Gene Expression Regulation and the Signal Transduction of Programmed Cell Death
Abstract
1. Introduction
2. Overview of Programmed Cell Death
2.1. History of Programmed Cell Death Research
2.2. Types of Programmed Cell Death
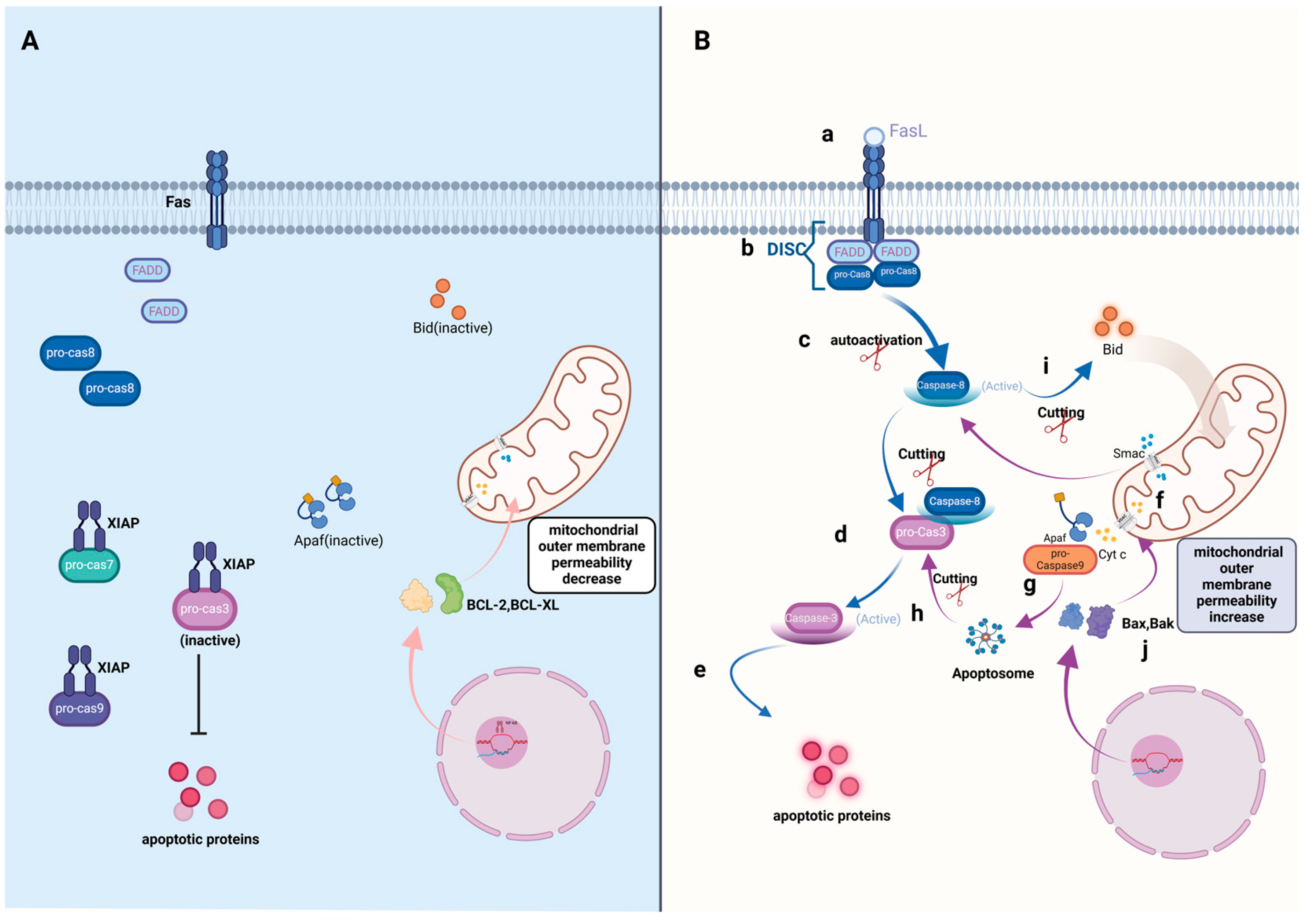
3. Apoptosis
3.1. Signal Transduction of Apoptosis
3.1.1. Caspase-Dependent Apoptosis
3.1.2. Caspase-Independent Apoptosis
3.2. Gene Regulation of Apoptosis
3.2.1. BCL-2 Family
3.2.2. NF-kB Signaling
3.2.3. p53
3.2.4. Other Regulatory Factors
3.3. Implications of Apoptosis in Cancer
4. Autophagy
4.1. Signal Transduction of Autophagy
4.1.1. Upstream Pathway of Autophagy
4.1.2. Autophagy Signal Transduction Pathway

4.2. Gene Regulation of Autophagy
4.2.1. ATG
4.2.2. mTOR
4.2.3. ULK1
4.2.4. Beclin-1
4.2.5. p62
4.2.6. AMPK
4.3. Role of Autophagy in Tumorigenesis and Cancer Treatment
5. Pyroptosis
5.1. Signal Transduction of Pyroptosis
5.1.1. Classic Pyroptotic Pathway
5.1.2. Non-Classical Pyroptotic Pathways
5.2. Gene Regulation of Pyroptosis
5.2.1. Gasdermin Family Proteins
5.2.2. GPX4
5.3. Aiming at Pyroptosis in Cancer
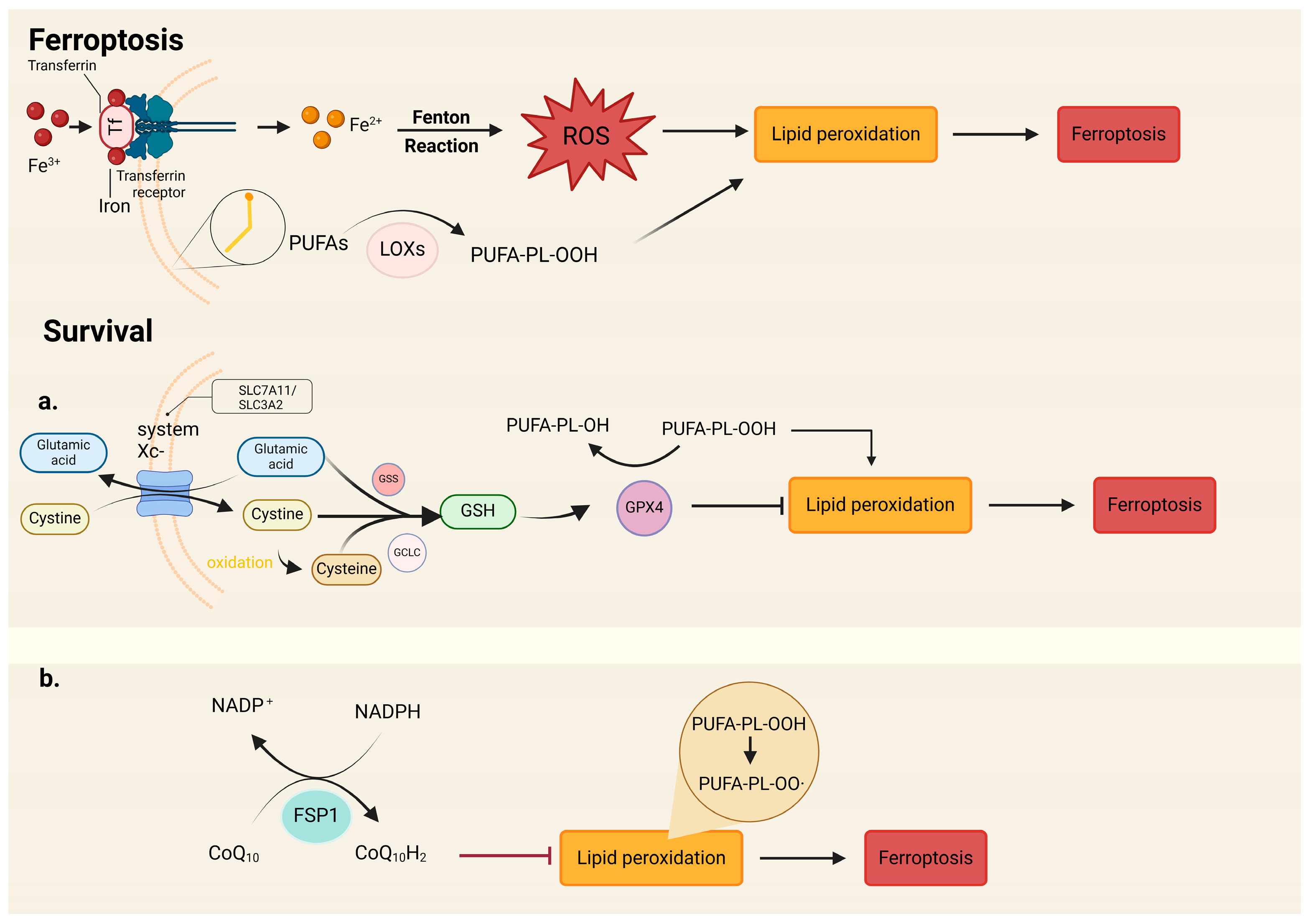
6. Ferroptosis
6.1. Signaling of Ferroptosis
6.2. Gene Regulation of Ferroptosis
6.2.1. p53
6.2.2. CoQ REDOX Enzyme FSP1
6.3. Ferroptosis Regulation in Cancer
7. Cuproptosis
7.1. Signaling of Cuproptosis
7.2. Gene Regulation of Cuproptosis
7.2.1. FDX1
7.2.2. Other Protein Families
7.3. Epigenetic Regulation of Different Types of Cell Death
7.4. Targeting Cell Death as Cancer Therapy
8. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F. Shrinkage necrosis: A distinct mode of cellular death. J. Pathol. 1971, 105, 13–20. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Colussi, P.A.; Kumar, S. Targeted disruption of caspase genes in mice: What they tell us about the functions of individual caspases in apoptosis. Immunol. Cell Biol. 1999, 77, 58–63. [Google Scholar] [CrossRef]
- Aravind, L.; Dixit, V.M.; Koonin, E.V. The domains of death: Evolution of the apoptosis machinery. Trends Biochem. Sci. 1999, 24, 47–53. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef]
