. Investig. Dermatol. 2022, 10. [Google Scholar] [CrossRef]
- Yang, L.-L.; Huang, H.-Y.; Chen, Z.-Z.; Chen, R.; Ye, R.; Zhang, W.; Yu, B. Keratin 17 is induced in prurigo nodularis lesions. Open Chem. 2020, 18, 463–471. [Google Scholar] [CrossRef]
- Yang, L.L.; Jiang, B.; Chen, S.H.; Liu, H.Y.; Chen, T.T.; Huang, L.H.; Yang, M.; Ding, J.; He, J.J.; Li, J.J.; et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Ski. Health Dis. 2022, 2, e75. [Google Scholar] [CrossRef]
- Alkon, N.; Assen, F.P.; Arnoldner, T.; Bauer, W.M.; Medjimorec, M.A.; Shaw, L.E.; Rindler, K.; Holzer, G.; Weber, P.; Weninger, W.; et al. Single-cell RNA sequencing defines disease-specific differences between chronic nodular prurigo and atopic dermatitis. J. Allergy Clin. Immunol. 2023, 152, 420–435. [Google Scholar] [CrossRef]
- Baraks, G.; Tseng, R.; Pan, C.-H.; Kasliwal, S.; Leiton, C.V.; Shroyer, K.R.; Escobar-Hoyos, L.F. Dissecting the Oncogenic Roles of Keratin 17 in the Hallmarks of Cancer. Cancer Res. 2022, 82, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Torabinia, N.; Razavi, S.M.; Sarrafpour, B.; Ziaei-Rad, E. The comparative evaluation of CK17 expression in histologic and cytological sections of oral squamous-cell carcinoma using immunohistochemistry. Diagn. Cytopathol. 2023, 51, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Sanguansin, S.; Kosanwat, T.; Juengsomjit, R.; Poomsawat, S. Diagnostic Value of Cytokeratin 17 during Oral Carcinogenesis: An Immunohistochemical Study. Int. J. Dent. 2021, 2021, 4089549. [Google Scholar] [CrossRef]
- Bai, J.D.K.; Babu, S.; Roa-Peña, L.; Hou, W.; Akalin, A.; Escobar-Hoyos, L.F.; Shroyer, K.R. Keratin 17 is a negative prognostic biomarker in high-grade endometrial carcinomas. Hum. Pathol. 2019, 94, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lozar, T.; Wang, W.; Gavrielatou, N.; Christensen, L.; Lambert, P.F.; Harari, P.M.; Rimm, D.L.; Burtness, B.; Grasic Kuhar, C.; Carchman, E.H. Emerging Prognostic and Predictive Significance of Stress Keratin 17 in HPV-Associated and Non HPV-Associated Human Cancers: A Scoping Review. Viruses 2023, 15, 2320. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Feng, Z.; Lu, L.; Li, Y.; Liu, Y.; Chen, Y. Analysis of the Expression and Role of Keratin 17 in Human Tumors. Front. Genet. 2022, 13, 801698. [Google Scholar] [CrossRef]
- Li, C.; Teng, Y.; Wu, J.; Yan, F.; Deng, R.; Zhu, Y.; Li, X. A pan-cancer analysis of the oncogenic role of Keratin 17 (KRT17) in human tumors. Transl. Cancer Res. 2021, 10, 4489–4501. [Google Scholar] [CrossRef]
- Hu, H.; Xu, D.; Huang, X.; Zhu, C.; Xu, J.; Zhang, Z.; Zhao, G. Keratin17 Promotes Tumor Growth and is Associated with Poor Prognosis in Gastric Cancer. J. Cancer 2018, 9, 346–357. [Google Scholar] [CrossRef]
- Lozar, T.; Laklouk, I.; Golfinos, A.E.; Gavrielatou, N.; Xu, J.; Flynn, C.; Keske, A.; Yu, M.; Bruce, J.Y.; Wang, W.; et al. Stress Keratin 17 Is a Predictive Biomarker Inversely Associated with Response to Immune Check-Point Blockade in Head and Neck Squamous Cell Carcinomas and Beyond. Cancers 2023, 15, 4905. [Google Scholar] [CrossRef]
