Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agents
2.2. Animals and Ethical Approval
2.3. Mucuna pruriens (L.) DC. and L-DOPA with Dose Verification
2.4. Parameters to Validate PD Induction and Treatment
2.4.1. Behavioral Assessment
Beam Balance Test
Olfactory Test/Buried Pellet Test
2.4.2. Enzyme-Linked Immunosorbent Assay
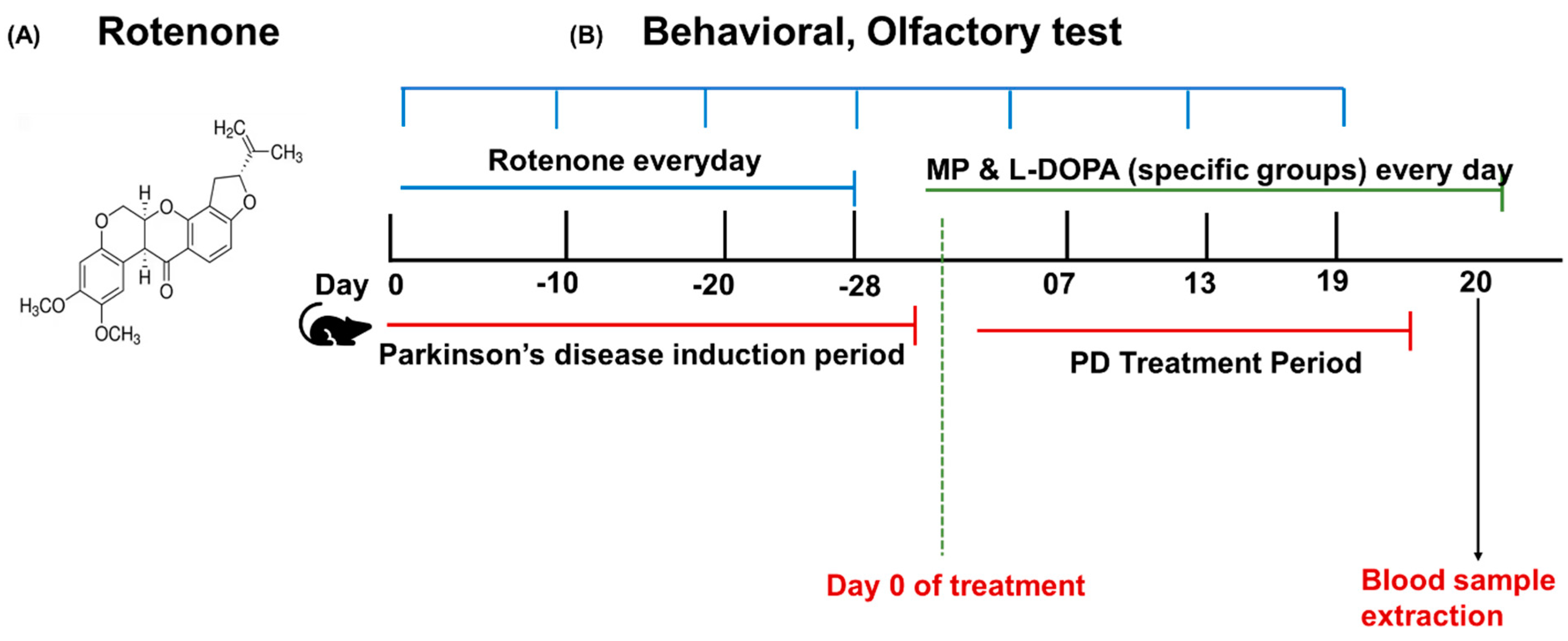
2.5. Rotenone Model Induction and Treatment Plan
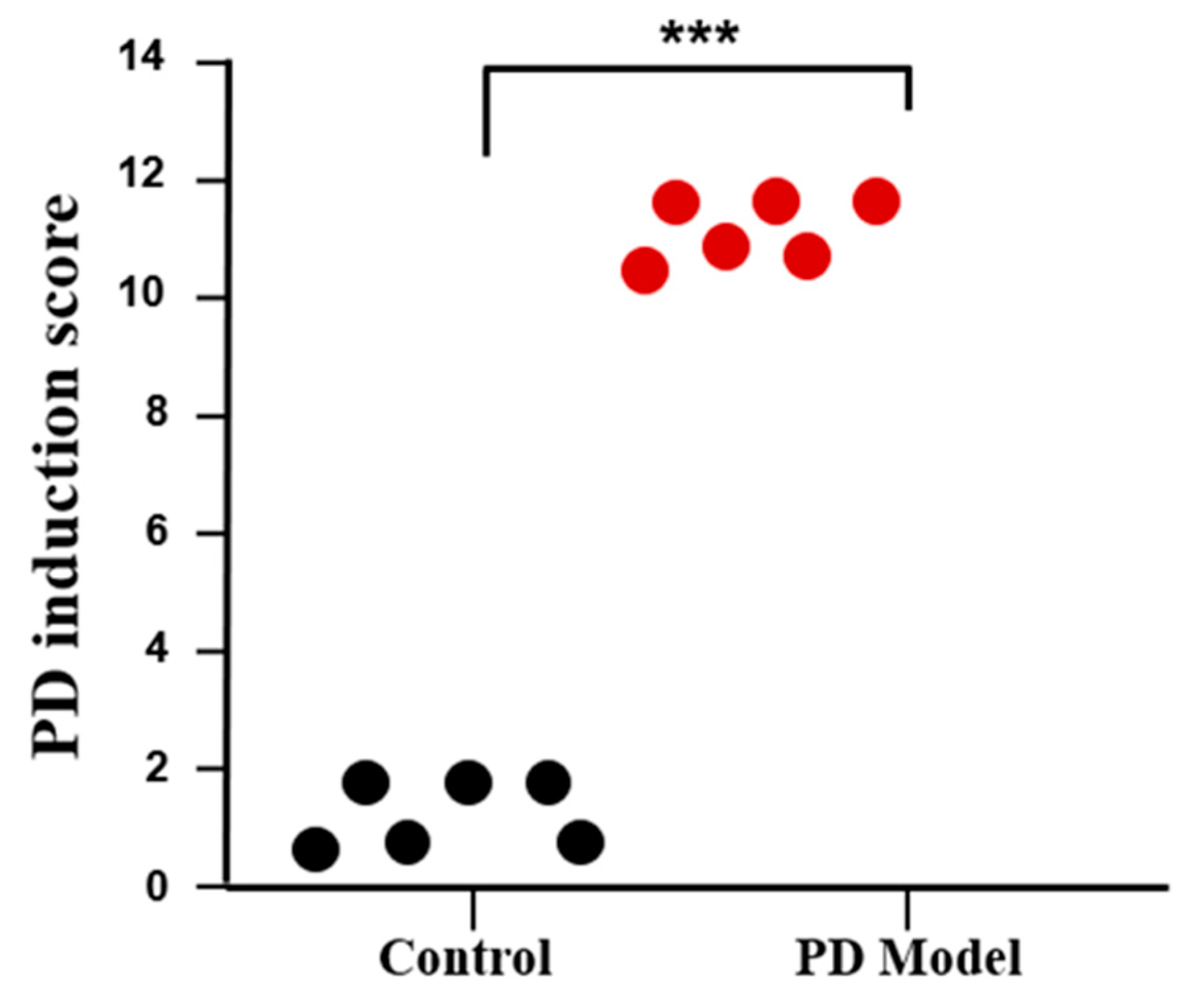
2.6. PD Induction Score
2.7. Experimental Design
2.8. Statistical Analysis
3. Results
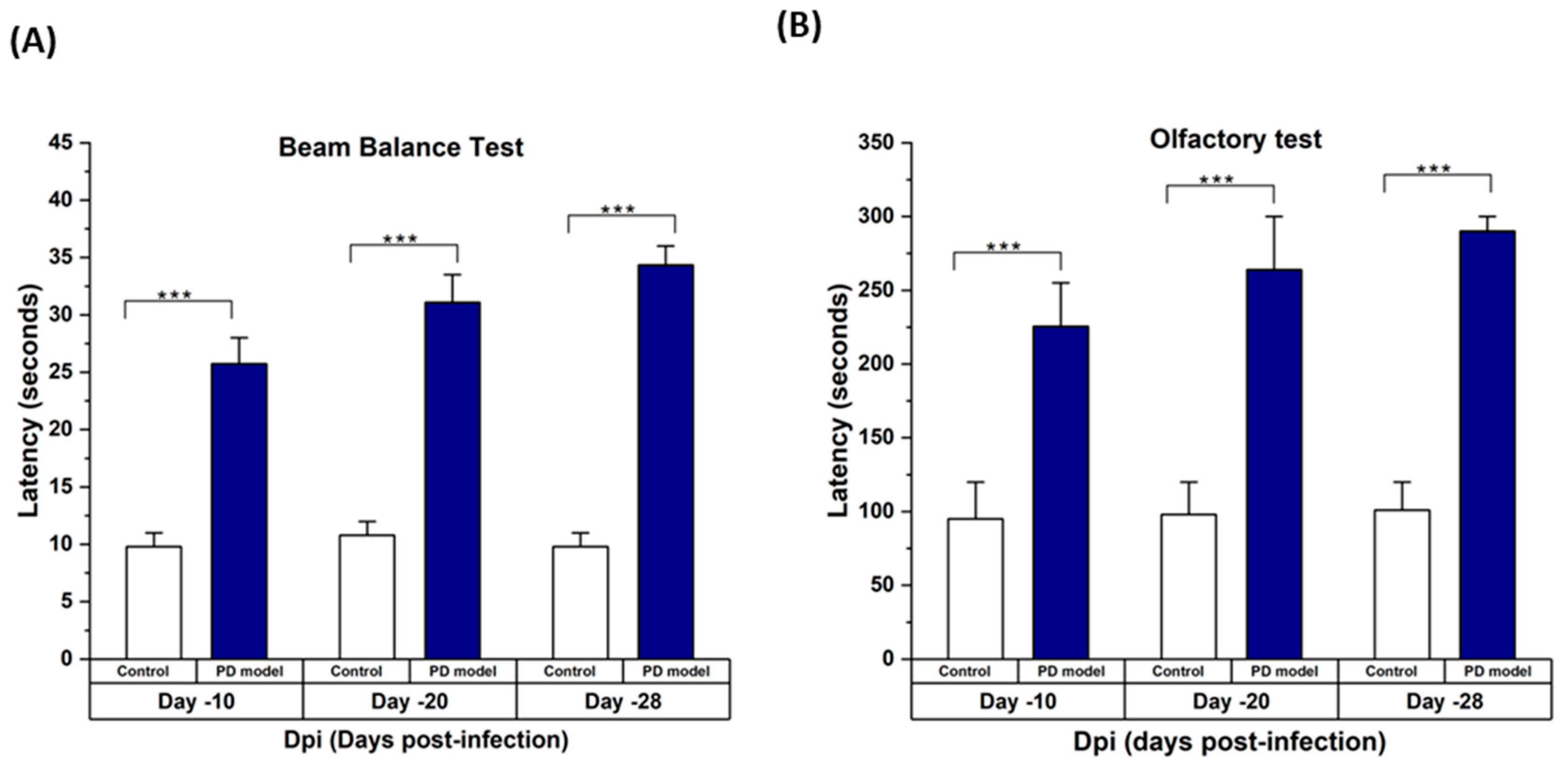
3.1. Rotenone-Induced Parkinson’s Disease (PD) Symptoms in Mice
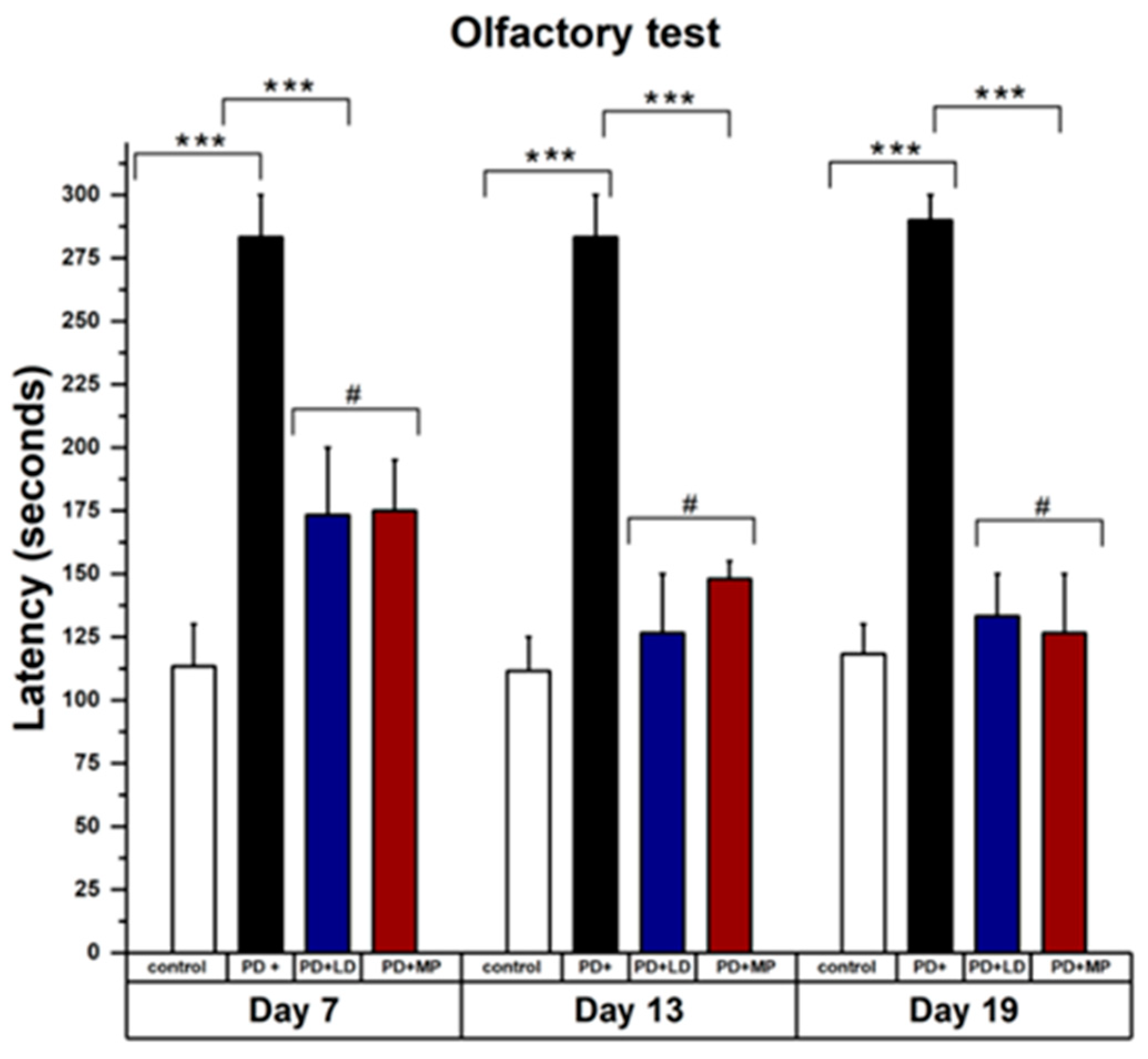
3.2. Comparative Study of MP and L-DOPA in Improving the Symptoms of Parkinson’s Disease
3.2.1. Effects of MP and Improving the Physical Symptoms of PD in Comparison to L-DOPA
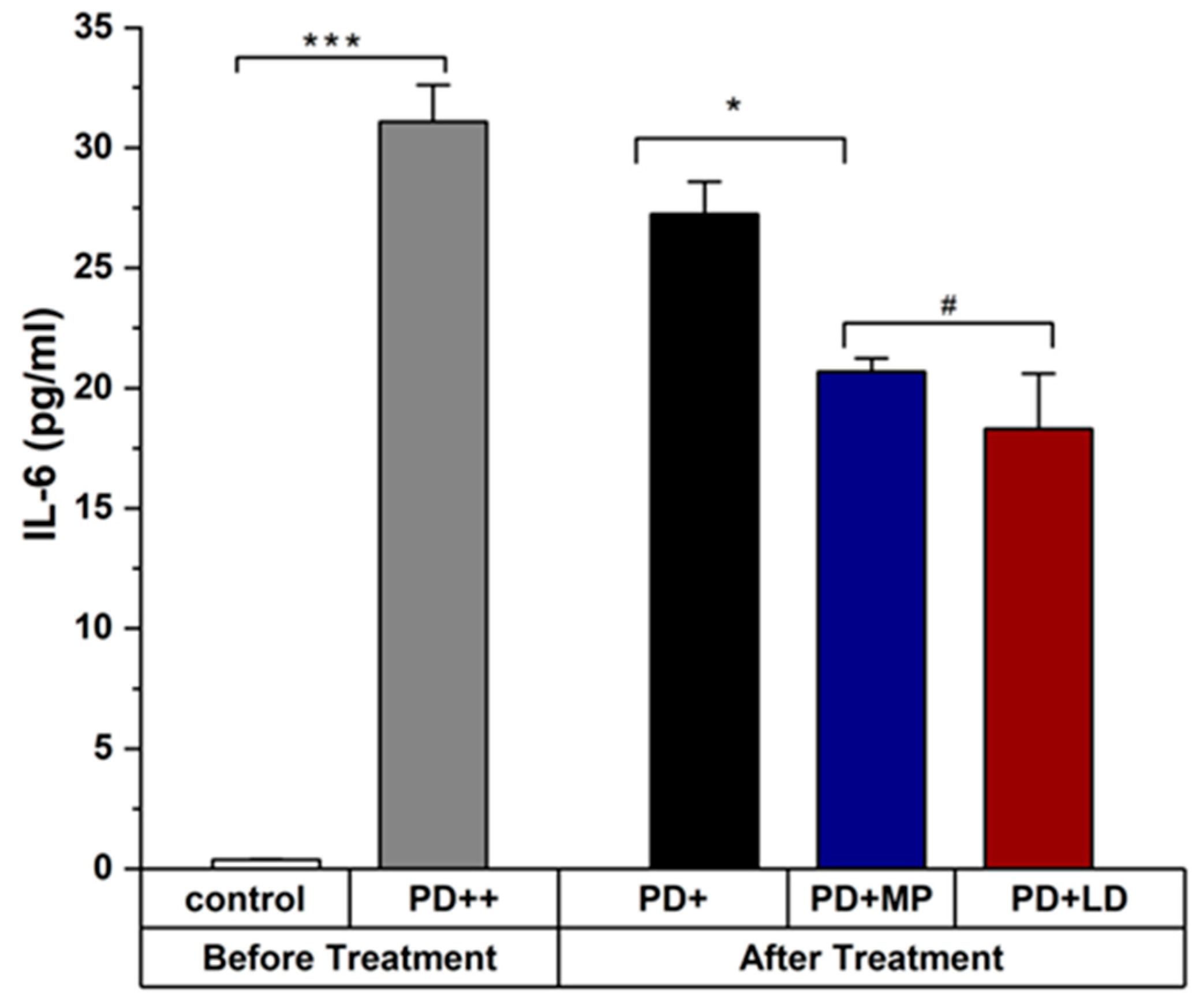
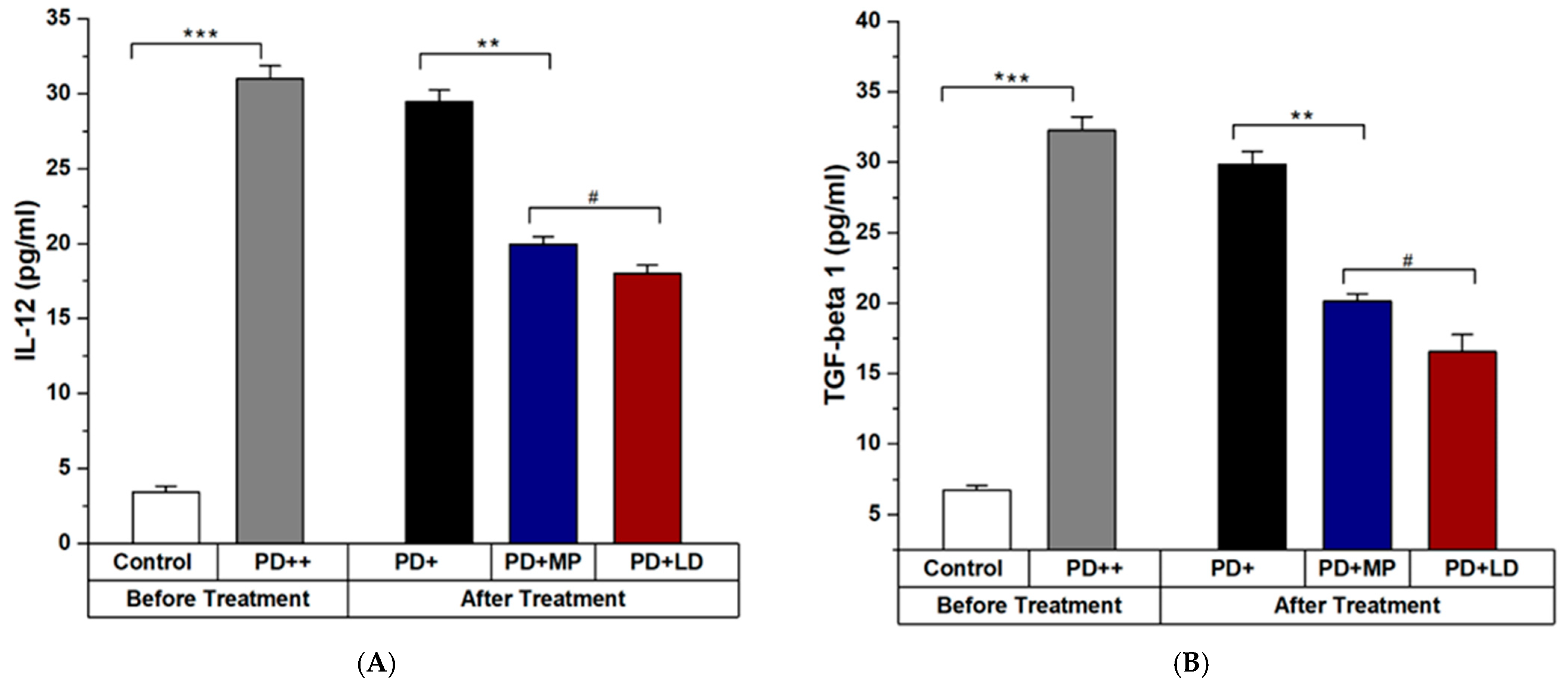
