Apoptotic Receptors and CD107a Expression by NK Cells in an Interaction Model with Trophoblast Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Inductors
2.3. Conditioned Media (CM) of Chorionic Villi Explants
2.4. Determination of NK-92 Cell Expression of Proapoptotic Receptors after Incubation with JEG-3 Trophoblast Cells and Inducers
2.5. Statistical Data Analysis
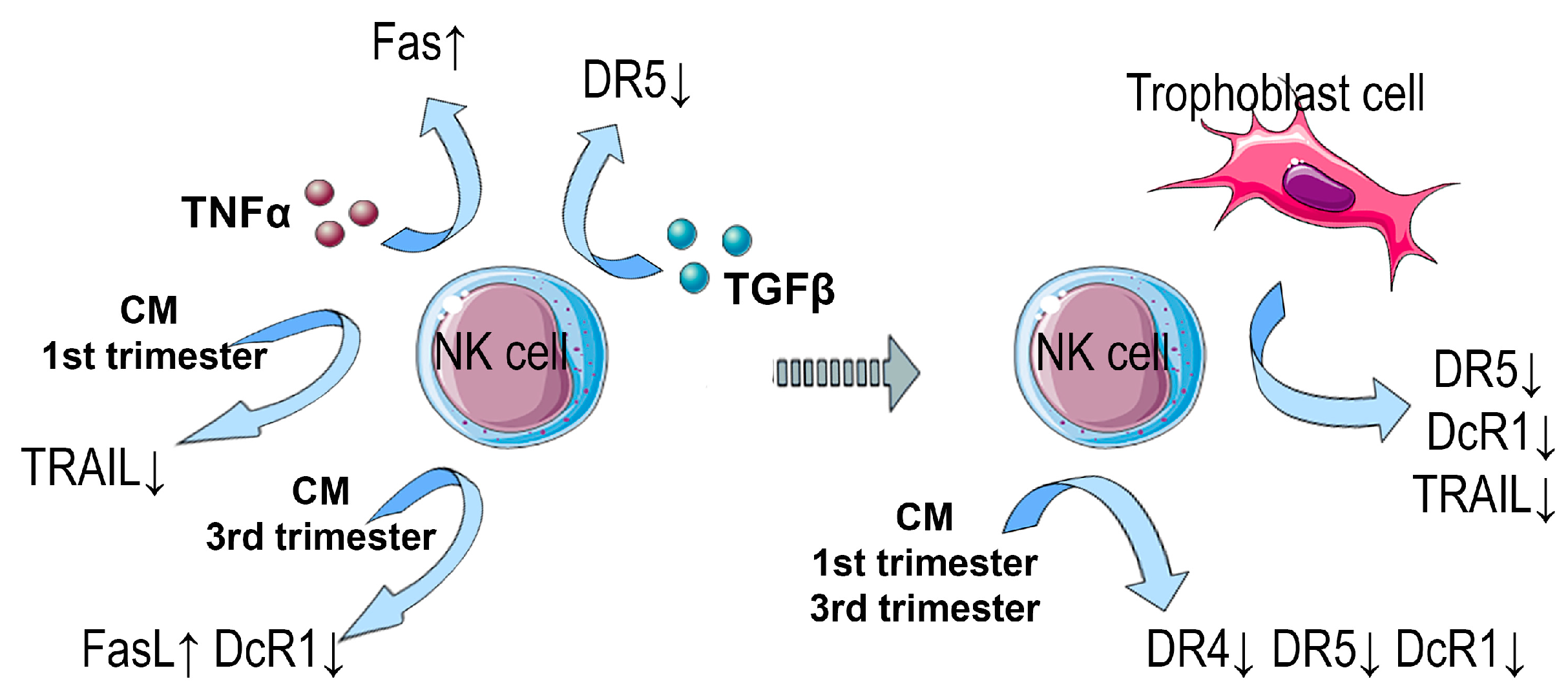
3. Results
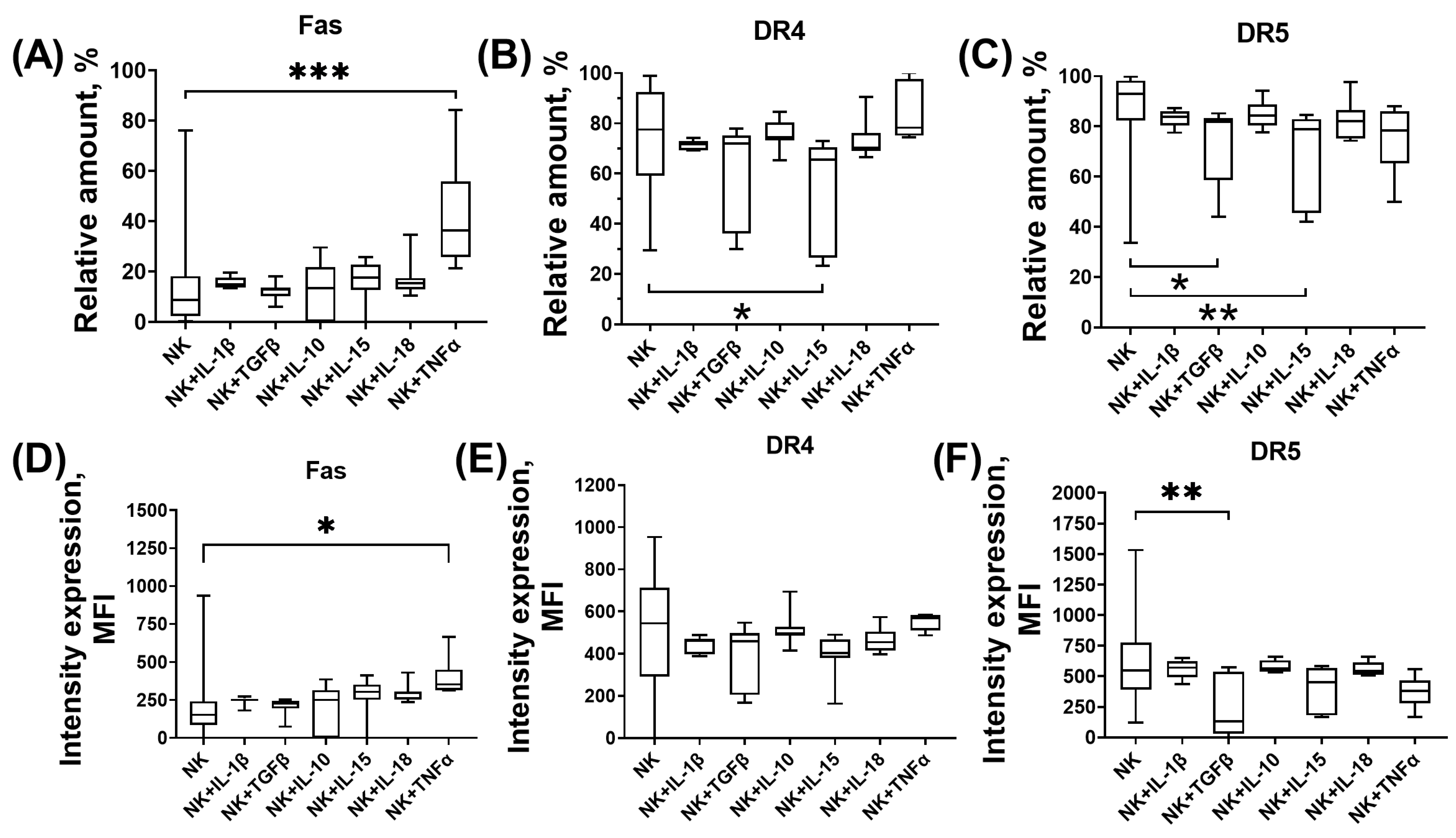
3.1. Expression of Proapoptotic Receptors and Their Ligands by NK-92 Cells in the Presence of Cytokines
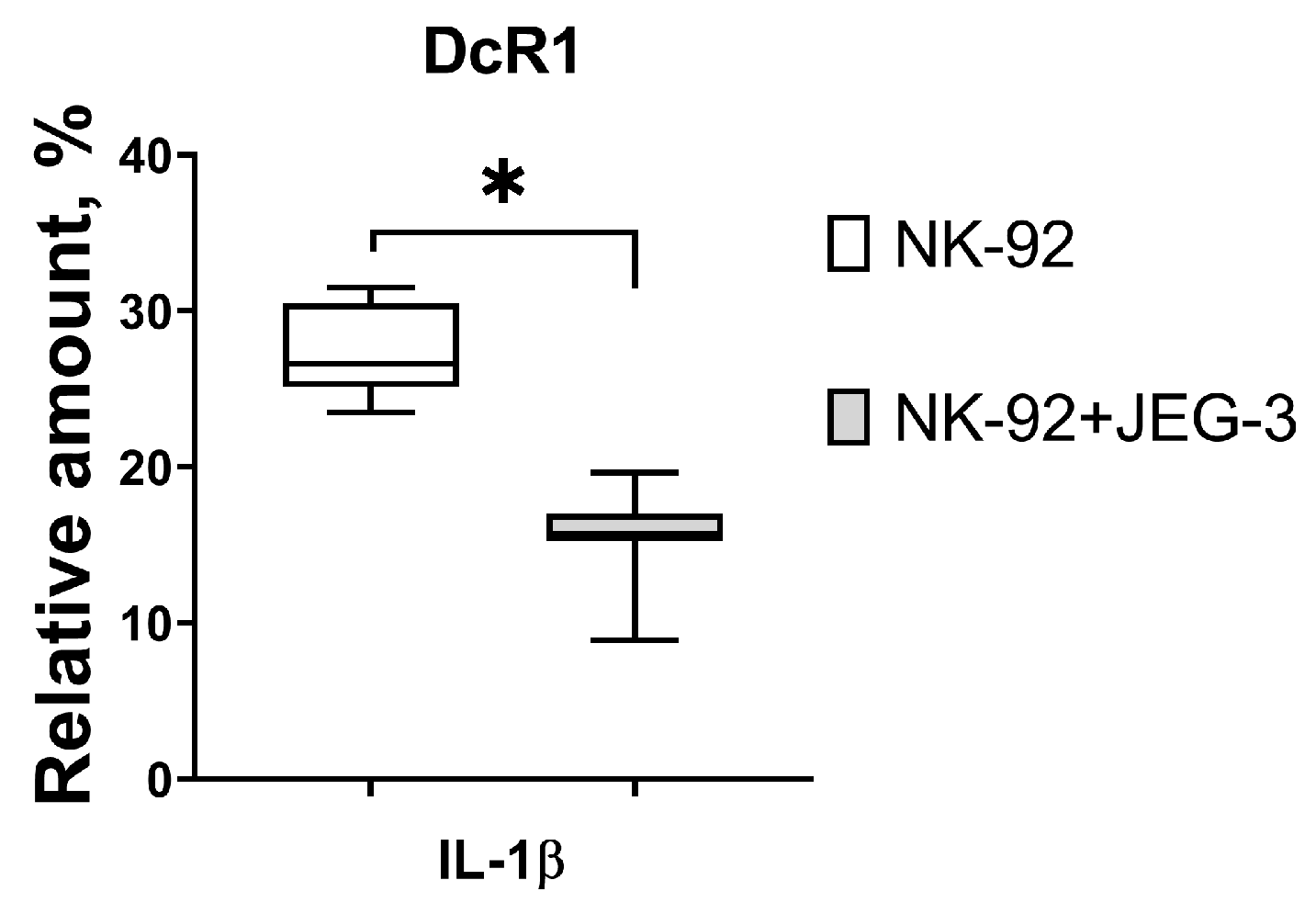
3.2. Expression of Proapoptotic Receptors and Their Ligands by NK-92 Cells in the Presence of Trophoblast Cells and Cytokines
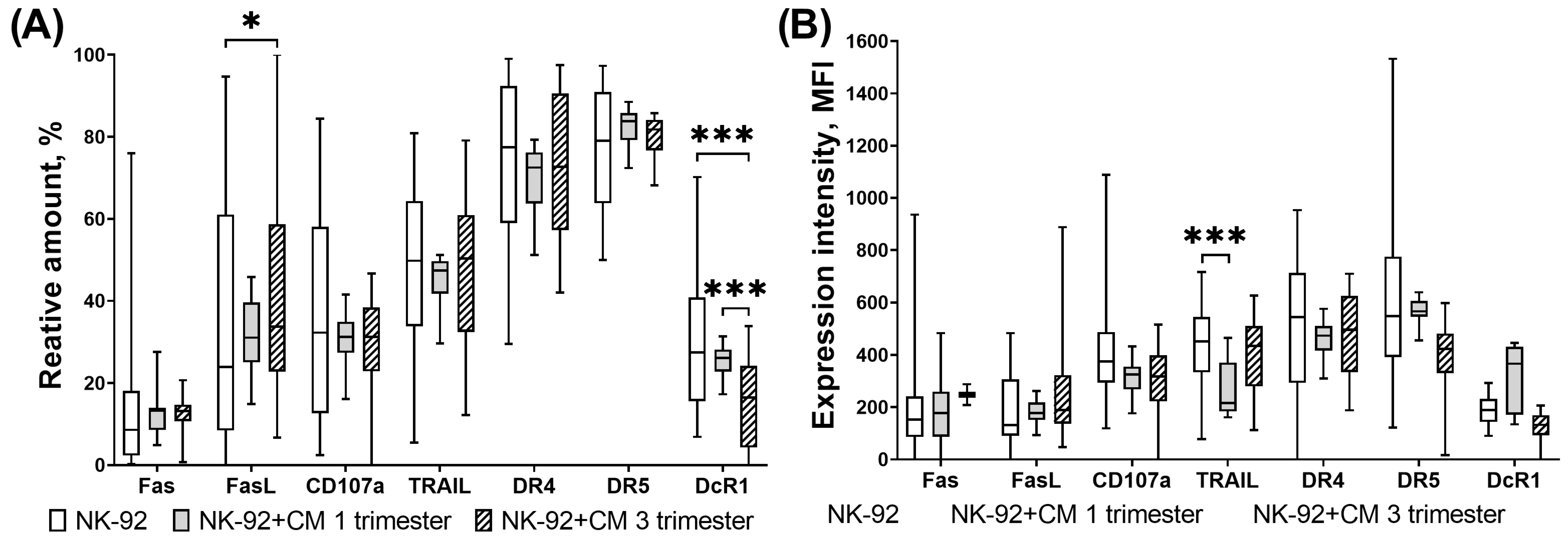
3.3. Expression of Proapoptotic Receptors and Their Ligands by NK-92 Cells in the Presence of Chorionic Villi CM
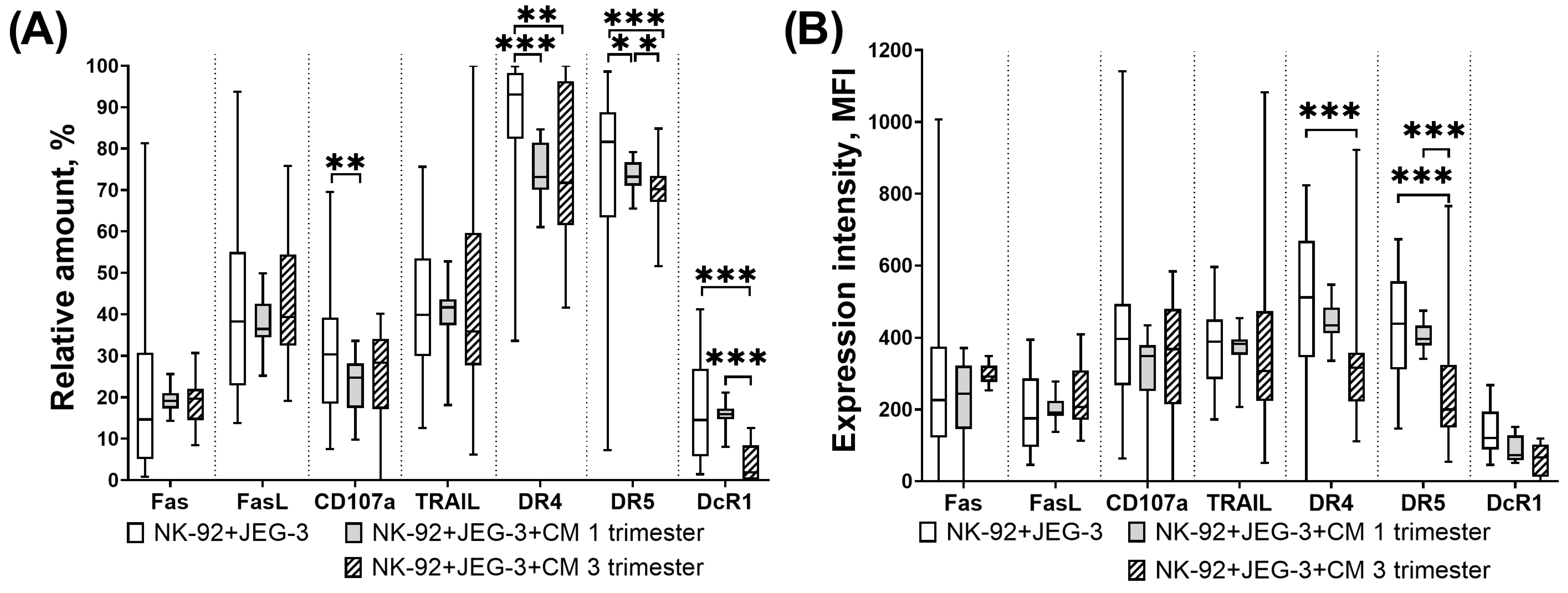
3.4. Expression of Proapoptotic Receptors and Ligands of NK-92 Cells in the Presence of Trophoblast Cells and Chorionic Villi CM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, A.R.; Gonzalez, S.; Lopez-Soto, A. Mechanisms of Apoptosis Resistance to NK Cell-Mediated Cytotoxicity in Cancer. Int. J. Mol. Sci. 2020, 21, 3726. [Google Scholar] [CrossRef]
- Tuomela, K.; Ambrose, A.R.; Davis, D.M. Escaping Death: How Cancer Cells and Infected Cells Resist Cell-Mediated Cytotoxicity. Front. Immunol. 2022, 13, 867098. [Google Scholar] [CrossRef]
- Krzewski, K.; Gil-Krzewska, A.; Nguyen, V.; Peruzzi, G.; Coligan, J.E. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood 2013, 121, 4672–4683. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Singh, H.D.; Otano, I.; Rombouts, K.; Singh, K.P.; Peppa, D.; Gill, U.S.; Bottcher, K.; Kennedy, P.T.F.; Oben, J.; Pinzani, M.; et al. TRAIL regulatory receptors constrain human hepatic stellate cell apoptosis. Sci. Rep. 2017, 7, 5514. [Google Scholar] [CrossRef]
