Dysregulated Inflammatory Cytokine Levels May Be Useful Markers in a Better Up-Dated Management of COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study and Population Design
2.2. Multiplex Cytokine Assay
2.3. Human Cytokines Sandwich ELISA Array
2.4. Principal Component Analysis (PCA)
2.5. Statistical Analysis
2.6. Ethics
3. Result
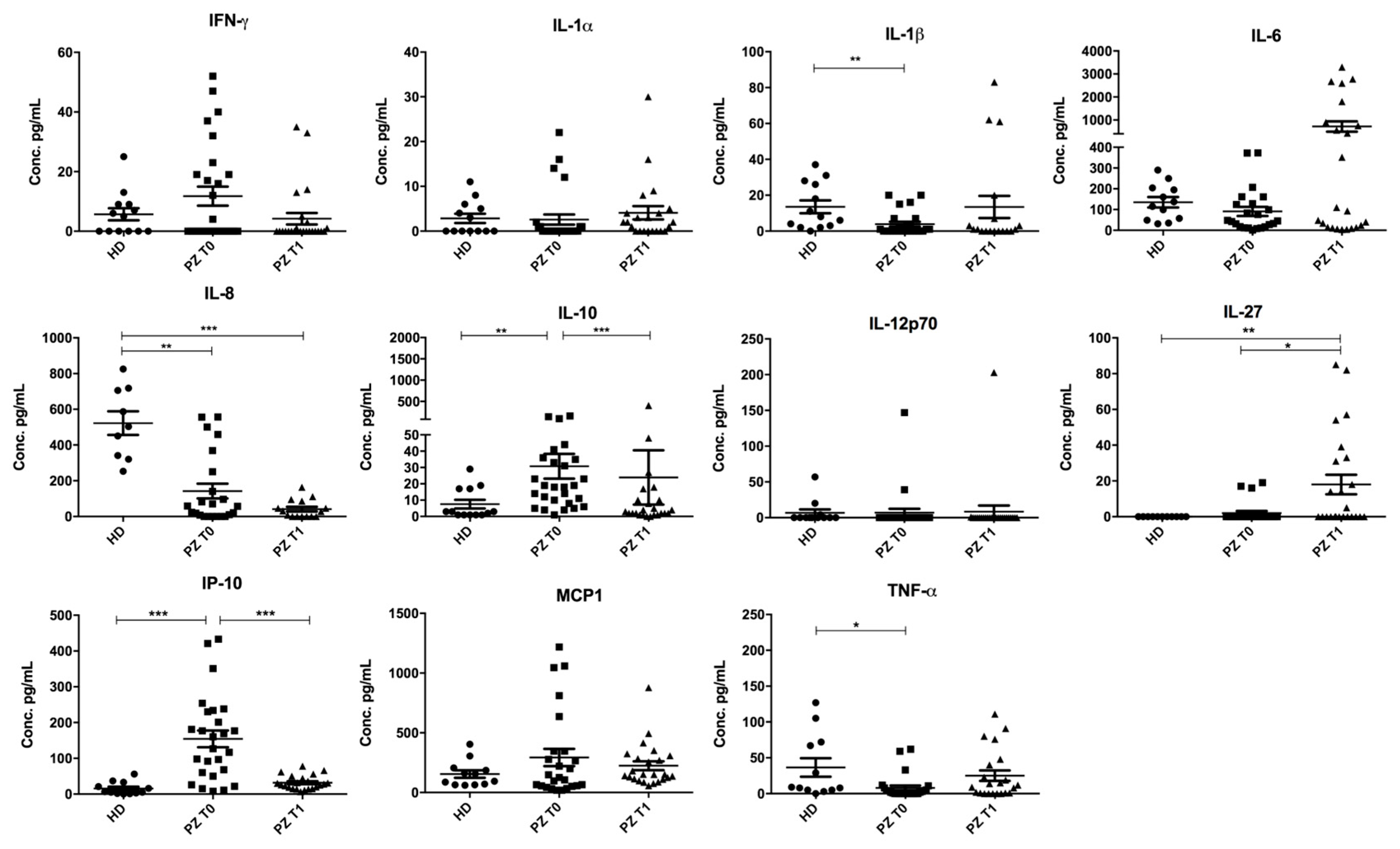
3.1. Concentrations of IL-10, IP-10, TNF-α, IL-1β, IL-27, and IL-8 Are Deregulated in Serum from Patients with COVID-19
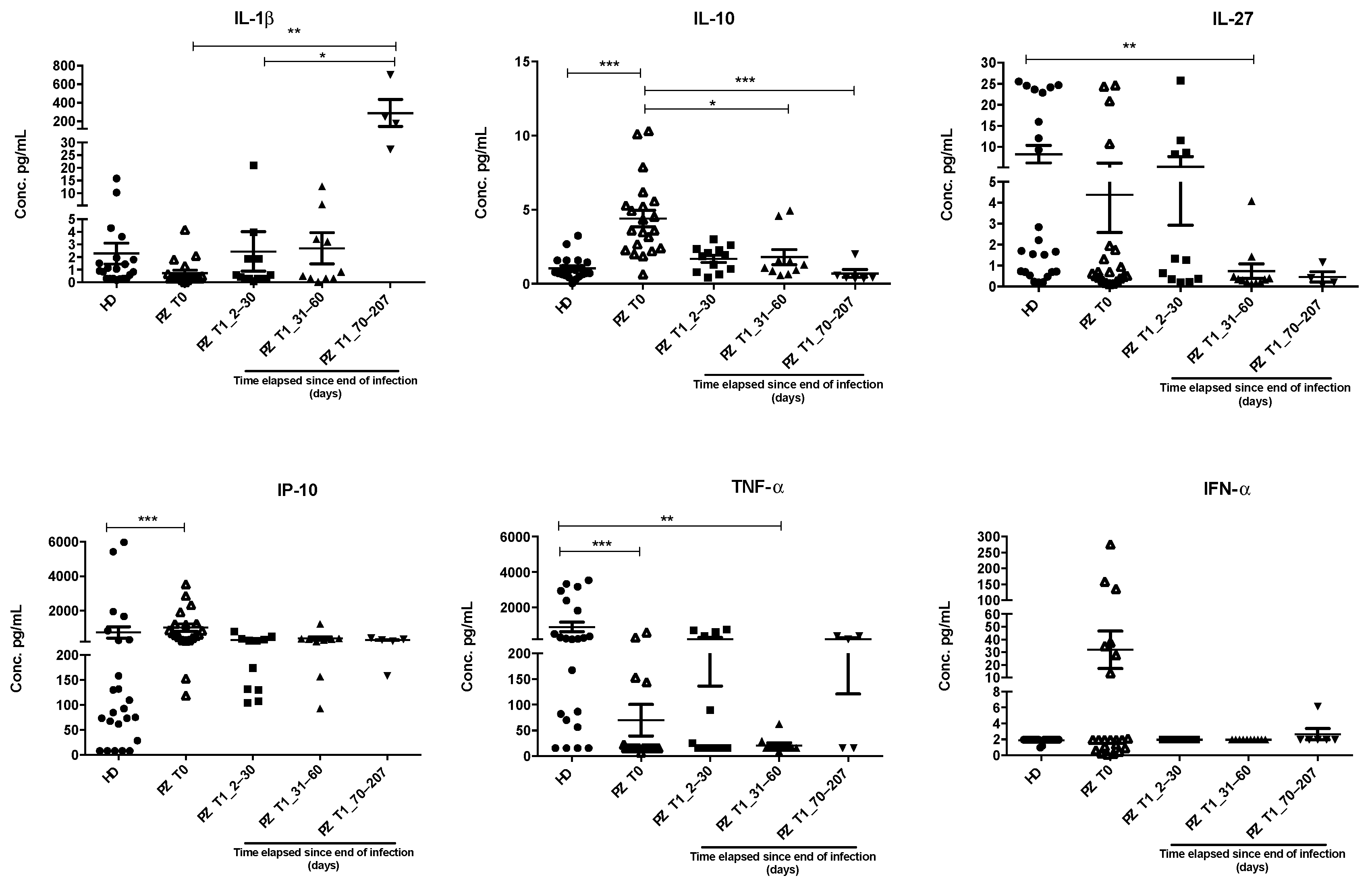
3.2. IL-1β, IL-10, IL-27, IP-10, and TNF-α Dysregulations Are Confirmed by ELISA Assay in an Extended Cohort
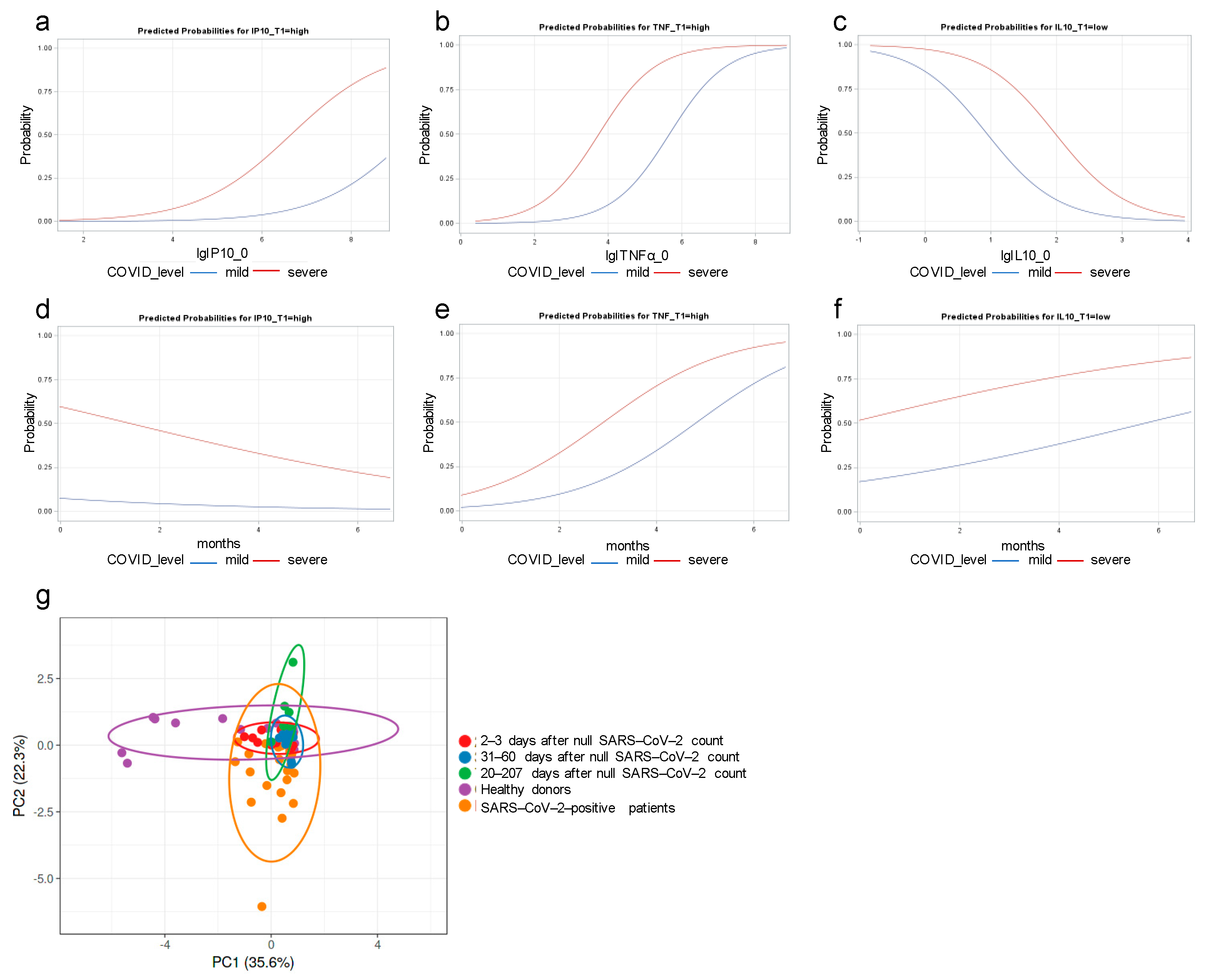
3.3. IP-10 and TNF-α Dysregulations Are Associated with COVID Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shen, Q.; Chang, H. Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects. Front. Immunol. 2022, 13, 843928. [Google Scholar] [CrossRef] [PubMed]
- Hunsawong, T.; Fernandez, S.; Buathong, R.; Khadthasrima, N.; Rungrojchareonkit, K.; Lohachanakul, J.; Suthangkornkul, R.; Tayong, K.; Huang, A.T.; Klungthong, C.; et al. Limited and Short-Lasting Virus Neutralizing Titers Induced by Inactivated SARS-CoV-2 Vaccine. Emerg. Infect. Dis. 2021, 27, 3178–3180. [Google Scholar] [CrossRef] [PubMed]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Dedkov, V.G.; Gladkikh, A.S.; Sharova, A.A.; Adish, Z.; Chernykh, E.I.; Kaschenko, V.A.; et al. Cytokine Profiling in Different SARS-CoV-2 Genetic Variants. Int. J. Mol. Sci. 2022, 23, 14146. [Google Scholar] [CrossRef] [PubMed]
