Investigating the Phytochemical Composition, Antioxidant, and Anti-Inflammatory Potentials of Cassinopsis ilicifolia (Hochst.) Kuntze Extract against Some Oxidative Stress and Inflammation Molecular Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Method of Extraction
2.2. Antioxidant Assays
2.2.1. Azino-bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS•+) Radical Scavenging Assay
2.2.2. Diphenyl-1-Picrylhydrazyl (DPPH•) Radical Scavenging Assay
2.2.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.3. Anti-Inflammatory Assays
2.3.1. Soybean 15-Lipoxygenase Inhibitory Assay
2.3.2. Culture of RAW 264.7 Murine Macrophages and Cytotoxicity Assay
2.3.3. Nitric Oxide Production Inhibitory Assay
2.4. Quantification of the Levels of Inflammatory Mediators in LPS-Activated RAW264.7 Cells
2.5. Determination of Intracellular Reactive Oxygen Species Levels
2.6. Phytochemical Analysis and Tentative Identification of Compounds in Extract
2.6.1. Total Phenolic Content
2.6.2. Total Flavonoid Content
2.6.3. Liquid Chromatography–Mass Spectrometric (LC–MS) Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity of C. ilicifolia Crude Extract
3.2. Anti-Inflammatory Activity of C. ilicifolia Crude Extract
3.2.1. Lipoxygenase Inhibitory Activity
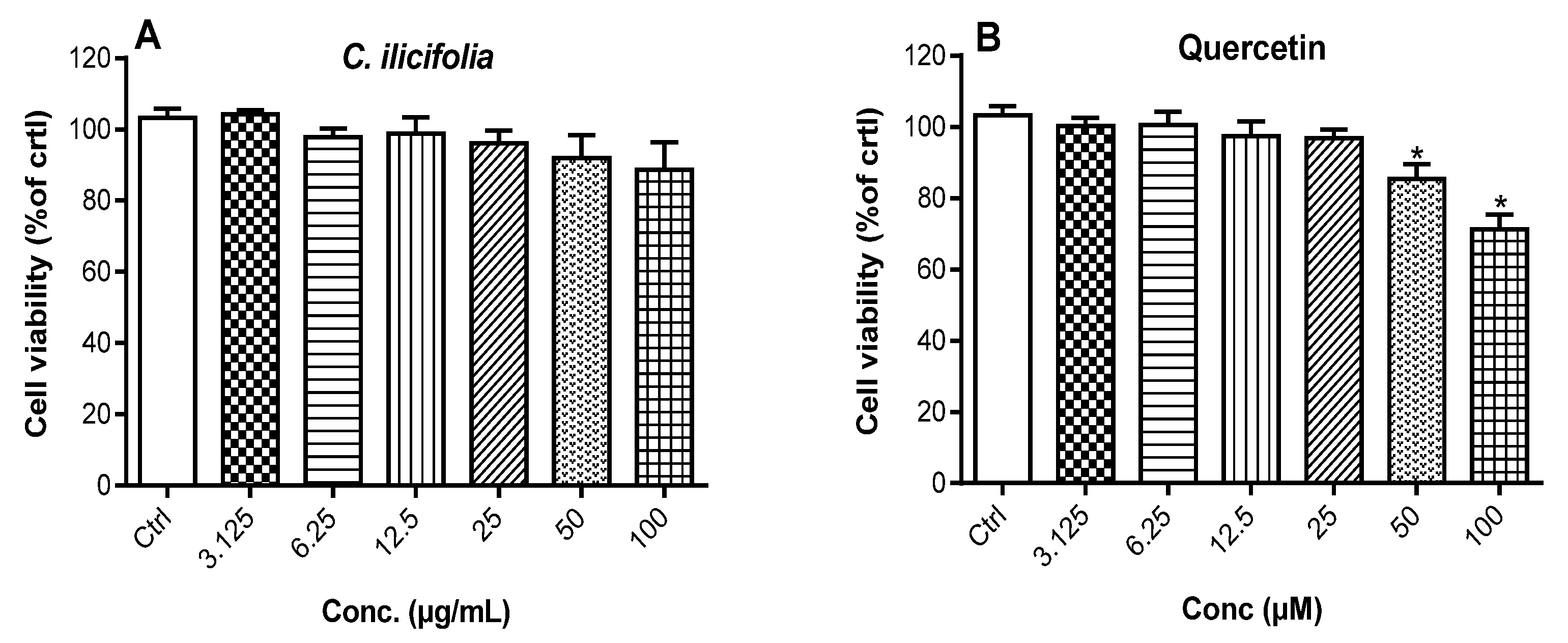
3.2.2. Cytotoxic Potency of C. ilicifolia Extract on RAW 264.7 Murine Macrophages
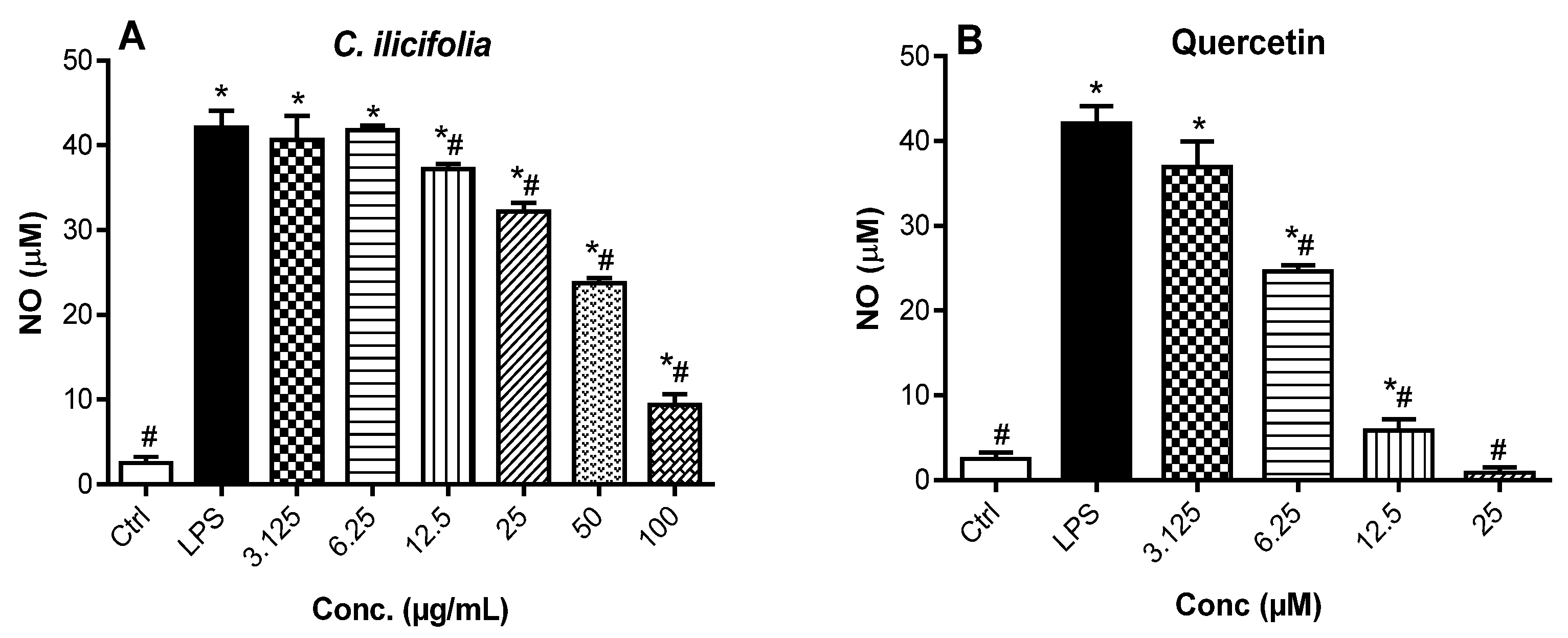
3.2.3. Nitric Oxide Production Inhibitory Effects of C. ilicifolia Crude Extract
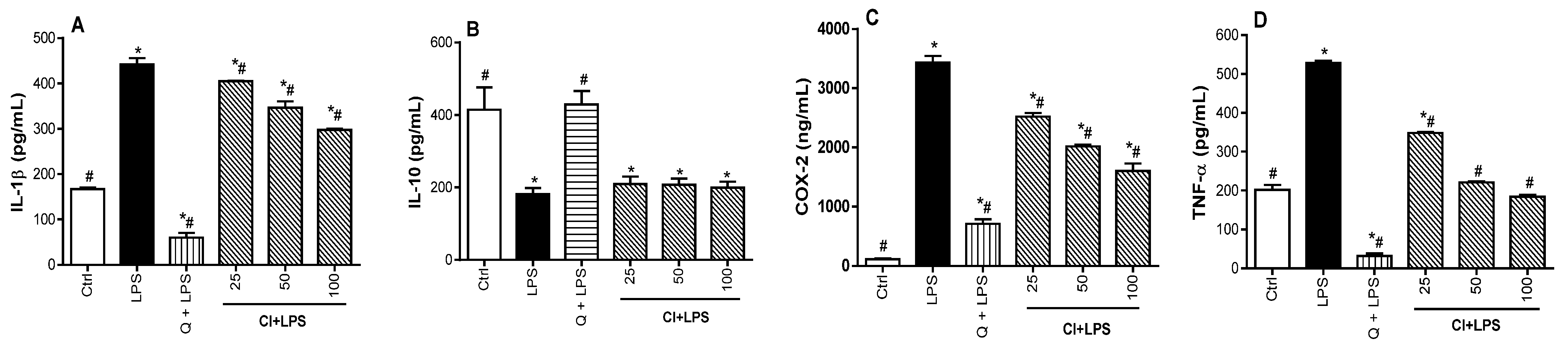
3.3. Regulatory Effect of C. ilicifolia Extract on the Levels of Inflammatory Mediators
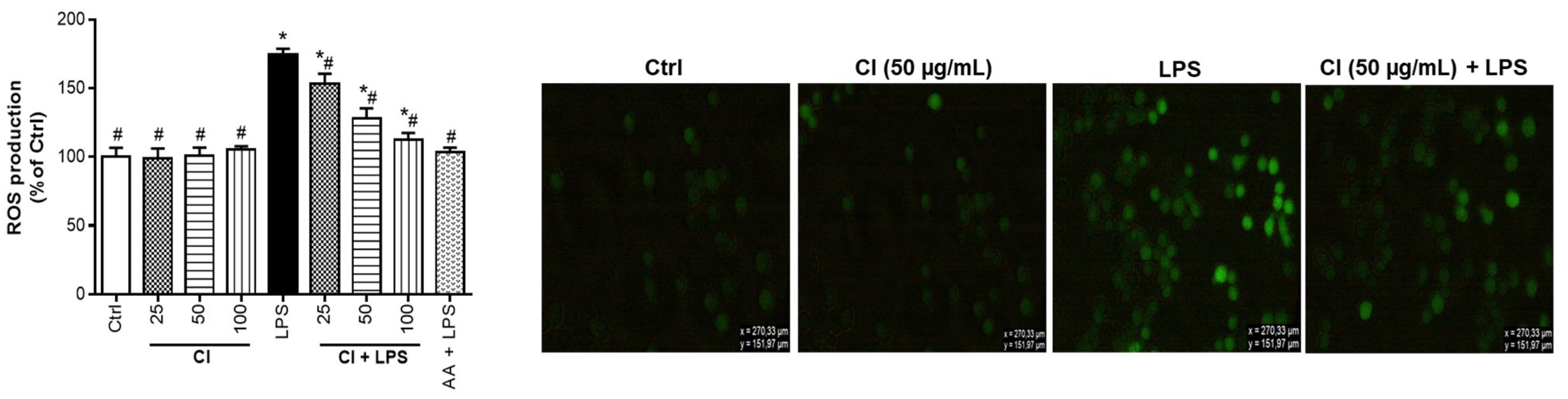
3.4. Preventive Action of C. ilicifolia Extract on the Production of Reactive Oxygen Species
3.5. Phytochemical Composition of C. ilicifolia