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s path in the immune system. Immunology 2009, 127, 145–154. [Google Scholar] [CrossRef]
- Hofle, J.; Trenkner, T.; Kleist, N.; Schwane, V.; Vollmers, S.; Barcelona, B.; Niehrs, A.; Fittje, P.; Huynh-Tran, V.H.; Sauter, J.; et al. Engagement of TRAIL triggers degranulation and IFNgamma production in human natural killer cells. EMBO Rep. 2022, 23, e54133. [Google Scholar] [CrossRef]
- Granzin, M.; Stojanovic, A.; Miller, M.; Childs, R.; Huppert, V.; Cerwenka, A. Highly efficient IL-21 and feeder cell-driven ex vivo expansion of human NK cells with therapeutic activity in a xenograft mouse model of melanoma. Oncoimmunology 2016, 5, e1219007. [Google Scholar] [CrossRef]
- Mirandola, P.; Ponti, C.; Gobbi, G.; Sponzilli, I.; Vaccarezza, M.; Cocco, L.; Zauli, G.; Secchiero, P.; Manzoli, F.A.; Vitale, M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood 2004, 104, 2418–2424. [Google Scholar] [CrossRef]
- Lee, J.; Dieckmann, N.M.G.; Edgar, J.R.; Griffiths, G.M.; Siegel, R.M. Fas Ligand localizes to intraluminal vesicles within NK cell cytolytic granules and is enriched at the immune synapse. Immun. Inflamm. Dis. 2018, 6, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Chakraborty, S.; Islam, S.; Ain, R. Trans-differentiation of trophoblast stem cells: Implications in placental biology. Life Sci. Alliance 2023, 6, e202201583. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Niimi, K.; Yamamoto, E.; Ikeda, Y.; Nishino, K.; Suzuki, S.; Kajiyama, H.; Kikkawa, F. Core 2 beta1,6-N-acetylglucosaminyltransferases accelerate the escape of choriocarcinoma from natural killer cell immunity. Biochem. Biophys. Rep. 2021, 26, 100951. [Google Scholar] [CrossRef]
