Engineering of Substrate-Binding Domain to Improve Catalytic Activity of Chondroitin B Lyase with Semi-Rational Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
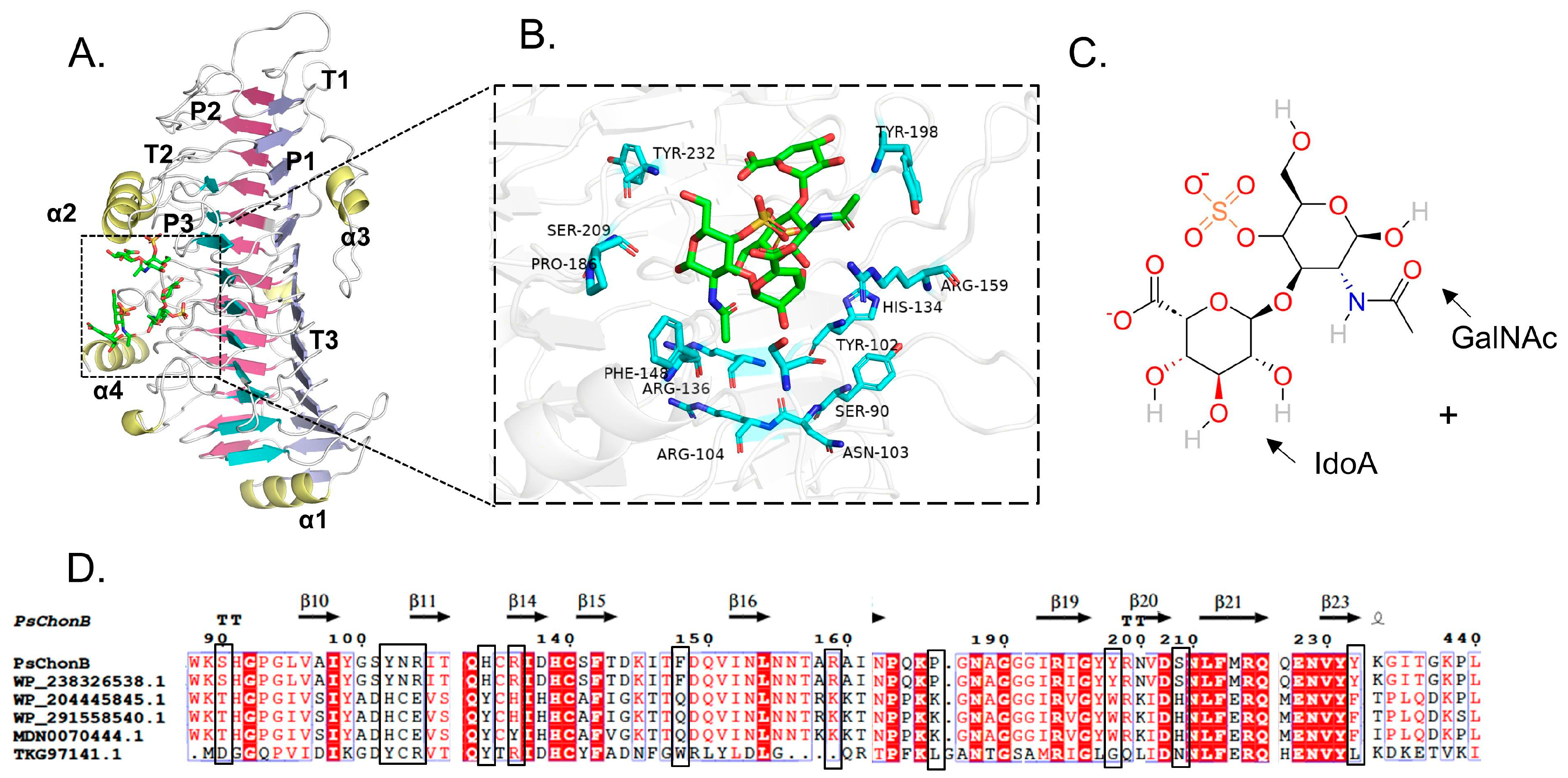
2.2. Homologous Modeling and Molecular Docking
2.3. Construction of the Mutation Library
2.4. Mutagenesis of PsChonB
2.5. Expression and Purification of PsChonB and Mutants
2.6. Assay of Enzyme Activity
2.7. Kinetic Parameters of PsChonB
3. Results
3.1. Site-Directed Mutagenesis of PsChonB
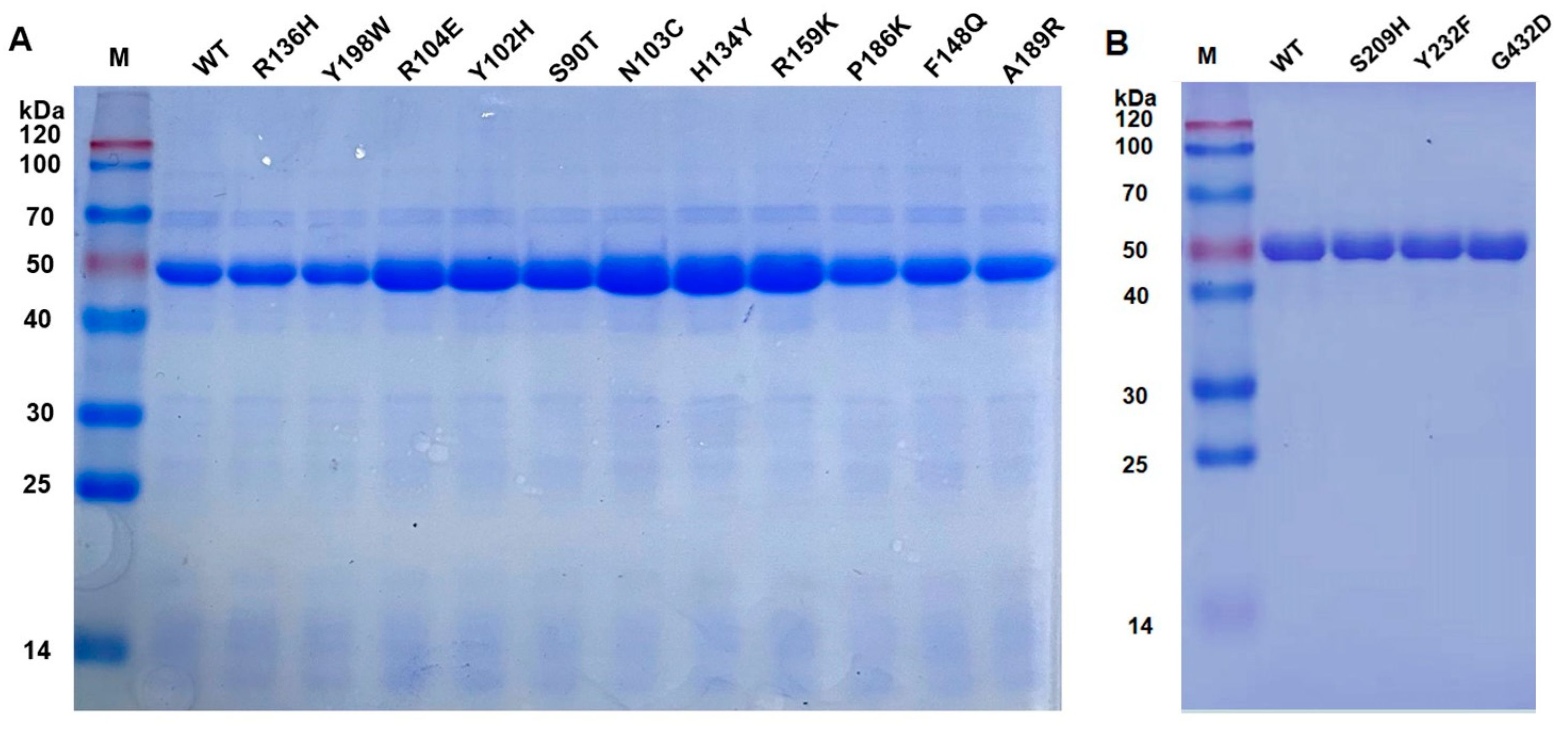
3.2. Expression of Wild-Type PsChonB and Its Mutants
3.3. Characterization of the PsChonB
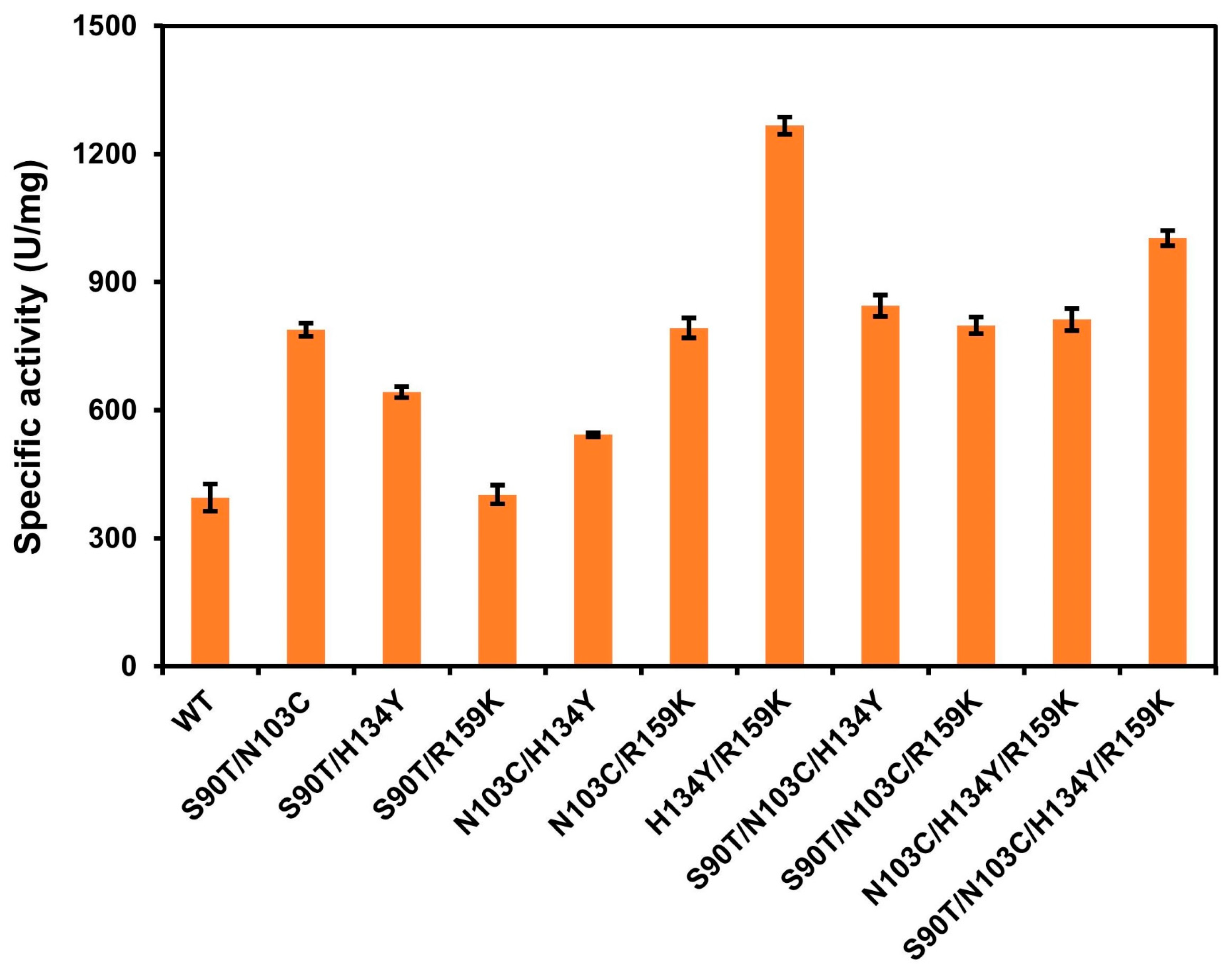
3.4. Iterative Mutation and Characterization of the Combinatorial Mutants
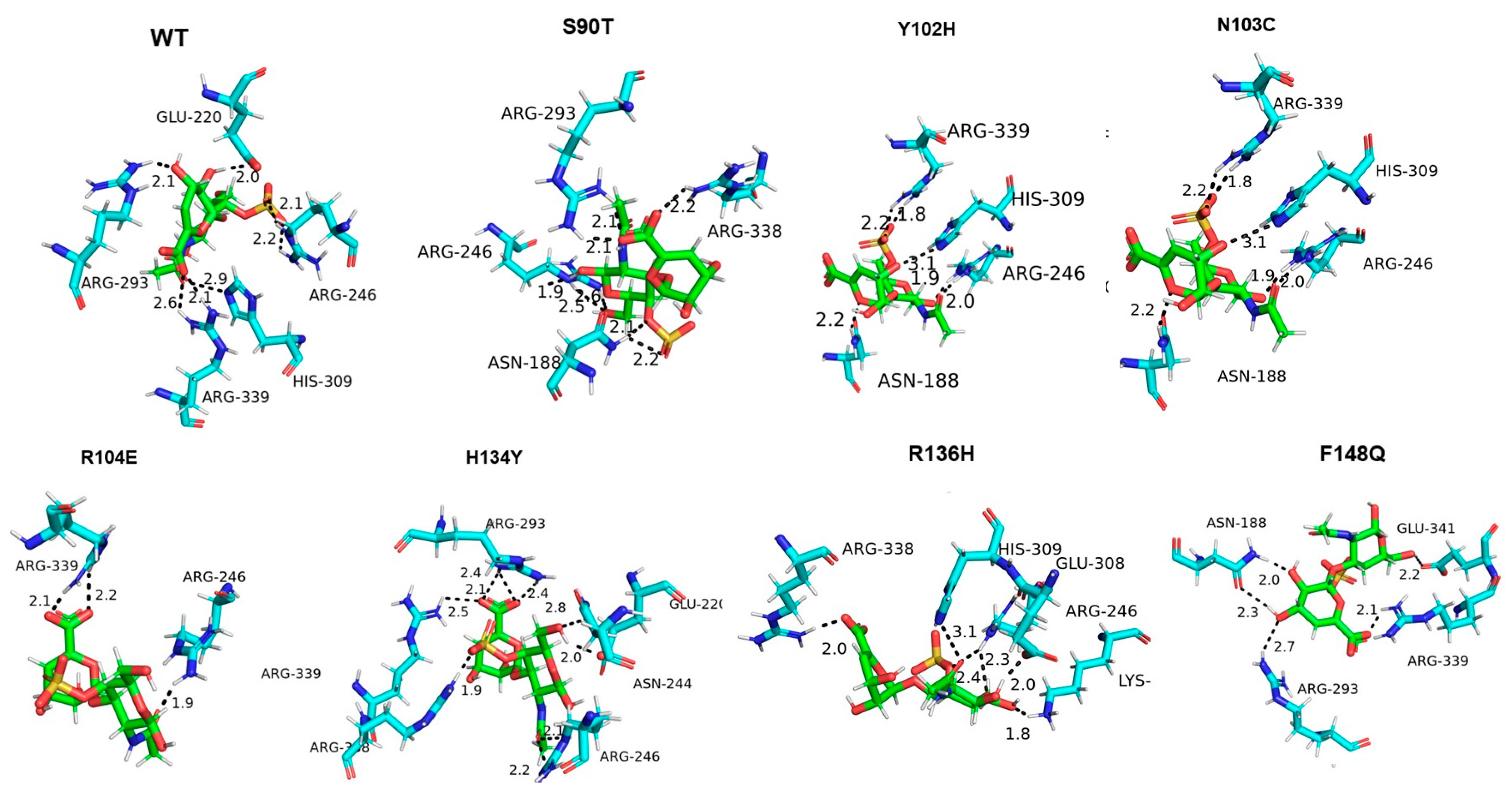
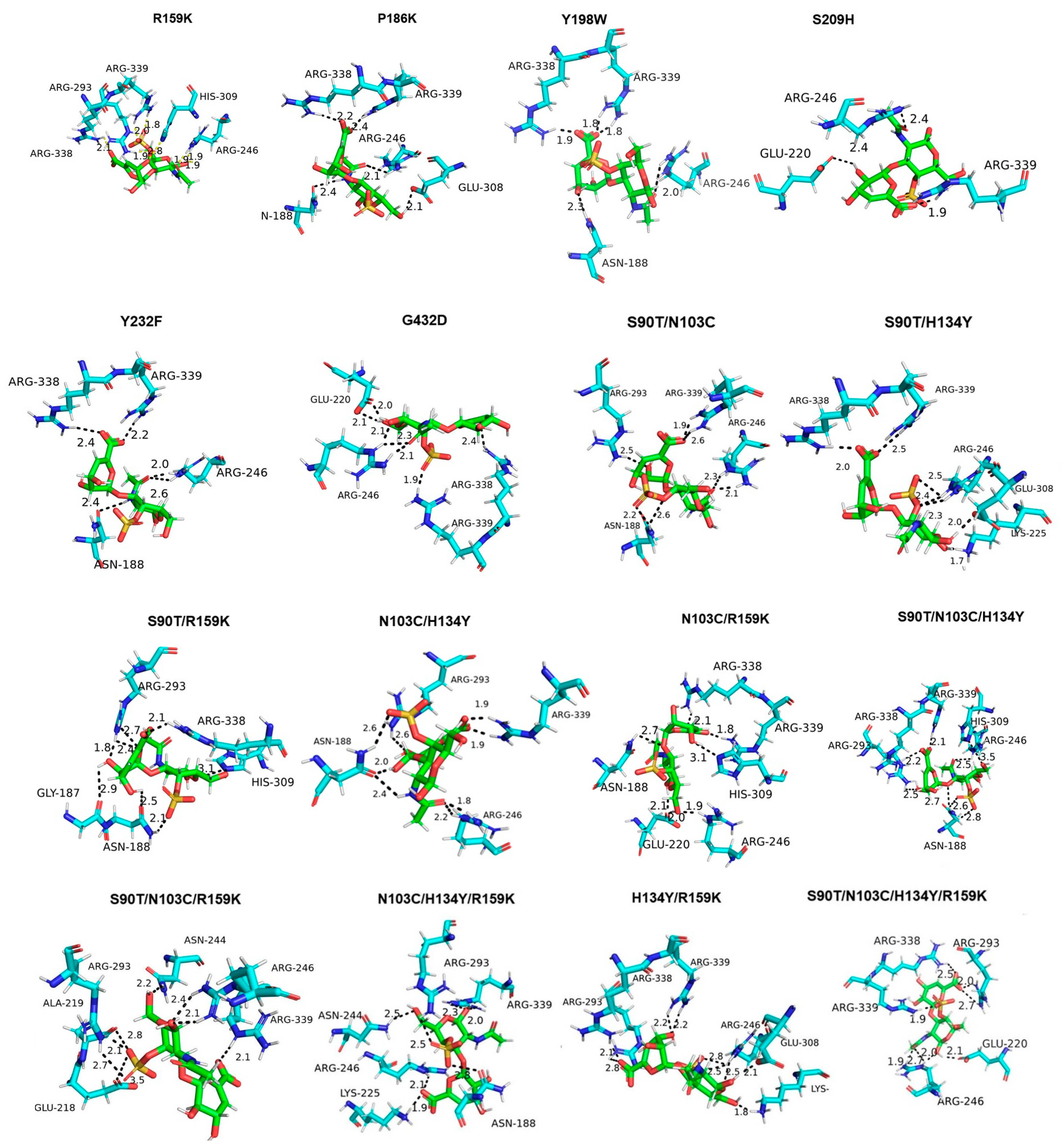
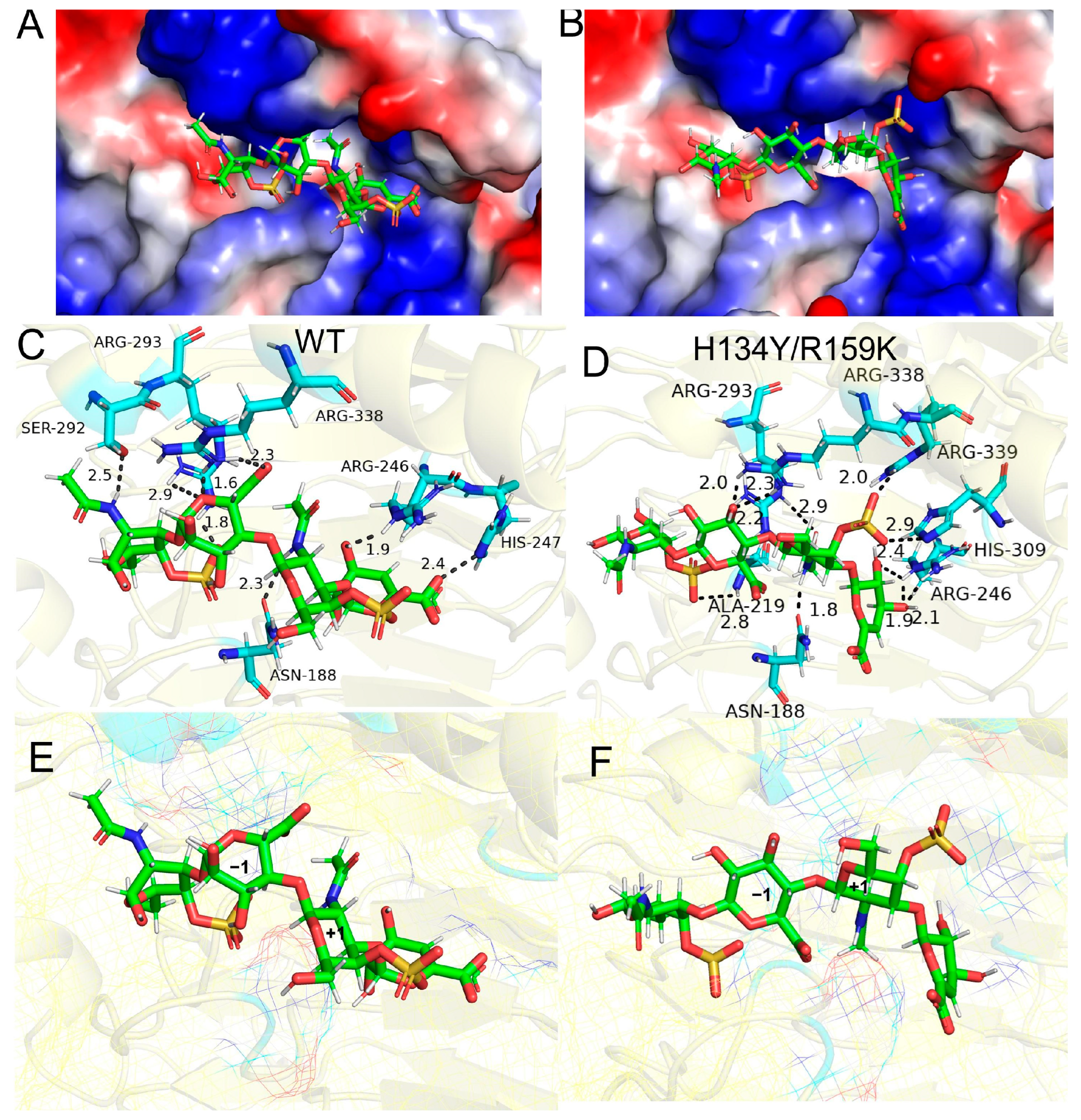
3.5. Molecular Docking of Enzyme and Substrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linhardt, R.J.; Galliher, P.M.; Cooney, C.L. Polysaccharide Lyases. Appl. Biochem. Biotechnol. 1986, 12, 135–176. [Google Scholar] [CrossRef] [PubMed]
- Michelacci, Y.M.; Dietrich, C.P. Structure of Chondroitin Sulfates. Analyses of the Products Formed from Chondroitin Sulfates A and C by the Action of the Chondroitinases C and AC from Flavobacterium heparinum. Biochim. Biophys. Acta 1976, 451, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Su, H.; Wang, X.; Tang, L.; Hu, J.; Yu, W.; Han, F. Cloning and Characterization of a Novel Chondroitinase ABC Categorized into a New Subfamily of Polysaccharide Lyase Family 8. Int. J. Biol. Macromol. 