Assessing the COX-2/PGE2 Ratio and Anti-Nucleosome Autoantibodies as Biomarkers of Autism Spectrum Disorders: Using Combined ROC Curves to Improve Diagnostic Values
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Sampling
2.2. Biochemical Assays
2.2.1. Anti-Nucleosome-Specific Antibodies
2.2.2. Cyclooxygenase-2 (COX-2)
2.2.3. Prostaglandin E2
2.2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Khachadourian, V.; Mahjani, B.; Sandin, S.; Kolevzon, A.; Buxbaum, J.D.; Reichenberg, A.; Janecka, M. Comorbidities in autism spectrum disorder and their etiologies. Transl. Psychiatry 2023, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Zoccante, L.; Ciceri, M.L.; Gozzi, L.A.; Gennaro, G.D.; Zerman, N. The “connectivome theory”: A new model to understand autism spectrum disorders. Front. Psychiatry 2022, 12, 794516. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Velasco, C.; Grahame, R.; Bravo, J.F. A connective tissue disorder may underlie ESSENCE problems in childhood. Res. Dev. Disabil. 2017, 60, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, C.; Penge, R.; Morlino, S.; Sperduti, I.; Terzani, A.; Giannini, M.T.; Colombi, M.; Grammatico, P.; Cardona, F.; Castori, M. Exploring relationships between joint hypermobility and neurodevelopment in children (4–13 years) with hereditary connective tissue disorders and developmental coordination disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2018, 177, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Elamin, N.E.; Al-Ayadhi, L.Y. Brain autoantibodies in autism spectrum disorder. Biomark. Med. 2014, 8, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.N.; Ferguson, B.J.; Hawkins, E.; Coman, A.; Schauer, J.; Ramirez-Celis, A.; Hecht, P.M.; Bruce, D.; Tilley, M.; Talebizadeh, Z. The Relationship between Maternal Antibodies to Fetal Brain and Prenatal Stress Exposure in Autism Spectrum Disorder. Metabolites 2023, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Amoura, Z.; Piette, J.C.; Bach, J.F.; Koutouzov, S. The key role of nucleosomes in lupus. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1999, 42, 833–843. [Google Scholar] [CrossRef]
- Mohan, C.; Liu, F.; Xie, C.; Williams, R., Jr. Anti-subnucleosome reactivities in systemic lupus erythematosus (SLE) patients and their first-degree relatives. Clin. Exp. Immunol. 2001, 123, 119–126. [Google Scholar] [CrossRef]
- Yap, D.Y.; Lai, K.N. Pathogenesis of renal disease in systemic lupus erythematosus—The role of autoantibodies and lymphocytes subset abnormalities. Int. J. Mol. Sci. 2015, 16, 7917–7931. [Google Scholar] [CrossRef] [PubMed]