- Han, M.; Jiang, Y.; Lao, K.; Xu, X.; Zhan, S.; Wang, Y.; Hu, X. sHLA-G involved in the apoptosis of decidual natural killer cells following Toxoplasma gondii infection. Inflammation 2014, 37, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Long, E.O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 1999, 189, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for study of human embryo implantation: Choice of cell lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar] [PubMed]
- Komatsu, F.; Kajiwara, M. Relation of natural killer cell line NK-92-mediated cytolysis (NK-92-lysis) with the surface markers of major histocompatibility complex class I antigens, adhesion molecules, and Fas of target cells. Oncol. Res. 1998, 10, 483–489. [Google Scholar] [PubMed]
- Melsted, W.N.; Matzen, S.H.; Andersen, M.H.; Hviid, T.V.F. The choriocarcinoma cell line JEG-3 upregulates regulatory T cell phenotypes and modulates pro-inflammatory cytokines through HLA-G. Cell. Immunol. 2018, 324, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.; Bork, J.B.S.; Isgaard, C.; Larsen, T.G.; Bordoy, A.M.; Bengtsson, M.S.; Hviid, T.V.F. Cytokine stimulation of the choriocarcinoma cell line JEG-3 leads to alterations in the HLA-G expression profile. Cell. Immunol. 2020, 352, 104110. [Google Scholar] [CrossRef]
- Eikmans, M.; van der Keur, C.; Anholts, J.D.H.; Drabbels, J.J.M.; van Beelen, E.; de Sousa Lopes, S.M.C.; van der Hoorn, M.L. Primary Trophoblast Cultures: Characterization of HLA Profiles and Immune Cell Interactions. Front. Immunol. 2022, 13, 814019. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Spitz, B.; Hanssens, M.; Luyten, C.; Pijnenborg, R. Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum. Reprod. 2006, 21, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Poloski, E.; Oettel, A.; Ehrentraut, S.; Luley, L.; Costa, S.D.; Zenclussen, A.C.; Schumacher, A. JEG-3 Trophoblast Cells Producing Human Chorionic Gonadotropin Promote Conversion of Human CD4+FOXP3- T Cells into CD4+FOXP3+ Regulatory T Cells and Foster T Cell Suppressive Activity. Biol. Reprod. 2016, 94, 106. [Google Scholar] [CrossRef]