- Kaidashev, I.; Shlykova, O.; Izmailova, O.; Torubara, O.; Yushchenko, Y.; Tyshkovska, T.; Kyslyi, V.; Belyaeva, A.; Maryniak, D. Host gene variability and SARS-CoV-2 infection: A review article. Heliyon 2021, 7, e07863. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cao, J.; Yu, Y.; Ding, J.; Eshak, E.S.; Liu, K.; Mubarik, S.; Shi, F.; Wen, H.; Zeng, Z.; et al. Epidemiological characteristics of patients with severe COVID-19 infection in Wuhan, China: Evidence from a retrospective observational study. Int. J. Epidemiol. 2021, 49, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Piazza, M.; Boner, A.L.; Bellanti, J.A. Long COVID: A proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. 2022, 43, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Hashemi, V.; Shomali, N.; Asghari, F.; Gharibi, T.; Akbari, M.; Gholizadeh, S.; Jafari, A. Innate and adaptive immune responses against coronavirus. Biomed. Pharmacother. 2020, 132, 110859. [Google Scholar] [CrossRef]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34. [Google Scholar] [CrossRef] [PubMed]
- Zamani, B.; Najafizadeh, M.; Motedayyen, H.; Arefnezhad, R. Predicting roles of IL-27 and IL-32 in determining the severity and outcome of COVID-19. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221145827. [Google Scholar] [CrossRef] [PubMed]
- Carreto-Binaghi, L.E.; Herrera, M.T.; Guzmán-Beltrán, S.; Juárez, E.; Sarabia, C.; Salgado-Cantú, M.G.; Juarez-Carmona, D.; Guadarrama-Pérez, C.; González, Y. Reduced IL-8 Secretion by NOD-like and Toll-like Receptors in Blood Cells from COVID-19 Patients. Biomedicines 2023, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.S.; Vishwakarma, S.; Trimbake, D.; Gurav, Y.K.; Potdar, V.A.; Mokashi, N.D.; Patsute, S.D.; Kaushal, H.; Choudhary, M.L.; Tilekar, B.N.; et al. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: Biomarkers of SARS-CoV-2 infection. Arch. Virol. 2021, 166, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Merza, M.Y.; Hwaiz, R.A.; Hamad, B.K.; Mohammad, K.A.; Hama, H.A.; Karim, A.Y. Analysis of cytokines in SARS-CoV-2 or COVID-19 patients in Erbil city, Kurdistan Region of Iraq. PLoS ONE 2021, 16, e0250330. [Google Scholar] [CrossRef] [PubMed]