2020, 164, 3762–3770. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, A.L.; Fink, D.; Blain, F.; Zhang-Sun, G.; Laliberte, M.; Bennett, D.C.; Gu, K.; Zimmermann, J.J.F.; Su, H. Isolation and Expression in Escherichia coli of cslA and cslB, Genes Coding for the Chondroitin Sulfate-Degrading Enzymes Chondroitinase AC and Chondroitinase B, Respectively, from Flavobacterium heparinum. Appl. Environ. Microbiol. 2000, 66, 29–35. [Google Scholar] [CrossRef]
- Volpi, N.; Sandri, I.; Venturelli, T. Activity of Chondroitin ABC Lyase and Hyaluronidase on Free-Radical Degraded Chondroitin Sulfate. Carbohydr. Res. 1995, 279, 193–200. [Google Scholar] [CrossRef]
- Sugimura, T.; Kato, F.; Mimatsu, K.; Takenaka, O.; Iwata, H. Experimental Chemonucleolysis with Chondroitinase ABC in Monkeys. Spine 1996, 21, 161–165. [Google Scholar] [CrossRef]
- Denholm, E.M.; Lin, Y.-Q.; Silver, P.J. Anti-Tumor Activities of Chondroitinase AC and Chondroitinase B: Inhibition of Angiogenesis, Proliferation and Invasion. Eur. J. Pharmacol. 2001, 416, 213–221. [Google Scholar] [CrossRef]
- Prabhakar, V.; Capila, I.; Soundararajan, V.; Raman, R.; Sasisekharan, R. Recombinant Expression, Purification, and Biochemical Characterization of Chondroitinase ABC II from Proteus vulgaris. J. Biol. Chem. 2009, 284, 974–982. [Google Scholar] [CrossRef]
