Efficacy of SGPP2 Modulation-Mediated Materials in Ameliorating Facial Wrinkles and Pore Sagging
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Preparation
2.2. Transfection of Keratinocytes with siRNA for RNAi Experiments
2.3. RNA Extraction and RT-qPCR
2.4. Conditioned-Media Experiment
2.5. Reconstructed Three-Dimensional (3D) Skin Experiment
2.6. Human Clinical Trial
2.7. Statistical Analysis
3. Results and Discussion
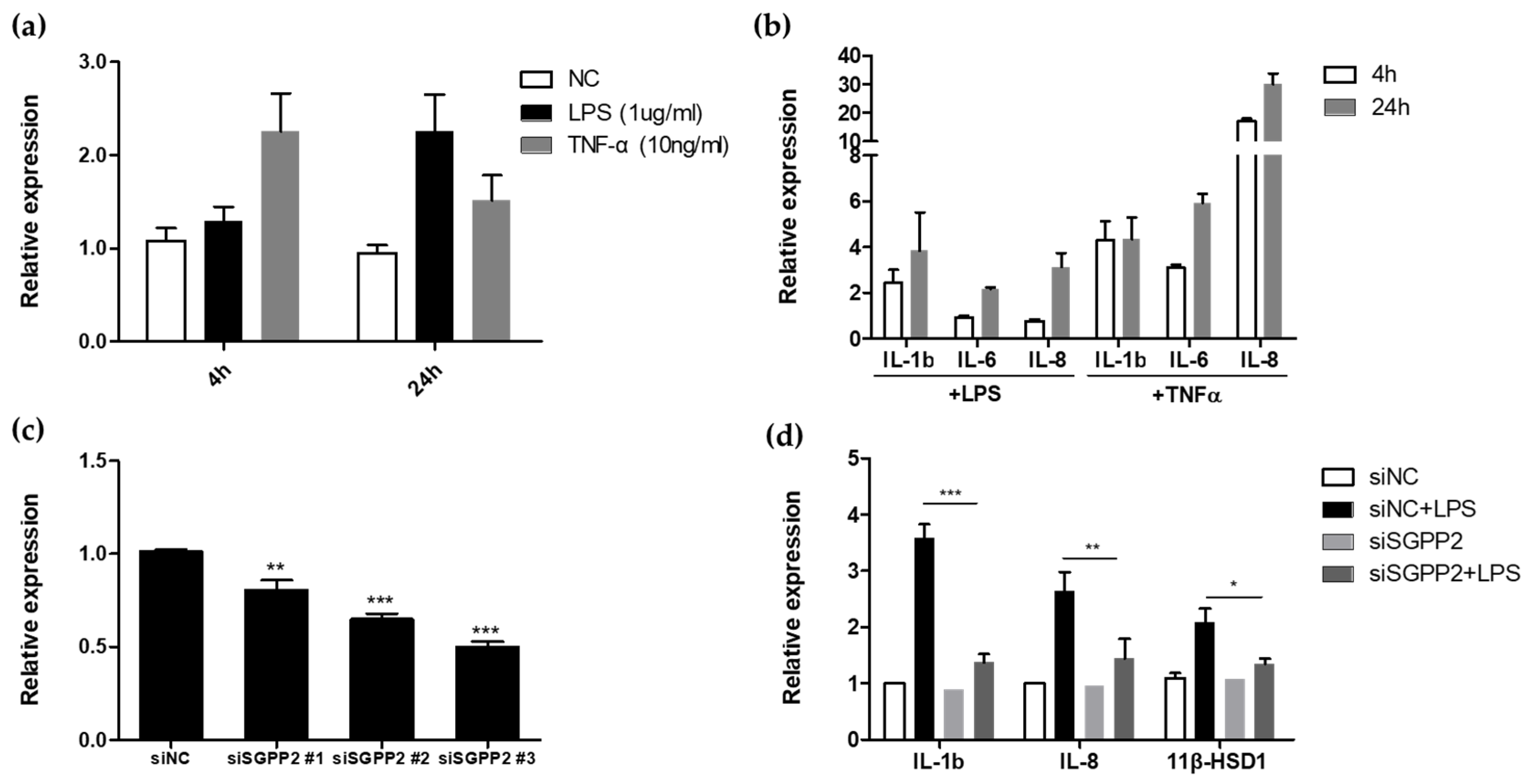
3.1. Functional Study of Wrinkle-Related Gene SGPP2 in HaCaT Cells
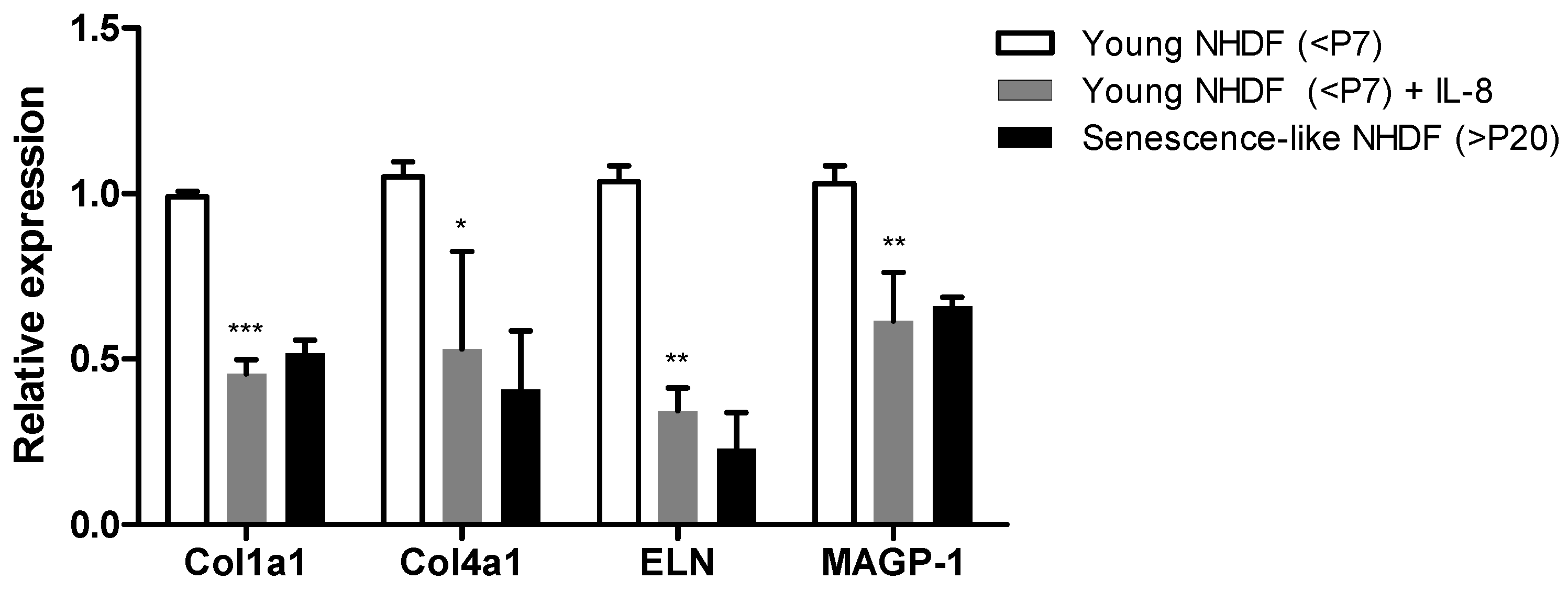
3.2. Inflammation Exacerbates Skin Fibroblast-Mediated Wrinkle Formation
3.3. Screening Active Materials That Regulate SGPP2 Expression
3.4. Antiaging Effect of SGPP2 Expression-Regulating Materials In Vitro
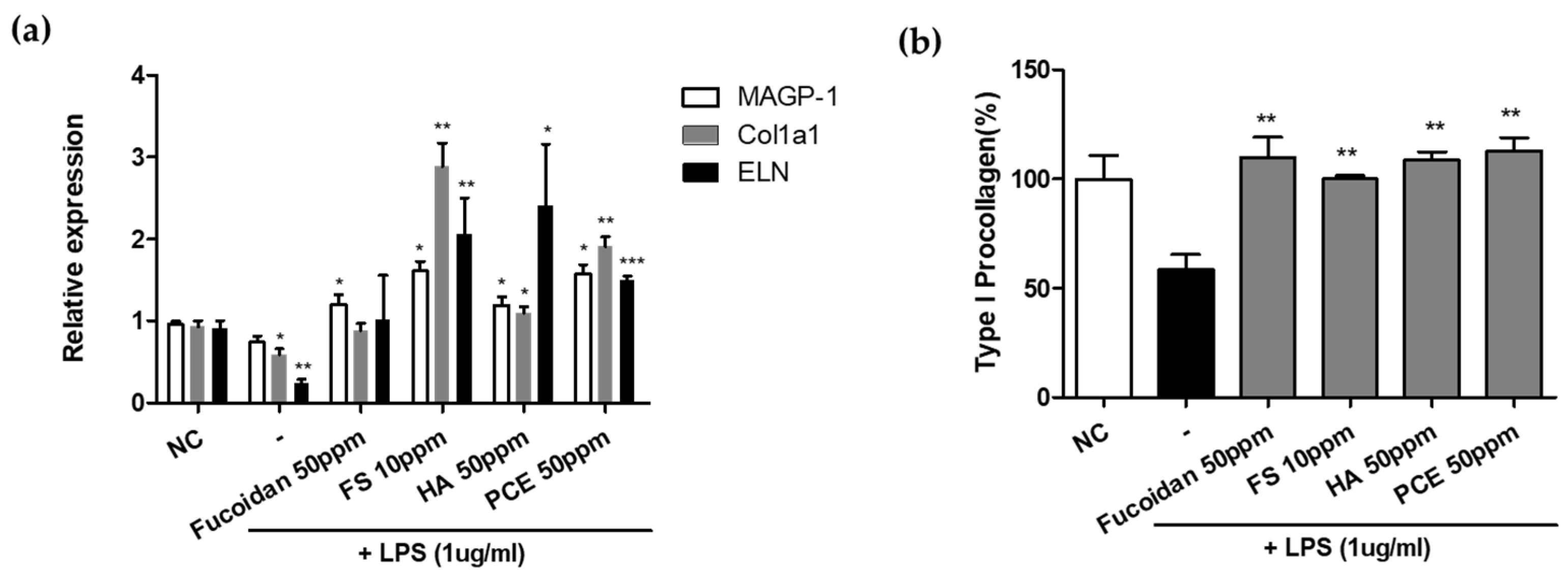
3.4.1. Recovery of Inflammation-Induced Decrease in ECM-Related Gene in NHDFs
3.4.2. Effect of Enhanced Type I Procollagen Synthesis
3.5. Dermal Collagen Enhances the Efficacy of the Selected Materials within 3D Skin Model
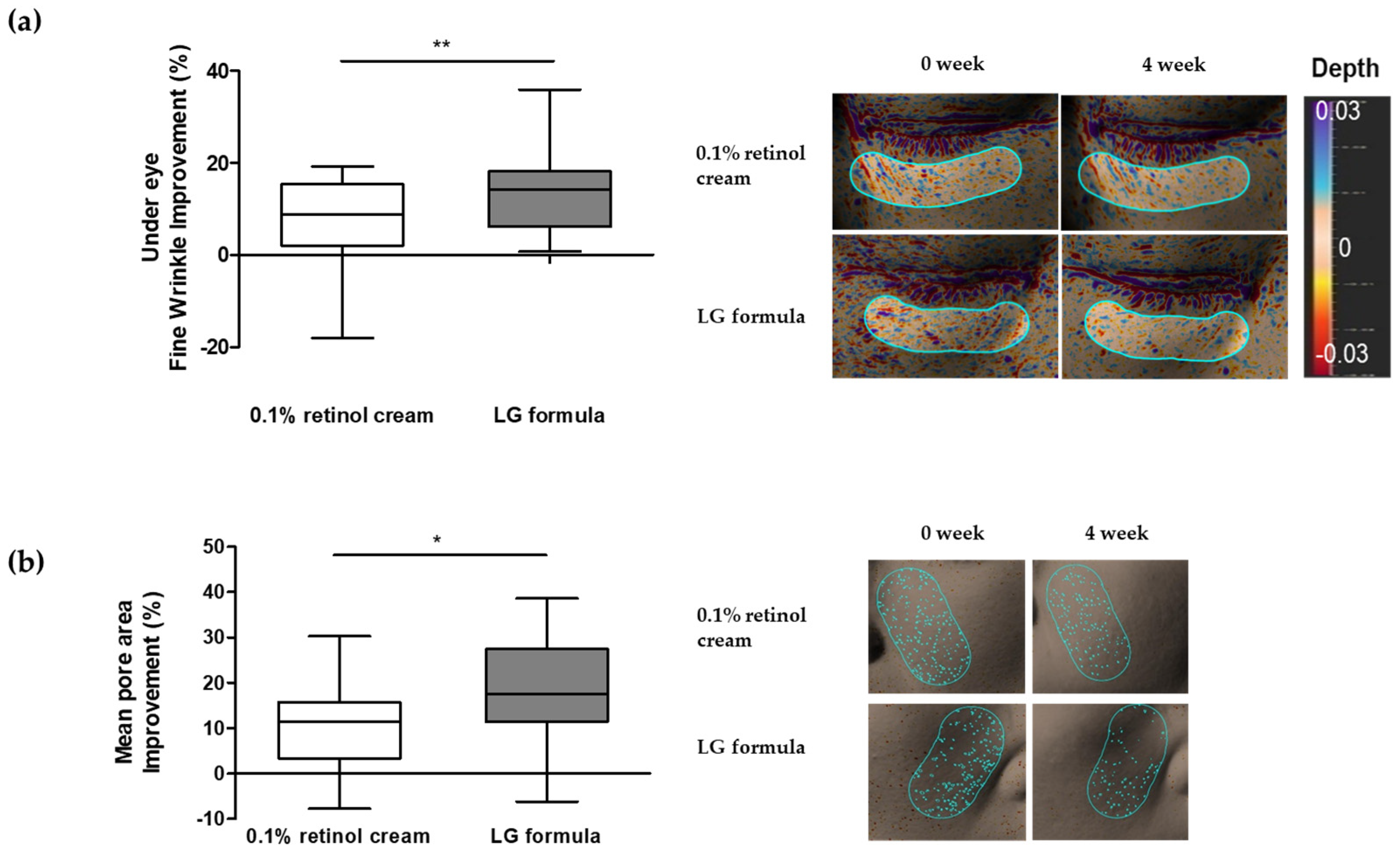
3.6. Improvement in Skin Wrinkles and Pores Using LG Formula-Containing Materials That Modulated SGPP2 Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-kappaB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef]
- Budamagunta, V.; Manohar-Sindhu, S.; Yang, Y.; He, Y.; Traktuev, D.O.; Foster, T.C.; Zhou, D. Senescence-associated hyper-activation to inflammatory stimuli in vitro. Aging 2021, 13, 19088–19107. [Google Scholar] [CrossRef] [PubMed]
