Green Tea Polyphenol (-)-Epicatechin Pretreatment Mitigates Hepatic Steatosis in an In Vitro MASLD Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. HepG2 Cell Culturing
2.2. Pretreatment with (-)-Epicatechin and Treatment with OA
2.3. MTS Assay
2.4. ELISA
2.5. Oil Red O Staining
Oil Red O Quantification
2.6. Measuring Intracellular GSH Levels
2.7. Statistical Analysis
3. Results
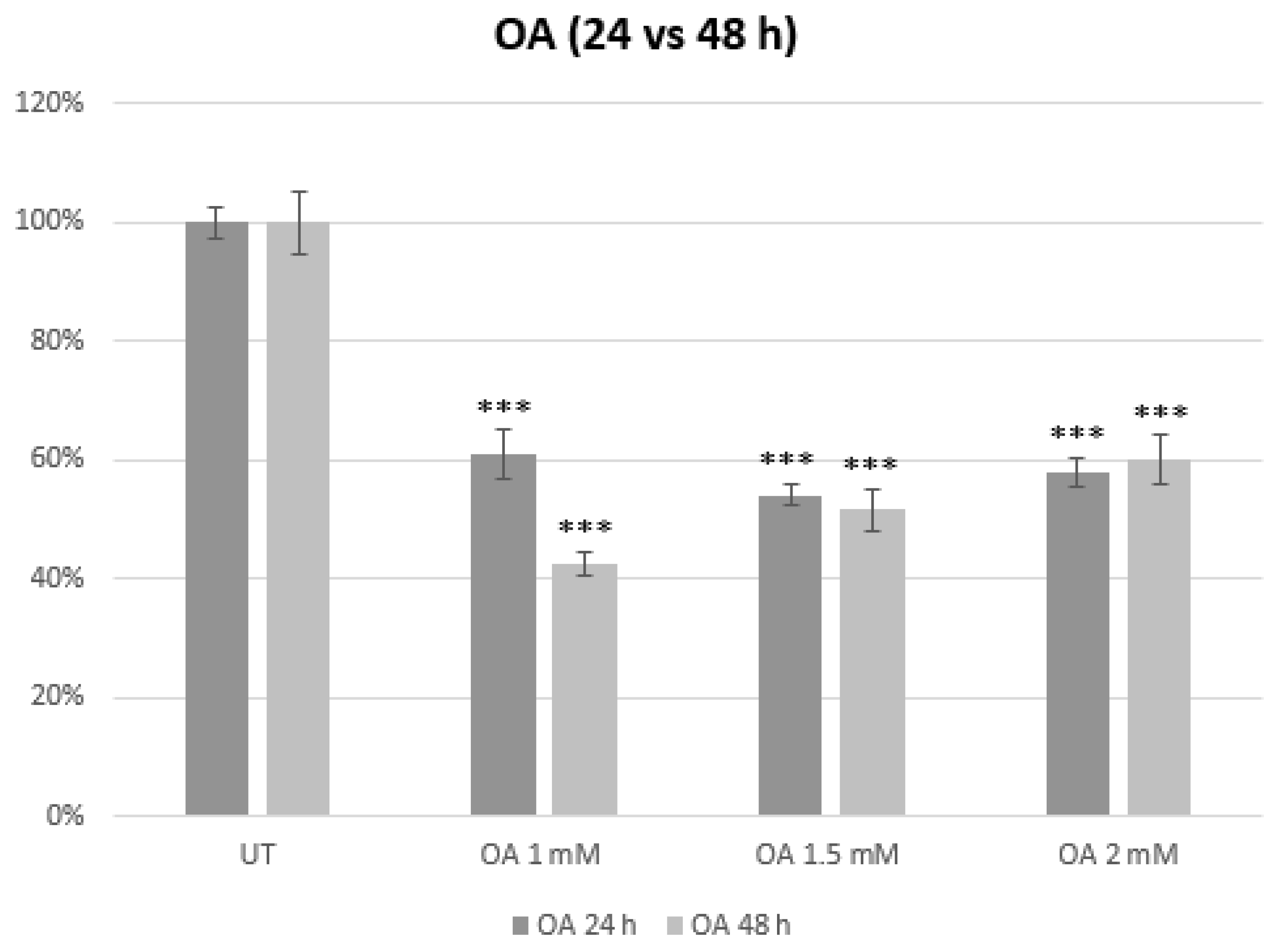
3.1. Establishment of an In Vitro Model of MASLD
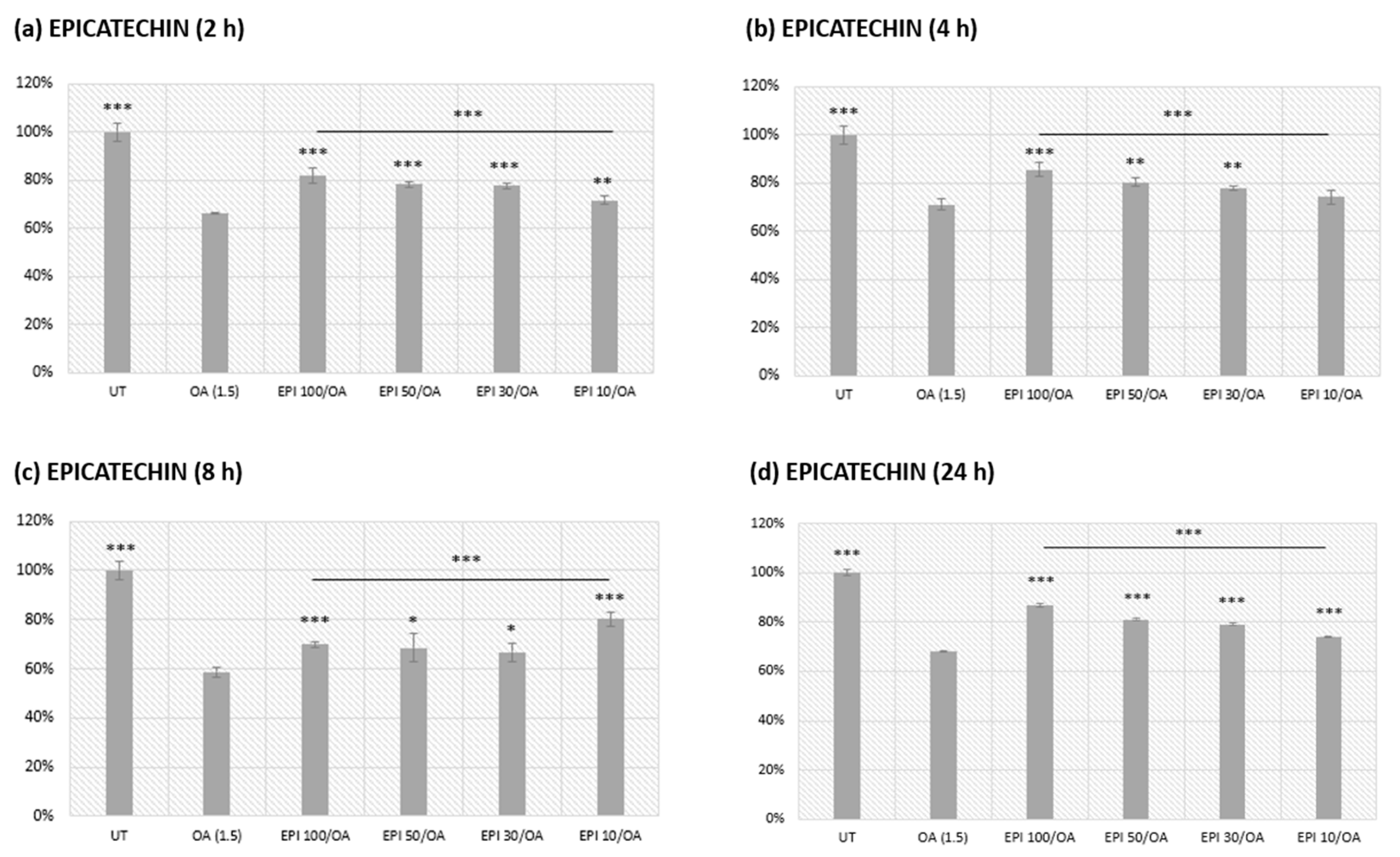
3.2. EPI Pretreatment and Subsequent Oleic Acid Exposure in HepG2 Cells
Assessment of a 4 h EPI Pretreatment Alone on HepG2 Cell Metabolic Viability
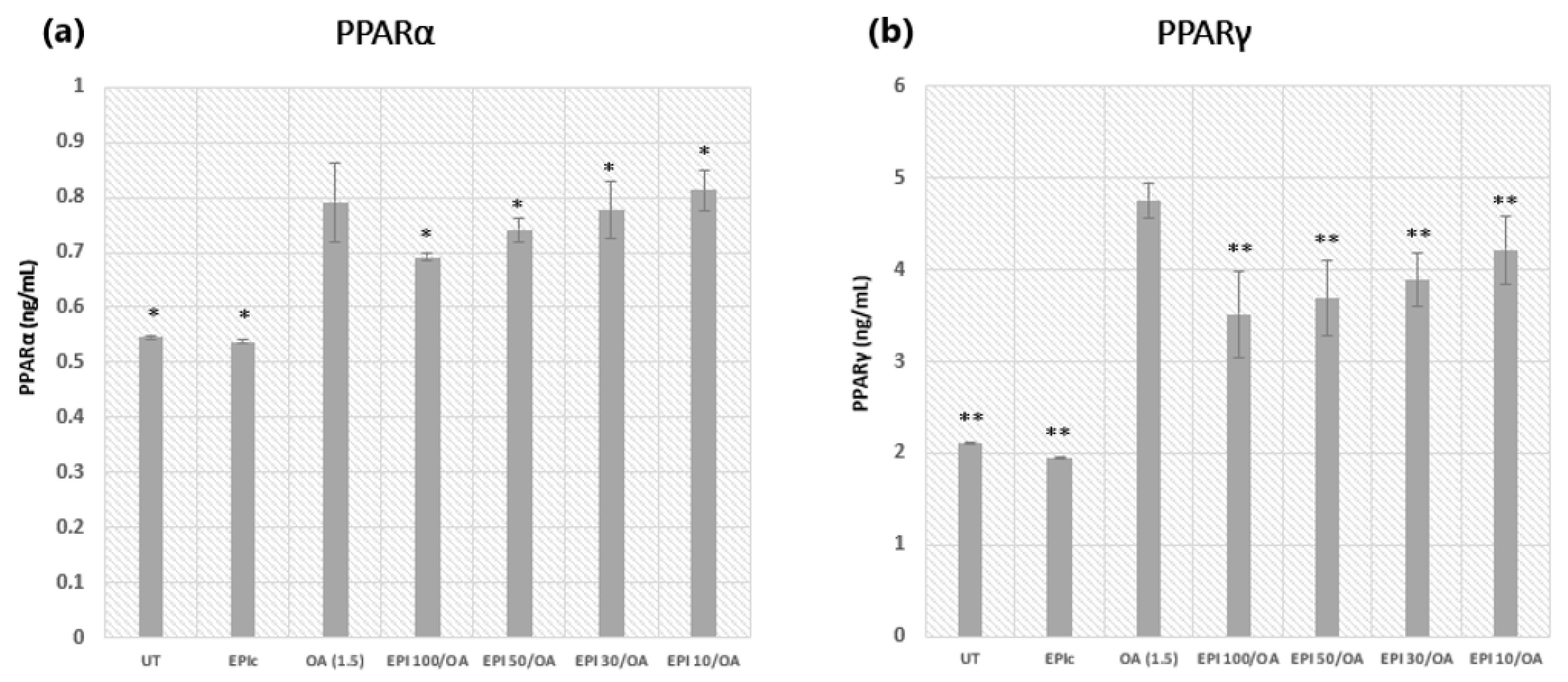
3.3. Peroxisome Proliferator-Activated Receptors: PPARα and PPARγ
3.4. Visualization of Fatty Changes and ORO Quantification in HepG2 Cells
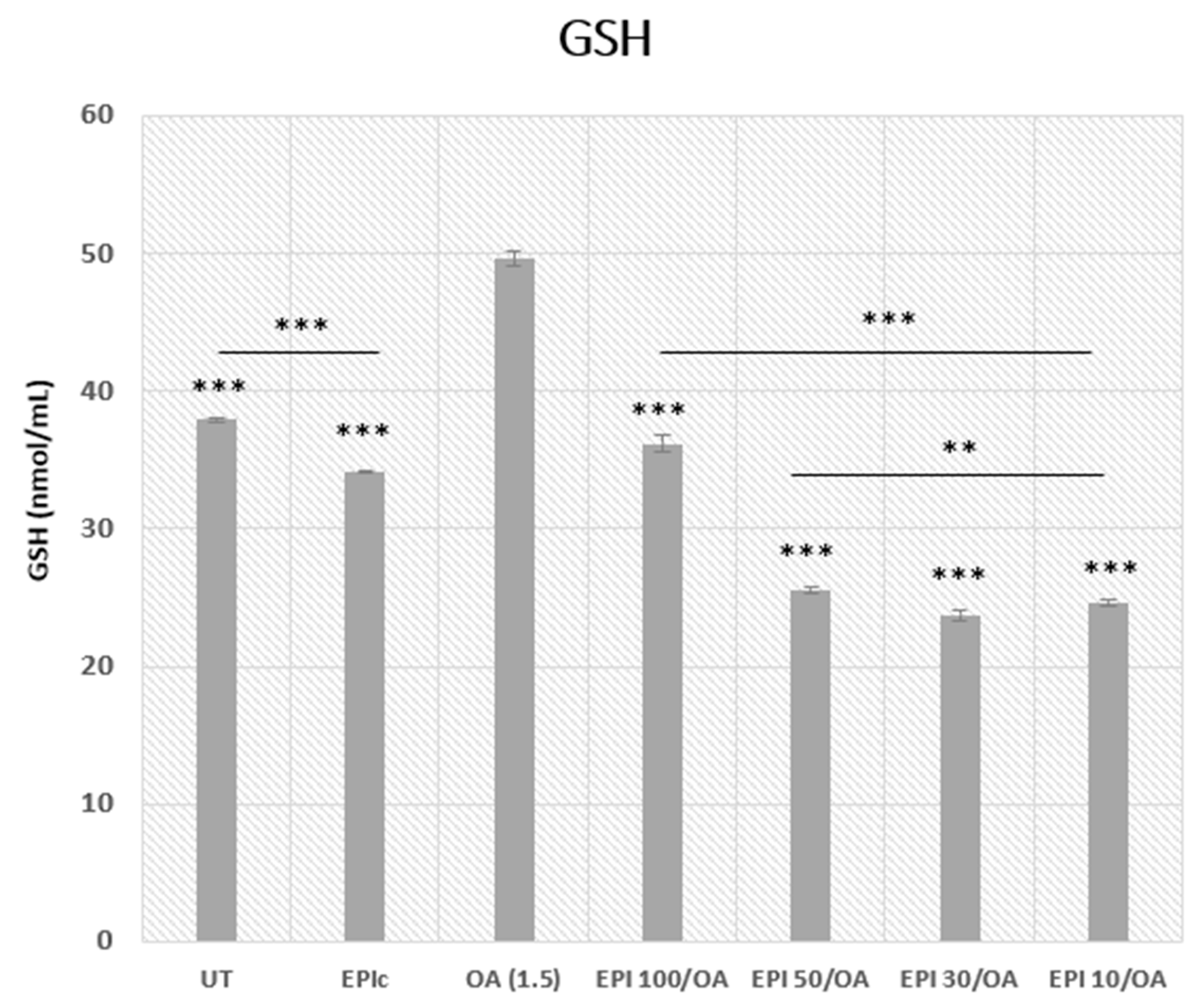
3.5. Intracellular GSH Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Anthony Sinha, R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. (Landmark Ed.) 2021, 26, 206–237. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Kolaric, T.O.; Kuna, L.; Covic, M.; Roguljic, H.; Matic, A.; Sikora, R.; Hefer, M.; Petrovic, A.; Mihaljevic, V.; Smolic, R.; et al. Preclinical Models and Promising Pharmacotherapeutic Strategies in Liver Fibrosis: An Update. Curr. Issues Mol. Biol. 2023, 45, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- Gensluckner, S.; Wernly, B.; Datz, C.; Aigner, E. Iron, Oxidative Stress, and Metabolic Dysfunction-Associated Steatotic Liver Disease. Antioxidants 2024, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Kolaric, T.O.; Nincevic, V.; Kuna, L.; Duspara, K.; Bojanic, K.; Vukadin, S.; Raguz-Lucic, N.; Wu, G.Y.; Smolic, M. Drug-induced Fatty Liver Disease: Pathogenesis and Treatment. J. Clin. Transl. Hepatol. 2021, 9, 731–737. [Google Scholar] [CrossRef]
