Development of a Multiplex Real-Time PCR to Disambiguate Culicoides sonorensis within Culicoides variipennis Complex, the Proven Vector of Bluetongue and Epizootic Hemorrhagic Disease Viruses in North America
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference and Field-Collected Specimens
2.2. Destructive or Non-Destructive DNA Extraction
2.3. Development of Multiplex Real-Time PCR Assay Using Species-Specific Primers and Probes
2.4. Validation of Multiplex Real-Time PCR Using Field-Collected and Reference Specimens
2.5. Specificity and Sensitivity of Multiplex Real-Time PCR
2.6. Sequencing and GenBank Submission
2.7. Phylogenetic Tree Construction
3. Results
3.1. Differentiation of Species within the C. variipennis Complex Using Multiplex Real-Time PCR
3.2. Specificity of Multiplex Real-Time PCR
3.3. Sensitivity of Multiplex Real-Time PCR
3.3.1. Sensitivity Analysis in Pools of Species outside the C. variipennis Complex
3.3.2. Sensitivity Analysis in Pools of Species within the C. variipennis Complex
3.4. Further Validation of Multiplex Real-Time PCR Using Field-Collected Specimens of C. variipennis Complex in Canada
3.5. Species Delineation by Phylogenetic Tree
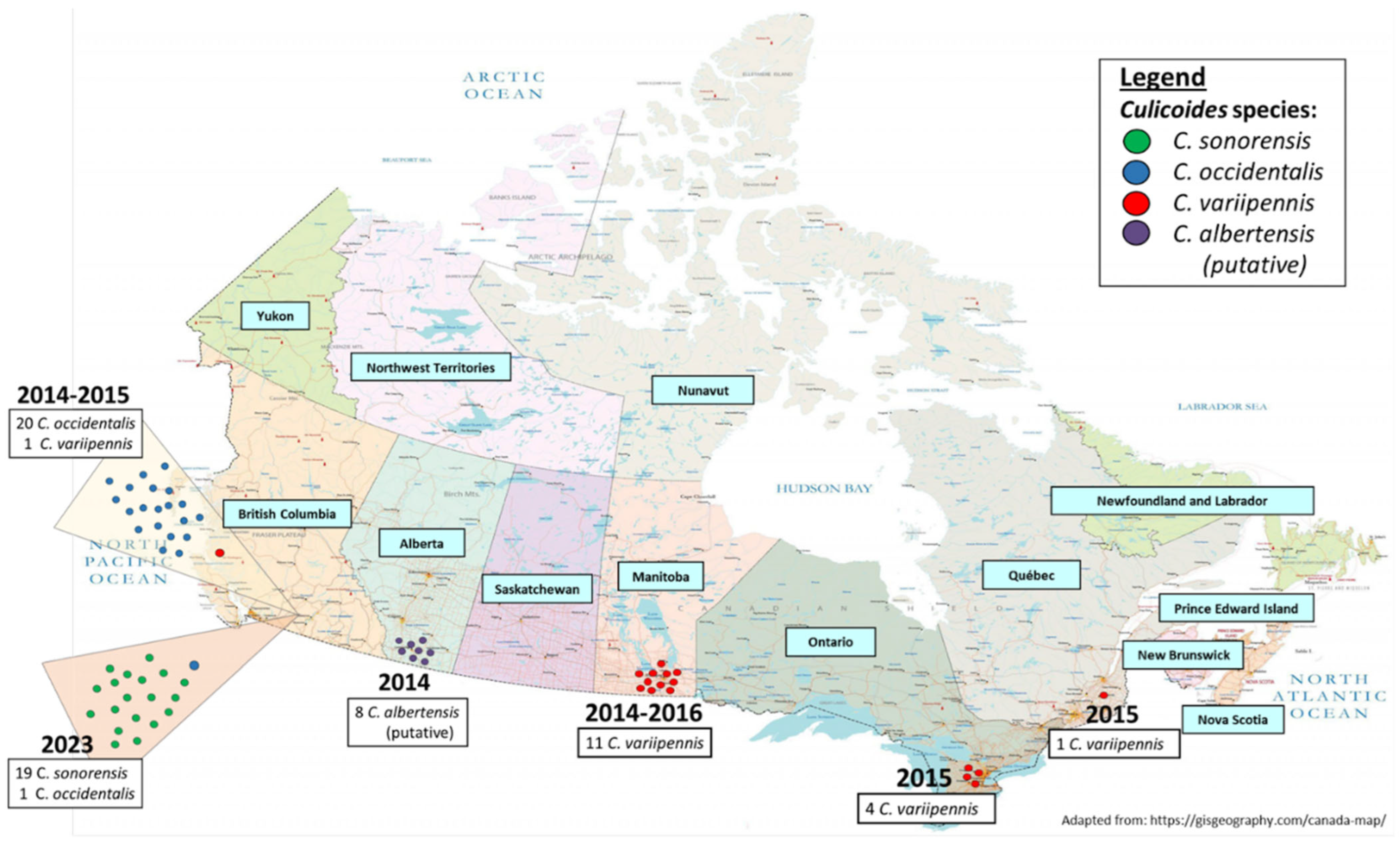
3.6. Geographical Distribution of Species within the C. variipennis Complex in Canada
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellor, P.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides biting midges—Underestimated vectors for arboviruses of public health and veterinary importance. Viruses 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Sunantaraporn, S.; Thepparat, A.; Phumee, A.; Sor-Suwan, S.; Boonserm, R.; Bellis, G.; Siriyasatien, P. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0010014. [Google Scholar] [CrossRef]
- Maclachlan, N.J. Bluetongue: History, global epidemiology, and pathogenesis. Prev. Vet. Med. 2011, 102, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Massolo, A.; Lysyk, T.; Johnson, G.; Marshall, S.; Berger, K.; Cork, S.C. Modelling the northward expansion of Culicoides sonorensis (Diptera: Ceratopogonidae) under future climate scenarios. PLoS ONE 2015, 10, e0130294. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Guthrie, A.J. Re-emergence of bluetongue, African horse sickness, and other orbivirus diseases. Vet. Res. 2010, 41, 35. [Google Scholar] [CrossRef]
- Jewiss-Gaines, A.; Barelli, L.; Hunter, F. First records of Culicoides sonorensis (Diptera: Ceratopogonidae), a known vector of bluetongue virus, in southern Ontario. J. Med. Entomol. 2017, 54, 757–762. [Google Scholar]
- Pybus, M.J.; Ravi, M.; Pollock, C. Epizootic hemorrhagic disease in Alberta, Canada. J. Wildl. Dis. 2014, 50, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.E.; Rothenburger, J.L.; Jardine, C.M.; Ambagala, A.; Hooper-McGrevy, K.; Colucci, N.; Furukawa-Stoffer, T.; Vigil, S.; Ruder, M.; Nemeth, N.M. Epizootic hemorrhagic disease in white-tailed deer, Canada. Emerg. Infect. Dis. 2019, 25, 832. [Google Scholar] [CrossRef]
- Holbrook, F.R.; Tabachnick, W.J.; Schmidtmann, E.T.; McKinnon, C.N.; Bobian, R.J.; Grogan, W.L. Sympatry in the Culicoides variipennis complex (Diptera: Ceratopogonidae): A taxonomic reassessment. J. Med. Entomol. 2000, 37, 65–76. [Google Scholar] [CrossRef]
