The Potential Therapeutic Effects of Tadalafil on the Endothelium in a Subarachnoid Hemorrhage Animal Model: Insights from Immunohistochemical Staining
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Grouping
2.2. Induction of SAH in Experimental Animals and Administration of Drugs
2.3. H&E and IHC Staining
2.4. IHC Analysis and Statistical Analysis
3. Results
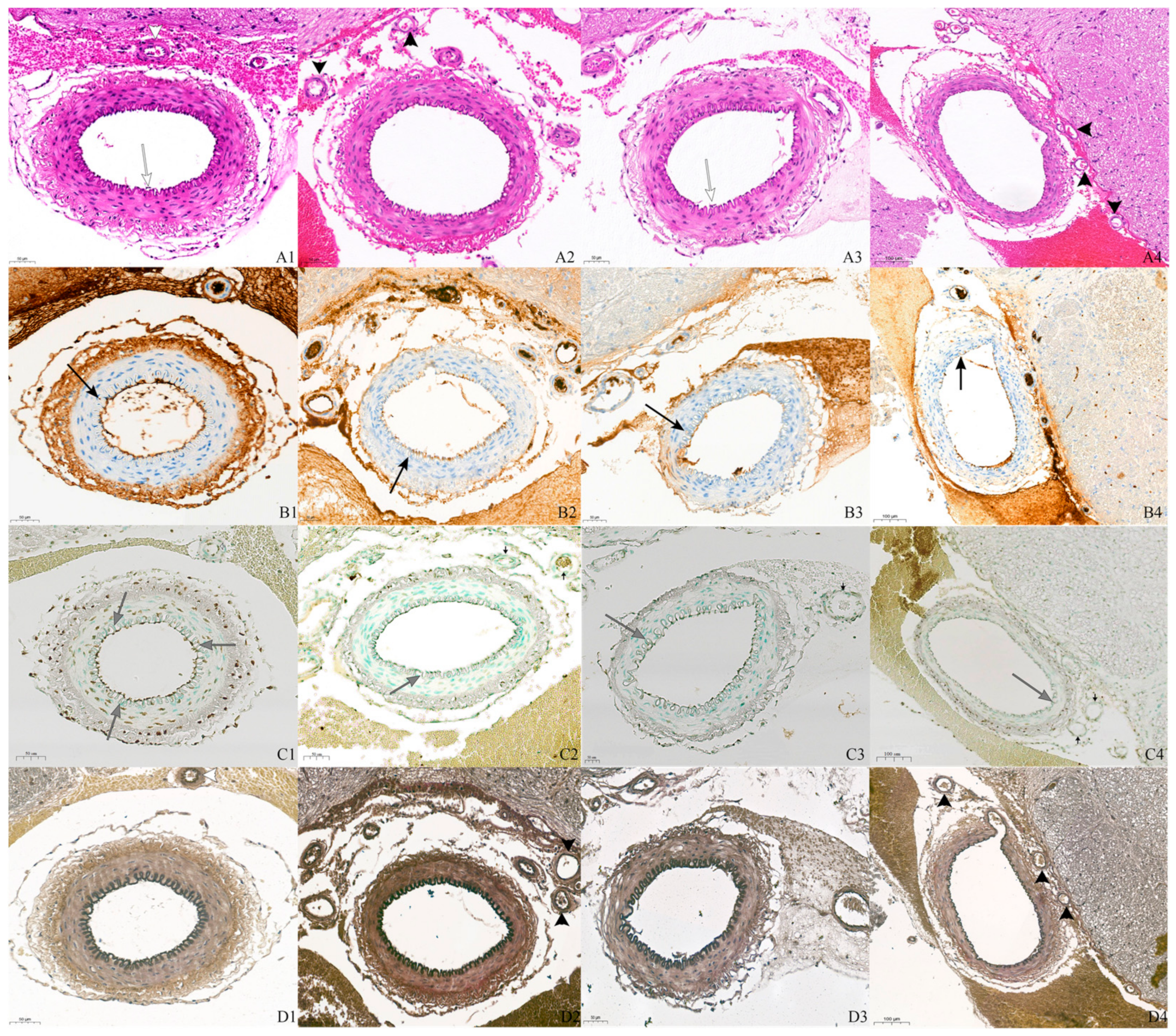
3.1. H&E and IHC Staining Findings
3.2. Phenotypic Scoring of BCL2 and Bax Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, V.A.; Gonzalez, L.F.; Suarez, J.I. Therapies for Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2023, 39, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Caylor, M.M.; Macdonald, R.L. Pharmacological Prevention of Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2024, 40, 159–169. [Google Scholar] [CrossRef]
- Geraghty, J.R.; Testai, F.D. Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Beyond Vasospasm and Towards a Multifactorial Pathophysiology. Curr. Atheroscler. Rep. 2017, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S.; Kaste, M.; Hillbom, M. Effect of nimodipine on platelet function in patients with subarachnoid hemorrhage. Stroke 1990, 21, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.D.; Walker, V.; Vile, J.; Perry, S.; Smythe, P.J.; Hunt, R. Oral nimodipine reduces prostaglandin and thromboxane production by arteries chronically exposed to a periarterial haematoma and the antifibrinolytic agent tranexamic acid. J. Neurol. Neurosurg. Psychiatry 1987, 50, 727–731. [Google Scholar] [CrossRef]
- Dreier, J.P.; Windmuller, O.; Petzold, G.; Lindauer, U.; Einhaupl, K.M.; Dirnagl, U. Ischemia triggered by red blood cell products in the subarachnoid space is inhibited by nimodipine administration or moderate volume expansion/hemodilution in rats. Neurosurgery 2002, 51, 1457–1465; discussion 1465–1467. [Google Scholar] [CrossRef]
- Cho, Y.D.; Byoun, H.S.; Park, K.H.; Won, Y.I.; Lim, J. The Impact of Enteral Nimodipine on Endothelial Cell Apoptosis in an Animal Subarachnoid Hemorrhage Model. Neurocrit. Care 2024. [Google Scholar] [CrossRef] [PubMed]