3.5.1. Total Phenolic and Total Flavonoid Contents
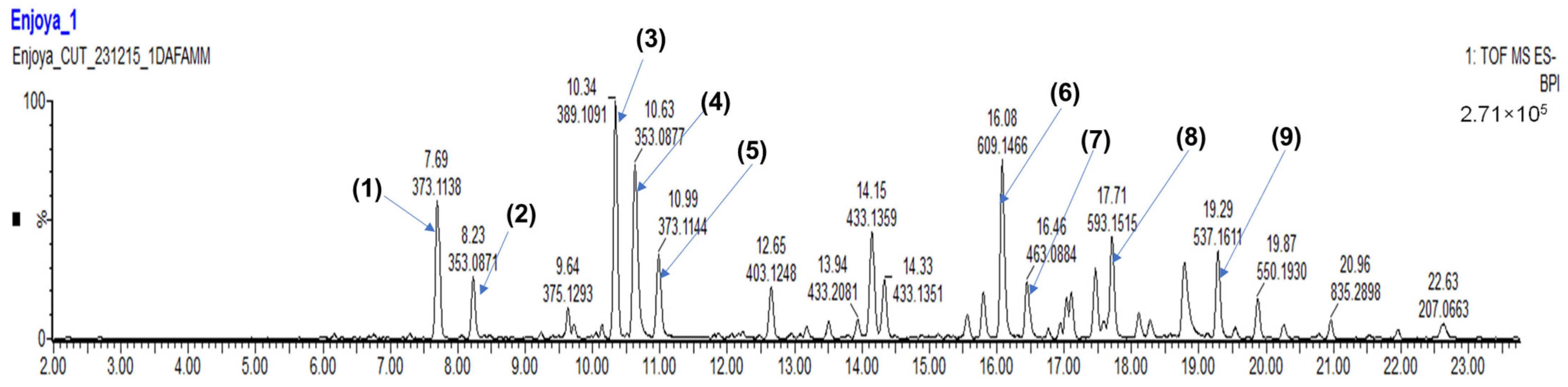
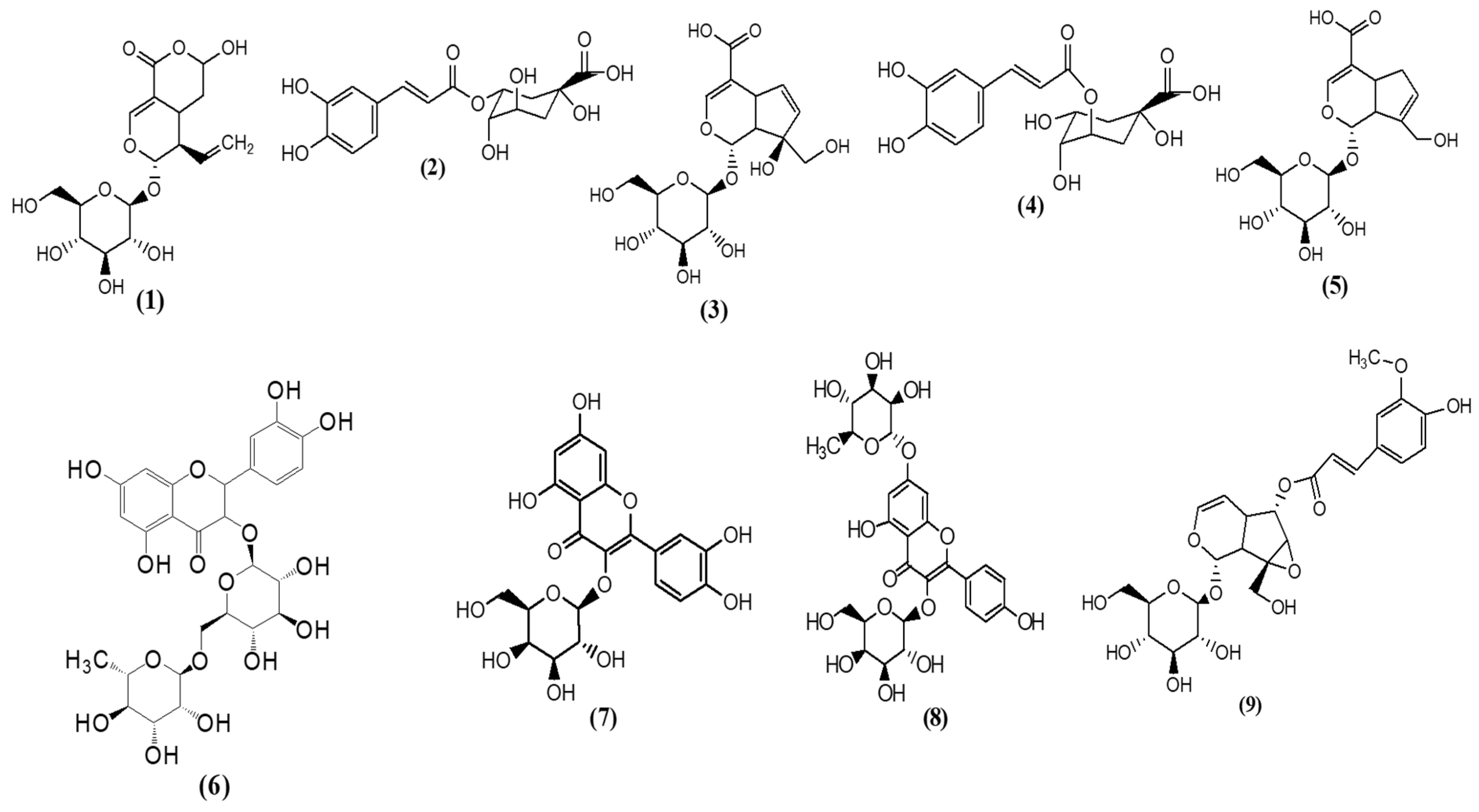
3.5.2. LC–MS Profile of C. ilicifolia Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Biswas, S. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 9, 5698931. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Basheer, A.S.; Abas, F.; Othman, I.; Naidu, R. Role of Inflammatory Mediators, Macrophages, and Neutrophils in Glioma Maintenance and Progression: Mechanistic Understanding and Potential Therapeutic Applications. Cancers 2021, 13, 4226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C.C. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis. Int. 2014, 13, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappaB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Brune, B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Front. Pharmacol. 2019, 10, 719. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Nwozo, O.S.; Effionga, E.M.; Aja, P.M.; Awuchi, C.G. Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review. Int. J. Food Prop. 2023, 26, 359–388. [Google Scholar] [CrossRef]
- Semenya, S.S.; Maroyi, A. Ethnobotanical survey of plants used by Bapedi traditional healers to treat tuberculosis and its opportunistic infections in the Limpopo Province, South Africa. S. Afr. J. Bot. 2019, 122, 401–421. [Google Scholar] [CrossRef]
- Okem, A.; Finnie, J.F.; Van Staden, J. Pharmacological, genotoxic and phytochemical properties of selected South African medicinal plants used in treating stomach-related ailments. J. Ethnopharmacol. 2012, 139, 712–720. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Mfotie Njoya, E. Medicinal plants, antioxidant potential, and cancer. In Cancer, 2nd ed.; Oxidative Stress and Dietary Antioxidants: Preedy, V.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 349–357. [Google Scholar]