- van der Smissen, A.; Hintze, V.; Scharnweber, D.; Moeller, S.; Schnabelrauch, M.; Majok, A.; Simon, J.C.; Anderegg, U. Growth Promoting Substrates for Human Dermal Fibroblasts Provided by Artificial Extracellular Matrices Composed of Collagen I and Sulfated Glycosaminoglycans. Biomaterials 2011, 32, 8938–8946. [Google Scholar] [CrossRef]
- Wang, S.-M.; Su, T.-T.; Zhang, Q.-D.; Guan, J.-W.; He, J.; Gu, L.-C.; Li, F.-C. Comparative Study of Two Chondroitin Sulfate/Dermatan Sulfate 4-O-Sulfatases with High Identity. Front. Microbiol. 2019, 10, 1309. [Google Scholar] [CrossRef]
- Uygun, B.E.; Stojsih, S.E.; Matthew, H.W.T. Effects of Immobilized Glycosaminoglycans on the Proliferation and Differentiation of Mesenchymal Stem Cells. Tissue Eng. Part A 2009, 15, 3499–3512. [Google Scholar] [CrossRef] [PubMed]
- Oberkersch, R.; Maccari, F.; Bravo, A.I.; Volpi, N.; Gazzaniga, S.; Calabrese, G.C. Atheroprotective Remodelling of Vascular Dermatan Sulfate Proteoglycans in Response to Hypercholesterolaemia in a Rat Model. Int. J. Exp. Pathol. 2014, 95, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.P.; Carneiro, G.D.; Sielski, M.S.; Barbosa, G.O.; Werneck, C.C.; Vicente, C.P. Combined Dermatan Sulfate and Endothelial Progenitor Cell Treatment: Action on the Initial Inflammatory Response after Arterial Injury in C57BL/6 Mice. Cytotherapy 2015, 17, 1447–1464. [Google Scholar] [CrossRef] [PubMed]
- Bucay, V.; Gold, M.H.; Andriessen, A. Low Molecular Weight Heparan Sulfate Containing Facial Skin Care for Reducing Inflammation and Restoring Aged-Skin Homeostasis. J. Cosmet. Dermatol. 2020, 19, 1851–1856. [Google Scholar] [CrossRef]
- Ma, C.; Yu, M.; Huang, Z.; Wang, J.; Zhao, X.; Kang, C.; Xu, H.; Wang, Y.; Hou, H. Oral Administration of Hydrolysates of Cartilage Extract in the Prevention of Osteoarthritis. J. Funct. Foods 2021, 78, 104376. [Google Scholar] [CrossRef]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The Current Use and Evolving Landscape of Nutraceuticals. Pharmacol. Res. 2022, 175, 10600. [Google Scholar] [CrossRef]
- Wang, H.; Betti, M. Supplementation of Chondroitin Sulfate-Oligosaccharides in Skim Bovine Milk Improves Fe Uptake in a Human Intestinal Caco-2 Cell Line. J. Funct. Foods 2018, 46, 556–566. [Google Scholar] [CrossRef]
- Wu, J.; Ji, Y.; Su, N.; Li, Y.; Liu, X.; Mei, X.; Zhou, Q.; Zhang, C.; Xing, X. Establishment of Chondroitin B Lyase-Based Analytical Methods for Sensitive and Quantitative Detection of Dermatan Sulfate in Heparin. Carbohydr. Polym. 2016, 144, 338–345. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Wan, J.; Zhao, L.; Liu, Y.; Wei, Y.; Ouyang, Z. Cloning, Prokaryotic Expression, and Enzyme Activity of a UDP-Glucose Flavonoid 3-o-Glycosyltransferase from Mulberry (Morus alba L.) Leaves. Pharmacogn. Mag. 2020, 16, 441–447. [Google Scholar]
- Tian, X.; Chen, Y.; Peng, Z.; Lin, Q.; Sun, A. NEDD4 E3 Ubiquitin Ligases: Promising Biomarkers and Therapeutic Targets for Cancer. Biochem. Pharmacol. 2023, 214, 115641. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Piccinocchi, R.; Buccato, D.G.; De Lellis, L.F.; Baldi, A.; El-Seedi, H.R.; Khalifa, S.A.M.; Piccinocchi, G.; Xiao, X.; et al. Efficacy of Digestive Enzyme Supplementation in Functional Dyspepsia: A Monocentric, Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Biomed. Pharmacother. 2023, 169, 115858. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, T.; Saito, H.; Habuchi, O.; Suzuki, S. Purification and Properties of Bacterial Chondroitinases and Chondrosulfatases. J. Biol. Chem. 1968, 243, 1523–1535. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, D.; Wang, S.; Wei, L.; Wang, W.; Li, F. Identification and Biochemical Characterization of a Novel Chondroitin Sulfate/Dermantan Sulfate Lyase from Photobacterium sp. Int. J. Biol. Macromol. 2020, 165, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.C.; Mu, Y.L.; Gu, X.Y.; Xu, X.N.; Guo, T.T.; Kong, J. Site-Directed Mutation of β-Galactosidase from Streptococcus Thermophilus for Galactooligosaccharide-Enriched Yogurt Making. J. Dairy Sci. 2022, 105, 940–949. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, J.; Zhu, J.; Chen, S.; Han, T.; Zhang, Y.; Gao, R.; Xie, G.; Guo, Z. Molecular Dynamics Simulation Guided Distal Mutation of Thermotoga Naphthophila β-Glucosidase for Significantly Enhanced Synthesis of Galactooligosaccharides and Expanded Product Scope. Int. J. Biol. Macromol. 2022, 210, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ratananikom, K.; Choengpanya, K.; Tongtubtim, N.; Charoenrat, T.; Withers, S.G.; Kongsaeree, P.T. Mutational Analysis in the Glycone Binding Pocket of Dalbergia Cochinchinensis β-Glucosidase to Increase Catalytic Efficiency toward Mannosides. Carbohydr. Res. 2013, 373, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Peng, J.; Zhao, Y.; Wu, M.; Chen, S.; Shao, J.; Wang, X.; Xia, G.; Shen, Y. Obtaining Acid-Sensitive Prosaikogenin F by Enzymatic Hydrolysis of Saikosaponin A. Pharmacogn. Mag. 2023, 19, 689–699. [Google Scholar] [CrossRef]
- Su, W.-B.; Zhu, C.-Y.; Zhou, H.-P.; Gao, J.; Zhang, Y.-W. A Single Site Mutation Significantly Improves the Thermostability and Activity of Heparinase I from Bacteroides eggerthii. Biocatal. Biotransform. 2022, 40, 226–232. [Google Scholar] [CrossRef]
- Zhou, H.-P.; Wang, D.-R.; Xu, C.-L.; Zhang, Y.-W. Combination of Engineering the Substrate and Ca2+ Binding Domains of Heparinase I to Improve the Catalytic Activity. Prep. Biochem. Biotechnol. 2023, 53, 1297–1305. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Fan, X.-M.; Yu, S.; Zhang, J.-Y.; Gao, J.; Zhang, Y.-W. Engineering the Terminal Regions of Chondroitinase AC to Improve the Thermostability and Activity. Mol. Catal. 2024, 557, 113994. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, Y.; Zhao, L.; Cao, R. Improvement of the Catalytic Performance of Chitosanase Csn-PD from Paenibacillus Dendritiformis by Semi-Rational Design. Int. J. Biol. Macromol. 2024, 264, 130753. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gu, S.; Zhu, J.; Ro, K.