- Didier, K.; Bolko, L.; Giusti, D.; Toquet, S.; Robbins, A.; Antonicelli, F.; Servettaz, A. Autoantibodies associated with connective tissue diseases: What meaning for clinicians? Front. Immunol. 2018, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Baratelli, F.; Krysan, K.; Heuzé-Vourc’h, N.; Zhu, L.; Escuadro, B.; Sharma, S.; Reckamp, K.; Dohadwala, M.; Dubinett, S.M. PGE2 confers survivin-dependent apoptosis resistance in human monocyte-derived dendritic cells. J. Leukoc. Biol. 2005, 78, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Fogel-Petrovic, M.; Long, J.A.; Knight, D.A.; Thompson, P.J.; Upham, J.W. Activated human dendritic cells express inducible cyclo-oxygenase and synthesize prostaglandin E2 but not prostaglandin D2. Immunol. Cell Biol. 2004, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kalled, S.L.; Cutler, A.H.; Burkly, L.C. Apoptosis and altered dendritic cell homeostasis in lupus nephritis are limited by anti-CD154 treatment. J. Immunol. 2001, 167, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.R.; Anderson, D. Ratios and the statistical analysis of biological data. Syst. Zool. 1978, 27, 71–78. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Kitchener, N. Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr. Neurol. 2009, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, P.M.; Ghezzo, A.; Bolotta, A.; Ferreri, C.; Minguzzi, R.; Vignini, A.; Visconti, P.; Marini, M. Perspective biological markers for autism spectrum disorders: Advantages of the use of receiver operating characteristic curves in evaluating marker sensitivity and specificity. Dis. Markers 2015, 2015, 329607. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.E. Basic principles of ROC analysis. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 1978; Volume 8, pp. 283–298. [Google Scholar]
- Alexander, J.M.; Pirone, A.; Jacob, M.H. Excessive β-catenin in excitatory neurons results in reduced social and increased repetitive behaviors and altered expression of multiple genes linked to human autism. Front. Synaptic Neurosci. 2020, 12, 14. [Google Scholar] [CrossRef]
- Chen, C.; Magee, J.C.; Bazan, N.G. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 2002, 87, 2851–2857. [Google Scholar] [CrossRef]
- Chen, C.; Bazan, N.G. Lipid signaling: Sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005, 77, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Chen, C. Lipid signaling and synaptic plasticity. Neurosci. 2006, 12, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.; Bestard-Lorigados, I.; Crawford, D.A. Autism-related behaviors in the cyclooxygenase-2-deficient mouse model. Genes Brain Behav. 2019, 18, e12506. [Google Scholar] [CrossRef]