- Kohler, P.O.; Bridson, W.E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J. Clin. Endocrinol. Metab. 1971, 32, 683–687. [Google Scholar] [CrossRef]
- Gustafsson, C.; Hummerdal, P.; Matthiesen, L.; Berg, G.; Ekerfelt, C.; Ernerudh, J. Cytokine secretion in decidual mononuclear cells from term human pregnancy with or without labour: ELISPOT detection of IFN-gamma, IL-4, IL-10, TGF-beta and TNF-alpha. J. Reprod. Immunol. 2006, 71, 41–56. [Google Scholar] [CrossRef]
- Beceriklisoy, H.B.; Schafer-Somi, S.; Kucukaslan, I.; Agaoglu, R.; Gultiken, N.; Ay, S.S.; Kaya, D.; Aslan, S. Cytokines, growth factors and prostaglandin synthesis in the uterus of pregnant and non-pregnant bitches: The features of placental sites. Reprod. Domest. Anim. = Zuchthyg. 2009, 44 (Suppl. S2), 115–119. [Google Scholar] [CrossRef]
- Lin, L.; Bai, K.; Li, J.; Chiu, P.C.N.; Lee, C.L. Regulatory role of human endometrial gland secretome on macrophage differentiation. J. Reprod. Immunol. 2023, 160, 104158. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Bose, R.; Paul, M.; Nandy, D.; Basak, T.; Ain, R. IL1beta-NFkappabeta-Myocardin signaling axis governs trophoblast-directed plasticity of vascular smooth muscle cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23637. [Google Scholar] [CrossRef]
- Toth, B.; Haufe, T.; Scholz, C.; Kuhn, C.; Friese, K.; Karamouti, M.; Makrigiannakis, A.; Jeschke, U. Placental interleukin-15 expression in recurrent miscarriage. Am. J. Reprod. Immunol. 2010, 64, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, S.; Pritz, S.; Amann, N.; Meister, S.; Beyer, S.; Jegen, M.; Kuhn, C.; Hutter, S.; Knabl, J.; Mahner, S.; et al. Sex Specific Expression of Interleukin 7, 8 and 15 in Placentas of Women with Gestational Diabetes. Int. J. Mol. Sci. 2020, 21, 8026. [Google Scholar] [CrossRef]