- Bacil, G.P.; Cogliati, B.; Cardoso, D.R.; Barbisan, L.F.; Romualdo, G.R. Are isothiocyanates and polyphenols from. Food Funct. 2022, 13, 8348–8362. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Lakshmi, T. A Molecular Insight into the Role of Antioxidants in Nonalcoholic Fatty Liver Diseases. Oxid. Med. Cell Longev. 2022, 2022, 9233650. [Google Scholar] [CrossRef]
- Schwärzler, J.; Grabherr, F.; Grander, C.; Adolph, T.E.; Tilg, H. The pathophysiology of MASLD: An immunometabolic perspective. Expert. Rev. Clin. Immunol. 2024, 20, 375–386. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Gopal, T.; Kalari Kandy, R.R.; Boopathy, L.K.; Perumal, S.K.; Ganesan, M.; Rasineni, K.; Donohue, T.M.; Osna, N.A.; Kharbanda, K.K. Mitochondrial Dysfunction-Associated Mechanisms in the Development of Chronic Liver Diseases. Biology 2023, 12, 1311. [Google Scholar] [CrossRef]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Pettinelli, P.; Del Pozo, T.; Araya, J.; Rodrigo, R.; Araya, A.V.; Smok, G.; Csendes, A.; Gutierrez, L.; Rojas, J.; Korn, O.; et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: Correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim. Biophys. Acta 2009, 1792, 1080–1086. [Google Scholar] [CrossRef]
- Poornima, M.S.; Sindhu, G.; Billu, A.; Sruthi, C.R.; Nisha, P.; Gogoi, P.; Baishya, G.; Raghu, K.G. Pretreatment of hydroethanolic extract of Dillenia indica L. attenuates oleic acid induced NAFLD in HepG2 cells via modulating SIRT-1/p-LKB-1/AMPK, HMGCR & PPAR-α signaling pathways. J. Ethnopharmacol. 2022, 292, 115237. [Google Scholar]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef]