-S.; Zhao, L.; Du, L.; Xie, J.; Wei, D. Screening, Cloning and Expression of β-Galactosidase from Lactic Acid Bacteria and Semi-Rational Design for the Improvement of Transgalactosylation Activity. Process Biochem. 2024, 140, 19–31. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Z.; Gu, Q.; Yu, X. Semi-Rational Design for Enhancing Thermostability of Culex Pipiens Acetylcholinesterase and Sensitivity Analysis of Acephate. Sci. Total Environ. 2024, 934, 173282. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, T.; Li, C.; Gao, W.; Liu, Z.; Miao, M. Semi-Rationally Designed Site-Saturation Mutation of Helicobacter Pylori α-1,2-Fucosyltransferase for Improved Catalytic Activity and Thermostability. Int. J. Biol. Macromol. 2024, 259, 129316. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Y.; Tian, M.; Tang, Y.-L.; Han, K.-K.; Yu, S.; Ma, X.-L.; Zhang, Y.-W. A Highly Active and Stable Chondroitin B Lyase from Pedobacter Schmidteae: Cloning, Expression, and Characterization. Biocatal. Agric. Biotechnol. 2023, 54, 102916. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Vmax (U/mg) | Km (mg/mL) | |
|---|---|---|
| WT | 406.72 ± 20.36 | 0.36 ± 0.03 |
| S90T | 895.41 ± 48.66 | 0.45 ± 0.07 |
| N103C | 956.22 ± 74.19 | 0.38 ± 0.09 |
| H134Y | 933.28 ± 40.00 | 0.31 ± 0.04 |
| R159K | 844.08 ± 52.82 | 0.43 ± 0.08 |
| S90T/N103C | 804.01 ± 75.51 | 0.42 ± 0.12 |
| S90T/H134Y | 664.59 ± 70.53 | 0.57 ± 0.09 |
| S90T/R159K | 417.61 ± 20.02 | 0.20 ± 0.04 |
| N103C/H134Y | 586.53 ± 21.46 | 0.31 ± 0.04 |
| N103C/R159K | 800.00 ± 30.20 | 0.33 ± 0.04 |