- Yudhawati, R.; Sakina, S.; Fitriah, M. Interleukin-1β and Interleukin-10 Profiles and Ratio in Serum of COVID-19 Patients and Correlation with COVID-19 Severity: A Time Series Study. Int. J. Gen. Med. 2022, 15, 8043–8054. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef] [PubMed]
- Zuccalà, P.; Latronico, T.; Marocco, R.; Savinelli, S.; Vita, S.; Mengoni, F.; Tieghi, T.; Borgo, C.; Kertusha, B.; Carraro, A.; et al. Longitudinal Assessment of Multiple Immunological and Inflammatory Parameters during Successful DAA Therapy in HCV Monoinfected and HIV/HCV Coinfected Subjects. Int. J. Mol. Sci. 2022, 23, 11936. [Google Scholar] [CrossRef] [PubMed]
- Mascia, C.; Lichtner, M.; Zuccalà, P.; Vita, S.; Tieghi, T.; Marocco, R.; Savinelli, S.; Rossi, R.; Iannetta, M.; Campagna, M.; et al. Active HCV infection is associated with increased circulating levels of interferon-gamma (IFN-γ)-inducible protein-10 (IP-10), soluble CD163 and inflammatory monocytes regardless of liver fibrosis and HIV coinfection. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 644–655. [Google Scholar] [CrossRef]
- Lichtner, M.; Mastroianni, C.M.; Rossi, R.; Russo, G.; Belvisi, V.; Marocco, R.; Mascia, C.; Del Borgo, C.; Mengoni, F.; Sauzullo, I.; et al. Severe and persistent depletion of circulating plasmacytoid dendritic cells in patients with 2009 pandemic H1N1 infection. PLoS ONE 2011, 6, e19872. [Google Scholar] [CrossRef]
