Efficacy of Vitamin B12 and Adenosine Triphosphate in Enhancing Skin Radiance: Unveiled with a Drug–Target Interaction Deep Learning-Based Model
Abstract
1. Introduction
2. Materials and Methods
2.1. MT–DTI Model for Hit Prediction of EDNRB Antagonist and ADIPOR1 Agonist
2.2. Materials
2.3. Cell Culture
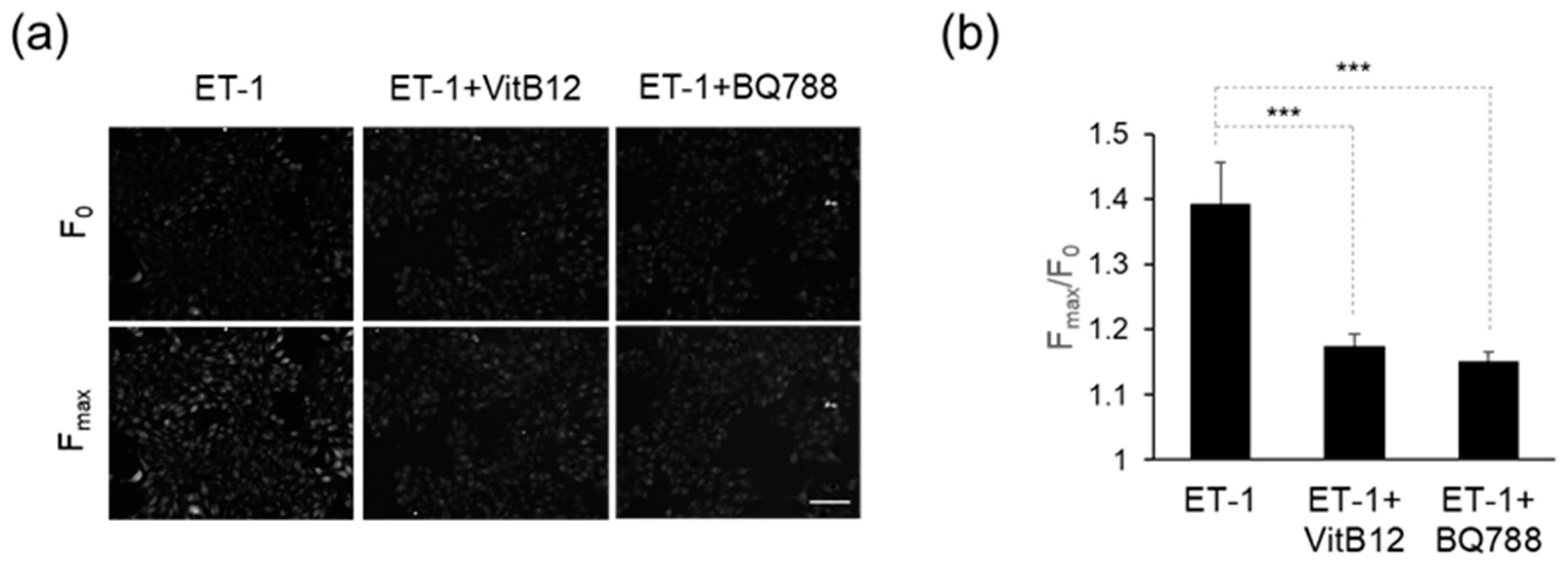
2.4. Intracellular Calcium Measurement
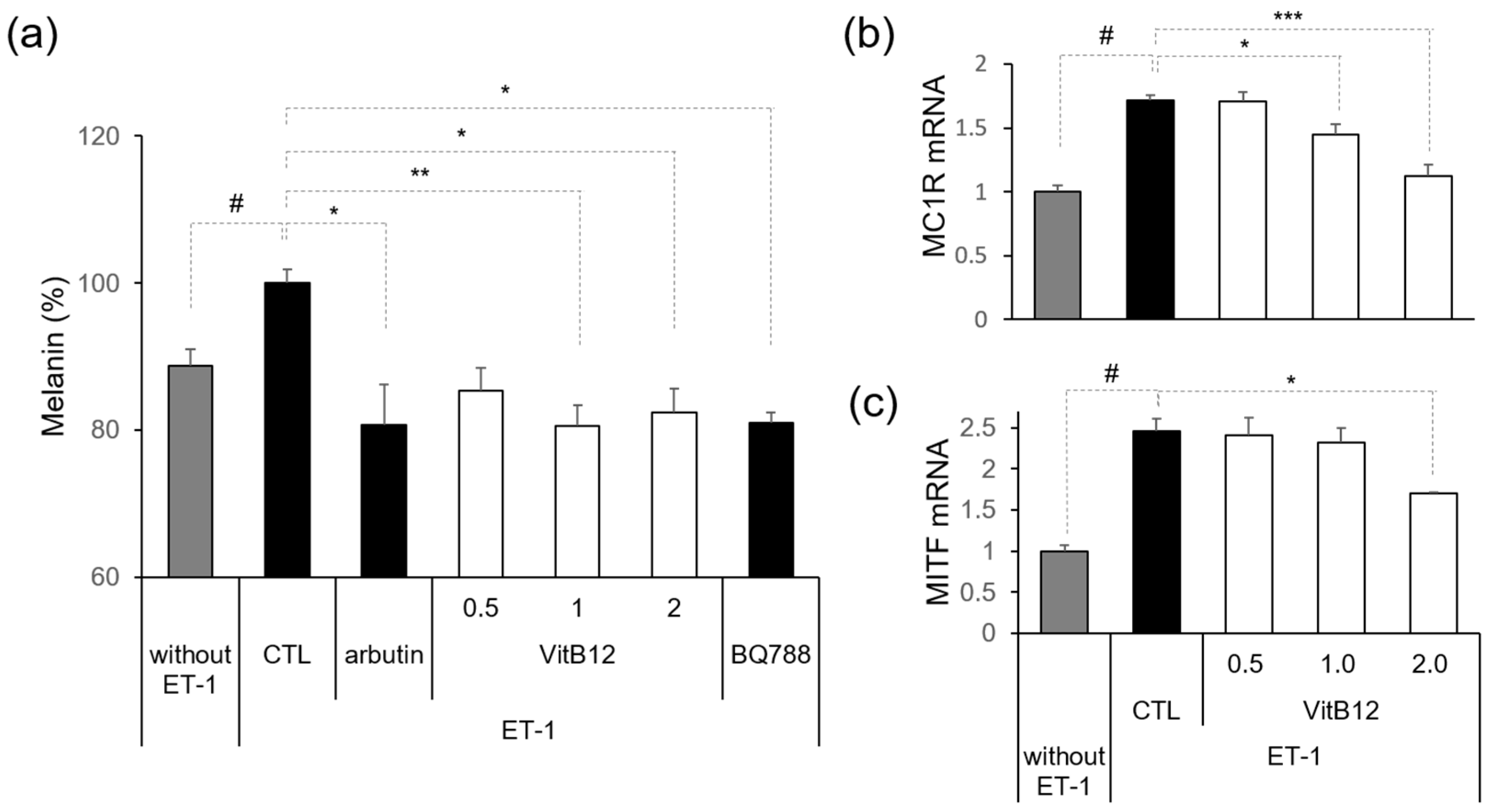
2.5. Melanin Content Assay
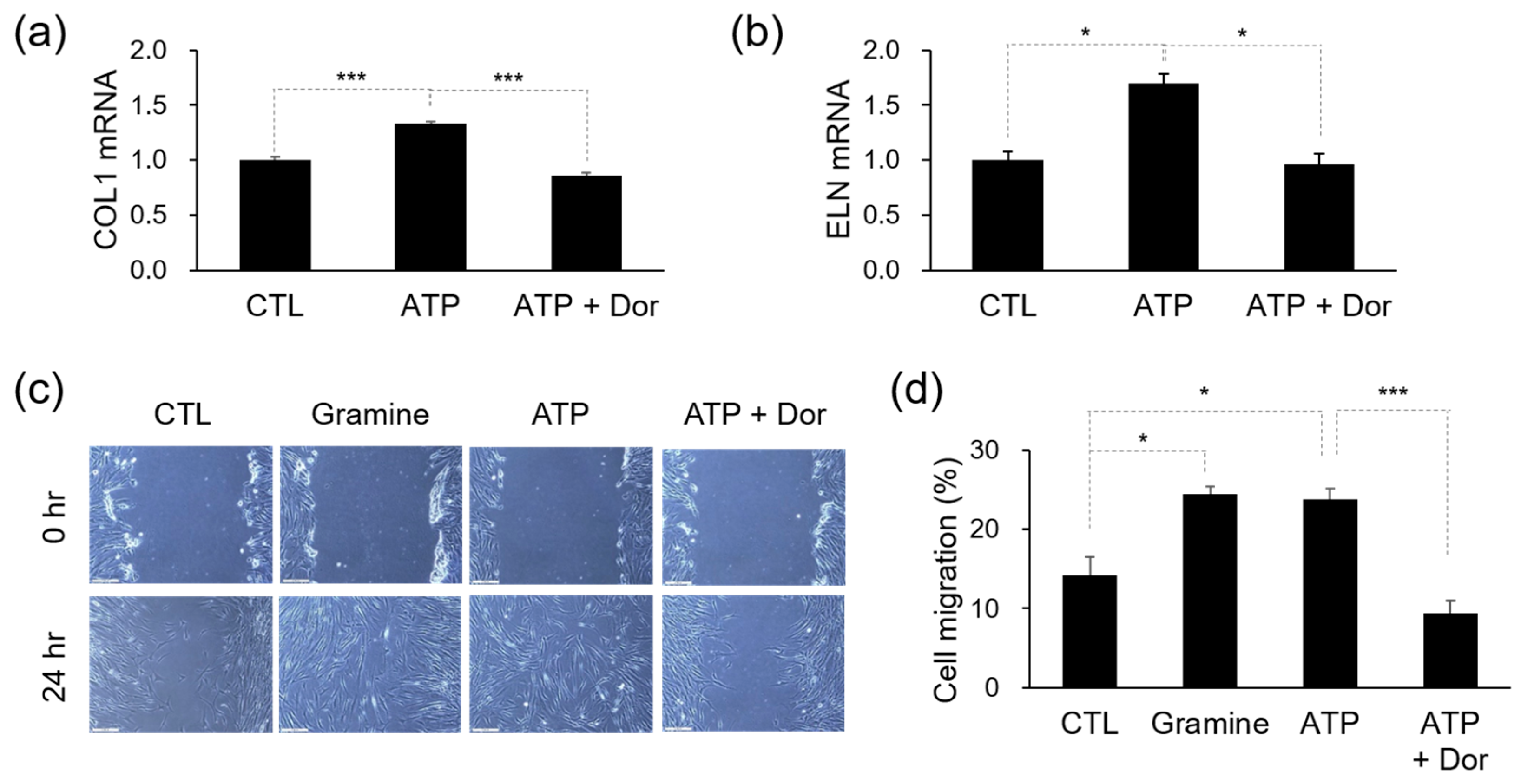
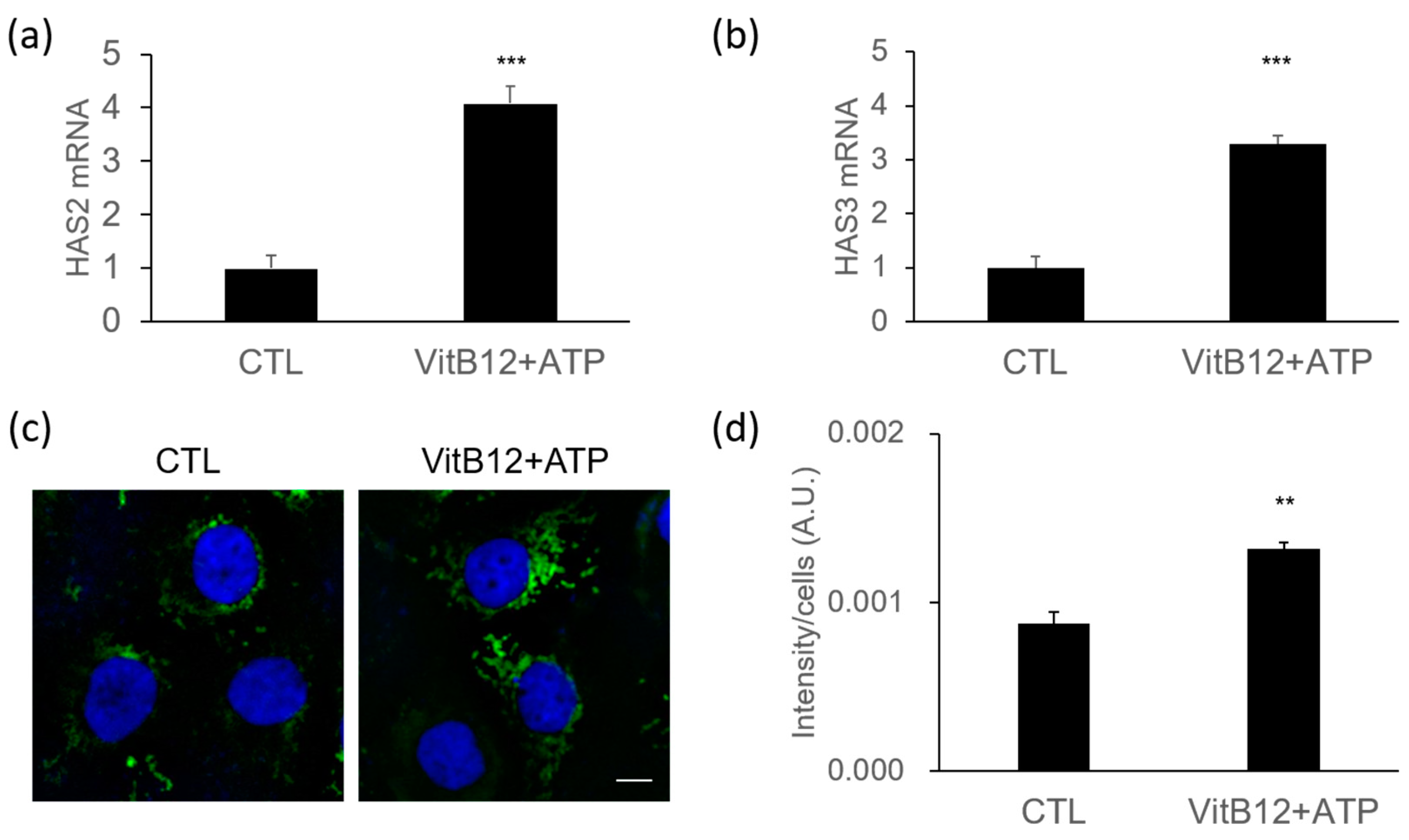
2.6. Isolation of Total RNA and Quantitative PCR (q-PCR)
2.7. Cell Migration Assay
2.8. Immunofluorescence Analysis
2.9. Clinical Studies
2.10. Statistical Analysis
3. Results
3.1. EDNRB Antagonist and ADIPOR Agonist Prediction and Compound Selection
3.2. Efficacy of VitB12 as an Endothelin Receptor Antagonist
3.3. Efficacy of ATP in Skin Regeneration
3.4. Increase of Hyaluronic Acid Synthases by a Complex of VitB12 and ATP
3.5. Skin Enhancement by the VitB12 and ATP Complex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirao, N.; Koizumi, K.; Ikeda, H.; Ohira, H. Reliability of Online Surveys in Investigating Perceptions and Impressions of Faces. Front. Psychol. 2021, 12, 733405. [Google Scholar] [CrossRef]
- Flament, F.; Abric, A.; Adam, A.S. Evaluating the respective weights of some facial signs on perceived ages in differently aged women of five ethnic origins. J. Cosmet. Dermatol. 2021, 20, 842–853. [Google Scholar] [CrossRef]
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to manage facial hyperpigmentation in skin of colour. Drugs Context 2022, 11, 2. [Google Scholar] [CrossRef]
- Saldana-Caboverde, A.; Kos, L. Roles of endothelin signaling in melanocyte development and melanoma. Pigment. Cell Melanoma Res. 2010, 23, 160–170. [Google Scholar] [CrossRef]
- Murase, D.; Hachiya, A.; Kikuchi-Onoe, M.; Fullenkamp, R.; Ohuchi, A.; Kitahara, T.; Moriwaki, S.; Hase, T.; Takema, Y. Cooperation of endothelin-1 signaling with melanosomes plays a role in developing and/or maintaining human skin hyperpigmentation. Biol. Open 2015, 4, 1213–1221. [Google Scholar] [CrossRef]
- Imokawa, G.; Kobayashi, T.; Miyagishi, M.; Higashi, K.; Yada, Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997, 10, 218–228. [Google Scholar] [CrossRef]
- Regazzetti, C.; De Donatis, G.M.; Ghorbel, H.H.; Cardot-Leccia, N.; Ambrosetti, D.; Bahadoran, P.; Chignon-Sicard, B.; Lacour, J.P.; Ballotti, R.; Mahns, A.; et al. Endothelial Cells Promote Pigmentation through Endothelin Receptor B Activation. J. Investig. Dermatol. 2015, 135, 3096–3104. [Google Scholar] [CrossRef]
- Hara, Y.; Shibata, T. Characteristics of dermal vascularity in melasma and solar lentigo. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12953. [Google Scholar] [CrossRef] [PubMed]
- Sjerobabski-Masnec, I.; Situm, M. Skin aging. Acta Clin. Croat 2010, 49, 515–518. [Google Scholar] [PubMed]
- Ezure, T.; Amano, S. Adiponectin and leptin up-regulate extracellular matrix production by dermal fibroblasts. Biofactors 2007, 31, 229–236. [Google Scholar] [CrossRef]
- Akazawa, Y.; Sayo, T.; Sugiyama, Y.; Sato, T.; Akimoto, N.; Ito, A.; Inoue, S. Adiponectin resides in mouse skin and upregulates hyaluronan synthesis in dermal fibroblasts. Connect. Tissue Res. 2011, 52, 322–328. [Google Scholar] [CrossRef]
- Oh, J.; Lee, Y.; Oh, S.W.; Li, T.; Shin, J.; Park, S.H.; Lee, J. The Role of Adiponectin in the Skin. Biomol. Ther. 2022, 30, 221–231. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, M.K.; Kim, S.; Kim, J.Y.; Lee, D.H.; Chung, J.H. UV-induced inhibition of adipokine production in subcutaneous fat aggravates dermal matrix degradation in human skin. Sci. Rep. 2016, 6, 25616. [Google Scholar] [CrossRef]
- Vatiwutipong, P.; Vachmanus, S.; Noraset, T.; Tuarob, S. Artificial Intelligence in Cosmetic Dermatology: A Systematic Literature Review. IEEE Access 2023, 11, 71407–71425. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, H.; Lee, Y.J.; Kim, T.-Y.; Bahn, G.; Kim, Y.-h.; Lim, J.-M.; Park, S.