- Babu, S.; Roa-Peña, L.; Akalin, A.; Escobar-Hoyos, L.; Shroyer, K. Keratin 17 is a Negative Prognostic Biomarker in Non-Small Cell Lung Cancer. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Roa-Peña, L.; Leiton, C.V.; Babu, S.; Pan, C.-H.; Vanner, E.A.; Akalin, A.; Bandovic, J.; Moffitt, R.A.; Shroyer, K.R.; Escobar-Hoyos, L.F. Keratin 17 identifies the most lethal molecular subtype of pancreatic cancer. Sci. Rep. 2019, 9, 11239. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, D.; Okayama, H.; Saito, K.; Ashizawa, M.; Thar Min, A.K.; Endo, E.; Kase, K.; Yamada, L.; Kikuchi, T.; Hanayama, H.; et al. KRT17 as a prognostic biomarker for stage II colorectal cancer. Carcinogenesis 2020, 41, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Khanom, R.; Nguyen, C.T.K.; Kayamori, K.; Zhao, X.; Morita, K.; Miki, Y.; Katsube, K.; Yamaguchi, A.; Sakamoto, K. Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth. PLoS ONE 2016, 11, e0161163. [Google Scholar] [CrossRef] [PubMed]
- Yuanhua, L.; Pudong, Q.; Wei, Z.; Yuan, W.; Delin, L.; Yan, Z.; Geyu, L.; Bo, S. TFAP2A Induced KRT16 as an Oncogene in Lung Adenocarcinoma via EMT. Int. J. Biol. Sci. 2019, 15, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhou, X.D.; Sens, M.A.; Garrett, S.H.; Zheng, Y.; Dunlevy, J.R.; Sens, D.A.; Somji, S. Keratin 6 Expression Correlates to Areas of Squamous Differentiation in Multiple Independent Isolates of As+3-Induced Bladder Cancer. J. Appl. Toxicol. 2010, 30, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Harada-Kagitani, S.; Kouchi, Y.; Shinomiya, Y.; Kodama, M.; Ohira, G.; Matsubara, H.; Ikeda, J.-I.; Kishimoto, T. Keratin 6A is expressed at the invasive front and enhances the progression of colorectal cancer. Lab. Investig. 2024, 104, 102075. [Google Scholar] [CrossRef]
- Kengkarn, S.; Petmitr, S.; Boonyuen, U.; Reamtong, O.; Poomsawat, S.; Sanguansin, S. Identification of Novel Candidate Biomarkers for Oral Squamous Cell Carcinoma Based on Whole Gene Expression Profiling. Pathol. Oncol. Res. 2020, 26, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Zhou, S.; He, B.; Du, Y.; Garmire, L.X. Deep-learning and transfer learning identify new breast cancer survival subtypes from single-cell imaging data. medRxiv 2023. [Google Scholar] [CrossRef]
- Merkin, R.D.; Vanner, E.A.; Romeiser, J.L.; Shroyer, A.L.W.; Escobar-Hoyos, L.F.; Li, J.; Powers, R.S.; Burke, S.; Shroyer, K.R. Keratin 17 is overexpressed and predicts poor survival in estrogen receptor-negative/human epidermal growth factor receptor-2-negative breast cancer. Hum. Pathol. 2017, 62, 23–32. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Lokman, N.A.; Pyragius, C.E.; Ween, M.P.; Macpherson, A.M.; Ruszkiewicz, A.; Hoffmann, P.; Oehler, M.K. Keratin 5 overexpression is associated with serous ovarian cancer recurrence and chemotherapy resistance. Oncotarget 2017, 8, 17819–17832. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lang, H.-Y.; Yuan, J.; Wang, J.; Wang, R.; Zhang, X.-H.; Zhang, J.; Zhao, T.; Li, Y.-R.; Liu, J.-Y.; et al. Overexpression of keratin 17 is associated with poor prognosis in epithelial ovarian cancer. Tumour Biol. 2013, 34, 1685–1689. [Google Scholar] [CrossRef]