- Shults, P.; Hopken, M.; Eyer, P.-A.; Blumenfeld, A.; Mateos, M.; Cohnstaedt, L.W.; Vargo, E.L. Species delimitation and mitonuclear discordance within a species complex of biting midges. Sci. Rep. 2022, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Shults, P.T.; McDermott, E.G. A review of the vector status of North American Culicoides (Diptera: Ceratopogonidae) for bluetongue virus, epizootic hemorrhagic disease virus, and other arboviruses of concern. Curr. Trop. Med. Rep. 2022, 9, 130–139. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Lassen, S.; Nielsen, S.A.; Skovgård, H.; Kristensen, M. Molecular differentiation of Culicoides biting midges (Diptera: Ceratopogonidae) from the subgenus Culicoides Latreille in Denmark. Parasitol. Res. 2012, 110, 1765–1771. [Google Scholar] [CrossRef]
- Ander, M.; Troell, K.; Chirico, J. Barcoding of biting midges in the genus Culicoides: A tool for species determination. Med. Vet. Entomol. 2013, 27, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Dowton, M.; Cooper, R.D.; Beebe, N.W. Intraspecific concerted evolution of the rDNA ITS1 in Anopheles farauti sensu stricto (Diptera: Culicidae) reveals recent patterns of population structure. J. Mol. Evol. 2008, 67, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Schierwater, B.; Hadrys, H. On the value of Elongation factor-1α for reconstructing pterygote insect phylogeny. Mol. Phylogenet. Evol. 2010, 54, 651–656. [Google Scholar] [CrossRef]
- Geiser, D.M.; del Mar Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Vezenegho, S.B.; Bass, C.; Puinean, M.; Williamson, M.S.; Field, L.M.; Coetzee, M.; Koekemoer, L.L. Development of multiplex real-time PCR assays for identification of members of the Anopheles funestus species group. Malar. J. 2009, 8, 282. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Kayedi, M.H.; Sedaghat, M.M.; Racine, T.; Kobinger, G.P.; Moosa-Kazemi, S.H. DNA barcodes corroborating identification of mosquito species and multiplex real-time PCR differentiating Culex pipiens complex and Culex torrentium in Iran. PLoS ONE 2018, 13, e0207308. [Google Scholar] [CrossRef]
- Wirth, W.W.; Dyce, A.; Peterson, B.V. An atlas of wing photographs, with a summary of the numerical characters of the Nearctic species of Culicoides (Diptera: Ceratopogonidae). Contrib. Am. Entomol. Inst. 1985, 22, 1–46. [Google Scholar]
- Janke, L.A.; Vigil, S.; Lindsay, K.G.; Furukawa-Stoffer, T.; Colucci, N.; Ambagala, A.; Hanner, R. Culicoides (Diptera: Ceratopogonidae) of Ontario: A Dichotomous Key and Wing Atlas. Can. J. Arthropod Identif. 2023, 50. [Google Scholar]
- Shahhosseini, N.; Friedrich, J.; Moosa-Kazemi, S.H.; Sedaghat, M.M.; Kayedi, M.H.; Tannich, E.; Schmidt-Chanasit, J.; Lühken, R. Host-feeding patterns of Culex mosquitoes in Iran. Parasites Vectors 2018, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, N.; Sedaghat, M.M.; Paquette, S.-J.; Abai, M.R.; Kayedi, M.H. Genotyping, bionomics and host-feeding behavior of Phlebotomus spp.(Diptera: Psychodidae) in Iran. Zool. Anz. 2024, 310, 34–42. [Google Scholar] [CrossRef]
- Cêtre-Sossah, C.; Baldet, T.; Delécolle, J.-C.; Mathieu, B.; Perrin, A.; Grillet, C.; Albina, E. Molecular detection of Culicoides spp. and Culicoides imicola, the principal vector of bluetongue (BT) and African horse sickness (AHS) in Africa and Europe. Vet. Res. 2004, 35, 325–337. [Google Scholar] [CrossRef]