- Vardeh, D.; Wang, D.; Costigan, M.; Lazarus, M.; Saper, C.B.; Woolf, C.J.; FitzGerald, G.A.; Samad, T.A. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J. Clin. Investig. 2009, 119, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, F.; Warner, T.D. Origins of prostaglandin E2: Involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J. Pharmacol. Exp. Ther. 2002, 303, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.D.; Gerdes, M.B.; Williams, Z.J.; Moore, D.J.; Cascio, C.J. Increased pain sensitivity and pain-related anxiety in individuals with autism. Pain Rep. 2020, 5, e861. [Google Scholar] [CrossRef] [PubMed]
- Fahad Ullah, M.; Bhat, S.H.; Tariq, M.; Abuduhier, F.M. Clinical significance of enzymes in disease and diagnosis. In Biocatalysis: Enzymatic Basics and Applications; Springer: Cham, Switzerland, 2019; pp. 213–231. [Google Scholar]
- El-Ansary, A.; Hassan, W.M.; Qasem, H.; Das, U.N. Identification of biomarkers of impaired sensory profiles among autistic patients. PLoS ONE 2016, 11, e0164153. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Crawford, D.A. Lipid signalling in the pathology of autism spectrum disorders. Compr. Guide Autism 2014, 18, 1259–1283. [Google Scholar]
- Wong, C.T.; Wais, J.; Crawford, D.A. Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur. J. Neurosci. 2015, 42, 2742–2760. [Google Scholar] [CrossRef]
- Bell, J.G.; Miller, D.; MacDonald, D.J.; MacKinlay, E.E.; Dick, J.R.; Cheseldine, S.; Boyle, R.M.; Graham, C.; O’Hare, A.E. The fatty acid compositions of erythrocyte and plasma polar lipids in children with autism, developmental delay or typically developing controls and the effect of fish oil intake. Br. J. Nutr. 2010, 103, 1160–1167. [Google Scholar] [CrossRef]
- Jory, J. Abnormal fatty acids in Canadian children with autism. Nutrition 2016, 32, 474–477. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.K.; Ben Bacha, A.G.; Al-Ayadhi, L.Y. Impaired plasma phospholipids and relative amounts of essential polyunsaturated fatty acids in autistic patients from Saudi Arabia. Lipids Health Dis. 2011, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.M.; Grünke, M.; Hieronymus, T.; Herrmann, M.; Kühnel, A.; Manger, B.; Kalden, J.R. In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1997, 40, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Amoura, Z.; Piette, J.C.; Chabre, H.; Cacoub, P.; Papo, T.; Wechsler, B.; Bach, J.F.; Koutouzov, S. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus. Correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1997, 40, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Voll, R.E.; Zoller, O.M.; Hagenhofer, M.; Ponner, B.B.; Kalden, J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998, 41, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Al-Ayadhi, L. Neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2012, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, F.G.; DuBois, R.N. Connecting COX-2 and Wnt in cancer. Cancer Cell 2006, 9, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Malenka, R.C. β-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 2003, 6, 1169–1177. [Google Scholar] [CrossRef]