- Hoggarth, Z.E.; Osowski, D.B.; Slusser-Nore, A.; Shrestha, S.; Pathak, P.; Solseng, T.; Garrett, S.H.; Patel, D.H.; Savage, E.; Sens, D.A.; et al. Enrichment of genes associated with squamous differentiation in cancer initiating cells isolated from urothelial cells transformed by the environmental toxicant arsenite. Toxicol. Appl. Pharmacol. 2019, 374, 41–52. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Ying, J.; Sun, Y.; Liu, J.; Yin, G. KRT6A expedites bladder cancer progression, regulated by miR-31-5p. Cell Cycle 2022, 21, 1479–1490. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Zhang, X.; Fang, J. Five hub genes contributing to the oncogenesis and trastuzumab-resistance in gastric cancer. Gene 2023, 851, 146942. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Shen, J.; Lu, Y.; Lin, K.; Wang, H.; Li, Y.; Chang, P.; Walker, M.G.; Li, D. RNA sequencing analyses reveal novel differentially expressed genes and pathways in pancreatic cancer. Oncotarget 2017, 8, 42537–42547. [Google Scholar] [CrossRef]
- Williams, H.L.; Dias Costa, A.; Zhang, J.; Raghavan, S.; Winter, P.S.; Kapner, K.S.; Ginebaugh, S.P.; Väyrynen, S.A.; Väyrynen, J.P.; Yuan, C.; et al. Spatially-resolved single-cell assessment of pancreatic cancer expression subtypes reveals co-expressor phenotypes and extensive intra-tumoral heterogeneity. Cancer Res. 2023, 83, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, S.; Ye, S.; Shen, Z.; Gao, L.; Han, Z.; Zhang, P.; Luo, F.; Chen, S.; Kang, M. Keratin 17 activates AKT signalling and induces epithelial-mesenchymal transition in oesophageal squamous cell carcinoma. J. Proteom. 2020, 211, 103557. [Google Scholar] [CrossRef]
- Maruthappu, T.; Chikh, A.; Fell, B.; Delaney, P.J.; Brooke, M.A.; Levet, C.; Moncada-Pazos, A.; Ishida-Yamamoto, A.; Blaydon, D.; Waseem, A.; et al. Rhomboid family member 2 regulates cytoskeletal stress-associated Keratin 16. Nat. Commun. 2017, 8, 14174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, G.; Li, T.; Ai, J.; Li, W.; Zeng, S.; Ye, M.; Liu, Q.; Xiao, J.; Li, Y.; et al. LncRNA NR120519 Blocks KRT17 to Promote Cell Proliferation and Migration in Hypopharyngeal Squamous Carcinoma. Cancers 2023, 15, 603. [Google Scholar] [CrossRef]
- Escobar-Hoyos, L.F.; Yang, J.; Zhu, J.; Cavallo, J.-A.; Zhai, H.; Burke, S.; Koller, A.; Chen, E.I.; Shroyer, K.R. Keratin 17 in premalignant and malignant squamous lesions of the cervix: Proteomic discovery and immunohistochemical validation as a diagnostic and prognostic biomarker. Mod. Pathol. 2014, 27, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Dai, L. Transcription factors TP63 facilitates malignant progression of thyroid cancer by upregulating KRT17 expression and inducing epithelial-mesenchymal transition. Growth Factors 2023, 41, 71–81. [Google Scholar] [CrossRef] [PubMed]
- DePianto, D.; Kerns, M.; Dlugosz, A.A.; Coulombe, P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 2010, 42, 910–914. [Google Scholar] [CrossRef]
- Wang, W.; Lozar, T.; Golfinos, A.E.; Lee, D.; Gronski, E.; Ward-Shaw, E.; Hayes, M.; Bruce, J.Y.; Kimple, R.J.; Hu, R.; et al. Stress Keratin 17 expression in head and neck cancer contributes to immune evasion and resistance to immune checkpoint blockade. Clin. Cancer Res. 2022, 28, 2953–2968. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.R.; Derr, P.; Derr, K.; Doudican, N.; Michael, S.; Lish, S.R.; Taylor, N.A.; Krueger, J.G.; Ferrer, M.; Carucci, J.A.; et al. A 3D biofabricated cutaneous squamous cell carcinoma tissue model with multi-channel confocal microscopy imaging biomarkers to quantify antitumor effects of chemotherapeutics in tissue. Oncotarget 2020, 11, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Hameetman, L.; Commandeur, S.; Bavinck, J.N.B.; Wisgerhof, H.C.; de Gruijl, F.R.; Willemze, R.; Mullenders, L.; Tensen, C.P.; Vrieling, H. Molecular profiling of cutaneous squamous cell carcinomas and actinic keratoses from organ transplant recipients. BMC Cancer 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-J.; Chen, H.; Chen, J.-Q.; Lei, Q.-H.; Zheng, M.; Shao, Z.-R. Immunolocalization of vimentin, keratin 17, Ki-67, involucrin, β-catenin and E-cadherin in cutaneous squamous cell carcinoma. Pathol. Oncol. Res. 2014, 20, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, C.; Hu, W.; Chen, T.; Wang, Q.; Pan, F.; Qiu, B.; Tang, B. Knockdown of KRT17 decreases osteosarcoma cell proliferation and the Warburg effect via the AKT/mTOR/HIF1α pathway. Oncol. Rep. 2020, 44, 103–114. [Google Scholar] [CrossRef]
- Li, D.; Ni, X.-F.; Tang, H.; Zhang, J.; Zheng, C.; Lin, J.; Wang, C.; Sun, L.; Chen, B. KRT17 Functions as a Tumor Promoter and Regulates Proliferation, Migration and Invasion in Pancreatic Cancer via mTOR/S6k1 Pathway. Cancer Manag. Res. 2020, 12, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Hoyos, L.F.; Shah, R.; Roa-Peña, L.; Vanner, E.A.; Najafian, N.; Banach, A.; Nielsen, E.; Al-Khalil, R.; Akalin, A.; Talmage, D.; et al. Keratin-17 Promotes p27KIP1 Nuclear Export and Degradation and Offers Potential Prognostic Utility. Cancer Res. 2015, 75, 3650–3662. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Hsu, J.; Jacob, J.T.; Pineda, C.M.; Hobbs, R.P.; Coulombe, P.A. A role for keratin 17 during DNA damage response and tumor initiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2020150118. [Google Scholar] [CrossRef]
- Delgado-Coka, L.; Horowitz, M.; Torrente-Goncalves, M.; Roa-Peña, L.; Leiton, C.V.; Hasan, M.; Babu, S.; Fassler, D.; Oentoro, J.; Bai, J.-D.K.; et al. Keratin 17 modulates the immune topography of pancreatic cancer. J. Transl. Med. 2024, 22, 443. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liu, H.; Zeng, Z.; Liang, Z.; Xie, H.; Li, W.; Xiong, L.; Liu, Z.; Chen, M.; Jie, H.; et al. KRT17 Promotes T-lymphocyte Infiltration Through the YTHDF2-CXCL10 Axis in Colorectal Cancer. Cancer Immunol. Res. 2023, 11, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.M.; Arutyunov, A.; Ilagan, E.; Yao, N.; Wills-Karp, M.; Coulombe, P.A. Regulation of C-X-C chemokine gene expression by keratin 17 and hnRNP K in skin tumor keratinocytes. J. Cell Biol. 2015, 208, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Uberoi, A.; Spurgeon, M.; Gronski, E.; Majerciak, V.; Lobanov, A.; Hayes, M.; Loke, A.; Zheng, Z.-M.; Lambert, P.F. Stress keratin 17 enhances papillomavirus infection-induced disease by downregulating T cell recruitment. PLoS Pathog. 2020, 16, e1008206. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shan, H. Keratin 6A gene silencing suppresses cell invasion and metastasis of nasopharyngeal carcinoma via the β-catenin cascade. Mol. Med. Rep. 2019, 19, 3477–3484. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.-C.; Song, X.; Lu, J.-R.; Jin, Z. KRT6 interacting with notch1 contributes to progression of renal cell carcinoma, and aliskiren inhibits renal carcinoma cell lines proliferation in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 9182–9188. [Google Scholar]