- Pinto, M.C.; Tejeda, A.; Duque, A.L.; Macias, P. Determination of lipoxygenase activity in plant extracts using a modified ferrous oxidation-xylenol orange assay. J. Agric. Food Chem. 2007, 55, 5956–5959. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mfotie Njoya, E.; Ndemangou, B.; Akinyelu, J.; Munvera, A.M.; Chukwuma, C.I.; Mkounga, P.; Mashele, S.S.; Makhafola, T.J.; McGaw, L.J. In vitro antiproliferative, anti-inflammatory effects and molecular docking studies of natural compounds isolated from Sarcocephalus pobeguinii (Hua ex Pobeg). Front. Pharmacol. 2023, 14, 1205414. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; Angeloni, C.; Freschi, M.; Lorenzini, A.; Prata, C.; Maraldi, T.; Hrelia, S. Combination of Epigallocatechin Gallate and Sulforaphane Counteracts In Vitro Oxidative Stress and Delays Stemness Loss of Amniotic Fluid Stem Cells. Oxid. Med. Cell Longev. 2018, 2018, 5263985. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Orthofer, R.; Lamuela-Raventós, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.; Barrow, C. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. BioMed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef] [PubMed]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of Reactive Oxygen Species in Aging and Age-Related Diseases: A Review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Gegotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Makhafola, T.J.; Elgorashi, E.E.; McGaw, L.J.; Verschaeve, L.; Eloff, J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef]
- Mannoubi, I.E. Impact of different solvents on extraction yield, phenolic composition, in vitro antioxidant and antibacterial activities of deseeded Opuntia stricta fruit. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 176–184. [Google Scholar] [CrossRef]