- Pimentel-Muiños, F.X.; Seed, B. Regulated commitment of TNF receptor signaling: A molecular switch for death or activation. Immunity 1999, 11, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.S.; Dixit, V.; Ashkenazi, A. Death receptor signal transducers: Nodes of coordination in immune signaling networks. Nat. Immunol. 2009, 10, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Read, S.H.; Baliga, B.C.; Ekert, P.G.; Vaux, D.L.; Kumar, S. A novel Apaf-1-independent putative caspase-2 activation complex. J. Cell Biol. 2002, 159, 739–745. [Google Scholar] [CrossRef]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Oppenheim, R.W.; Flavell, R.A.; Vinsant, S.; Prevette, D.; Kuan, C.Y.; Rakic, P. Programmed cell death of developing mammalian neurons after genetic deletion of caspases. J. Neurosci. 2001, 21, 4752–4760. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Follis, A.V.; Kriwacki, R.W.; Green, D.R. Many players in BCL-2 family affairs. Trends Biochem. Sci. 2014, 39, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Thor Straten, P.; Andersen, M.H. The anti-apoptotic members of the Bcl-2 family are attractive tumor-associated antigens. Oncotarget 2010, 1, 239–245. [Google Scholar] [CrossRef]
- Naranmandura, H.; Chen, X.; Tanaka, M.; Wang, W.W.; Rehman, K.; Xu, S.; Chen, Z.; Chen, S.Q.; Suzuki, N. Release of apoptotic cytochrome C from mitochondria by dimethylarsinous acid occurs through interaction with voltage-dependent anion channel in vitro. Toxicol. Sci. 2012, 128, 137–146. [Google Scholar] [CrossRef][Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Umezawa, K.; Higashihara, M.; Horie, R. Combined inhibition of NF-κB and Bcl-2 triggers synergistic reduction of viability and induces apoptosis in melanoma cells. Oncol. Res. 2013, 21, 173–180. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, G.; Zhu, X.; Jiang, K.; Wu, H.; Deng, G.; Qiu, C. Sodium selenite induces apoptosis via ROS-mediated NF-κB signaling and activation of the Bax-caspase-9-caspase-3 axis in 4T1 cells. J. Cell. Physiol. 2019, 234, 2511–2522. [Google Scholar] [CrossRef]
- Baratchian, M.; Davis, C.A.; Shimizu, A.; Escors, D.; Bagnéris, C.; Barrett, T.; Collins, M.K. Distinct Activation Mechanisms of NF-κB Regulator Inhibitor of NF-κB Kinase (IKK) by Isoforms of the Cell Death Regulator Cellular FLICE-like Inhibitory Protein (cFLIP). J. Biol. Chem. 2016, 291, 7608–7620. [Google Scholar] [CrossRef]