- Vanbinst, T.; Vandenbussche, F.; Vandemeulebroucke, E.; De Leeuw, I.; Deblauwe, I.; De Deken, G.; Madder, M.; Haubruge, E.; Losson, B.; De Clercq, K.J.T.; et al. Bluetongue virus detection by real-time RT-PCR in Culicoides captured during the 2006 epizootic in Belgium and development of an internal control. Transbound. Emerg. Dis. 2009, 56, 170–177. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Wong, G.; Frederick, C.; Kobinger, G.P. Mosquito species composition and abundance in Quebec, Eastern Canada. J. Med. Entomol. 2020, 57, 1025–1031. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Mayo, C.E. Potential strategies for control of bluetongue, a globally emerging, Culicoides-transmitted viral disease of ruminant livestock and wildlife. Antivir. Res. 2013, 99, 79–90. [Google Scholar] [CrossRef]
- Jones, R.; Roughton, R.; Foster, N.; Bando, B. Culicoides, the vector of epizootic hemorrhagic disease in white-tailed deer in Kentucky in 1971. J. Wildl. Dis. 1977, 13, 2–8. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Paquette, S.-J.; Kayedi, M.H.; Abaei, M.R.; Sedaghat, M.M. Genetic Characterization of Sandfly-Borne Viruses in Phlebotomine Sandflies in Iran. Microorganisms 2023, 11, 2754. [Google Scholar] [CrossRef]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Hopken, M.W. Pathogen Vectors at the Wildlife-Livestock Interface: Molecular Approaches to Elucidating Culicoides (Diptera: Ceratopogonidae) Biology. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2016. [Google Scholar]
- Shults, P.; Moran, M.; Blumenfeld, A.J.; Vargo, E.L.; Cohnstaedt, L.W.; Eyer, P.-A. Development of microsatellite markers for population genetics of biting midges and a potential tool for species identification of Culicoides sonorensis Wirth & Jones. Parasites 2022, 15, 69. [Google Scholar]
- Muniesa, A.; Ferreira, C.; Fuertes, H.; Halaihel, N.; de Blas, I. Estimation of the relative sensitivity of qPCR analysis using pooled samples. PLoS ONE 2014, 9, e93491. [Google Scholar] [CrossRef] [PubMed]


| Probe Fluorophore | ||||||
|---|---|---|---|---|---|---|
| Samples | Collection Year | Collection Site | CY5— C. sonorensis | HEX— C. variipennis | FAM— C. occidentalis | TAMRA— 18S Internal Control |
| C. sonorensis #1 | 1973 | Idaho, United States | + | − | − | + |
| C. sonorensis #2 | 1973 | Idaho, United States | + | − | − | + |
| C. sonorensis #3 | 1973 | Idaho, United States | + | − | − | + |
| C. sonorensis #4 | 1973 | Idaho, United States | + | − | − | + |
| C. variipennis #1 | 2015 | Ontario, Canada | − | + | − | + |
| C. variipennis #2 | 2015 | Ontario, Canada | − | + | − | + |
| C. variipennis #3 | 2015 | Ontario, Canada | − | + | − | + |
| C. variipennis #4 | 2015 | Ontario, Canada | − | + | − | + |
| C. occidentalis #1 | 2015 | British Columbia, Canada | − | − | + | + |
| C. occidentalis #2 | 2015 | British Columbia, Canada | − | − | + | + |
| C. occidentalis #3 | 2015 | British Columbia, Canada | − | − | + | + |
| Samples | Collection Year | Collection Site | Probe Fluorophore | |||
|---|---|---|---|---|---|---|
| CY5— C. sonorensis | HEX— C. variipennis | FAM— C. occidentalis | TAMRA—18S Internal Control | |||
| subgenus Avaritia * | 2016 | Nova Scotia, Canada | − | − | − | + |
| subgenus Avaritia ** | 2016 | Nova Scotia, Canada | − | − | − | + |
| C. biguttatus | 2016 | Nova Scotia, Canada | − | − | − | + |
| C. travisi | 2022 | British Columbia, Canada | − | − | − | + |
| C. stellifer | 2022 | Ontario, Canada | − | − | − | + |
| C. crepuscularis | 2014 | British Columbia, Canada | − | − | − | + |