- Cosme, P.; Rodriguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Jalde, S.S.; Patel, R.V. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22002. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free. Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 2022, 178, 106163. [Google Scholar] [CrossRef]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-kappaB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tirpude, N.V.; Kumari, M.; Padwad, Y. Rutin prevents inflammation-associated colon damage via inhibiting the p38/MAPKAPK2 and PI3K/Akt/GSK3beta/NF-kappaB signalling axes and enhancing splenic Tregs in DSS-induced murine chronic colitis. Food Funct. 2021, 12, 8492–8506. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Yang, M.; Liu, M. Rutin prevents inflammation induced by lipopolysaccharide in RAW 264.7 cells via conquering the TLR4-MyD88-TRAF6-NF-kappaB signalling pathway. J. Pharm. Pharmacol. 2021, 73, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Um, J.Y.; Lee, J.Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-kappaB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H.; et al. Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces Cerevisiae as a Model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Zheng, B.; Zhang, L.; Zhang, F.; Zhang, Y.; Li, J.; Pu, G.; Zhang, L.; Wu, H. Network Pharmacology-Based Identification of Potential Targets of Lonicerae japonicae Flos Acting on Anti-Inflammatory Effects. BioMed Res. Int. 2021, 2021, 5507003. [Google Scholar] [CrossRef]
- He, Y.Q.; Yang, H.; Shen, Y.; Zhang, J.H.; Zhang, Z.G.; Liu, L.L.; Song, H.T.; Lin, B.; Hsu, H.Y.; Qin, L.P.; et al. Monotropein attenuates ovariectomy and LPS-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of NF-kappaB pathway. Chem. Biol. Interact. 2018, 291, 128–136. [Google Scholar] [CrossRef]
- Wu, M.; Lai, H.; Peng, W.; Zhou, X.; Zhu, L.; Tu, H.; Yuan, K.; Yang, Z. Monotropein: A comprehensive review of biosynthesis, physicochemical properties, pharmacokinetics, and pharmacology. Front. Pharmacol. 2023, 14, 1109940. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Xu, X.R.; Li, W.M.; Xia, K.; Wang, L.F.; Yang, X.C. Monotropein alleviates H2O2-induced inflammation, oxidative stress and apoptosis via NF-kappaB/AP-1 signaling. Mol. Med. Rep. 2020, 22, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Jia, H.; Zhang, Y.; Zhou, J.; Chen, X.; Wu, L.; Wang, J. Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling. Molecules 2022, 27, 8568. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gu, Q.; Nie, S.M.; Wang, J.D.; Zhao, H.; Zhai, B.W.; Zhang, M.Y.; Fu, Y.J. Untargeted metabolomics reveals the regulatory effect of geniposidic acid on lipid accumulation in HepG2 cells and Caenorhabditis elegans and validation in hyperlipidemic hamsters. Phytomed. Int. J. Phytother. Phytopharm. 2024, 125, 155295. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Li, X.; Chen, J. Geniposidic acid attenuates DSS-induced colitis through inhibiting inflammation and regulating gut microbiota. Phytother. Res. PTR 2023, 37, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Zhai, R.; Takemura, T.; Ouhara, K.; Taniguchi, Y.; Hamamoto, Y.; Fujimori, R.; Kajiya, M.; Matsuda, S.; Munenaga, S.; et al. Anti-Inflammatory Effects of Geniposidic Acid on Porphyromonas gingivalis-Induced Periodontitis in Mice. Biomedicines 2022, 10, 3096. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative iridoid glycosides and phenolic compounds from Veronica peregrina. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Zhu, G.; Wang, Y.; Zheng, G.; Kan, Q. Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2. Cell Cycle 2019, 18, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
| Plant Name | IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | ROS | NO | 15-LOX | |
| C. ilicifolia | 31.61 ± 1.35 | 21.29 ± 1.36 | >200 | 43.07 ± 2.08 | 21.10 ± 1.20 | 40.28 ± 1.35 |
| Ascorbic acid | 4.99 ± 0.20 * | 4.64 ± 0.85 * | 26.68 ± 1.34 * | 5.76 ± 0.87 * | n.d. | n.d. |
| Quercetin | n.d. | n.d. | n.d. | n.d. | 2.11 ± 1.01 * | n.d. |
| Gallic acid | n.d. | n.d. | n.d. | n.d. | n.d. | 22.08 ± 1.96 * |
| Plant Name | % Extraction Yield (g Extract/100 g Dry Material) | Phenolic Content (mgGAE/g Extract) | Flavonoid Content (mgQE/g Extract) |
|---|---|---|---|
| C. ilicifolia | 15.66 | 109.32 ± 5.26 | 23.63 ± 2.03 |
| Peak N° | RT (min) | [M-H]-(m/z) | Tentative Assignment (Compound Name) | Ontology | Molecular Formula | Total Score | Peak Height Intensity | Conc. in Extract vs. 3CQA (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.77 | 315.07 | Gentesic acid 5-O-glucoside | Phenolic glycosides | C13H16O9 | 7.20 | 2096 | 77 |
| 2 | 7.28 | 473.13 | Trans-Caffeic acid [apiosyl-(-glucosyl] ester | Hydroxycinnamic acid glycosides | C20H26O13 | 6.32 | 4933 | 182 |
| 3 | 7.69 | 373.11 | Secologanic acid | Terpene glycosides | C16H22O10 | 7.13 | 144,505 | 5342 |
| 4 | 8.23 | 353.08 | Chlorogenic acid 3CQA | Quinic acids and derivatives | C16H18O9 | 7.83 | 13,644 | 1191 |
| 5 | 9.23 | 373.11 | Geniposidic acid | Iridoid O-glycosides | C16H22O10 | 7.55 | 6376 | 236 |
| 6 | 9.64 | 375.12 | Loganic acid | Iridoid O-glycosides | C16H24O10 | 8.61 | 32,220 | 504 |
| 7 | 10.34 | 389.10 | Monotropein | Iridoid O-glycosides | C16H22O11 | 7.46 | 107,470 | 3973 |
| 8 | 10.63 | 353.08 | Chlorogenic acid 5CQA | Quinic acids and derivatives | C16H18O9 | 8.82 | 64,238 | 2375 |
| 9 | 10.99 | 373.11 | Geniposidic acid | Iridoid O-glycosides | C16H22O10 | 7.63 | 40,505 | 1497 |
| 10 | 13.51 | 403.12 | Oleoside 11-methyl ester | Terpene glycosides | C17H24O11 | 7.43 | 17,541 | 648 |
| 11 | 13.79 | 435.22 | Amphipaniculoside E | Fatty acyl glycosides of mono- and disaccharides | C20H36O10 | 5.52 | 4102 | 152 |
| 12 | 14.51 | 625.14 | Quercetin 3-glucosyl-galactoside | Flavonoid-3-O-glycosides | C27H29O17 | 7.22 | 625 | 23 |
| 13 | 15.47 | 581.22 | (7′R)-(+)-Lyoniresinol 9′-glucoside | Lignan glycosides | C28H38O13 | 6.25 | 636 | 24 |
| 14 | 15.81 | 609.14 | Quercetin robinobioside | Flavonoid-3-O-glycosides | C27H30O16 | 7.07 | 48,294 | 785 |
| 15 | 16.08 | 609.14 | Rutin | Flavonoid-3-O-glycosides | C27H30O16 | 8.09 | 225,648 | 8342 |
| 16 | 16.14 | 463.08 | Kaempferol 3-alpha-L-arabinopyranoside | Flavonoid-3-O-glycosides | C20H18O10 | 6.13 | 4716 | 174 |
| 17 | 16.46 | 463.08 | Quercetin 3-galactoside | Flavonoid-3-O-glycosides | C21H20O12 | 7.90 | 41,638 | 1539 |
| 18 | 17.15 | 515.12 | 3,4-Dicaffeoylquinic acid | Isoflavonoid O-glycosides | C25H24O12 | 6.67 | 800 | 30 |
| 19 | 17.46 | 503.25 | 4,7-Megastigmadiene-3,9-diol-9-[apiosyl-(1-6)-glucoside] | Fatty acyl glycosides of mono- and disaccharides | C24H40O11 | 5.94 | 73,694 | 724 |
| 20 | 17.59 | 515.12 | 3,5-Dicaffeoylquinic acid | Quinic acids and derivatives | C25H24O12 | 6.11 | 18,935 | 700 |
| 21 | 17.71 | 593.15 | Astragalin 7-rhamnoside | Flavonoid-7-O-glycosides | C27H30O15 | 8.12 | 109,553 | 4050 |
| 22 | 18.11 | 447.09 | Astragalin | Flavonoid-3-O-glycosides | C21H20O11 | 8.09 | 22,404 | 828 |
| 23 | 18.27 | 447.09 | Quercitrin | Flavonoid-3-O-glycosides | C21H20O11 | 6.76 | 12,741 | 471 |