- Thein, S.; Pham, A.; Bayer, K.U.; Tao-Cheng, J.H.; Dosemeci, A. IKK regulates the deubiquitinase CYLD at the postsynaptic density. Biochem. Biophys. Res. Commun. 2014, 450, 550–554. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L. The transcriptional targets of p53 in apoptosis control. Biochem. Biophys. Res. Commun. 2005, 331, 851–858. [Google Scholar] [CrossRef]
- Kenzelmann Broz, D.; Attardi, L.D. In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis 2010, 31, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Seervi, M.; Xue, D. Mitochondrial Cell Death Pathways in Caenorhabiditis elegans. Curr. Top. Dev. Biol. 2015, 114, 43–65. [Google Scholar]
- Ndubaku, C.; Varfolomeev, E.; Wang, L.; Zobel, K.; Lau, K.; Elliott, L.O.; Maurer, B.; Fedorova, A.V.; Dynek, J.N.; Koehler, M.; et al. Antagonism of c-IAP and XIAP proteins is required for efficient induction of cell death by small-molecule IAP antagonists. ACS Chem. Biol. 2009, 4, 557–566. [Google Scholar] [CrossRef]
- Bélanger, C.; Gravel, A.; Tomoiu, A.; Janelle, M.E.; Gosselin, J.; Tremblay, M.J.; Flamand, L. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J. Hum. Virol. 2001, 4, 62–73. [Google Scholar]
- Wang, X.; Su, G.Y.; Zhao, C.; Qu, F.Z.; Wang, P.; Zhao, Y.Q. Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J. Ginseng Res. 2018, 42, 133–143. [Google Scholar] [CrossRef]
- Ren, J.; Huang, J.; Yang, Z.; Sun, M.; Yang, J.; Lin, C.; Jin, F.; Liu, Y.; Tang, L.; Hu, J.; et al. Cytoplasmic TP53INP2 acts as an apoptosis partner in TRAIL treatment: The synergistic effect of TRAIL with venetoclax in TP53INP2-positive acute myeloid leukemia. J. Exp. Clin. Cancer Res. 2024, 43, 176. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lin, X.; Leng, S.; Hou, Y.; Dang, Z.; Xue, S.; Li, N.; Zhang, F. The PRC2 complex epigenetically silences GATA4 to suppress cellular senescence and promote the progression of breast cancer. Transl. Oncol. 2024, 46, 102014. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zeng, W.; Tao, K.; E, Z.; Wang, B.; Yang, Q. Chaperone-mediated autophagy: Roles in neuroprotection. Neurosci. Bull. 2015, 31, 452–458. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar]
- Wang, H.; Liu, Y.; Wang, D.; Xu, Y.; Dong, R.; Yang, Y.; Lv, Q.; Chen, X.; Zhang, Z. The Upstream Pathway of mTOR-Mediated Autophagy in Liver Diseases. Cells 2019, 8, 1597. [Google Scholar] [CrossRef]
