Gibberellins Play an Essential Role in the Bud Growth of Petunia hybrida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Hormonal Application
2.3. Isolation of Genes
2.4. Analysis of Gene Expression
2.5. Arabidopsis Transformation and Phenotype Analysis
2.6. Data Analysis
3. Results
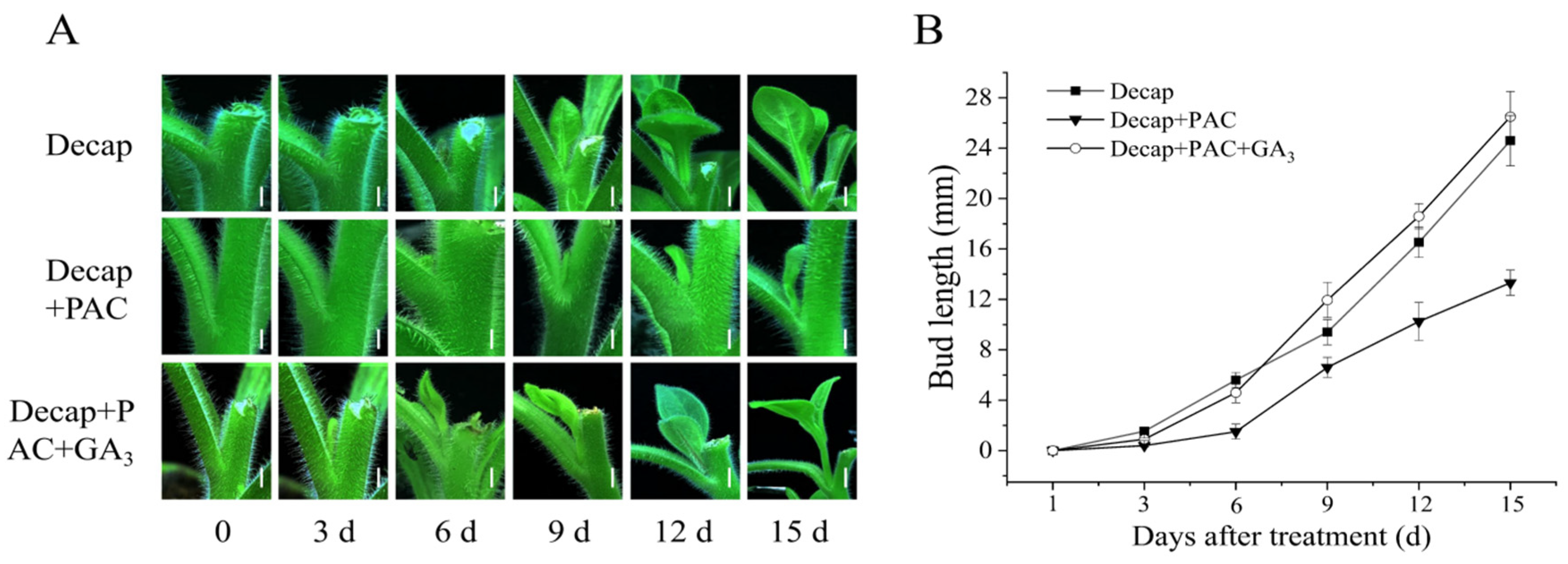
3.1. Exogenous GA3 Could Not Promote Axillary Bud Germination in Petunia
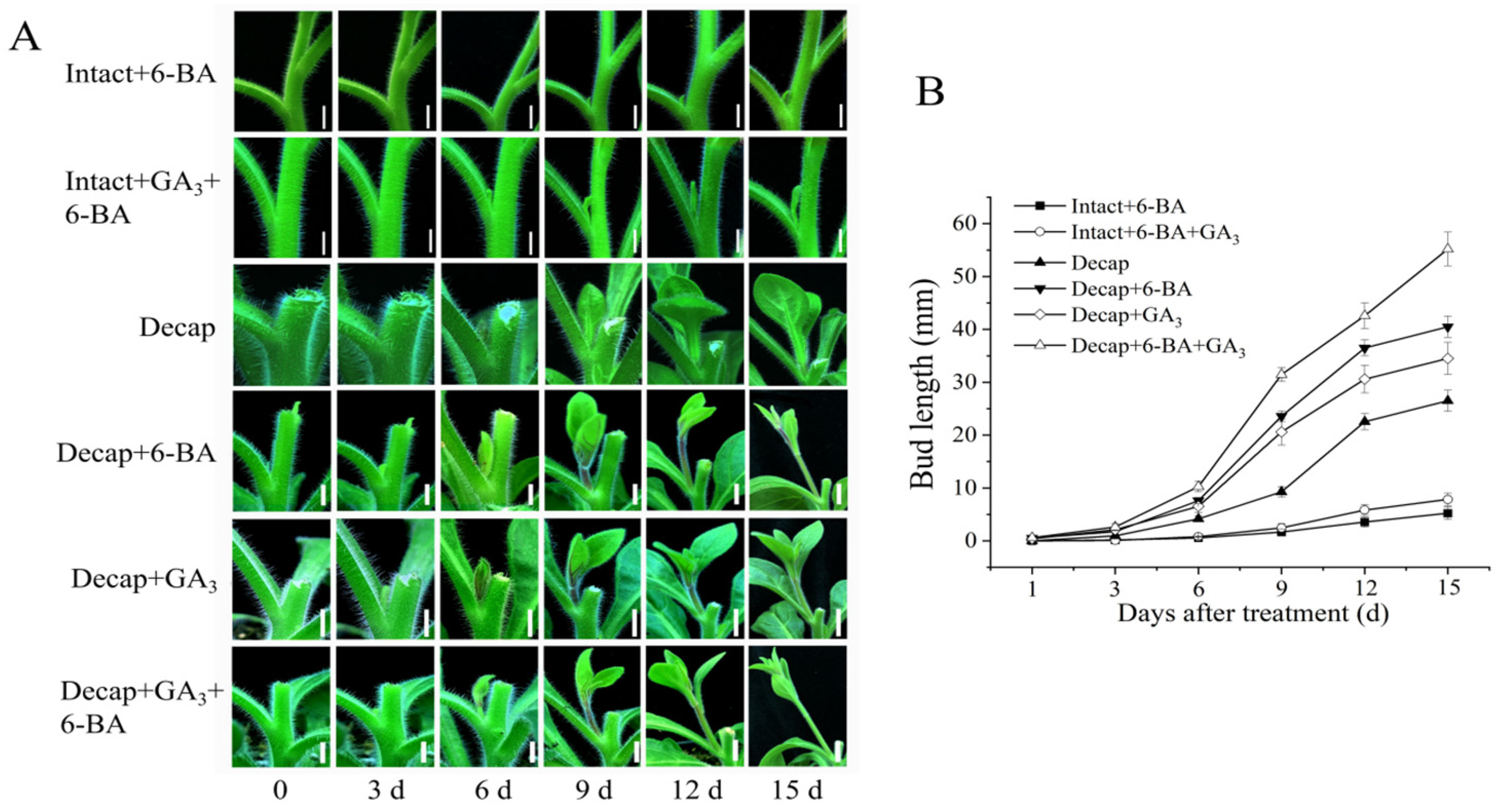
3.2. GA Promotes Bud Elongation
3.3. GA and CK Synergistically Promote Bud Elongation
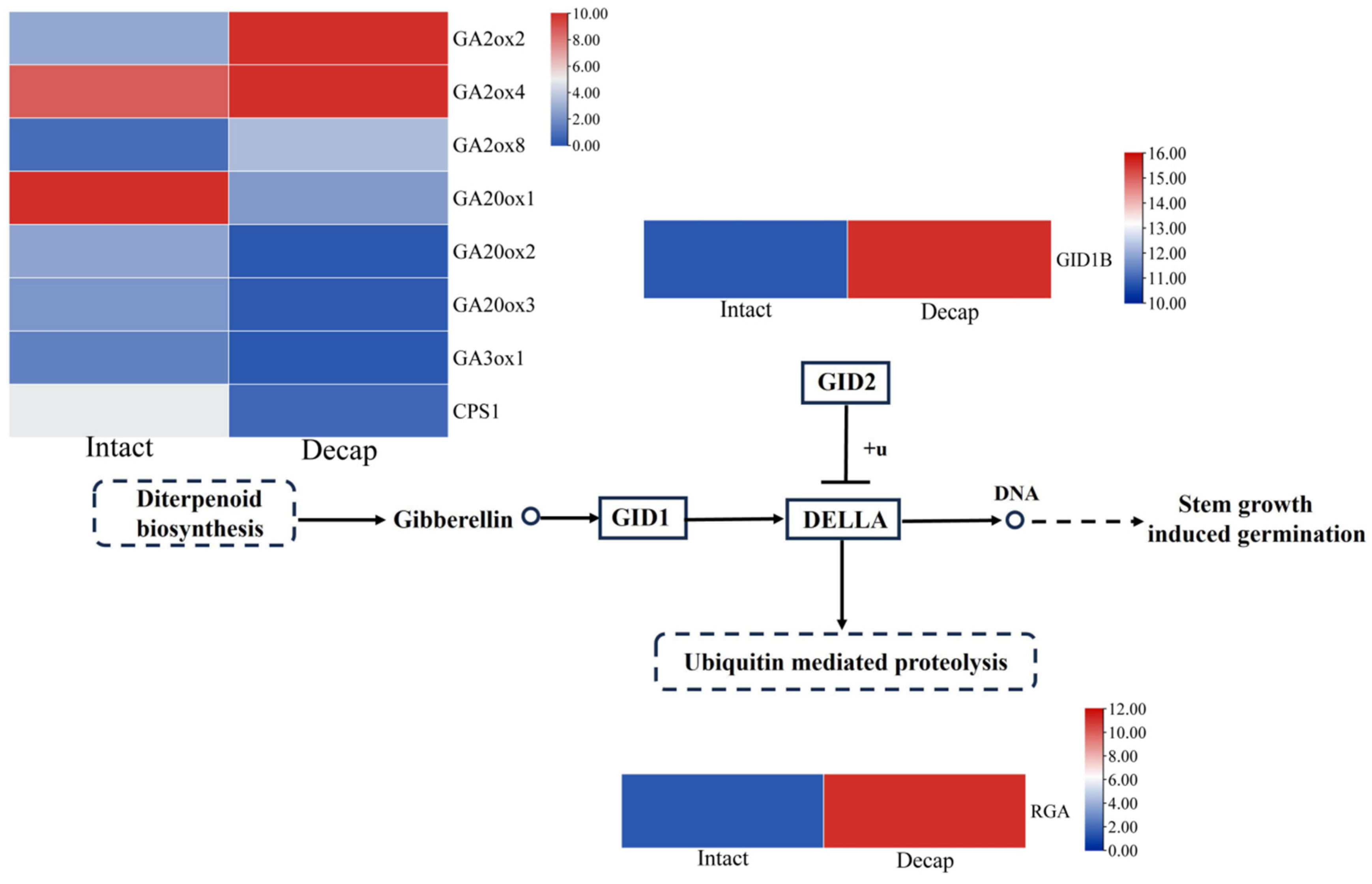
3.4. GA Pathway Genes Respond to Decapitation
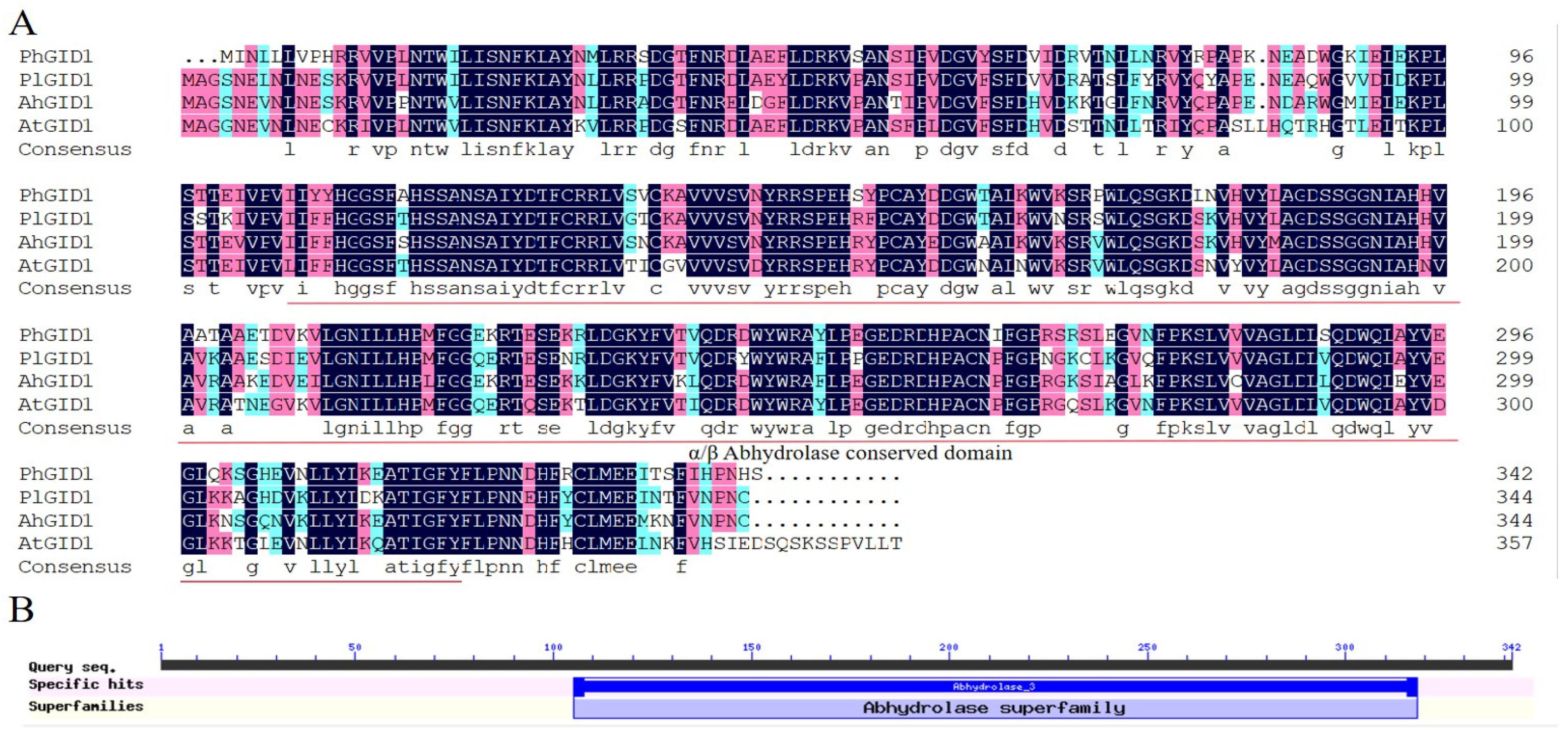
3.5. Cloning of PhGID1 and Sequence Analysis
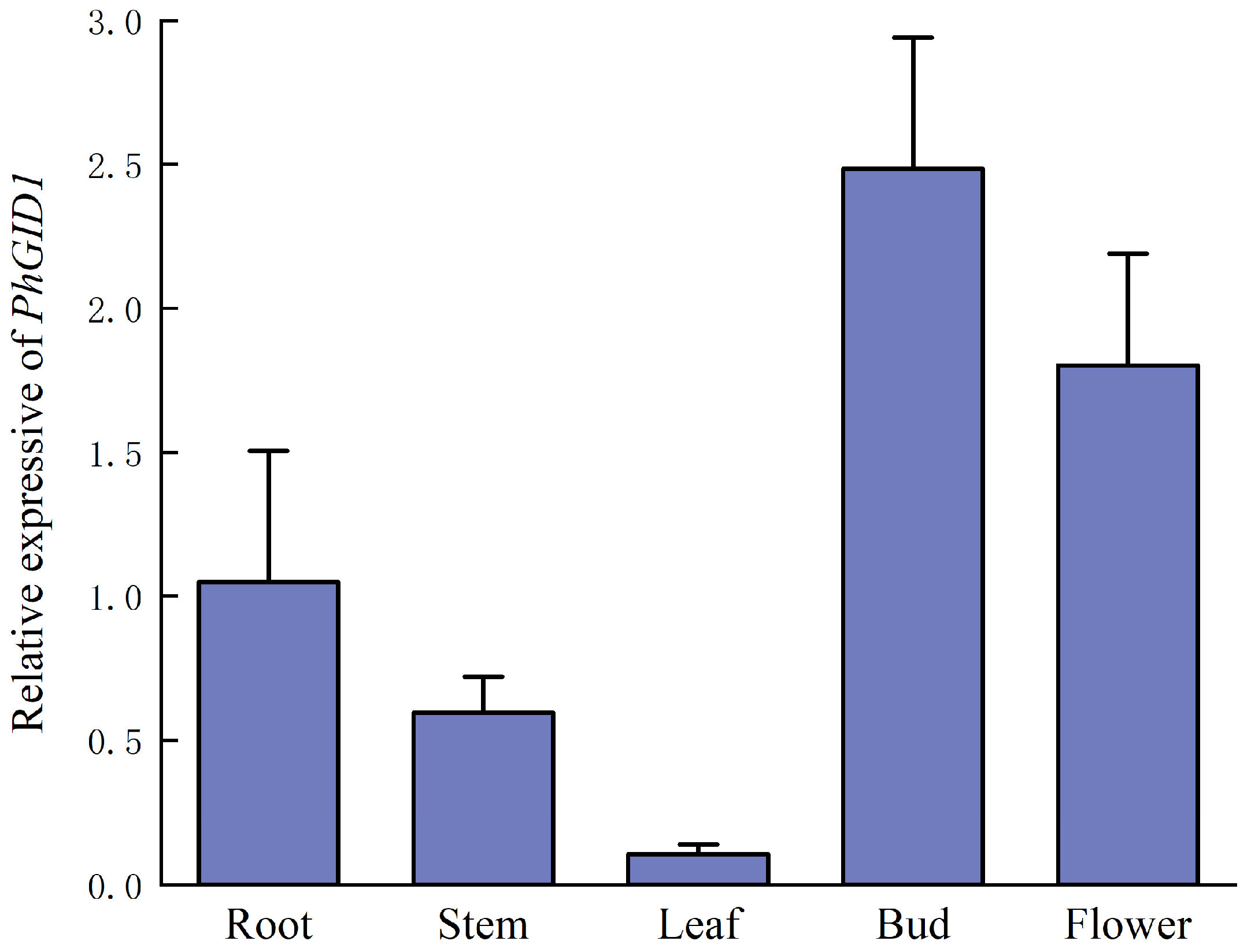
3.6. Analysis of Tissue-Specific Expression of PhGID1
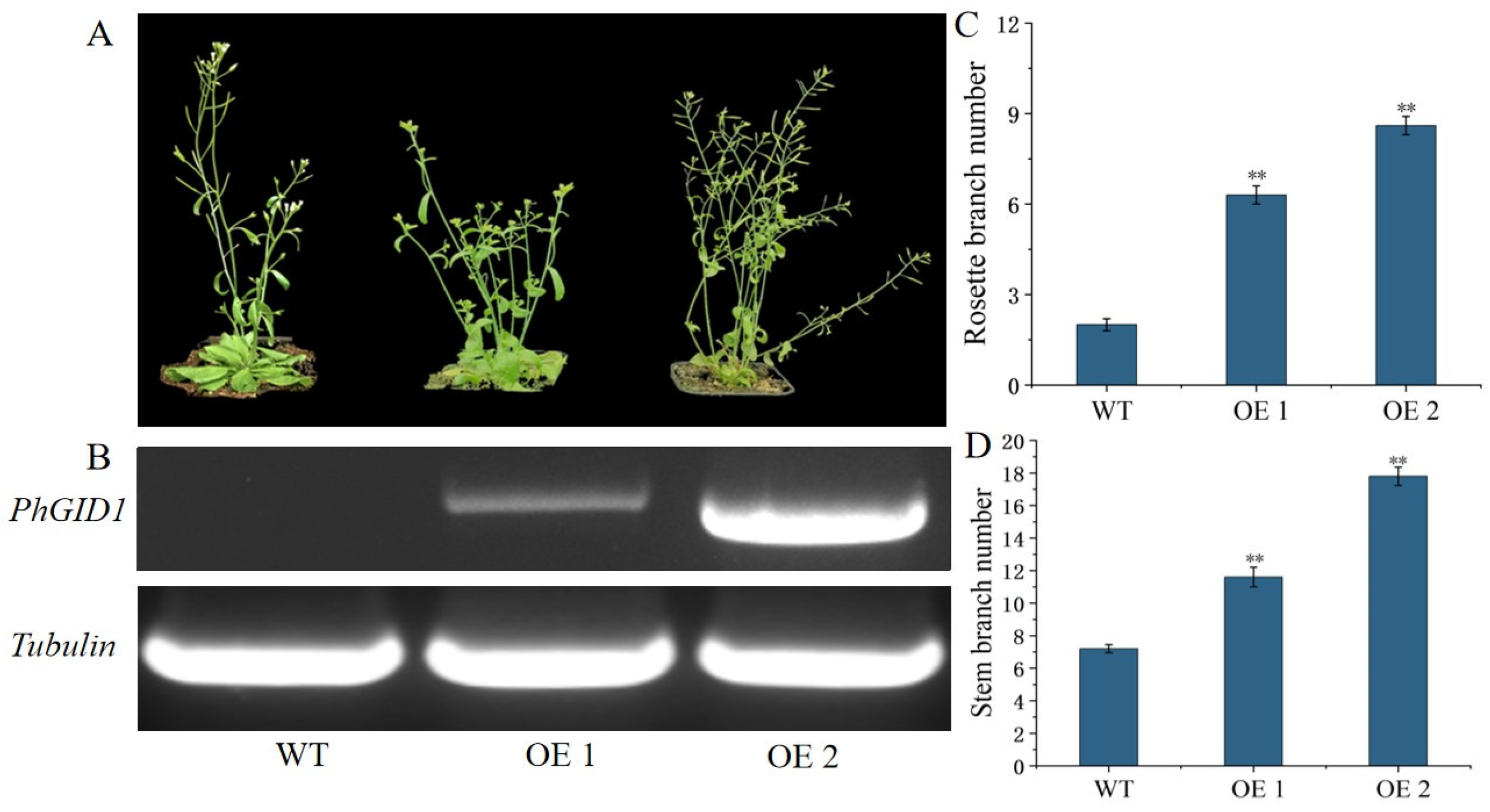
3.7. Overexpression of PhGID1 Increases Branch Number