- Wang, W.; Zhu, L.; Zhou, J.; Liu, X.; Xiao, M.; Chen, N.; Huang, X.; Chen, H.; Pei, X.; Zhang, H. Targeting the KRT16-vimentin axis for metastasis in lung cancer. Pharmacol. Res. 2023, 193, 106818. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wong, P.; Coulombe, P.A. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 2006, 441, 362–365. [Google Scholar] [CrossRef]
- Hobbs, R.P.; DePianto, D.J.; Jacob, J.T.; Han, M.C.; Chung, B.-M.; Batazzi, A.S.; Poll, B.G.; Guo, Y.; Han, J.; Ong, S.; et al. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat. Genet. 2015, 47, 933–938. [Google Scholar] [CrossRef]
- Jacob, J.T.; Nair, R.R.; Poll, B.G.; Pineda, C.M.; Hobbs, R.P.; Matunis, M.J.; Coulombe, P.A. Keratin 17 regulates nuclear morphology and chromatin organization. J. Cell Sci. 2020, 133, jcs254094. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Lin, J.; Lu, X.; Gao, Q.; Pan, M.; Zhang, Y.; Shen, S.; Zhu, W.-G.; Paus, R. Keratin 17 Impacts Global Gene Expression and Controls G2/M Cell Cycle Transition in Ionizing Radiation–Induced Skin Damage. J. Investig. Dermatol. 2023, 143, 2436–2446.e13. [Google Scholar] [CrossRef] [PubMed]
- Steen, K.; Chen, D.; Wang, F.; Majumdar, R.; Chen, S.; Kumar, S.; Lombard, D.B.; Weigert, R.; Zieman, A.G.; Parent, C.A.; et al. A role for keratins in supporting mitochondrial organization and function in skin keratinocytes. MBoC 2020, 31, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Wang, L.; Zhang, Y.; Pan, Q.; Xie, X.; Du, H. Application of Human Keratin 6A in Stem Cell Culture and Product. CN Patent CN110643571A, 22 October 2019. [Google Scholar]
- Wang, Y.-N.; Chang, W.-C. Induction of disease-associated keratin 16 gene expression by epidermal growth factor is regulated through cooperation of transcription factors Sp1 and c-Jun. J. Biol. Chem. 2003, 278, 45848–45857. [Google Scholar] [CrossRef] [PubMed]
- MA, S.; RAO, L.; FREEDBERG, I.M.; BLUMENBERG, M. Transcriptional Control of K5, K6, K14, and K17 Keratin Genes by AP-1 and NF-κB Family Members. Gene Expr. 1997, 6, 361–370. [Google Scholar] [PubMed]
- Zhang, J.; Li, X.; Wei, J.; Chen, H.; Lu, Y.; Li, L.; Han, L.; Lu, C. Gallic acid inhibits the expression of keratin 16 and keratin 17 through Nrf2 in psoriasis-like skin disease. Int. Immunopharmacol. 2018, 65, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, S.; Wang, G. Keratin 17 in disease pathogenesis: From cancer to dermatoses. J. Pathol. 2019, 247, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kurtović, M.; Piteša, N.; Bartoniček, N.; Ozretić, P.; Musani, V.; Čonkaš, J.; Petrić, T.; King, C.; Sabol, M. RNA-seq and ChIP-seq Identification of Unique and Overlapping Targets of GLI Transcription Factors in Melanoma Cell Lines. Cancers 2022, 14, 4540. [Google Scholar] [CrossRef]
- Gu, L.-H.; Coulombe, P.A. Hedgehog Signaling, Keratin 6 Induction, and Sebaceous Gland Morphogenesis. Am. J. Pathol. 2008, 173, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Paramio, J.M.; Segrelles, C.; Laín, S.; Gómez-Casero, E.; Lane, D.P.; Lane, E.B.; Jorcano, J.L. p53 is phosphorylated at the carboxyl terminus and promotes the differentiation of human HaCaT keratinocytes. Mol. Carcinog. 2000, 29, 251–262. [Google Scholar] [CrossRef]