- Malik, N.; Macartney, T.; Hornberger, A.; Anderson, K.E.; Tovell, H.; Prescott, A.R.; Alessi, D.R. Mechanism of activation of SGK3 by growth factors via the Class 1 and Class 3 PI3Ks. Biochem. J. 2018, 475, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, L. Regulation of ATG and Autophagy Initiation. Adv. Exp. Med. Biol. 2019, 1206, 41–65. [Google Scholar] [PubMed]
- Sun, Q.; Fan, W.; Chen, K.; Ding, X.; Chen, S.; Zhong, Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 2008, 105, 19211–19216. [Google Scholar] [CrossRef]
- Ktistakis, N.T.; Tooze, S.A. Digesting the Expanding Mechanisms of Autophagy. Trends Cell Biol. 2016, 26, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Legouis, R.; Culetto, E. ESCRT and autophagies: Endosomal functions and beyond. Semin. Cell Dev. Biol. 2018, 74, 21–28. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Li, X.-X.; He, Z.-X.; Pan, S.-T.; Yang, Y.; Zhang, X.; Chow, K.; Yang, T.; Qiu, J.-X.; Zhou, Q.; et al. Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des. Dev. Ther. 2015, 9, 1511–1554, Retracted in Drug Des. Dev. Ther. 2022, 16, 2437–2438. [Google Scholar] [CrossRef]
- Deng, L.; Chen, L.; Zhao, L.; Xu, Y.; Peng, X.; Wang, X.; Ding, L.; Jin, J.; Teng, H.; Wang, Y.; et al. Ubiquitination of Rheb governs growth factor-induced mTORC1 activation. Cell Res. 2019, 29, 136–150. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Wei, W.; Lv, Y.; Zhang, X.; Yao, Y.; Wang, L.; Ling, T.; Zou, X. Effects of proton pump inhibitors on reversing multidrug resistance via downregulating V-ATPases/PI3K/Akt/mTOR/HIF-1α signaling pathway through TSC1/2 complex and Rheb in human gastric adenocarcinoma cells in vitro and in vivo. OncoTargets Ther. 2018, 11, 6705–6722. [Google Scholar] [CrossRef]
- Emerling, B.M.; Weinberg, F.; Snyder, C.; Burgess, Z.; Mutlu, G.M.; Viollet, B.; Budinger, G.S.; Chandel, N.S. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic. Biol. Med. 2009, 46, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar] [PubMed]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef]
- Ganley, I.G.; Lam du, H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, C.H.; Seo, M.; Otto, N.M.; Grunwald, D.; Kim, K.H.; Moriarity, B.; Kim, Y.M.; Starker, C.; Nho, R.S.; et al. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy 2016, 12, 547–564. [Google Scholar] [CrossRef]