| H134Y/R159K | 1392.30 ± 103.98 | 0.37 ± 0. 08 |
| S90T/N103C/H134Y | 867.63 ± 61.70 | 0.29 ± 0.07 |
| S90T/N103C/R159K | 837.51 ± 31.82 | 0.32 ± 0.04 |
| N103C/H134Y/R159K | 860.98 ± 33.26 | 0.39 ± 0.05 |
| S90T/N103C/H134Y/R159K | 1051.98 ± 43.09 | 0.35 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Xu, Y.-Y.; Li, Y.-N.; Yu, S.; Wang, Y.-L.; Ma, X.-L.; Zhang, Y.-W. Engineering of Substrate-Binding Domain to Improve Catalytic Activity of Chondroitin B Lyase with Semi-Rational Design. Curr. Issues Mol. Biol. 2024, 46, 9916-9927. https://doi.org/10.3390/cimb46090591
Tian M, Xu Y-Y, Li Y-N, Yu S, Wang Y-L, Ma X-L, Zhang Y-W. Engineering of Substrate-Binding Domain to Improve Catalytic Activity of Chondroitin B Lyase with Semi-Rational Design. Current Issues in Molecular Biology. 2024; 46(9):9916-9927. https://doi.org/10.3390/cimb46090591
Chicago/Turabian StyleTian, Miao, Yuan-Yuan Xu, Yang-Nan Li, Shen Yu, Yi-Lin Wang, Xiao-Lai Ma, and Ye-Wang Zhang. 2024. "Engineering of Substrate-Binding Domain to Improve Catalytic Activity of Chondroitin B Lyase with Semi-Rational Design" Current Issues in Molecular Biology 46, no. 9: 9916-9927. https://doi.org/10.3390/cimb46090591
APA StyleTian, M., Xu, Y.-Y., Li, Y.-N., Yu, S., Wang, Y.-L., Ma, X.-L., & Zhang, Y.-W. (2024). Engineering of Substrate-Binding Domain to Improve Catalytic Activity of Chondroitin B Lyase with Semi-Rational Design. Current Issues in Molecular Biology, 46(9), 9916-9927. https://doi.org/10.3390/cimb46090591


_Kim.png)