3.2.2. Effects of MP in Improving the Inflammation of PD in Comparison to L-DOPA
4. Discussion
Future Work and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm. Ther. 2015, 40, 504. [Google Scholar]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Lee, H.M.; Koh, S.-B. Many faces of Parkinson’s disease: Non-motor symptoms of Parkinson’s disease. J. Mov. Disord. 2015, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S.; Hartmann, A. Neuroinflammatory processes in Parkinson’s disease. Park. Relat. Disord. 2005, 11, S9–S15. [Google Scholar] [CrossRef]
- Ma, K.; Xiong, N.; Shen, Y.; Han, C.; Liu, L.; Zhang, G.; Wang, L.; Guo, S.; Guo, X.; Xia, Y. Weight loss and malnutrition in patients with Parkinson’s disease: Current knowledge and future prospects. Front. Aging Neurosci. 2018, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Chang, S.-C.; Lee, J. Significant roles of neuroinflammation in Parkinson’s disease: Therapeutic targets for PD prevention. Arch. Pharmacal Res. 2019, 42, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Cytokines in Parkinson’s disease. Adv. Res. Neurodegener. 2000, 7, 143–151. [Google Scholar]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Deets, K.A.; Vance, R.E. Inflammasomes and adaptive immune responses. Nat. Immunol. 2021, 22, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; König, I.R.; Delcambre, S.; Petrucci, S.; Balck, A.; Brüggemann, N.; Zimprich, A.; Wasner, K.; Pereira, S.L.; Avenali, M. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 2020, 143, 3041–3051. [Google Scholar] [CrossRef]
- Arena, G.; Sharma, K.; Agyeah, G.; Krüger, R.; Grünewald, A.; Fitzgerald, J. Neurodegeneration and neuroinflammation in Parkinson’s disease: A self-sustained loop. Curr. Neurol. Neurosci. Rep. 2022, 22, 427–440. [Google Scholar] [CrossRef]
- Brodacki, B.; Staszewski, J.; Toczyłowska, B.; Kozłowska, E.; Drela, N.; Chalimoniuk, M.; Stępien, A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFα, and INFγ concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 2008, 441, 158–162. [Google Scholar] [CrossRef]
- Alharthy, K.M.; Althurwi, H.N.; Albaqami, F.F.; Altharawi, A.; Alzarea, S.I.; Al-Abbasi, F.A.; Nadeem, M.S.; Kazmi, I. Barbigerone potentially alleviates rotenone-activated Parkinson’s disease in a rodent model by reducing oxidative stress and neuroinflammatory cytokines. ACS Omega 2023, 8, 4608–4615. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in Parkinson’s disease. In Parkinson’s Disease and Related Disorders; Springer: Vienna, Austria, 2006; pp. 373–381. [Google Scholar]
- Gao, H.-M.; Liu, B.; Zhang, W.; Hong, J.-S. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003, 17, 1–22. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, S. Do studies in humans better depict Th17 cells? Blood J. Am. Soc. Hematol. 2009, 114, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Wyss-Coray, T. A role for TGF-β signaling in neurodegeneration: Evidence from genetically engineered models. Curr. Alzheimer Res. 2006, 3, 505–513. [Google Scholar] [CrossRef]
- Samantasinghar, A.; Ahmed, F.; Rahim, C.S.A.; Kim, K.H.; Kim, S.; Choi, K.H. Artificial intelligence-assisted repurposing of lubiprostone alleviates tubulointerstitial fibrosis. Transl. Res. 2023, 262, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the Context of an Inflammatory Cytokine Milieu Supports De Novo Differentiation of IL-17-Producing T Cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Karampetsou, M.; Vekrellis, K.; Melachroinou, K. The promise of the TGF-β superfamily as a therapeutic target for Parkinson’s disease. Neurobiol. Dis. 2022, 171, 105805. [Google Scholar] [CrossRef] [PubMed]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming growth factor-β induces development of the TH17 lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Evans, A.; Manson, A.; Patsalos, P.; Ratnaraj, N.; Watt, H.; Timmermann, L.; Van der Giessen, R.; Lees, A. Mucuna pruriens in Parkinson’s disease: A double blind clinical and pharmacological study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1672–1677. [Google Scholar] [CrossRef]
- Sakata, M.; Miyamoto, K.; Koh, J.; Nagashima, Y.; Kondo, T.; Ito, H. Japanese Mucuna pruriens (Hasshou beans) Showed Fast-acting and Long-lasting Effects in Parkinson’s Disease. Intern. Med. 2024, 3171-23. [Google Scholar] [CrossRef]
- Mahajani, S.; Doshi, V.; Parikh, K.; Manyam, B. Bioavailability of l-DOPA from HP-200—A Formulation of Seed Powder of Mucuna pruriens (Bak): A Pharmacokinetic and Pharmacodynamic Study. Phytother. Res. 1996, 10, 254–256. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Greene, J.G.; Miller, G.W. Behavioral phenotyping of mouse models of Parkinson’s disease. Behav. Brain Res. 2010, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.; Mo, M.; Song, C.; Wang, X.; Chen, S.; Liu, Y. Minocycline protects against rotenone-induced neurotoxicity correlating with upregulation of Nurr1 in a Parkinson’s disease rat model. BioMed Res. Int. 2019, 2019, 6843265. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Beneficial effect of vitamin E in rotenone induced model of PD: Behavioural, neurochemical and biochemical study. Exp. Neurobiol. 2013, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Thakur, P.; Nehru, B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience 2013, 231, 420–431. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaigham, S.B.; Paeng, D.-G. Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice. Curr. Issues Mol. Biol. 2024, 46, 9234-9244. https://doi.org/10.3390/cimb46080545
Zaigham SB, Paeng D-G. Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice. Current Issues in Molecular Biology. 2024; 46(8):9234-9244. https://doi.org/10.3390/cimb46080545
Chicago/Turabian StyleZaigham, Sheher Bano, and Dong-Guk Paeng. 2024. "Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice" Current Issues in Molecular Biology 46, no. 8: 9234-9244. https://doi.org/10.3390/cimb46080545