- Xu, H.D.; Qin, Z.H. Beclin 1, Bcl-2 and Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 109–126. [Google Scholar]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Overmeyer, J.H.; Maltese, W.A. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J. Cell Sci. 2006, 119 Pt 2, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Seo, M.; Jung, C.H.; Grunwald, D.; Stone, M.; Otto, N.M.; Toso, E.; Ahn, Y.; Kyba, M.; Griffin, T.J.; et al. ULK1 phosphorylates Ser30 of BECN1 in association with ATG14 to stimulate autophagy induction. Autophagy 2018, 14, 584–597. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic Regulation of p62 is Critical for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Guo, J.Y.; Chen, H.-Y.; Mathew, R.; Fan, J.; Strohecker, A.M.; Karsli-Uzunbas, G.; Kamphorst, J.J.; Chen, G.; Lemons, J.M.; Karantza, V.; et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011, 25, 460–470. [Google Scholar] [CrossRef]
- Li, Z.; Chen, B.; Wu, Y.; Jin, F.; Xia, Y.; Liu, X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer 2010, 10, 98. [Google Scholar] [CrossRef]
- Jia, Y.L.; Xu, M.; Dou, C.W.; Liu, Z.K.; Xue, Y.M.; Yao, B.W.; Ding, L.L.; Tu, K.S.; Zheng, X.; Liu, Q.G. P300/CBP-associated factor (PCAF) inhibits the growth of hepatocellular carcinoma by promoting cell autophagy. Cell Death Dis. 2016, 7, e2400. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-P.; Hu, L.-F.; Zheng, H.-F.; Mao, C.-J.; Hu, W.-D.; Xiong, K.-P.; Wang, F.; Liu, C.-F. Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol. Sin. 2013, 34, 625–635. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.A.; Heitman, J. Dismantling the Cryptococcus coat. Trends Microbiol. 2001, 9, 112–113. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye 2018, 32, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef]
- Feng, S.; Fox, D.; Man, S.M. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J. Mol. Biol. 2018, 430 Pt B, 3068–3080. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Chen, X.; He, W.-T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Kang, R.; Zeng, L.; Zhu, S.; Xie, Y.; Liu, J.; Wen, Q.; Cao, L.; Xie, M.; Ran, Q.; Kroemer, G.; et al. Lipid Peroxidation Drives Gasdermin D-Mediated Pyroptosis in Lethal Polymicrobial Sepsis. Cell Host Microbe 2018, 24, 97–108.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Zhang, T.; Pu, X.; Li, Y.; Wu, Z. Genomic analysis quantifies pyroptosis in the immune microenvironment of HBV-related hepatocellular carcinoma. