The Impact of Modern Bone Markers in Multiple Myeloma: Prospective Analyses Pre and Post-First Line Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Patient Inclusion
2.2. Immunoenzymatically Testing (ELISA)
2.3. Statistical Analysis
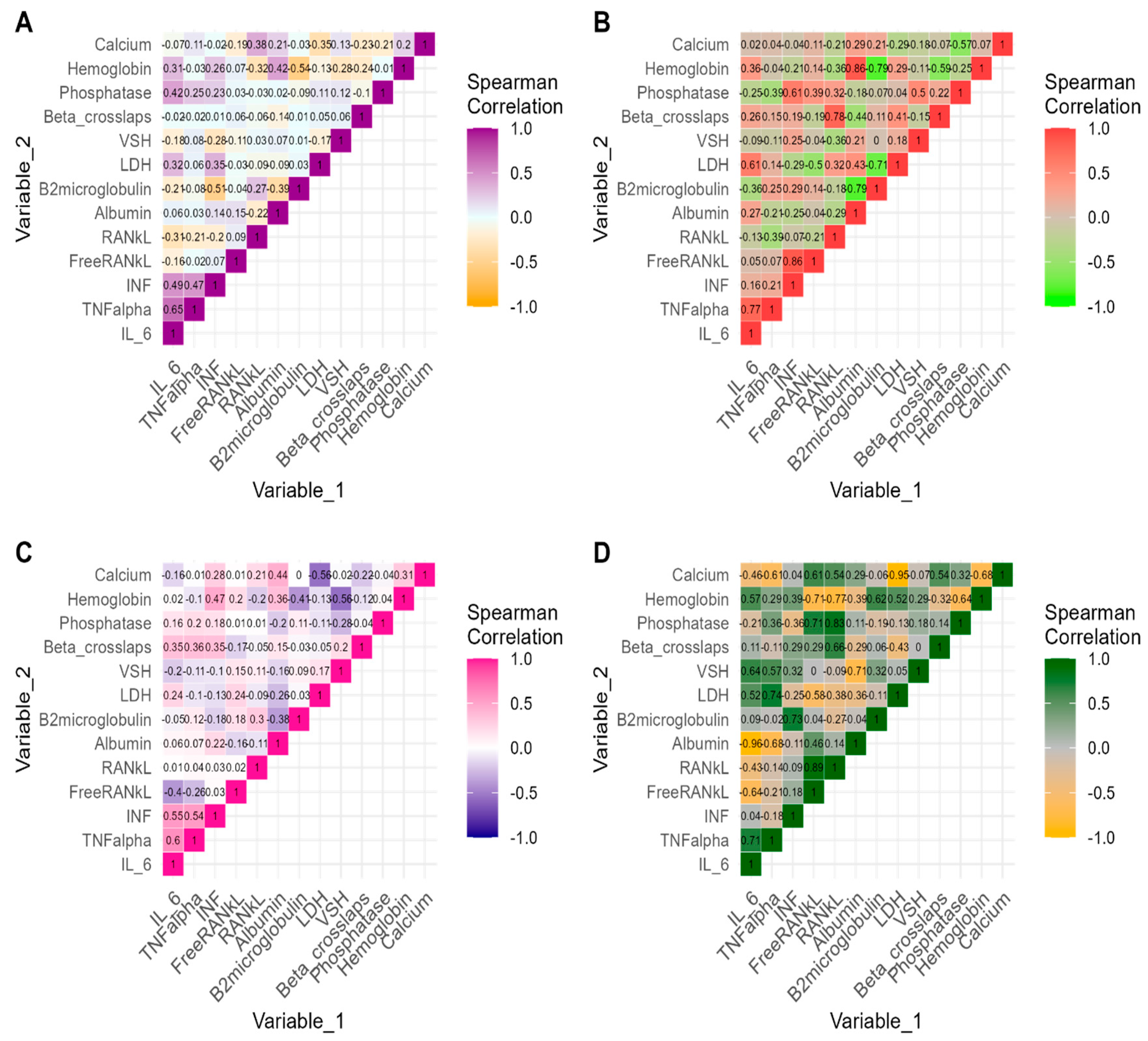
3. Results
Baseline Sample Characteristics
4. Discussion
Limits of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maes, H.; Delforge, M. Optimizing quality of life in multiple myeloma patients: Current options, challenges and recommendations. Expert. Rev. Hematol. 2015, 8, 355–366. [Google Scholar] [CrossRef] [PubMed]
- de Wet, R.; Lane, H.; Tandon, A.; Augustson, B.; Joske, D. ‘It is a journey of discovery’: Living with myeloma. Support. Care Cancer 2019, 27, 2435–2442. [Google Scholar] [CrossRef]
- Pop, V.; Parvu, A.; Craciun, A.; Farcas, A.D.; Tomoaia, G.; Bojan, A. Modern markers for evaluating bone disease in multiple myeloma (Review). Exp. Ther. Med. 2021, 22, 1329. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Mikhael, J.; Hajek, R.; Chari, A.; Zweegman, S.; Lee, H.C.; Mateos, M.-V.; Larocca, A.; Ramasamy, K.; Kaiser, M.; et al. Management of patients with multiple myeloma beyond the clinical-trial setting: Understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 2021, 11, 40. [Google Scholar] [CrossRef]
- Kaweme, N.M.; Changwe, G.J.; Zhou, F. Approaches and Challenges in the Management of Multiple Myeloma in the Very Old: Future Treatment Prospects. Front. Med. 2021, 8, 612696. [Google Scholar] [CrossRef]
- Ebied, M.; Chan, V. Multidisciplinary Professional Roles Addressing Needs in Multiple Myeloma: An Innovative ‘Virtual’ Pharmacist Surveillance Clinic. Semin. Oncol. Nurs. 2021, 37, 151173. [Google Scholar] [CrossRef] [PubMed]
- Du, J.S.; Yen, C.H.; Hsu, C.M.; Hsiao, H.H. Management of Myeloma Bone Lesions. Int. J. Mol. Sci. 2021, 22, 3389. [Google Scholar] [CrossRef]
- Jasrotia, S.; Gupta, R.; Sharma, A.; Halder, A.; Kumar, L. Cytokine profile in multiple myeloma. Cytokine 2020, 136, 155271. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, X.; Zhang, Y.; Bao, C.; Zhou, Z.; Jin, J. Cytokine profiles in patients with newly diagnosed multiple myeloma: Survival is associated with IL-6 and IL-17A levels. Cytokine 2021, 138, 155358. [Google Scholar] [CrossRef]
- Melaccio, A.; Reale, A.; Saltarella, I.; Desantis, V.; Lamanuzzi, A.; Cicco, S.; Frassanito, M.A.; Vacca, A.; Ria, R. Pathways of Angiogenic and Inflammatory Cytokines in Multiple Myeloma: Role in Plasma Cell Clonal Expansion and Drug Resistance. J. Clin. Med. 2022, 11, 6491. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef]
- Peng, C.; Yang, Q.; Kong, X.; Sun, Z.; Wang, L.; Xiao, L. Association of lymphocyte subsets and cytokines with bone metabolism: A retrospective, cross-sectional study. BMC Musculoskelet. Disord. 2024, 25, 43. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Rossi, D.; Fangazio, M.; De Paoli, L.; Puma, A.; Riccomagno, P.; Pinto, V.; Zigrossi, P.; Ramponi, A.; Monga, G.; Gaidano, G. Beta-2-microglobulin is an independent predictor of progression in asymptomatic multiple myeloma. Cancer 2010, 116, 2188–2200. [Google Scholar] [CrossRef]
- Salama, S.; Yousef, R.; Olama, A.; Marashi, M.; Salama, H.; Al-Daour, R.; Salim, N. Impact of Serum Albumin and B2-Microglobulin level on 2-Year Overall Survival among Newly Diagnosed Multiple Myeloma Patients: A retrospective Study. RAS Med. Sci. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- D’Anastasi, M.; Notohamiprodjo, M.; Schmidt, G.P.; Dürr, H.-R.; Reiser, M.F.; Baur-Melnyk, A. Tumor Load in Patients With Multiple Myeloma: β2-Microglobulin Levels Versus Whole-Body MRI. Am. J. Roentgenol. 2014, 203, 854–862. [Google Scholar] [CrossRef]
- Banaszkiewicz, M.; Małyszko, J.; Vesole, D.H.; Woziwodzka, K.; Jurczyszyn, A.; Żórawski, M.; Krzanowski, M.; Batko, K.; Kuźniewski, M.; Krzanowska, K. New Biomarkers of Ferric Management in Multiple Myeloma and Kidney Disease-Associated Anemia. J. Clin. Med. 2019, 8, 1828. [Google Scholar] [CrossRef]
- Mohammed, N.; Kompella, S.B.S.S.; Bhodramoni, Y.; Gundeti, S.; Sree, B.R. Biochemical Characterization of Multiple Myeloma Patients across ISS Stages—A Data Base Workup from a Tertiary Care Hospital in India. Asian Pac. J. Cancer Care 2019, 4, 77–82. [Google Scholar] [CrossRef]
- Vallet, S.; Hoyle, N.R.; Kyle, R.A.; Podar, K.; Pecherstorfer, M. A role for bone turnover markers β-CrossLaps (CTX) and amino-terminal propeptide of type I collagen (PINP) as potential indicators for disease progression from MGUS to multiple myeloma. Leuk. Lymphoma 2018, 59, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.G.; Passam, F.H.; Ganotakis, E.S.; Sfiridaki, K.; Xilouri, I.; Perisinakis, K.; Kyriakou, D.S. The clinical and prognostic significance of erythrocyte sedimentation rate (ESR), serum interleukin-6 (IL-6) and acute phase protein levels in multiple myeloma. Clin. Lab. Haematol. 2003, 25, 41–46. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2018, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Oh, J.I.; Park, J.; Choi, J.H.; Bae, E.K.; Lee, H.J.; Jung, W.J.; Lee, D.S.; Ahn, K.S.; Yoon, S.S. TNF α mediated IL-6 secretion is regulated by JAK/STAT pathway but not by MEK phosphorylation and AKT phosphorylation in U266 multiple myeloma cells. BioMed Res. Int. 2013, 2013, 580135. [Google Scholar] [CrossRef] [PubMed]
- Basmaci, C.; Pehlivan, M.; Tomatir, A.; Sever, T.; Okan, V.; Yilmaz, M.; Oguzkan-Balci, S.; Pehlivan, S. Effects of TNFα, NOS3, MDR1 Gene Polymorphisms on Clinical Parameters, Prognosis and Survival of Multiple Myeloma Cases. Asian Pac. J. Cancer Prev. 2016, 17, 1009–1014. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [PubMed]
- Robak, P.; Weglowska, E.; Drozdz, I.; Mikulski, D.; Jarych, D.; Ferlinska, M.; Wawrzyniak, E.; Misiewicz, M.; Smolewski, P.; Fendler, W.; et al. Cytokine and Chemokine Profile in Patients with Multiple Myeloma Treated with Bortezomib. Mediat. Inflamm. 2020, 2020, 1835836. [Google Scholar] [CrossRef]
- Kastrinakis, N.G.; Gorgoulis, V.G.; Foukas, P.G.; Dimopoulos, M.A.; Kittas, C. Molecular aspects of multiple myeloma. Ann. Oncol. 2000, 11, 1217–1228. [Google Scholar] [CrossRef]
- Isola, I.; Brasó-Maristany, F.; Moreno, D.F.; Mena, M.-P.; Oliver-Calders, A.; Paré, L.; Rodríguez-Lobato, L.G.; Martin-Antonio, B.; Cibeira, M.T.; Bladé, J.; et al. Gene Expression Analysis of the Bone Marrow Microenvironment Reveals Distinct Immunotypes in Smoldering Multiple Myeloma Associated to Progression to Symptomatic Disease. Front. Immunol. 2021, 12, 792609. [Google Scholar] [CrossRef]
| Status of Osteolytic Lesions after First-Line Treatment | ||||
|---|---|---|---|---|
| MM Patients (n = 35) | Non-Changed (n1 = 28) | Changed (n2 = 7) | p-Value | |
| Demographics characteristics | ||||
| Age at MM diagnosis (years), mean (SD) | 63.7(10.9) | 66.1 (10.4) | 53.7 (6.9) | 0.0052 * |
| Sex, n (%) | 1.0000 | |||
| Male | 16 (45.7) | 13 (46.4) | 3 (42.9) | |
| Female | 19 (54.3) | 15 (53.6) | 4 (57.1) | |
| Clinical Characteristics | ||||
| Immune classifications, n (%) | ||||
| Ig A type | 11 (31.4) | 10 (35.7) | 1 (14.3) | 0.3916 |
| Ig G type | 19 (54.3) | 16 (57.1) | 3 (42.9) | 0.6772 |
| Ig M type | 1 (2.9) | 0 (0.0) | 1 (14.3) | 0.2000 |
| Treatment methods, n (%) | 0.4341 | |||
| DVD | 1 (2.9) | 0 (0.0) | 1 (14.3) | |
| DVMP | 4 (11.4) | 4 (14.3) | 0 (0.0) | |
| DVTd | 5 (14.3) | 4 (14.3) | 1 (14.3) | |
| DRd | 2 (5.7) | 2 (7.1) | 0 (0.0) | |
| VRd | 3 (8.6) | 2 (7.1) | 1 (14.3) | |
| VCD | 12 (34.3) | 9 (32.1) | 3 (42.9) | |
| VD | 5 (14.3) | 5 (17.9) | 0 (0.0) | |
| VEL_DEX | 3 (8.6) | 2 (7.1) | 1 (14.3) | |
| FLC type (%) | ||||
| Kappa | 19 (54.3) | 14 (50.0) | 5 (71.4) | 0.4150 |
| Lambda | 9 (25.7) | 8 (28.6) | 1 (14.3) | 0.6478 |
| Pre-Treatment | Post-Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Changed Status of Lytic Lesions (n1 = 28) | Changed Status of Lytic Lesions (n2 = 7) | p-Value | Non-Changed Status of Lytic Lesions (n1 = 28) | Changed Status of Lytic Lesions (n2 = 7) | p-Value | Non-Changed Status of Lytic Lesions (n1 = 28) | Changed Status of Lytic Lesions (n2 = 7) | |
| Median [IQR] or Mean (SD) | Median [IQR] or Mean (SD) | Median [IQR] or Mean (SD) | Median [IQR] or Mean (SD) | p-Value Time Effect | p-Value Time Effect | |||
| IL-6 (pg/mL) | 52.7 [49.1, 97.9] | 56.4 [47.6, 136.6] | 1.000 (a) | 54.1 [48.5, 91.9] | 50.5 [48.1, 91.2] | 0.9179 (a) | 0.1049 (c) | 0.2969 (c) |
| TNF-α (pg/mL) | 77.6 [76.5, 141.7] | 77.6 [76.4, 98.5] | 0.9015 (a) | 78.5 [76.7, 149.8] | 77.2 [76.8, 93.9] | 0.5919 (a) | 0.6223 (c) | 0.8125 (c) |
| IFN (pg/mL) | 12.1 [7.1, 25.3] | 9.4 [6.6, 14.9] | 0.5580 (a) | 13.9 [6.8, 35.9] | 9.7 [7.9, 12.2] | 0.4540 (a) | 0.7793 (c) | 0.1000 (c) |
| FreeRANKL (pg/mL) | 190.2 # (124.6) | 288.2 (179.8) | 0.1059(b) | 219.4 ## (152.6) | 274.6 (124.0) | 0.3898 (b) | 0.2466 (d) | 0.8632 (d) |
| RANKL (pg/mL) | 148.1 (133.4) | 116.1 ### (104.7) | 0.5031(b) | 154.5 # (141.6) | 169.9 (147.1) | 0.8135 (b) | 0.1383 (d) | 0.1206 (d) |
| Albumin (g/dL) | 3.2 [2.8, 3.7] | 3.8 [3.6, 4.4] | 0.0549 (a) | 3.5 [3.1, 3.7] | 4.2 [4.0, 4.3] | 0.0029 * (a) | 0.0077 * (c) | 0.4688 (c) |
| B2microglobulin (mg/L) | 4.9 [4.2, 6.2] | 4.6 [4.1, 5.7] | 0.5051 (a) | 4.4 [3.9, 4.7] | 4.5 [3.8, 4.6] | 0.4729 (a) | 0.0011 * (c) | 0.0469 * (c) |
| Beta crosslaps (ng/mL) | 0.6 [0.4, 1.1] | 0.5 [0.2, 1.1] | 0.5494 (a) | 0.4 [0.2, 0.9] | 0.4 [0.2, 1.4] | 0.3221 (a) | 0.3426 (c) | 0.4688 (c) |
| Calcium (mg/dL) | 9.0 [8.5, 9.6] | 9.3 [9.0, 10.6] | 0.1735 (a) | 8.7 [8.3, 9.0] | 9.2 [8.9, 9.5] | 0.0304 * (a) | 0.0190 * (c) | 0.1563 (c) |
| Bone alkaline phosphatase (μg/L) | 14.1 [10.4, 18.1] | 18.9 [11.9, 28.4] | 0.3024 (a) | 11.6 [9.9, 12.8] | 12.6 [11.7, 13.0] | 0.1726 (a) | 0.2438 (c) | 0.4688 (c) |
| Hgb (g/dL) | 10.1 (2.4) | 11.5 (3.1) | 0.1938 (b) | 11.4 (2.3) | 12.4 (0.9) | 0.1146 (b) | 0.0045 * (d) | 0.4832 (d) |
| LDH (U/L) | 310.5 [255.0, 395.8] | 339.0 [285.5, 387.0] | 0.7570 (a) | 276.0 [206.8, 401.5] | 319.0 [260.0, 358.0] | 0.9343 (a) | 0.7241 (c) | 1.000 (c) |
| VSH | 43.0 [27.0, 56.5] | 33.0 [25.5, 54.5] | 0.4453 (a) | 29.5 [20.5, 36.8] | 21.0 [21.0, 26.5] | 0.0987 (a) | 0.0201 * (c) | 0.1056 (c) |
| Post-Treatment | Non-Changed Status of Lytic Lesions Group (n1 = 28) | Changed Status of Lytic Lesions Group (n2 = 7) | ||
|---|---|---|---|---|
| Changes * in Variables | Rho (ρ) | p-Value | Rho (ρ) | p-Value |
| IL-6 | ||||
| Albumin | −0.20 | 0.4230 | 0.21 | 0.6445 |
| Beta2microglobulin | −0.02 | 0.9405 | −0.29 | 0.5345 |
| LDH | −0.37 | 0.0497 * | 0.14 | 0.7599 |
| VSH | −0.24 | 0.2185 | 0.11 | 0.2682 |
| Calcium | 0.30 | 0.1188 | 0.32 | 0.4821 |
| Hemoglobin | −0.16 | 0.3094 | −0.75 | 0.0522 |
| TNF-alpha | ||||
| Albumin | 0.11 | 0.5859 | −0.11 | 0.8192 |
| Beta2microglobulin | −0.22 | 0.2557 | −0.66 | 0.0938 |
| LDH | −0.33 | 0.0830 | −0.43 | 0.3374 |
| VSH | 0.16 | 0.4164 | 0.22 | 0.6414 |
| Calcium | 0.19 | 0.3274 | 0.25 | 0.5887 |
| Hemoglobin | 0.18 | 0.3533 | −0.54 | 0.2152 |
| IFN-beta | ||||
| Albumin | −0.19 | 0.3459 | 0.39 | 0.3833 |
| Beta2microglobulin | −0.07 | 0.7336 | −0.11 | 0.8192 |
| LDH | −0.41 | 0.0315 * | −0.50 | 0.2532 |
| VSH | 0.02 | 0.9207 | 0.11 | 0.8175 |
| Calcium | 0.05 | 0.8184 | 0.39 | 0.3833 |
| Hemoglobin | −0.08 | 0.6698 | −0.18 | 0.7017 |
| Free RANKL | ||||
| Albumin | 0.23 (a) | 0.2949 | 0.50 | 0.2532 |
| Beta2microglobulin | 0.19 (a) | 0.3878 | 0.36 | 0.4316 |
| LDH | 0.15 (a) | 0.5128 | 0.61 | 0.1482 |
| VSH | −0.21 (a) | 0.3386 | 0.0 | 1.0000 |
| Calcium | −0.38 (a) | 0.0772 | 0.71 | 0.0713 |
| Hemoglobin | −0.29 (a) | 0.1892 | 0.39 | 0.3833 |