- Liss, K.H.; Finck, B.N. PPARs and nonalcoholic fatty liver disease. Biochimie 2017, 136, 65–74. [Google Scholar] [CrossRef]
- Tailleux, A.; Wouters, K.; Staels, B. Roles of PPARs in NAFLD: Potential therapeutic targets. Biochim. Biophys. Acta 2012, 1821, 809–818. [Google Scholar] [CrossRef]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Azzam, H.; Malnick, S. Non-alcoholic fatty liver disease-the heart of the matter. World J. Hepatol. 2015, 7, 1369–1376. [Google Scholar] [CrossRef]
- Mattson, M.P. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp. Gerontol. 2009, 44, 625–633. [Google Scholar] [CrossRef]
- Sokal-Dembowska, A.; Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Can Nutraceuticals Support the Treatment of MASLD/MASH, and thus Affect the Process of Liver Fibrosis? Int. J. Mol. Sci. 2024, 25, 5238. [Google Scholar] [CrossRef]
- Yang, Z.; Song, S.; Li, L.; Yuan, Z.; Li, Y. Association between the composite dietary antioxidant index and metabolic dysfunction-associated steatotic liver disease in adults: A cross-sectional study from NHANES 2017–2020. Sci. Rep. 2024, 14, 13801. [Google Scholar] [CrossRef]
- Chen, J.; Jian, L.; Guo, Y.; Tang, C.; Huang, Z.; Gao, J. Liver Cell Mitophagy in Metabolic Dysfunction-Associated Steatotic Liver Disease and Liver Fibrosis. Antioxidants 2024, 13, 729. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Mei, G.; Chen, H.; Peng, S.; Zhao, Y.; Yao, P.; Tang, Y. Quercetin and non-alcoholic fatty liver disease: A review based on experimental data and bioinformatic analysis. Food Chem. Toxicol. 2021, 154, 112314. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, T.; Li, J.; Wang, S.; Qiu, F.; Yu, H.; Zhang, Y. Effects of Natural Products on Fructose-Induced Nonalcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 9, 96. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef]
- Lin, J.K.; Lin-Shiau, S.Y. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol. Nutr. Food Res. 2006, 50, 211–217. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Q.; Liu, L.; Hu, Y.Y.; Feng, Q. Potential Biological Effects of (-)-Epigallocatechin-3-gallate on the Treatment of Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2018, 62, 1700483. [Google Scholar] [CrossRef]
- Masterjohn, C.; Bruno, R.S. Therapeutic potential of green tea in nonalcoholic fatty liver disease. Nutr. Rev. 2012, 70, 41–56. [Google Scholar] [CrossRef]
- Cremonini, E.; Iglesias, D.E.; Kang, J.; Lombardo, G.E.; Mostofinejad, Z.; Wang, Z.; Zhu, W.; Oteiza, P.I. (−)-Epicatechin and the comorbidities of obesity. Arch. Biochem. Biophys. 2020, 690, 108505. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Kasprzak, P.; Kondracka, A.; Oniszczuk, T.; Rusinek, A.; Oniszczuk, A. Does Resveratrol Improve Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)? Int. J. Mol. Sci. 2024, 25, 3746. [Google Scholar] [CrossRef]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, metabolism, distribution and excretion of (-)-epicatechin: A review of recent findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar]
- Barnett, C.F.; Moreno-Ulloa, A.; Shiva, S.; Ramirez-Sanchez, I.; Taub, P.R.; Su, Y.; Ceballos, G.; Dugar, S.; Schreiner, G.; Villarreal, F. Pharmacokinetic, partial pharmacodynamic and initial safety analysis of (-)-epicatechin in healthy volunteers. Food Funct. 2015, 6, 824–833. [Google Scholar] [CrossRef]
- Alkinani, K.B.; Ali, E.M.M.; Al-Shaikh, T.M.; Awlia Khan, J.A.; Al-Naomasi, T.M.; Ali, S.S.; Abduljawad, A.A.; Mosa, O.F.; Zafar, T.A. Hepatoprotective Effects of (-) Epicatechin in CCl. Evid. Based Complement. Altern. Med. 2021, 2021, 4655150. [Google Scholar]
- Hid, E.J.; Mosele, J.I.; Prince, P.D.; Fraga, C.G.; Galleano, M. (-)-Epicatechin and cardiometabolic risk factors: A focus on potential mechanisms of action. Pflug. Arch. 2022, 474, 99–115. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults-Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Sikkeland, J.; Jin, Y.; Saatcioglu, F. Methods to assess lipid accumulation in cancer cells. Methods Enzymol. 2014, 542, 407–423. [Google Scholar] [PubMed]
- Omanovic Kolaric, T.; Kizivat, T.; Mihaljevic, V.; Zjalic, M.; Bilic-Curcic, I.; Kuna, L.; Smolic, R.; Vcev, A.; Wu, G.Y.; Smolic, M. Liraglutide Exerts Protective Effects by Downregulation of PPARγ, ACSL1 and SREBP-1c in Huh7 Cell Culture Models of Non-Alcoholic Steatosis and Drug-Induced Steatosis. Curr. Issues Mol. Biol. 2022, 44, 3465–3480. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary Polyphenols Protect Against Oleic Acid-Induced Steatosis in an in Vitro Model of NAFLD by Modulating Lipid Metabolism and Improving Mitochondrial Function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Hajighasem, A.; Farzanegi, P.; Mazaheri, Z. Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2019, 125, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Layman, J.I.; Pereira, D.L.; Chellan, N.; Huisamen, B.; Kotzé, S.H. A histomorphometric study on the hepatoprotective effects of a green rooibos extract in a diet-induced obese rat model. Acta Histochem. 2019, 121, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sozio, M.S.; Lu, C.; Zeng, Y.; Liangpunsakul, S.; Crabb, D.W. Activated AMPK inhibits PPAR-{alpha} and PPAR-{gamma} transcriptional activity in hepatoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G739–G747. [Google Scholar] [CrossRef]
- Sotoudeheian, M.; Hoseini, S.; Mirahmadi, S.-M.-S. Therapeutic Properties of Polyphenols Affect AMPK Molecular Pathway in Hyperlipidemia. Preprints 2023, 2023010528. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK signaling by polyphenols: A novel strategy for tackling aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef]
- Cooreman, M.P.; Vonghia, L.; Francque, S.M. MASLD/MASH and type 2 diabetes: Two sides of the same coin? From single PPAR to pan-PPAR agonists. Diabetes Res. Clin. Pract. 2024, 212, 111688. [Google Scholar] [CrossRef]
- Boliaki, N.; Henin, G.; Bale, G.; Lanthier, N. Impact of Peroxisome Proliferator-Activated Receptor Agonists on Myosteatosis in the Context of Metabolic Dysfunction-Associated Steatotic Liver Disease. Discov. Med. 2024, 36, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Bustamante, A.; Sambra, V.; Álvarez, D.; Videla, L.; Valenzuela, R. Beneficial Effects of Dietary Polyphenols in the Prevention and Treatment of NAFLD: Cell-Signaling Pathways Underlying Health Effects. Curr. Med. Chem. 2022, 29, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, D.; Zeng, R.; Xian, T.; Lu, Y.; Zeng, G.; Sun, Z.; Huang, B.; Huang, Q. Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. Eur. J. Pharmacol. 2017, 795, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, B.; Chen, L.; Jiang, H. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. J. Transl. Med. 2023, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Guo, X.; Zhu, N.; Niu, L.; Ding, X.; Xie, Z.; Chen, X.; Yang, F. Oleic Acid and Eicosapentaenoic Acid Reverse Palmitic Acid-induced Insulin Resistance in Human HepG2 Cells via the Reactive Oxygen Species/JUN Pathway. Genom. Proteom. Bioinform. 2021, 19, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Zeigerer, A.; Kory, N.; Krahmer, N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020, 108, 72–81. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.C.; Amankwa-Sakyi, M.; Flynn, T.J. Cellular glutathione in fatty liver in vitro models. Toxicol. In Vitro 2011, 25, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport. Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Malfa, G.A.; Tomasello, B.; Bianchi, S.; Acquaviva, R. Natural Compounds and Glutathione: Beyond Mere Antioxidants. Antioxidants 2023, 12, 1445. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 367–398. [Google Scholar] [CrossRef] [PubMed]
- Mohs, A.; Otto, T.; Schneider, K.M.; Peltzer, M.; Boekschoten, M.; Holland, C.H.; Hudert, C.A.; Kalveram, L.; Wiegand, S.; Saez-Rodriguez, J.; et al. Hepatocyte-specific NRF2 activation controls fibrogenesis and carcinogenesis in steatohepatitis. J. Hepatol. 2021, 74, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Dey, A.; Sen, R.; Chatterjee, M.; Chattopadhyay, S.; Bandyopadhyay, S.K. Intracellular GSH depletion triggered mitochondrial Bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J. Pharmacol. Exp. Ther. 2011, 336, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, H.A.; Mahbub, A.A.; Le Maitre, C.L.; Jordan-Mahy, N. Molecular Action of Polyphenols in Leukaemia and Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 3085. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.E.; Choi-Kwon, S.; Park, K.A.; Lee, H.J.; Park, M.S.; Lee, J.H.; Kwon, S.B.; Park, K.C. Dietary supplementation of (+)-catechin protects against UVB-induced skin damage by modulating antioxidant enzyme activities. Photodermatol. Photoimmunol. Photomed. 2003, 19, 235–241. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: Protection against oxidative and electrophilic injury. Eur. J. Pharmacol. 2004, 489, 39–48. [Google Scholar] [CrossRef]