-W.; Song, Y.-S.; Kim, M.-S. Drug–Target Interaction Deep Learning-Based Model Identifies the Flavonoid Troxerutin as a Candidate TRPV1 Antagonist. Appl. Sci. 2023, 13, 5617. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Li, Y.C.; Chen, C.R.; Young, T.H. Pearl extract enhances the migratory ability of fibroblasts in a wound healing model. Pharm. Biol. 2013, 51, 289–297. [Google Scholar] [CrossRef]
- Bianchini, J.M.; Zhang, Q.; Hanna, G.; Flach, C.R.; Wang, H.; Southall, M.D.; Mendelsohn, R.; Randhawa, M. A unique gel matrix moisturizer delivers deep hydration resulting in significant clinical improvement in radiance and texture. Clin. Cosmet. Investig. Dermatol. 2019, 12, 229–239. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef]
- Grissom, C.B.; Chagovetz, A.M.; Wang, Z. Use of viscosigens to stabilize vitamin B12 solutions against photolysis. J. Pharm. Sci. 1993, 82, 641–643. [Google Scholar] [CrossRef]
- Shin, B.; Park, S.; Kang, K.; Ho, J.C. Self-Attention Based Molecule Representation for Predicting Drug-Target Interaction. In Proceedings of the 4th Machine Learning for Healthcare Conference, Proceedings of Machine Learning Research, Ann Arbor, MI, USA, 9–10 August 2019; pp. 230–248. [Google Scholar]