- Okuda, T.; Yu, L.M.; Cingolani, L.A.; Kemler, R.; Goda, Y. β-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc. Natl. Acad. Sci. USA 2007, 104, 13479–13484. [Google Scholar] [CrossRef]
- Sherigar, D.K. ORAL PAPERS FINAL. Indian J. Psychiatry 2019, 61, S452–S520. [Google Scholar]
- Kharrazian, D.; Herbert, M.; Lambert, J. The relationships between intestinal permeability and target antibodies for a spectrum of autoimmune diseases. Int. J. Mol. Sci. 2023, 24, 16352. [Google Scholar] [CrossRef] [PubMed]
- Carlson, N.G.; Rojas, M.A.; Redd, J.W.; Tang, P.; Wood, B.; Hill, K.E.; Rose, J.W. Cyclooxygenase-2 expression in oligodendrocytes increases sensitivity to excitotoxic death. J. Neuroinflamm. 2010, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.; Sammaritano, L.; Nass, R.; Lockshin, M. Effects of mothers’ autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch. Pediatr. Adolesc. Med. 2003, 157, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Chimini, L.; Bonomi, F.; Filippini, E.; Motta, M.; Faden, D.; Lojacono, A.; Rebaioli, C.B.; Frassi, M.; Danieli, E. Neuropsychological development of children born to patients with systemic lupus erythematosus. Lupus 2004, 13, 805–811. [Google Scholar] [CrossRef]
- Lee, J.Y.; Huerta, P.T.; Zhang, J.; Kowal, C.; Bertini, E.; Volpe, B.T.; Diamond, B. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat. Med. 2009, 15, 91–96. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, L.A.; Konstantinov, K.N.; Lee, S.C.; Hardin, J.A.; Volpe, B.T.; Diamond, B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001, 7, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Kowal, C.; DeGiorgio, L.A.; Lee, J.Y.; Edgar, M.A.; Huerta, P.T.; Volpe, B.T.; Diamond, B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl. Acad. Sci. USA 2006, 103, 19854–19859. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, D.; Lee, J.; Niu, H.; Faust, T.W.; Frattini, S.; Kowal, C.; Huerta, P.T.; Volpe, B.T.; Diamond, B. Female mouse fetal loss mediated by maternal autoantibody. J. Exp. Med. 2012, 209, 1083–1089. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; Maestro, R. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for therapeutic intervention. Leukemia 2014, 28, 15–33. [Google Scholar] [CrossRef]

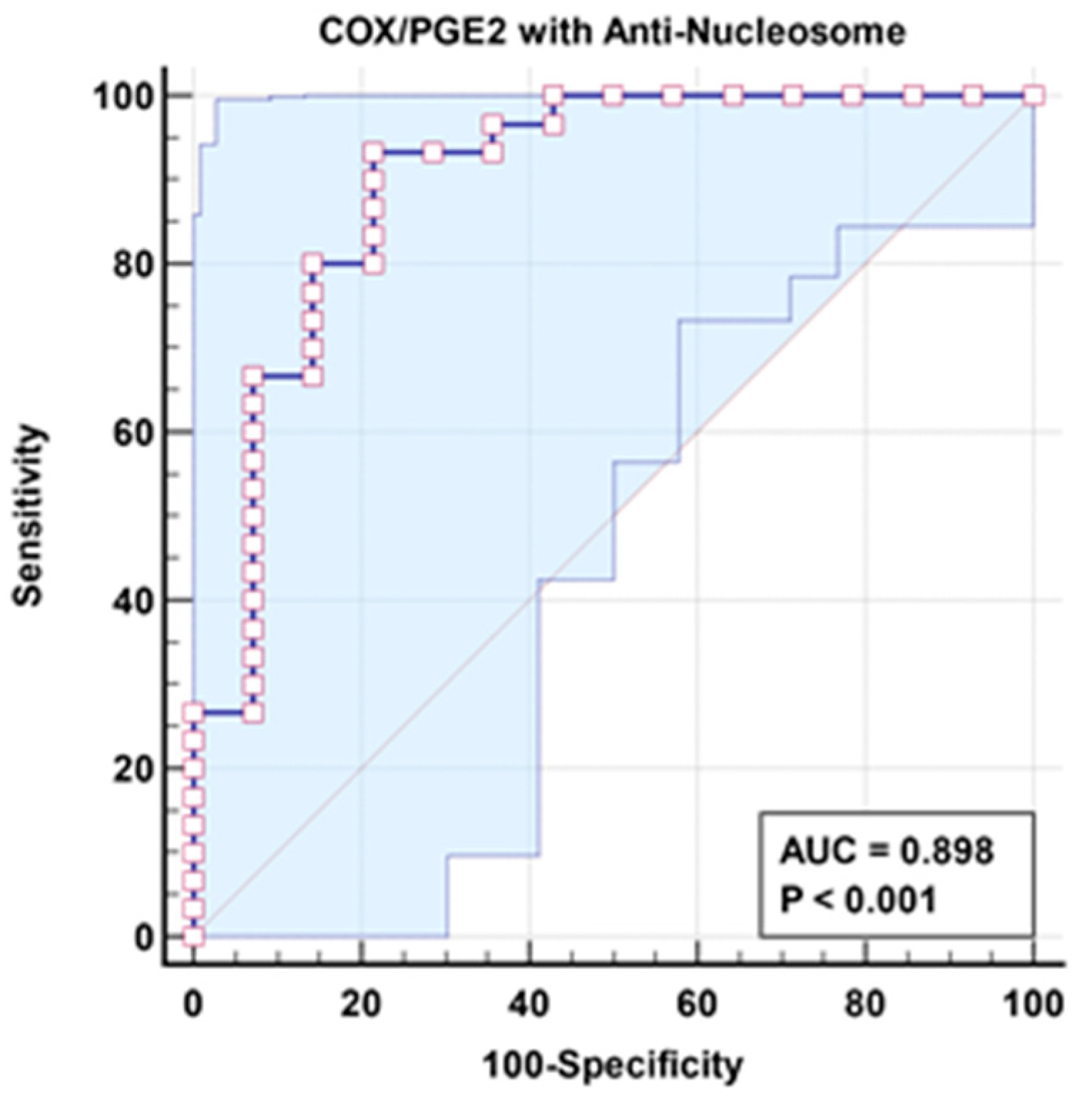
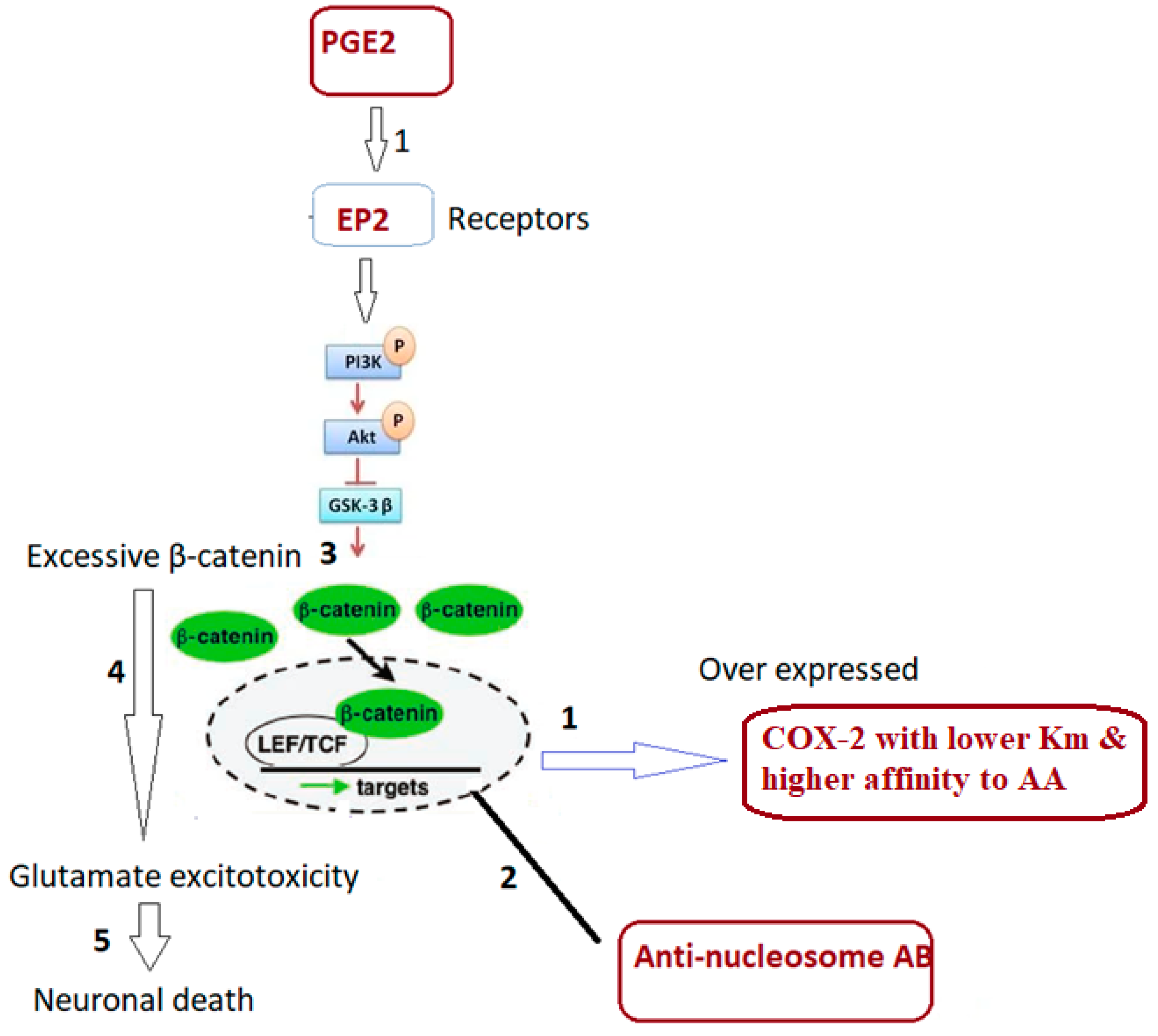
| Variables | Autistic (N = 40) | Controls (N = 42) |
|---|---|---|
| Age | 2–12 years (7.98 ± 2.59) | 2–14 years (7.83 ± 2.64) |
| Sex | All males | All males |
| Weight | 29.24 ± 11.45 kg | 28.81 ± 11.04 kg |