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nahas, Z.; Ticchiarelli, F.; van Rongen, M.; Dillon, J.; Leyser, O. The activation of Arabidopsis axillary buds involves a switch from slow to rapid committed outgrowth regulated by auxin and strigolactone. New Phytol. 2024, 242, 1084–1097. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L. Epigenetic regulation of gibberellin metabolism and signaling. Plant Cell Physiol. 2020, 61, 1912–1918. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef]
- Hirano, K.; Ueguchi-Tanaka, M.; Matsuoka, M. GID1-mediated gibberellin signaling in plants. Trends Plant Sci. 2008, 13, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Koorneef, M.; Elgersma, A.; Hanhart, C.J.; van Loenen-Martinet, E.P.; van Rijn, L.; Zeevaart, J.A.D. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol. Plant. 1985, 65, 33–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Wang, L.; Wang, J.; Wang, Q.; Yu, P.; Bai, M.; Fan, M. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity. J. Integr. Plant Biol. 2020, 62, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.F.; Yang, S.Y.; Chen, K.T.; Hsing, Y.I.; Zeevaart, J.A.; Chen, L.J.; Yu, S.M. A Novel Class of Gibberellin 2-Oxidases Control Semidwarfism, Tillering, and Root Development in Rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bello, L.; Moritz, T.; López-Díaz, I. Silencing C19-GA2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. J. Exp. Bot. 2015, 66, 5897–5910. [Google Scholar] [CrossRef]
- Agharkar, M.; Lomba, P.; Altpeter, F.; Zhang, H.; Kenworthy, K.; Lange, T. Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnol. J. 2007, 5, 791–801. [Google Scholar] [CrossRef]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Tenreira, T.; Lange, M.J.P.; Lange, T.; Bres, C.; Labadie, M.; Monfort, A.; Hernould, M.; Rothan, C.; Denoyes, B. A Specific Gibberellin 20-Oxidase Dictates the Flowering-Runnering Decision in Diploid Strawberry. Plant Cell 2017, 29, 2168–2182. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.; Chen, M.S.; Pan, B.Z.; Ye, K.; Xu, Z.F. Gibberellin Promotes Shoot Branching in the Perennial Woody Plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Elfving, D.C.; Visser, D.B.; Henry, J.L. Gibberellins Stimulate Lateral Branch Development in Young Sweet Cherry Trees in the Orchard. Int. J. Fruit Sci. 2011, 11, 41–54. [Google Scholar] [CrossRef]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of Meristem Activity and Sprout Growth in Potato Tubers Require Both Cytokinin and Gibberellin. Plant Physiol. 2010, 155, 776–796. [Google Scholar] [CrossRef]
- O’Neill, D.P.; Ross, J.J. Auxin Regulation of the Gibberellin Pathway in Pea. Plant Physiol. 2002, 130, 1974–1982. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhang, J.Y.; Peng, L.; Dong, S.T.; Liu, P.; Zhao, B. Effects of endogenous hormones on tiller development process of different maize varieties. Sci. Agric. Sin. 2012, 45, 840–847. [Google Scholar] [CrossRef]
- Nakamura, H.; Xue, Y.-L.; Miyakawa, T.; Hou, F.; Qin, H.-M.; Fukui, K.; Shi, X.; Ito, E.; Ito, S.; Park, S.-H.; et al. Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 2013, 4, 2613. [Google Scholar] [CrossRef]
- Tan, M.; Li, G.; Liu, X.; Cheng, F.; Ma, J.; Zhao, C.; Zhang, D.; Han, M. Exogenous application of GA3 inactively regulates axillary bud outgrowth by influencing of branching-inhibitors and bud-regulating hormones in apple (Malus domestica Borkh.). Mol. Genet. Genom. 2018, 293, 1547–1563. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Pramanik, K.; Pal, P.; Soren, T.; Mitra, S.; Ghosh, P.K.; Sarkar, A.; Maiti, T.K. In silico structural, functional and phylogenetic analysis of Klebsiella phytases. J. Plant Biochem. Biotechnol. 2018, 27, 362–372. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- González-Grandío, E.; Poza-Carrión, C.; Sorzano, C.O.S.; Cubas, P. BRANCHED1Promotes Axillary Bud Dormancy in Response to Shade in Arabidopsis. Plant Cell 2013, 25, 834–850. [Google Scholar] [CrossRef] [PubMed]