- Samaras, C.; Kyriazopoulou, E.; Poulakou, G.; Reiner, E.; Kosmidou, M.; Karanika, I.; Petrakis, V.; Adamis, G.; Gatselis, N.K.; Fragkou, A.; et al. Interferon gamma-induced protein 10 (IP-10) for the early prognosis of the risk for severe respiratory failure and death in COVID-19 pneumonia. Cytokine 2023, 162, 156111. [Google Scholar] [CrossRef] [PubMed]
- Trigo-Rodríguez, M.; Cárcel, S.; Navas, A.; Espíndola-Gómez, R.; Garrido-Gracia, J.C.; Esteban Moreno, M.; León-López, R.; Pérez-Crespo, P.M.M.; Alonso, E.A.; Vinuesa, D.; et al. Role of IP-10 to Predict Clinical Progression and Response to IL-6 Blockade With Sarilumab in Early COVID-19 Pneumonia. A Subanalysis of the SARICOR Clinical Trial. Open Forum Infect. Dis. 2023, 10, ofad133. [Google Scholar] [CrossRef]
- Ng, T.H.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.F.; Neves, P.F.M.D.; Lima, S.S.; Lopes, J.D.C.; Torres, M.K.D.S.; Vallinoto, I.M.V.C.; Bichara, C.D.A.; Dos Santos, E.F.; de Brito, M.T.F.M.; da Silva, A.L.S.; et al. Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome. Front. Cell Infect. Microbiol. 2022, 12, 922422. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.A.; Sánchez-Arcila, J.C.; Teva, A.; Perce-da-Silva, D.S.; Vasconcelos, M.P.; Lima, C.A.; Aprígio, C.J.; Rodrigues-da-Silva, R.N.; Santos, D.O.; Banic, D.M.; et al. IL10A genotypic association with decreased IL-10 circulating levels in malaria infected individuals from endemic area of the Brazilian Amazon. Malar. J. 2015, 14, 30. [Google Scholar] [CrossRef]
| 2 doses of BNT162b2 vaccinated healthy donors (HDs) (number) | 10 |
| Male (%) | 10 |
| Female (%) | 90 |
| Average age ± SD | 41.6 ± 12.06 |
| Not vaccinated patients (PZ) (number) | 29 |
| Male (%) | 65.51 |
| Female (%) | 62.07 |
| Average age ± SD (years) | 60.31 ± 15.89 |
| Time elapsed since end of infection (days) ± SD | 71.86 ± 29.94 |
| Mild (number) | 15 |
| Mild (%) | 51.7 |
| Severe (number) | 14 |