| Height | 129.19 ± 15.73 cm | 128.96 ± 16.28 cm |
| Family history of autoimmune | 16/40 (40%) | 3/42 (7%) |
| Parameters | Groups | N * | Min. | Max. | Mean ± S.D. | Median | Percent Change | p Value |
|---|---|---|---|---|---|---|---|---|
| COX/PGE2 | Control | 40 | 0.180 | 3.070 | 0.87 ± 0.67 | 0.601 | 100.00 | 0.001 |
| Patient | 42 | 0.000 | 1.130 | 0.35 ± 0.28 | 0.231 | 39.99 | ||
| Anti-nucleosome | Control | 14 | 0.050 | 5.940 | 1.20 ± 1.80 | 0.220 | 100.00 | 0.001 |
| Patient | 32 | 0.050 | 34.130 | 4.43 ± 5.85 | 3.261 | 368.69 |
| Parameters | R (Correlation Coefficient) | p Value | |
|---|---|---|---|
| COX/PGE2 with anti-nucleosome | −0.178 | 0.247 | N a |
| Parameters | AUC | Cut-Off Value | Sensitivity % | Specificity % | p Value | 95% CI |
|---|---|---|---|---|---|---|
| COX/PGE2 | 0.808 | 0.252 | 57.1% | 97.5% | 0.001 | 0.716–0.900 |
| Anti-nucleosome | 0.830 | 0.340 | 96.9% | 64.3% | 0.001 | 0.692–0.969 |
| Parameters | AUC | Sensitivity % | Specificity % | p Value | 95% CI |
|---|---|---|---|---|---|
| COX/PGE2 with anti-nucleosome | 0.898 | 93.3% | 78.6% | 0.001 | 0.785–1.000 |
| Parameters | Regression Coefficient | Standard Error | Odds Ratio | 95% CI for Odds Ratio | p Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| COX/PGE2 | −6.099 | 2.306 | 0.002 | 0.000 | 0.206 | 0.008 |
| Anti-nucleosome | 0.600 | 0.268 | 1.821 | 1.078 | 3.077 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ansary, A.; Alfawaz, H.A.; Ben Bacha, A.; AL-Ayadhi, L. Assessing the COX-2/PGE2 Ratio and Anti-Nucleosome Autoantibodies as Biomarkers of Autism Spectrum Disorders: Using Combined ROC Curves to Improve Diagnostic Values. Curr. Issues Mol. Biol. 2024, 46, 8699-8709. https://doi.org/10.3390/cimb46080513
El-Ansary A, Alfawaz HA, Ben Bacha A, AL-Ayadhi L. Assessing the COX-2/PGE2 Ratio and Anti-Nucleosome Autoantibodies as Biomarkers of Autism Spectrum Disorders: Using Combined ROC Curves to Improve Diagnostic Values. Current Issues in Molecular Biology. 2024; 46(8):8699-8709. https://doi.org/10.3390/cimb46080513
Chicago/Turabian StyleEl-Ansary, Afaf, Hanan A. Alfawaz, Abir Ben Bacha, and Laila AL-Ayadhi. 2024. "Assessing the COX-2/PGE2 Ratio and Anti-Nucleosome Autoantibodies as Biomarkers of Autism Spectrum Disorders: Using Combined ROC Curves to Improve Diagnostic Values" Current Issues in Molecular Biology 46, no. 8: 8699-8709. https://doi.org/10.3390/cimb46080513
APA StyleEl-Ansary, A., Alfawaz, H. A., Ben Bacha, A., & AL-Ayadhi, L. (2024). Assessing the COX-2/PGE2 Ratio and Anti-Nucleosome Autoantibodies as Biomarkers of Autism Spectrum Disorders: Using Combined ROC Curves to Improve Diagnostic Values. Current Issues in Molecular Biology, 46(8), 8699-8709. https://doi.org/10.3390/cimb46080513