- Bielak-Zmijewska, A.; Grabowska, W.; Ciolko, A.; Bojko, A.; Mosieniak, G.; Bijoch, L.; Sikora, E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019, 20, 1239. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H. Prostaglandin E2 Induces Skin Aging via E-Prostanoid 1 in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 20, 5555. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Jang, S.I.; Kim, E.J.; Lee, H.K. A method of evaluating facial pores using optical 2D images and analysis of age-dependent changes in facial pores in Koreans. Skin Res. Technol. 2018, 24, 304–308. [Google Scholar] [CrossRef]
- Jung, H.J.; Ahn, J.Y.; Lee, J.I.; Bae, J.Y.; Kim, H.L.; Suh, H.Y.; Youn, J.I.; Park, M.Y. Analysis of the number of enlarged pores according to site, age, and sex. Skin Res. Technol. 2018, 24, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, B.; Miyamoto, K.; Purwar, A.; Chye, R.; Matsubara, A. New image analysis tool for facial pore characterization and assessment. Skin Res. Technol. 2019, 25, 631–638. [Google Scholar] [CrossRef]
- Lee, S.; Cherel, M.; Gougeon, S.; Jeong, E.; Lim, J.M.; Park, S.G. Identifying patterns behind the changes in skin pores using 3-dimensional measurements and K-means clustering. Skin Res. Technol. 2022, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Lanoue, J.; Goldenberg, G. Enlarged facial pores: An update on treatments. Cutis 2016, 98, 33–36. [Google Scholar]
- Messaraa, C.; Metois, A.; Walsh, M.; Flynn, J.; Doyle, L.; Robertson, N.; Mansfield, A.; O’Connor, C.; Mavon, A. Antera 3D capabilities for pore measurements. Skin Res. Technol. 2018, 24, 606–613. [Google Scholar] [CrossRef]