| RANKL | ||||
| Albumin | −0.26 (b) | 0.2143 | 0.37 (d) | 0.4685 |
| Beta2microglobulin | −0.01 (b) | 0.9796 | 0.49 (d) | 0.3287 |
| LDH | −0.67 (b) | 0.0003 * | −0.03 (d) | 0.9572 |
| VSH | −0.15 (b) | 0.4672 | −0.81 (d) | 0.0499 * |
| Calcium | 0.06 (b) | 0.7757 | −0.26 (d) | 0.6228 |
| Hemoglobin | −0.20 (b) | 0.3284 | −0.03 (d) | 0.9572 |
| Bone alkaline phosphatase | ||||
| Albumin | −0.17 (c) | 0.3877 | 0.25 | 0.5887 |
| Beta2microglobulin | −0.09 (c) | 0.6572 | 0.43 | 0.3374 |
| LDH | −0.30 (c) | 0.1254 | −0.39 | 0.3833 |
| VSH | 0.07 (c) | 0.7311 | −0.49 | 0.2682 |
| Calcium | 0.27 (c) | 0.1774 | 0.00 | 1.0000 |
| Hemoglobin | 0.29 (c) | 0.1444 | 0.29 | 0.5345 |
| Beta crosslaps | ||||
| Albumin | −0.20 | 0.3018 | 0.86 | 0.0137 * |
| Beta2microglobulin | 0.02 | 0.9229 | 0.43 | 0.3374 |
| LDH | −0.17 | 0.3879 | 0.21 | 0.6445 |
| VSH | 0.07 | 0.7313 | 0.50 | 0.2482 |
| Calcium | −0.07 | 0.7210 | 0.96 | 0.0005 * |
| Hemoglobin | 0.21 | 0.2759 | 0.21 | 0.6445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, V.S.; Iancu, M.; Țigu, A.B.; Adam, A.; Tomoaia, G.; Farcas, A.D.; Bojan, A.S.; Parvu, A. The Impact of Modern Bone Markers in Multiple Myeloma: Prospective Analyses Pre and Post-First Line Treatment. Curr. Issues Mol. Biol. 2024, 46, 9330-9341. https://doi.org/10.3390/cimb46090552
Pop VS, Iancu M, Țigu AB, Adam A, Tomoaia G, Farcas AD, Bojan AS, Parvu A. The Impact of Modern Bone Markers in Multiple Myeloma: Prospective Analyses Pre and Post-First Line Treatment. Current Issues in Molecular Biology. 2024; 46(9):9330-9341. https://doi.org/10.3390/cimb46090552
Chicago/Turabian StylePop, Vlad Stefan, Mihaela Iancu, Adrian Bogdan Țigu, Anda Adam, Gheorghe Tomoaia, Anca Daniela Farcas, Anca Simona Bojan, and Andrada Parvu. 2024. "The Impact of Modern Bone Markers in Multiple Myeloma: Prospective Analyses Pre and Post-First Line Treatment" Current Issues in Molecular Biology 46, no. 9: 9330-9341. https://doi.org/10.3390/cimb46090552
APA StylePop, V. S., Iancu, M., Țigu, A. B., Adam, A., Tomoaia, G., Farcas, A. D., Bojan, A. S., & Parvu, A. (2024). The Impact of Modern Bone Markers in Multiple Myeloma: Prospective Analyses Pre and Post-First Line Treatment. Current Issues in Molecular Biology, 46(9), 9330-9341. https://doi.org/10.3390/cimb46090552