- Abulhasan, Y.B.; Ortiz Jimenez, J.; Teitelbaum, J.; Simoneau, G.; Angle, M.R. Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J. Neurosurg. 2020, 134, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Polin, R.S.; Hansen, C.A.; German, P.; Chadduck, J.B.; Kassell, N.F. Intra-arterially administered papaverine for the treatment of symptomatic cerebral vasospasm. Neurosurgery 1998, 42, 1256–1264; discussion 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ju, J.; Jiang, B.; Wang, Y.; He, W.; Yin, X.; Guo, W.; Wang, Z. Changes of the Expression and Activity of Phosphodiesterase V in the Basilar Artery Before and After Cerebral Vasospasm in a Rabbit Model. World Neurosurg. 2019, 132, e795–e801. [Google Scholar] [CrossRef] [PubMed]
- Pauls, M.M.; Moynihan, B.; Barrick, T.R.; Kruuse, C.; Madigan, J.B.; Hainsworth, A.H.; Isaacs, J.D. The effect of phosphodiesterase-5 inhibitors on cerebral blood flow in humans: A systematic review. J. Cereb. Blood Flow. Metab. 2018, 38, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Youm, J.Y.; Park, B.; Park, K.H.; Il Won, Y.; Byoun, H.S.; Lim, J. Vasodilatory effects of tadalafil in an animal model of cerebral vasospasm: Comparative analysis with oral nimodipine. Clin. Neurol. Neurosurg. 2023, 232, 107857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Zhang, R.L.; Cui, Y.; LaPointe, M.C.; Silver, B.; Chopp, M. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006, 1118, 192–198. [Google Scholar] [CrossRef]
- Saber, H.; Desai, A.; Palla, M.; Mohamed, W.; Seraji-Bozorgzad, N.; Ibrahim, M. Efficacy of Cilostazol in Prevention of Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 2979–2985. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Kostis, J.B. Overview of phosphodiesterase 5 inhibition in erectile dysfunction. Am. J. Cardiol. 2003, 92, 9M–18M. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Rigante, L.; van Lieshout, J.H.; Vergouwen, M.D.I.; van Griensven, C.H.S.; Vart, P.; van der Loo, L.; de Vries, J.; Vinke, R.S.; Etminan, N.; Aquarius, R.; et al. Time trends in the risk of delayed cerebral ischemia after subarachnoid hemorrhage: A meta-analysis of randomized controlled trials. Neurosurg. Focus. 2022, 52, E2. [Google Scholar] [CrossRef]