| Severe (%) | 48.2 |
| Healthy donors (HD) (number) | 25 |
| Not vaccinated | 6 |
| 3 doses of BNT162b2 vaccinated | 19 |
| Male (%) | 28 |
| Female (%) | 72 |
| Average age ± SD | 47.27 ± 16.18 |
| Patients (PZ) (number) | 33 |
| Not vaccinated | 24 |
| 3 doses of BNT162b2 vaccinated | 9 |
| Male (%) | 66.7 |
| Female (%) | 33.3 |
| Average age ± SD (years) | 47.48 ± 16.29 |
| Time elapsed since end of infection (days) ± SD | 63 ± 71.53 |
| (1° group) 2–30 days (number) | 14 |
| Average time elapsed since end of infection (days) | 15 ± 9.20 |
| (2° group) 31–60 days (number) | 12 |
| Average time elapsed since end of infection (days) | 39 ± 6.68 |
| (3° group) 70–207 days (number) | 7 |
| Average time elapsed since end of infection (days) | 161 ± 94.98 |
| Mild (number) | 7 |
| Mild (%) | 21.21 |
| Severe (number) | 3 |
| Severe (%) | 9.09 |
| Pauci-symptomatic (number) | 8 |
| Pauci-symptomatic (%) | 24.24 |
| Pneumonia (number) | 15 |
| Pneumonia (%) | 45.45 |
| (a) | ||||
| Odds Ratio of Event = Severe COVID-19 | ||||
| Variable | Estimate | 95% CL | p-Value | |
| IP10_T0 high vs. low * | 2.62 | 0.53 | 13.10 | 0.240 |
| * high: ≥the top quintile vs. low: <the top quintile (i.e., ≥873 vs. <873). | ||||
| (b) | ||||
| Odds Ratio of Event = Severe COVID-19 | ||||
| Variable | Estimate | 95% CL | p-Value | |
| TNF-α_T0 high vs. low * | 2.58 | 0.54 | 13.15 | 0.239 |
| * high: ≥the top quintile vs. low: <the top quintile (i.e., ≥153 vs. <153). | ||||
| (c) | ||||
| Odds Ratio of High IP-10 Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 11.33 | 1.05 | 122.55 | 0.046 |
| * high: ≥the top quintile vs. low: <the top quintile (i.e., ≥363 vs. <363). | ||||
| (d) | ||||
| Odds Ratio of High TNF-α Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| TNF-α high vs. low * | 18.00 | 1.69 | 191.52 | 0.017 |