- Baker, S.J.; Ignatius, M.; Johnson, S.; Vaish, S.K. Hyperpigmentation of skin. A sign of vitamin-B12 deficiency. Br. Med. J. 1963, 1, 1713–1715. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Gopinath, H. Reversible hyperpigmentation of vitamin B12 deficiency. Indian J. Med. Res. 2020, 152, S227–S228. [Google Scholar] [CrossRef]
- Januchowski, R. Evaluation of topical vitamin B(12) for the treatment of childhood eczema. J. Altern. Complement Med. 2009, 15, 387–389. [Google Scholar] [CrossRef]
- Kyawsoewin, M.; Manokawinchoke, J.; Namangkalakul, W.; Egusa, H.; Limraksasin, P.; Osathanon, T. Roles of extracellular adenosine triphosphate on the functions of periodontal ligament cells. BDJ Open 2023, 9, 28. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jinnah, H.A.; Kamatani, N. Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front. Pharmacol. 2019, 10, 98. [Google Scholar] [CrossRef]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E.; Greig, A.V. Purinergic signaling in healthy and diseased skin. J. Investig. Dermatol. 2012, 132, 526–546. [Google Scholar] [CrossRef]
- Taboubi, S.; Milanini, J.; Delamarre, E.; Parat, F.; Garrouste, F.; Pommier, G.; Takasaki, J.; Hubaud, J.C.; Kovacic, H.; Lehmann, M. G alpha(q/11)-coupled P2Y2 nucleotide receptor inhibits human keratinocyte spreading and migration. FASEB J. 2007, 21, 4047–4058. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, H.; Lee, H.; Kim, J.; Yeo, H.; Hyung, K.; Song, D.; Kim, M.; Jun, S.-H.; Kang, N.-G. Efficacy of Vitamin B12 and Adenosine Triphosphate in Enhancing Skin Radiance: Unveiled with a Drug–Target Interaction Deep Learning-Based Model. Curr. Issues Mol. Biol. 2024, 46, 9082-9092. https://doi.org/10.3390/cimb46080537
Chun H, Lee H, Kim J, Yeo H, Hyung K, Song D, Kim M, Jun S-H, Kang N-G. Efficacy of Vitamin B12 and Adenosine Triphosphate in Enhancing Skin Radiance: Unveiled with a Drug–Target Interaction Deep Learning-Based Model. Current Issues in Molecular Biology. 2024; 46(8):9082-9092. https://doi.org/10.3390/cimb46080537
Chicago/Turabian StyleChun, Hyeyeon, Hyejin Lee, Jongwook Kim, Hyerin Yeo, Kyongeun Hyung, Dayoung Song, Moonju Kim, Seung-Hyun Jun, and Nae-Gyu Kang. 2024. "Efficacy of Vitamin B12 and Adenosine Triphosphate in Enhancing Skin Radiance: Unveiled with a Drug–Target Interaction Deep Learning-Based Model" Current Issues in Molecular Biology 46, no. 8: 9082-9092. https://doi.org/10.3390/cimb46080537
APA StyleChun, H., Lee, H., Kim, J., Yeo, H., Hyung, K., Song, D., Kim, M., Jun, S.-H., & Kang, N.-G. (2024). Efficacy of Vitamin B12 and Adenosine Triphosphate in Enhancing Skin Radiance: Unveiled with a Drug–Target Interaction Deep Learning-Based Model. Current Issues in Molecular Biology, 46(8), 9082-9092. https://doi.org/10.3390/cimb46080537


_Kim.png)