- Cai, B.-H.; Hsu, P.-C.; Hsin, I.-L.; Chao, C.-F.; Lu, M.-H.; Lin, H.-C.; Chiou, S.-H.; Tao, P.-L.; Chen, J.-Y. p53 acts as a co-repressor to regulate keratin 14 expression during epidermal cell differentiation. PLoS ONE 2012, 7, e41742. [Google Scholar] [CrossRef]
- Cottle, D.L.; Kretzschmar, K.; Gollnick, H.P.; Quist, S.R. p53 activity contributes to defective interfollicular epidermal differentiation in hyperproliferative murine skin. Br. J. Dermatol. 2016, 174, 204–208. [Google Scholar] [CrossRef]
- Liao, C.; Xie, G.; Zhu, L.; Chen, X.; Li, X.; Lu, H.; Xu, B.; Ramot, Y.; Paus, R.; Yue, Z. p53 Is a Direct Transcriptional Repressor of Keratin 17: Lessons from a Rat Model of Radiation Dermatitis. J. Investig. Dermatol. 2016, 136, 680–689. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Cioce, A.; Cavani, A.; Cattani, C.; Scopelliti, F. Role of the Skin Immune System in Wound Healing. Cells 2024, 13, 624. [Google Scholar] [CrossRef]
- Gao, Y.; Gong, B.; Chen, Z.; Song, J.; Xu, N.; Weng, Z. Damage-Associated Molecular Patterns, a Class of Potential Psoriasis Drug Targets. Int. J. Mol. Sci. 2024, 25, 771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dang, E.; Shi, X.; Jin, L.; Feng, Z.; Hu, L.; Wu, Y.; Wang, G. The Pro-Inflammatory Cytokine IL-22 Up-Regulates Keratin 17 Expression in Keratinocytes via STAT3 and ERK1/2. PLoS ONE 2012, 7, e40797. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Magnaldo, T.; Freedberg, I.M.; Blumenberg, M. Expression of the carcinoma-associated keratin K6 and the role of AP-1 proto-oncoproteins. Gene Expr. 1993, 3, 187–199. [Google Scholar] [PubMed]
- Jiang, C.K.; Magnaldo, T.; Ohtsuki, M.; Freedberg, I.M.; Bernerd, F.; Blumenberg, M. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc. Natl. Acad. Sci. USA 1993, 90, 6786–6790. [Google Scholar] [CrossRef]
- Chung, B.-M.; Murray, C.I.; Eyk, J.E.V.; Coulombe, P.A. Identification of Novel Interaction between Annexin A2 and Keratin 17: EVIDENCE FOR RECIPROCAL REGULATION. J. Biol. Chem. 2012, 287, 7573–7581. [Google Scholar] [CrossRef]
- Yano, S.; Komine, M.; Fujimoto, M.; Okochi, H.; Tamaki, K. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J. Investig. Dermatol. 2004, 122, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Melzi, G.; Indino, S.; Piazza, S.; Sangiovanni, E.; Baruffaldi Preis, F.; Marabini, L.; Donetti, E. Keratin 17 as a Marker of UVB-Induced Stress in Human Epidermis and Modulation by Vitis vinifera Extract. Cells Tissues Organs 2022, 211, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Del Bino, S.; Asselineau, D. Regulation of keratin expression by ultraviolet radiation: Differential and specific effects of ultraviolet B and ultraviolet a exposure. J. Investig. Dermatol. 2001, 117, 1421–1429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romashin, D.; Arzumanian, V.; Poverennaya, E.; Varshaver, A.; Luzgina, N.; Rusanov, A. Evaluation of Cd-induced cytotoxicity in primary human keratinocytes. Hum. Exp. Toxicol. 2024, 43, 09603271231224458. [Google Scholar] [CrossRef]
- Romashin, D.; Rusanov, A.; Tolstova, T.; Varshaver, A.; Netrusov, A.; Kozhin, P.; Luzgina, N. Loss of mutant p53 in HaCaT keratinocytes promotes cadmium-induced keratin 17 expression and cell death. Biochem. Biophys. Res. Commun. 2024, 709, 149834. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).