- Nkengne, A.; Pellacani, G.; Ciardo, S.; De Carvalho, N.; Vie, K. Visible characteristics and structural modifications relating to enlarged facial pores. Skin Res. Technol. 2021, 27, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, F.; Feng, X.; Shang, J.; Luo, X.; Chen, Y. Potential role of inflammaging mediated by the complement system in enlarged facial pores. J. Cosmet. Dermatol. 2024, 23, 27–32. [Google Scholar] [CrossRef]
- Inui, S.; Mori, A.; Ito, M.; Hyodo, S.; Itami, S. Reduction of conspicuous facial pores by topical fullerene: Possible role in the suppression of PGE2 production in the skin. J. Nanobiotechnol. 2014, 12, 6. [Google Scholar] [CrossRef]
- Lee, S.G.; Shin, J.G.; Kim, Y.; Leem, S.; Park, S.G.; Won, H.H.; Kang, N.G. Identification of Genetic Loci Associated with Facial Wrinkles in a Large Korean Population. J. Investig. Dermatol. 2022, 142, 2824–2827. [Google Scholar] [CrossRef]
- Cha, M.-Y.; Choi, J.-E.; Lee, D.-S.; Lee, S.-R.; Lee, S.-I.; Park, J.-H.; Shin, J.-H.; Suh, I.S.; Kim, B.H.; Hong, K.-W. Novel Genetic Associations for Skin Aging Phenotypes and Validation of Previously Reported Skin GWAS Results. Appl. Sci. 2022, 12, 11422. [Google Scholar] [CrossRef]
- Lee, S.; Ye, S.; Kim, M.; Lee, H.; Jun, S.H.; Kang, N.G. Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression. Biomolecules 2024, 14, 279. [Google Scholar] [CrossRef]
- Lee, H.; Ye, S.; Kim, J.; Jun, S.H.; Kang, N.G. Improvement in Facial Wrinkles Using Materials Enhancing PPARGC1B Expression Related to Mitochondrial Function. Curr. Issues Mol. Biol. 2024, 46, 5037–5051. [Google Scholar] [CrossRef]
- Kleuser, B.; Baumer, W. Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023, 24, 1456. [Google Scholar] [CrossRef]