- Rae, G.M.; David, K.; Wood, M. The Dormancy Marker DRM1/ARP Associated with Dormancy but a Broader Role In Planta. Dev. Biol. J. 2013, 2013, 632524. [Google Scholar] [CrossRef]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An Update on the Signals Controlling Shoot Branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef]
- Cao, D.; Chabikwa, T.; Barbier, F.; Dun, E.A.; Fichtner, F.; Dong, L.; Kerr, S.C.; Beveridge, C.A. Auxin-independent effects of apical dominance induce changes in phytohormones correlated with bud outgrowth. Plant Physiol. 2023, 192, 1420–1434. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Wang, L.; Gong, C.; Chen, B.; Du, Q.; Zhang, D. Single nucleotide polymorphisms in two GID1 orthologs associate with growth and wood property traits in Populus tomentosa. Tree Genet. Genomes 2016, 12, 109. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′ to 3′) |
|---|---|
| PhGID1-F | ATGATCAATTTGCTTTTAGTACC |
| PhGID1-R | CTATGAATGGTTAGGATGGATGA |
| qRT-PhGID1-F | CGACGGTCACCTGAACATAG |
| qRT-PhGID1-R | TTCTCGGATTCGGTCCTTTT |
| PhGID1-1300-F | CCAAATCGACTCTAGAATGATCAATTTGCTTTTAGTACC |
| PhGID1-1300-R | CCACTAGTATTTAAATGCTATGAATGGTTAGGATGGATGA |
| PhBRC1-F | CCCATTTGCTCATCTTTATTC |
| PhBRC1-R | CTGCCACTTTGCTTACTCATA |
| PhDRM1-F | GAATGTGTGGAGGAGTGTTTTT |
| PhDRM1-R | CCATATTGCACCCCCTTTTGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Deng, X.; Yao, H.; Ji, S.; Dong, L. Gibberellins Play an Essential Role in the Bud Growth of Petunia hybrida. Curr. Issues Mol. Biol. 2024, 46, 9906-9915. https://doi.org/10.3390/cimb46090590
Deng J, Deng X, Yao H, Ji S, Dong L. Gibberellins Play an Essential Role in the Bud Growth of Petunia hybrida. Current Issues in Molecular Biology. 2024; 46(9):9906-9915. https://doi.org/10.3390/cimb46090590
Chicago/Turabian StyleDeng, Jichu, Xinyi Deng, Huanyu Yao, Shunhua Ji, and Lili Dong. 2024. "Gibberellins Play an Essential Role in the Bud Growth of Petunia hybrida" Current Issues in Molecular Biology 46, no. 9: 9906-9915. https://doi.org/10.3390/cimb46090590