| C. yukonensis | 2014 | Saskatchewan, Canada | − | − | − | + |
| C. downesi | 2015 | Nova Scotia, Canada | − | − | − | + |
| C. riethi | 2017 | Alberta, Canada | − | − | − | + |
| C. wisconsinensis | 2014 | Alberta, Canada | − | − | − | + |
| C. denticulatus | 2015 | Nova Scotia, Canada | − | − | − | + |
| C. paraimpunctatus | 2016 | Nova Scotia, Canada | − | − | − | + |
| C. haematopotus | 2015 | Ontario, Canada | − | − | − | + |
| C. cockerellii | 2022 | Saskatchewan, Canada | − | − | − | + |
| piliferus species group *** | 2022 | Quebec, Canada | − | − | − | + |
| C. occidentalis | 2015 | British Columbia, Canada | − | − | + | + |
| C. sonorensis | 2015 | Idaho, United States | + | − | − | + |
| C. variipennis | 2015 | Ontario, Canada | − | + | − | + |
| Probe Fluorophore | |||||
|---|---|---|---|---|---|
| Sample Pools | CY5—C. sonorensis | HEX—C. variipennis | FAM—C. occidentalis | TAMRA—18S Internal Control | |
| Sensitivity | 9 Culicoides plus 1 C. occidentalis | − | − | 34.95 | 27.58 |
| 19 Culicoides plus 1 C. occidentalis | − | − | 36.23 | 27.66 | |
| 29 Culicoides plus 1 C. occidentalis | − | − | 36.07 | 28.14 | |
| 9 Culicoides plus 1 C. sonorensis | 28.63 | − | − | 28.53 | |
| 19 Culicoides plus 1 C. sonorensis | 29.3 | − | − | 29.02 | |
| 29 Culicoides plus 1 C. sonorensis | 29.89 | − | − | 28.46 | |
| 9 Culicoides plus 1 C. variipennis | − | 28.97 | − | 27.41 | |
| 19 Culicoides plus 1 C. variipennis | − | 29.96 | − | 27.98 | |
| 29 Culicoides plus 1 C. variipennis | − | 30.31 | − | 28.65 | |
| C. occidentalis 1/10 dilution | − | − | 35.46 | 33.38 | |
| C. occidentalis 1/20 dilution | − | − | 36.23 | 34.69 | |
| C. occidentalis 1/30 dilution | − | − | 37.13 | 35.15 | |
| C. sonorensis 1/10 dilution | 28.56 | − | − | 28.31 | |
| C. sonorensis 1/20 dilution | 29.66 | − | − | 29.22 | |
| C. sonorensis 1/30 dilution | 29.93 | − | − | 29.81 | |
| C. variipennis 1/10 dilution | − | 28.8 | − | 24.24 | |
| C. variipennis 1/20 dilution | − | 29.88 | − | 27.87 | |
| C. variipennis 1/30 dilution | − | 30.42 | − | 28.65 | |
| 9 Culicoides plus water | − | − | − | 27.23 | |
| 19 Culicoides plus water | − | − | − | 27.78 | |
| 29 Culicoides plus water | − | − | − | 28.35 | |
| Probe Fluorophore | |||||
|---|---|---|---|---|---|
| Sample Pools | CY5—C. sonorensis | HEX—C. variipennis | FAM—C. occidentalis | TAMRA— 18S Internal Control | |
| Sensitivity | 9 C. sonorensis plus 1 C. occidentalis | 25.4 | − | 29.86 | 23.21 |
| 8 C. sonorensis plus 2 C. occidentalis | 25.46 | − | 29.2 | 23.15 | |
| 7 C. sonorensis plus 3 C. occidentalis | 25.7 | − | 29.2 | 23.43 | |
| 9 C. variipennis plus 1 C. occidentalis | − | 26.85 | 29.21 | 26.22 | |
| 8 C. variipennis plus 1 C. occidentalis | − | 26.64 | 28.83 | 26.44 | |
| 7 C. variipennis plus 3 C. occidentalis | − | 26.95 | 28.87 | 26.7 | |
| 9 C. sonorensis plus 1 C. variipennis | 25.06 | 34.53 | − | 23.15 | |
| 8 C. sonorensis plus 2 C. variipennis | 24.95 | 28.17 | − | 22.79 | |
| 7 C. sonorensis plus 3 C. variipennis | 25.49 | 28.06 | − | 23.22 | |
| 9 C. occidentalis plus 1 C. variipennis | − | 29.32 | 28.53 | 27.55 | |
| 8 C. occidentalis plus 2 C. variipennis | − | 26.14 | 29.11 | 24.97 | |
| 7 C. occidentalis plus 3 C. variipennis | − | 27.72 | 28.67 | 26.42 | |
| 9 C. occidentalis plus 1 C. sonorensis | 29.64 | − | 28.57 | 28.97 | |
| 8 C. occidentalis plus 2 C. sonorensis | 28.22 | − | 28.71 | 27.98 | |
| 7 C. occidentalis plus 3 C. sonorensis | 27.63 | − | 28.59 | 27.32 | |
| 9 C. variipennis plus 1 C. sonorensis | 29.9 | 25.82 | − | 23.06 | |
| 8 C. variipennis plus 2 C. sonorensis | 27.82 | 25.33 | − | 22.43 | |
| 7 C. variipennis plus 3 C. sonorensis | 26.87 | 25.16 | − | 22.12 | |
| 1 C. sonorensis, 1 C. variipennis and 1 C. occidentalis | 30.35 | 28.2 | 29.56 | 25.96 | |
| 2 C. sonorensis, 2 C. variipennis and 2 C. occidentalis | 28.41 | 27.96 | 29.34 | 25.87 | |
| 3 C. sonorensis, 3 C. variipennis and 3 C. occidentalis | 27.87 | 27.4 | 29.27 | 25.34 | |
| 3 C. occidentalis plus 7× water | − | − | 28.97 | 28.02 | |
| 2 C. occidentalis plus 8× water | − | − | 29.22 | 27.95 | |
| 1 C. occidentalis plus 9× water | − | − | 29.23 | 27.65 | |
| 3 C. sonorensis plus 7× water | 26.34 | − | − | 24.46 | |
| 2 C. sonorensis plus 8× water | 27.46 | − | − | 26.32 | |
| 1 C. sonorensis plus 9× water | 28.75 | − | − | 26.47 | |
| 3 C. variipennis plus 7× water | − | 26.93 | − | 23.99 | |
| 2 C. variipennis plus 8× water | − | 27.53 | − | 24.6 | |
| 1 C. variipennis plus 9× water | − | 29.34 | − | 25.21 | |
| Probe Fluorophore | |||||||
|---|---|---|---|---|---|---|---|
| Samples | Collection Year | Collection Site | CY5— C. sonorensis | HEX— C. variipennis | FAM— C. occidentalis | TAMRA— 18S Internal Control | |
| Archived Samples (Ethanol Preserved) | MB #1 | 2014 | Manitoba, Canada | − | + | − | + |
| MB #2 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #3 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #4 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #5 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #6 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #7 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #8 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #9 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #10 | 2016 | Manitoba, Canada | − | + | − | + | |
| MB #11 | 2016 | Manitoba, Canada | − | + | − | + | |
| AB #1 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #2 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #3 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #4 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #5 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #6 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #7 | 2014 | Alberta, Canada | − | − | − | + | |
| AB #8 | 2014 | Alberta, Canada | − | − | − | + | |
| BC #1 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #2 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #3 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #4 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #5 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #6 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #7 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #8 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #9 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #10 | 2014 | British Columbia, Canada | − | − | + | + | |
| BC #11 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #12 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #13 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #14 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #15 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #16 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #17 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #18 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #19 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #20 | 2015 | British Columbia, Canada | − | − | + | + | |