- Tauber, Z.; Cizkova, K. The anti-inflammatory role of placental Hofbauer cells is altered in patients with chorioamnionitis: Are CYP2C8 and soluble epoxide hydrolase involved in immunomodulation? Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2022, 166, 267–273. [Google Scholar] [CrossRef]
- Mercnik, M.H.; Schliefsteiner, C.; Fluhr, H.; Wadsack, C. Placental macrophages present distinct polarization pattern and effector functions depending on clinical onset of preeclampsia. Front. Immunol. 2022, 13, 1095879. [Google Scholar] [CrossRef] [PubMed]
- Lidstrom, C.; Matthiesen, L.; Berg, G.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: Implications for suppressor macrophages in decidua. Am. J. Reprod. Immunol. 2003, 50, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Lob, S.; Ochmann, B.; Ma, Z.; Vilsmaier, T.; Kuhn, C.; Schmoeckel, E.; Herbert, S.L.; Kolben, T.; Wockel, A.; Mahner, S.; et al. The role of Interleukin-18 in recurrent early pregnancy loss. J. Reprod. Immunol. 2021, 148, 103432. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, D.; Mikhailova, V.; Nikolaenkov, I.; Markova, K.; Salloum, Z.; Kogan, I.; Gzgzyan, A.; Selkov, S.; Sokolov, D. The uteroplacental contact zone cytokine influence on NK cell cytotoxicity to trophoblasts. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Galvan, L.; Guglielmo, M.; Cruz Amaya, J.; Smith-Gagen, J.; Lombardi, V.C.; Merica, R.; Hudig, D. Optimizing NK-92 serial killers: Gamma irradiation, CD95/Fas-ligation, and NK or LAK attack limit cytotoxic efficacy. J. Transl. Med. 2022, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.B.; Araujo Junior, E.; Ribeiro, J.U.; Rodrigues, D.B.; Castro, E.C.; Caldas, T.M.; Rodrigues Junior, V. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J. Matern.