- Kachadourian, R.; Day, B.J. Flavonoid-induced glutathione depletion: Potential implications for cancer treatment. Free Radic. Biol. Med. 2006, 41, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, O.; Galati, G.; Moridani, M.Y.; Siraki, A.; O’Brien, P.J. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem. Biol. Interact. 2004, 148, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B. Endocrine disrupting chemicals and metabolic disorders in the liver: What if we also looked at the female side? Chemosphere 2021, 268, 129212. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefer, M.; Petrovic, A.; Roguljic, L.K.; Kolaric, T.O.; Kizivat, T.; Wu, C.H.; Tabll, A.A.; Smolic, R.; Vcev, A.; Smolic, M. Green Tea Polyphenol (-)-Epicatechin Pretreatment Mitigates Hepatic Steatosis in an In Vitro MASLD Model. Curr. Issues Mol. Biol. 2024, 46, 8981-8994. https://doi.org/10.3390/cimb46080531
Hefer M, Petrovic A, Roguljic LK, Kolaric TO, Kizivat T, Wu CH, Tabll AA, Smolic R, Vcev A, Smolic M. Green Tea Polyphenol (-)-Epicatechin Pretreatment Mitigates Hepatic Steatosis in an In Vitro MASLD Model. Current Issues in Molecular Biology. 2024; 46(8):8981-8994. https://doi.org/10.3390/cimb46080531
Chicago/Turabian StyleHefer, Marija, Ana Petrovic, Lucija Kuna Roguljic, Tea Omanovic Kolaric, Tomislav Kizivat, Catherine H. Wu, Ashraf A. Tabll, Robert Smolic, Aleksandar Vcev, and Martina Smolic. 2024. "Green Tea Polyphenol (-)-Epicatechin Pretreatment Mitigates Hepatic Steatosis in an In Vitro MASLD Model" Current Issues in Molecular Biology 46, no. 8: 8981-8994. https://doi.org/10.3390/cimb46080531