- Harada, H.; Grant, S. Apoptosis regulators. Rev. Clin. Exp. Hematol. 2003, 7, 117–138. [Google Scholar]
- Morris, J.L.; Gillet, G.; Prudent, J.; Popgeorgiev, N. Bcl2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int. J. Mol. Sci. 2021, 22, 3730. [Google Scholar] [CrossRef] [PubMed]
- Soond, S.M.; Kozhevnikova, M.V.; Savvateeva, L.V.; Townsend, P.A.; Zamyatnin, A.A., Jr. Intrinsically Connected: Therapeutically Targeting the Cathepsin Proteases and the Bcl2 Family of Protein Substrates as Co-regulators of Apoptosis. Int. J. Mol. Sci. 2021, 22, 4669. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, M.; Lopez, M.G.; Carceller, F.; Garcia, A.G.; Roda, J.M. Combined nimodipine and citicoline reduce infarct size, attenuate apoptosis and increase Bcl2 expression after focal cerebral ischemia. Neuroscience 2003, 118, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yamaguchi, M.; Colohan, A.R.; Zhang, J.H. Role of p53 and apoptosis in cerebral vasospasm after experimental subarachnoid hemorrhage. J. Cereb. Blood Flow. Metab. 2005, 25, 572–582. [Google Scholar] [CrossRef]
- Srimaharaj, W.; Hemrungrote, S.; Chaisricharoen, R. Cloud service for detection of human skin color. In Proceedings of the 2015 15th International Symposium on Communications and Information Technologies (ISCIT), Nara, Japan, 7–9 October 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 9–12. [Google Scholar]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012, 43, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Peeyush Kumar, T.; McBride, D.W.; Dash, P.K.; Matsumura, K.; Rubi, A.; Blackburn, S.L. Endothelial Cell Dysfunction and Injury in Subarachnoid Hemorrhage. Mol. Neurobiol. 2019, 56, 1992–2006. [Google Scholar] [CrossRef]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Atalay, B.; Caner, H.; Cekinmez, M.; Ozen, O.; Celasun, B.; Altinors, N. Systemic administration of phosphodiesterase V inhibitor, sildenafil citrate, for attenuation of cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery 2006, 59, 1102–1107; discussion 1107–1108. [Google Scholar] [CrossRef]
- Pickard, J.D.; Graham, D.I.; Matear, E.; MacPherson, P.; Tamura, A.; Fitch, W. Ultrastructure of cerebral arteries following experimental subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 1985, 48, 256–262. [Google Scholar] [CrossRef]
- Zubkov, A.Y.; Ogihara, K.; Bernanke, D.H.; Parent, A.D.; Zhang, J. Apoptosis of endothelial cells in vessels affected by cerebral vasospasm. Surg. Neurol. 2000, 53, 260–266. [Google Scholar] [CrossRef]
- Wellman, G.C.; Koide, M. Impact of subarachnoid hemorrhage on parenchymal arteriolar function. Acta Neurochir. Suppl. 2013, 115, 173–177. [Google Scholar]
- Cui, Y.; Feng, N.; Gu, X.; Fu, F.; Li, J.; Guo, H.; Liu, Y.; Zhang, S.; Li, J.; Wang, Y.; et al. kappa-Opioid receptor stimulation reduces palmitate-induced apoptosis via Akt/eNOS signaling pathway. Lipids Health Dis. 2019, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Kramer, M.F.; Becker, R.E.; Burnett, A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. USA 2005, 102, 11870–11875. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef]
- Koh, S.H.; Lo, E.H. The Role of the PI3K Pathway in the Regeneration of the Damaged Brain by Neural Stem Cells after Cerebral Infarction. J. Clin. Neurol. 2015, 11, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Falasca, M.; Maffucci, T. Role of class II phosphoinositide 3-kinase in cell signalling. Biochem. Soc. Trans. 2007, 35 Pt 2, 211–214. [Google Scholar] [CrossRef]
- Nemazanyy, I.; Montagnac, G.; Russell, R.C.; Morzyglod, L.; Burnol, A.F.; Guan, K.L.; Pende, M.; Panasyuk, G. Class III PI3K regulates organismal glucose homeostasis by providing negative feedback on hepatic insulin signalling. Nat. Commun. 2015, 6, 8283. [Google Scholar] [CrossRef] [PubMed]