-Fetal Neonatal Med. 2016, 29, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Incebiyik, A.; Kocarslan, S.; Camuzcuoglu, A.; Hilali, N.G.; Incebiyik, H.; Camuzcuoglu, H. Trophoblastic E-cadherin and TGF-beta expression in placenta percreta and normal pregnancies. J. Matern.-Fetal Neonatal Med. 2016, 29, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Kurian, N.K.; Rao, K.A. Cytokines, NK cells and regulatory T cell functions in normal pregnancy and reproductive failures. Am. J. Reprod. Immunol. 2023, 89, e13667. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, M.; Ning, F.; Ye, Y.; Lu, Q.; Lu, S.; Duan, Y.; Gan, X.; Zhao, M.; Guo, K.; et al. Extravillous trophoblast cell-derived exosomes induce vascular smooth muscle cell apoptosis via a mechanism associated with miR-143-3p. Mol. Hum. Reprod. 2023, 29, gaad026. [Google Scholar] [CrossRef]
- Murakoshi, H.; Matsuo, H.; Laoag-Fernandez, J.B.; Samoto, T.; Maruo, T. Expression of Fas/Fas-ligand, Bcl-2 protein and apoptosis in extravillous trophoblast along invasion to the decidua in human term placenta. Endocr. J. 2003, 50, 199–207. [Google Scholar] [CrossRef]
- Ayala-Ramirez, P.; Machuca-Acevedo, C.; Gamez, T.; Quijano, S.; Barreto, A.; Silva, J.L.; Olaya, C.M.; Garcia-Robles, R. Assessment of Placental Extracellular Vesicles-Associated Fas Ligand and TNF-Related Apoptosis-Inducing Ligand in Pregnancies Complicated by Early and Late Onset Preeclampsia. Front. Physiol. 2021, 12, 708824. [Google Scholar] [CrossRef]
- Liu, J.; Dong, P.; Jia, N.; Wen, X.; Luo, L.; Wang, S.; Li, J. The expression of intracellular cytokines of decidual natural killer cells in unexplained recurrent pregnancy loss. J. Matern.-Fetal Neonatal Med. 2022, 35, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Vrekoussis, T.; Heublein, S.; Bayer, B.; Anz, D.; Knabl, J.; Navrozoglou, I.; Dian, D.; Friese, K.; Makrigiannakis, A.; et al. Decidual macrophages are significantly increased in spontaneous miscarriages and over-express FasL: A potential role for