- Noujarede, J.; Carrie, L.; Garcia, V.; Grimont, M.; Eberhardt, A.; Mucher, E.; Genais, M.; Schreuder, A.; Carpentier, S.; Segui, B.; et al. Sphingolipid paracrine signaling impairs keratinocyte adhesion to promote melanoma invasion. Cell Rep. 2023, 42, 113586. [Google Scholar] [CrossRef]
- Allende, M.L.; Sipe, L.M.; Tuymetova, G.; Wilson-Henjum, K.L.; Chen, W.; Proia, R.L. Sphingosine-1-phosphate phosphatase 1 regulates keratinocyte differentiation and epidermal homeostasis. J. Biol. Chem. 2013, 288, 18381–18391. [Google Scholar] [CrossRef]
- Baumer, W.; Rossbach, K.; Mischke, R.; Reines, I.; Langbein-Detsch, I.; Luth, A.; Kleuser, B. Decreased concentration and enhanced metabolism of sphingosine-1-phosphate in lesional skin of dogs with atopic dermatitis: Disturbed sphingosine-1-phosphate homeostasis in atopic dermatitis. J. Investig. Dermatol. 2011, 131, 266–268. [Google Scholar] [CrossRef]
- Mechtcheriakova, D.; Wlachos, A.; Sobanov, J.; Kopp, T.; Reuschel, R.; Bornancin, F.; Cai, R.; Zemann, B.; Urtz, N.; Stingl, G.; et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007, 19, 748–760. [Google Scholar] [CrossRef]
- Jeong, E.T.; Jin, M.H.; Kim, M.S.; Chang, Y.H.; Park, S.G. Inhibition of melanogenesis by piceid isolated from Polygonum cuspidatum. Arch. Pharm. Res. 2010, 33, 1331–1338. [Google Scholar] [CrossRef]
- Seok, J.K.; Boo, Y.C. p-Coumaric Acid Attenuates UVB-Induced Release of Stratifin from Keratinocytes and Indirectly Regulates Matrix Metalloproteinase 1 Release from Fibroblasts. Korean J. Physiol. Pharmacol. 2015, 19, 241–247. [Google Scholar] [CrossRef]
- Oh, S.R.; Park, S.K.; Lee, P.; Kim, Y.M. The ginsenoside Rg2 downregulates MMP-1 expression in keratinocyte (HaCaT)-conditioned medium-treated human fibroblasts (Hs68). Appl. Biol. Chem. 2023, 66, 85. [Google Scholar] [CrossRef]
- Cheong, R.; Hoffmann, A.; Levchenko, A. Understanding NF-kappaB signaling via mathematical modeling. Mol. Syst. Biol. 2008, 4, 192. [Google Scholar] [CrossRef]
- Choe, S.J.; Kim, D.; Kim, E.J.; Ahn, J.S.; Choi, E.J.; Son, E.D.; Lee, T.R.; Choi, E.H. Psychological Stress Deteriorates Skin Barrier Function by Activating 11beta-Hydroxysteroid Dehydrogenase 1 and the HPA Axis. Sci. Rep. 2018, 8, 6334. [Google Scholar] [CrossRef]
- Ishii-Yonemoto, T.; Masuzaki, H.; Yasue, S.; Okada, S.; Kozuka, C.; Tanaka, T.; Noguchi, M.; Tomita, T.; Fujikura, J.; Yuji Yamamoto, Y.; et al. Glucocorticoid reamplification within cells intensifies NF-κB and MAPK signaling and reinforces inflammation in activated preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E930–E940. [Google Scholar]
- Itoi, S.; Terao, M.; Murota, H.; Katayama, I. 11beta-Hydroxysteroid dehydrogenase 1 contributes to the pro-inflammatory response of keratinocytes. Biochem. Biophys. Res. Commun. 2013, 440, 265–270. [Google Scholar] [CrossRef]
- Cannarozzo, G.; Fazia, G.; Bennardo, L.; Tamburi, F.; Amoruso, G.F.; Del Duca, E.; Nistico, S.P. A New 675 nm Laser Device in the Treatment of Facial Aging: A Prospective Observational Study. Photobiomodul Photomed. Laser Surg. 2021, 39, 118–122. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Weinmullner, R.; Zbiral, B.; Becirovic, A.; Stelzer, E.M.; Nagelreiter, F.; Schosserer, M.; Lammermann, I.; Liendl, L.; Lang, M.; Terlecki-Zaniewicz, L.; et al. Organotypic human skin culture models constructed with senescent fibroblasts show hallmarks of skin aging. npj Aging Mech. Dis. 2020, 6, 4. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Lammermann, I.; Terlecki-Zaniewicz, L.; Weinmullner, R.; Schosserer, M.; Dellago, H.; de Matos Branco, A.D.; Autheried, D.; Sevcnikar, B.; Kleissl, L.; Berlin, I.; et al. Blocking negative effects of senescence in human skin fibroblasts with a plant extract. npj Aging Mech. Dis. 2018, 4, 4. [Google Scholar] [CrossRef]
- Kamiya, Y.; Odama, M.; Mizuguti, A.; Murakami, S.; Ito, T. Puerarin blocks the aging phenotype in human dermal fibroblasts. PLoS ONE 2021, 16, e0249367. [Google Scholar] [CrossRef]
- Yang, J.; Dungrawala, H.; Hua, H.; Manukyan, A.; Abraham, L.; Lane, W.; Mead, H.; Wright, J.; Schneider, B.L. Cell size and growth rate are major determinants of replicative lifespan. Cell Cycle 2011, 10, 144–155. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, S.; Chen, Y.; Lyga, J.; Wyborski, R.; Santhanam, U. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin. Cosmet. Investig. Dermatol. 2013, 6, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.J.; Lee, K.; Cheong, S.H.; Ahn, G. Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. Int. J. Biol. Macromol. 2021, 168, 620–630. [Google Scholar] [CrossRef]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.-S.; Gunasekara, U.K.D.S.S.; Park, Y.-J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.-J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Isaev, A.; Kudlay, D.; Vasileva, M.; Kopnin, P. Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods. Curr. Issues Mol. Biol. 2023, 45, 3829–3847. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Messaraa, C.; Metois, A.; Walsh, M.; Hurley, S.; Doyle, L.; Mansfield, A.; O’Connor, C.; Mavon, A. Wrinkle and roughness measurement by the Antera 3D and its application for evaluation of cosmetic products. Skin Res. Technol. 2018, 24, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, S.; Wang, Y.; Ni, J.; Zhao, T.; Xiao, G. Anti-skin aging effects and bioavailability of collagen tripeptide and elastin peptide formulations in young and middle-aged women. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100019. [Google Scholar]
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Samaras, S.; Zedayko, T.; Cole, C.; Miller, D.; Wallo, W.; Leyden, J.J. A stabilized 0.1% retinol facial moisturizer improves the appearance of photodamaged skin in an eight-week, double-blind, vehicle-controlled study. J. Drugs Dermatol. 2009, 8, 932–936. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ye, S.; Jun, S.-H.; Kang, N.-G. Efficacy of SGPP2 Modulation-Mediated Materials in Ameliorating Facial Wrinkles and Pore Sagging. Curr. Issues Mol. Biol. 2024, 46, 9122-9135. https://doi.org/10.3390/cimb46080539
Kim J, Ye S, Jun S-H, Kang N-G. Efficacy of SGPP2 Modulation-Mediated Materials in Ameliorating Facial Wrinkles and Pore Sagging. Current Issues in Molecular Biology. 2024; 46(8):9122-9135. https://doi.org/10.3390/cimb46080539
Chicago/Turabian StyleKim, Juhyun, Sanghyun Ye, Seung-Hyun Jun, and Nae-Gyu Kang. 2024. "Efficacy of SGPP2 Modulation-Mediated Materials in Ameliorating Facial Wrinkles and Pore Sagging" Current Issues in Molecular Biology 46, no. 8: 9122-9135. https://doi.org/10.3390/cimb46080539
APA StyleKim, J., Ye, S., Jun, S.-H., & Kang, N.-G. (2024). Efficacy of SGPP2 Modulation-Mediated Materials in Ameliorating Facial Wrinkles and Pore Sagging. Current Issues in Molecular Biology, 46(8), 9122-9135. https://doi.org/10.3390/cimb46080539