| * high: ≥the top quintile vs. low: <the top quintile (i.e., ≥212 vs. <212). | ||||
| (e) | ||||
| Adjusted Odds Ratio of High IP-10 Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 13.51 | 1.08 | 191.80 | 0.050 |
| IP-10 at T0 ** | 2.62 | 0.77 | 8.84 | 0.120 |
| * high: ≥top quintile vs. low: <top quintile (i.e., ≥363 vs. <363) | ||||
| ** for one natural log increment | ||||
| (f) | ||||
| Adjusted Odds Ratio of High TNF-α Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 16.52 | 0.83 | 328.37 | 0.066 |
| TNF-α at T0 ** | 35.70 | 1.41 | 906.61 | 0.030 |
| * high: ≥80th percentile vs. low: <80th perc. (i.e., ≥212 vs. <212) | ||||
| ** for one natural log increment | ||||
| (g) | ||||
| Adjusted Odds Ratio of High IP-10 Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVOD-19 vs. non-severe | 11.78 | 1.15 | 120.28 | 0.037 |
| months from negative test ** | 1.25 | 0.47 | 3.34 | 0.656 |
| * high: ≥top quintile vs. low: <top quintile (i.e., ≥363 vs. <363) | ||||
| ** for 3 months increments | ||||
| (h) | ||||
| Adjusted Odds Ratio of High TNF-α Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 4.62 | 0.24 | 90.05 | 0.312 |
| months from negative test ** | 10.90 | 0.50 | 253.71 | 0.128 |
| * high: ≥80th percentile vs. low: <80th perc. (i.e., ≥212 vs. <212) | ||||
| ** for 3 months increment | ||||
| (i) | ||||
| Odds Ratio of Event = Severe COVID-19 | ||||
| Variable | Estimate | 95% CL | p-Value | |
| IL-10 low vs. high * | 1.47 | 0.21 | 10.20 | 0.699 |
| * low: ≤second quintile vs. high: >second quintile (i.e., ≤1.3 vs. >1.3) | ||||
| (j) | ||||
| Odds Ratio of Low IL-10 Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 8.17 | 1.42 | 47.02 | 0.019 |
| * low: ≤second quintile vs. high: >second quintile (i.e., ≤1.3 vs. >1.3) | ||||
| (k) | ||||
| Odds Ratio of Low IL-10 Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 6.43 | 1.05 | 39.40 | 0.032 |
| Il-10 at T0 ** | 2.86 | 0.47 | 17.54 | 0.256 |
| * low: ≤second quintile vs. high: >second quintile (i.e., ≤3.9 vs. >3.9) ** for one natural log increment | ||||
| (l) | ||||
| Odds Ratio of Low IL-10 Concentration at T1 * | ||||
| Variable | Estimate | 95% CL | p-Value | |
| severe COVID-19 vs. non-severe | 4.47 | 0.51 | 39.52 | 0.178 |
| months from negative test ** | 2.27 | 0.18 | 29.05 | 0.528 |
| * low: ≤second quintile vs. high: >second quintile (i.e., ≤3.9 vs. >3.9) ** for 3 months increment | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuliano, M.; Mongiovì, R.M.; Parente, A.; Kertusha, B.; Carraro, A.; Marocco, R.; Mancarella, G.; Del Borgo, C.; Fondaco, L.; Grimaldi, L.; et al. Dysregulated Inflammatory Cytokine Levels May Be Useful Markers in a Better Up-Dated Management of COVID-19. Curr. Issues Mol. Biol. 2024, 46, 8890-8902. https://doi.org/10.3390/cimb46080525
Iuliano M, Mongiovì RM, Parente A, Kertusha B, Carraro A, Marocco R, Mancarella G, Del Borgo C, Fondaco L, Grimaldi L, et al. Dysregulated Inflammatory Cytokine Levels May Be Useful Markers in a Better Up-Dated Management of COVID-19. Current Issues in Molecular Biology. 2024; 46(8):8890-8902. https://doi.org/10.3390/cimb46080525
Chicago/Turabian StyleIuliano, Marco, Roberta Maria Mongiovì, Alberico Parente, Blerta Kertusha, Anna Carraro, Raffaella Marocco, Giulia Mancarella, Cosmo Del Borgo, Laura Fondaco, Lorenzo Grimaldi, and et al. 2024. "Dysregulated Inflammatory Cytokine Levels May Be Useful Markers in a Better Up-Dated Management of COVID-19" Current Issues in Molecular Biology 46, no. 8: 8890-8902. https://doi.org/10.3390/cimb46080525