macrophages in trophoblast apoptosis. Int. J. Mol. Sci. 2012, 13, 9069–9080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Cardoso Alves, L.; Berger, M.D.; Koutsandreas, T.; Kirschke, N.; Lauer, C.; Sporri, R.; Chatziioannou, A.; Corazza, N.; Krebs, P. Non-apoptotic TRAIL function modulates NK cell activity during viral infection. EMBO Rep. 2020, 21, e48789. [Google Scholar] [CrossRef]
- Ma, S.; Caligiuri, M.A.; Yu, J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 2022, 43, 833–847. [Google Scholar] [CrossRef]
- Bergman, H.; Lindqvist, C. Human IL-15 Inhibits NK Cells Specific for Human NK-92 Cells. Anticancer Res. 2021, 41, 3281–3285. [Google Scholar] [CrossRef]
- Foltz, J.A.; Moseman, J.E.; Thakkar, A.; Chakravarti, N.; Lee, D.A. TGFbeta Imprinting During Activation Promotes Natural Killer Cell Cytokine Hypersecretion. Cancers 2018, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Regis, S.; Dondero, A.; Caliendo, F.; Bottino, C.; Castriconi, R. NK Cell Function Regulation by TGF-beta-Induced Epigenetic Mechanisms. Front. Immunol. 2020, 11, 311. [Google Scholar] [CrossRef]
- Laskarin, G.; Redzovic, A.; Vukelic, P.; Veljkovic, D.; Gulic, T.; Haller, H.; Rukavina, D. Phenotype of NK cells and cytotoxic/apoptotic mediators expression in ectopic pregnancy. Am. J. Reprod. Immunol. 2010, 64, 347–358. [Google Scholar] [CrossRef]
- Bai, X.; Williams, J.L.; Greenwood, S.L.; Baker, P.N.; Aplin, J.D.; Crocker, I.P. A placental protective role for trophoblast-derived TNF-related apoptosis-inducing ligand (TRAIL). Placenta 2009, 30, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.J.; Harris, L.K.; Freeman, A.; Baker, P.N.; Aplin, J.D.; Whitley, G.S.; Cartwright, J.E. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ. Res. 2007, 100, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.A.; Ni, J.; Pan, G.; Ruben, S.M.; Wei, Y.F.; Pace, J.L.; Hunt, J.S. TRAIL (Apo-2L) and TRAIL receptors in human placentas: Implications for immune privilege. J. Immunol. 1999, 162, 6053–6059. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Chen, X.M.; Liu, X.Q.; He, J.L.; Feng, Q.; Lan, X.; Zhang, X.; Geng, Y.Q.; Wang, Y.X.; Ding, Y.B. Activation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor gene expression following DNA demethylation in placental choriocarcinoma and transformed cell lines. Reprod. Fertil. Dev. 2015, 28, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Han, Y.; Chen, J.H.; Yao, Y.Q. Down-regulation of HLA-G boosted natural killer cell-mediated cytolysis in JEG-3 cells cultured in vitro. Fertil. Steril. 2008, 90, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Ortega, J.; Zarate, A.; Saucedo, R.; Hernandez-Valencia, M.; Cruz, J.G.; Puello, E. Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol. Obstet. Investig. 2019, 84, 12–19. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, V.A.; Sokolov, D.I.; Grebenkina, P.V.; Bazhenov, D.O.; Nikolaenkov, I.P.; Kogan, I.Y.; Totolian, A.A. Apoptotic Receptors and CD107a Expression by NK Cells in an Interaction Model with Trophoblast Cells. Curr. Issues Mol. Biol. 2024, 46, 8945-8957. https://doi.org/10.3390/cimb46080528
Mikhailova VA, Sokolov DI, Grebenkina PV, Bazhenov DO, Nikolaenkov IP, Kogan IY, Totolian AA. Apoptotic Receptors and CD107a Expression by NK Cells in an Interaction Model with Trophoblast Cells. Current Issues in Molecular Biology. 2024; 46(8):8945-8957. https://doi.org/10.3390/cimb46080528
Chicago/Turabian StyleMikhailova, Valentina A., Dmitry I. Sokolov, Polina V. Grebenkina, Dmitry O. Bazhenov, Igor P. Nikolaenkov, Igor Yu. Kogan, and Areg A. Totolian. 2024. "Apoptotic Receptors and CD107a Expression by NK Cells in an Interaction Model with Trophoblast Cells" Current Issues in Molecular Biology 46, no. 8: 8945-8957. https://doi.org/10.3390/cimb46080528