| 24 | 18.79 | 515.11 | 4,5-Dicaffeoylquinic acid | Quinic acids and derivatives | C25H24O12 | 6.48 | 25,615 | 947 |
| 25 | 19.29 | 537.16 | Minecoside | Coumaric acids and derivatives | C25H30O13 | 5.95 | 93,070 | 3441 |
| 26 | 20.25 | 431.09 | Kaempferol rhamnoside | Flavonoid O-glycosides | C21H20O10 | 8.03 | 9675 | 358 |
| 27 | 20.78 | 535.18 | 8-Hydroxypinoresinol 8-glucoside | Lignan glycosides | C26H32O12 | 7.27 | 4710 | 174 |
| 28 | 20.96 | 835.28 | Sylvestroside II | Iridoid O-glycosides | C35H50O20 | 5.42 | 8788 | 325 |
| 29 | 21.95 | 759.23 | Adinoside D;(-)-Adinoside D | Terpene glycosides | C33H44O20 | 6.58 | 9653 | 357 |
| 30 | 22.63 | 207.06 | 6-Methoxymellein | 2-benzopyrans | C11H12O4 | 7.60 | 3160 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mfotie Njoya, E.; McGaw, L.J.; Makhafola, T.J. Investigating the Phytochemical Composition, Antioxidant, and Anti-Inflammatory Potentials of Cassinopsis ilicifolia (Hochst.) Kuntze Extract against Some Oxidative Stress and Inflammation Molecular Markers. Curr. Issues Mol. Biol. 2024, 46, 9639-9658. https://doi.org/10.3390/cimb46090573
Mfotie Njoya E, McGaw LJ, Makhafola TJ. Investigating the Phytochemical Composition, Antioxidant, and Anti-Inflammatory Potentials of Cassinopsis ilicifolia (Hochst.) Kuntze Extract against Some Oxidative Stress and Inflammation Molecular Markers. Current Issues in Molecular Biology. 2024; 46(9):9639-9658. https://doi.org/10.3390/cimb46090573
Chicago/Turabian StyleMfotie Njoya, Emmanuel, Lyndy J. McGaw, and Tshepiso J. Makhafola. 2024. "Investigating the Phytochemical Composition, Antioxidant, and Anti-Inflammatory Potentials of Cassinopsis ilicifolia (Hochst.) Kuntze Extract against Some Oxidative Stress and Inflammation Molecular Markers" Current Issues in Molecular Biology 46, no. 9: 9639-9658. https://doi.org/10.3390/cimb46090573