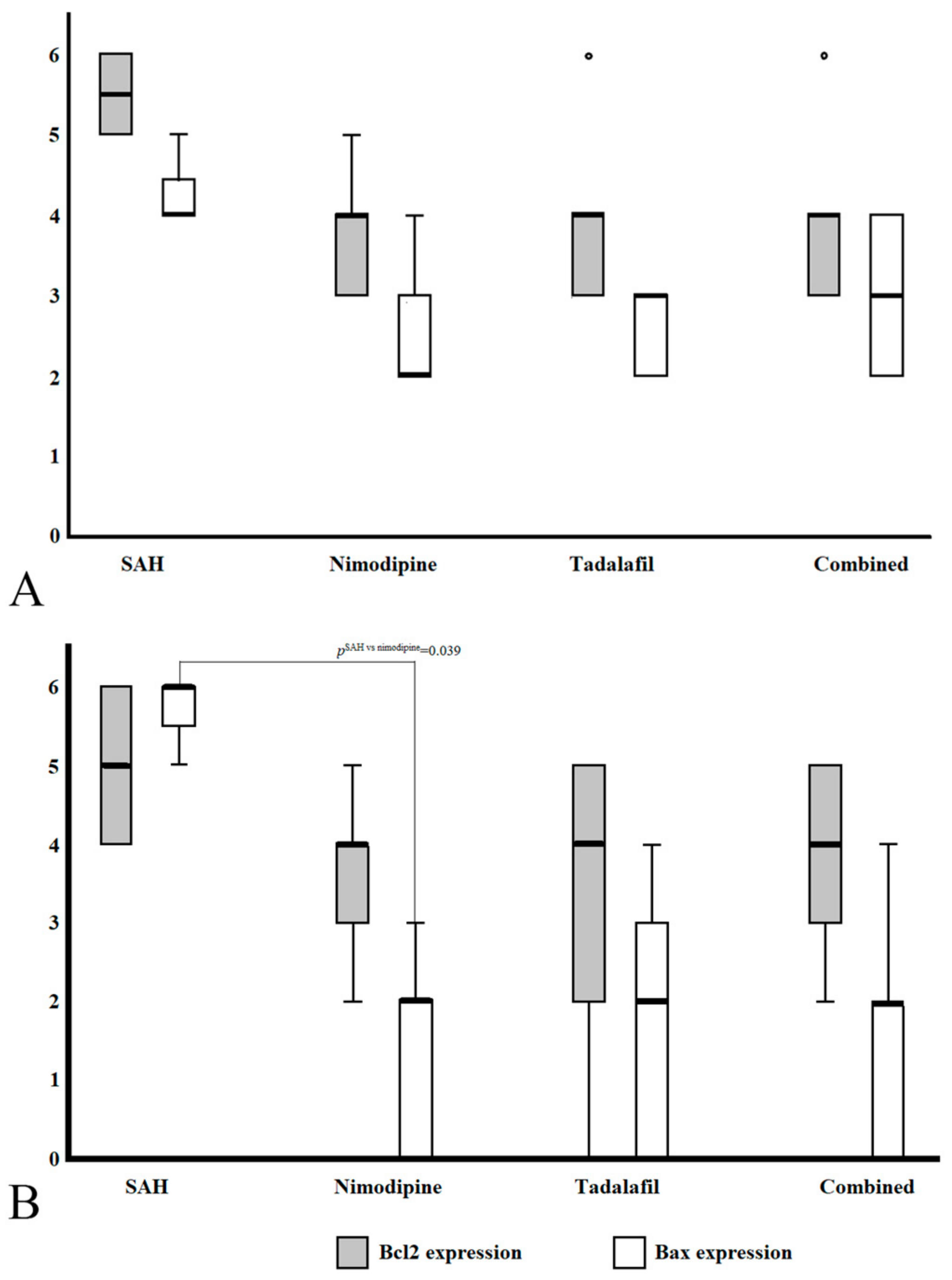
| Group (n) | Bcl2 Staining Score (Average ± SD) | p † | Bax Staining Score(Average ± SD) | p † | Groupwise Comparison | |||
|---|---|---|---|---|---|---|---|---|
| Group | p ‡ | p § | ||||||
| Basilar artery | C (15) | 1.07 ± 1.03 | <0.001 | 0 | <0.001 | C vs. S | <0.001 | <0.001 |
| S (13) | 4.77 ± 1.17 | 3.31 ± 1.49 | C vs. N | 0.002 | 0.001 | |||
| N (14) | 3.57 ± 1.40 | 2.43 ± 1. 65 | S vs. N | 0.311 | 0.720 | |||
| Penetrated arteriole | C (19) | 1.70 ± 0.95 | <0.001 | 0 | <0.001 | C vs. S | <0.001 | <0.001 |
| S (24) | 4.65 ± 1.27 | 4.00 ± 2.41 | C vs. N | 0.001 | 1.000 | |||
| N (21) | 4.15 ± 1.40 | 1.40 ± 1.39 | S vs. N | 0.724 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Kwon, H.-J.; Jeong, E.-O.; Koh, H.-S.; Lim, J. The Potential Therapeutic Effects of Tadalafil on the Endothelium in a Subarachnoid Hemorrhage Animal Model: Insights from Immunohistochemical Staining. Curr. Issues Mol. Biol. 2024, 46, 9555-9564. https://doi.org/10.3390/cimb46090567
Park KH, Kwon H-J, Jeong E-O, Koh H-S, Lim J. The Potential Therapeutic Effects of Tadalafil on the Endothelium in a Subarachnoid Hemorrhage Animal Model: Insights from Immunohistochemical Staining. Current Issues in Molecular Biology. 2024; 46(9):9555-9564. https://doi.org/10.3390/cimb46090567
Chicago/Turabian StylePark, Kwang Hyon, Hyon-Jo Kwon, Eun-Oh Jeong, Hyeon-Song Koh, and Jeongwook Lim. 2024. "The Potential Therapeutic Effects of Tadalafil on the Endothelium in a Subarachnoid Hemorrhage Animal Model: Insights from Immunohistochemical Staining" Current Issues in Molecular Biology 46, no. 9: 9555-9564. https://doi.org/10.3390/cimb46090567
APA StylePark, K. H., Kwon, H.-J., Jeong, E.-O., Koh, H.-S., & Lim, J. (2024). The Potential Therapeutic Effects of Tadalafil on the Endothelium in a Subarachnoid Hemorrhage Animal Model: Insights from Immunohistochemical Staining. Current Issues in Molecular Biology, 46(9), 9555-9564. https://doi.org/10.3390/cimb46090567