| BC #21 | 2014 | British Columbia, Canada | − | + | − | + | |
| Frozen Samples (−70 °C) | BC #22 | 2023 | British Columbia, Canada | + | − | − | + |
| BC #23 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #24 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #25 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #26 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #27 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #28 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #29 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #30 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #31 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #32 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #33 | 2023 | British Columbia, Canada | − | − | + | + | |
| BC #34 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #35 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #36 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #37 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #38 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #39 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #40 | 2023 | British Columbia, Canada | + | − | − | + | |
| BC #41 | 2023 | British Columbia, Canada | + | − | − | + | |
| Controls | C. occidentalis | 2015 | British Columbia, Canada | − | − | + | + |
| C. sonorensis | 2015 | Idaho, United States | + | − | − | + | |
| C. variipennis | 2015 | Ontario, Canada | − | + | − | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paquette, S.-J.; Czekay, D.; Manalaysay, J.; Furukawa-Stoffer, T.; Ambagala, A.; Vigil, S.; Shahhosseini, N. Development of a Multiplex Real-Time PCR to Disambiguate Culicoides sonorensis within Culicoides variipennis Complex, the Proven Vector of Bluetongue and Epizootic Hemorrhagic Disease Viruses in North America. Curr. Issues Mol. Biol. 2024, 46, 9534-9554. https://doi.org/10.3390/cimb46090566
Paquette S-J, Czekay D, Manalaysay J, Furukawa-Stoffer T, Ambagala A, Vigil S, Shahhosseini N. Development of a Multiplex Real-Time PCR to Disambiguate Culicoides sonorensis within Culicoides variipennis Complex, the Proven Vector of Bluetongue and Epizootic Hemorrhagic Disease Viruses in North America. Current Issues in Molecular Biology. 2024; 46(9):9534-9554. https://doi.org/10.3390/cimb46090566
Chicago/Turabian StylePaquette, Sarah-Jo, Dominic Czekay, Jessica Manalaysay, Tara Furukawa-Stoffer, Aruna Ambagala, Stacey Vigil, and Nariman Shahhosseini. 2024. "Development of a Multiplex Real-Time PCR to Disambiguate Culicoides sonorensis within Culicoides variipennis Complex, the Proven Vector of Bluetongue and Epizootic Hemorrhagic Disease Viruses in North America" Current Issues in Molecular Biology 46, no. 9: 9534-9554. https://doi.org/10.3390/cimb46090566
APA StylePaquette, S.-J., Czekay, D., Manalaysay, J., Furukawa-Stoffer, T., Ambagala, A., Vigil, S., & Shahhosseini, N. (2024). Development of a Multiplex Real-Time PCR to Disambiguate Culicoides sonorensis within Culicoides variipennis Complex, the Proven Vector of Bluetongue and Epizootic Hemorrhagic Disease Viruses in North America. Current Issues in Molecular Biology, 46(9), 9534-9554. https://doi.org/10.3390/cimb46090566








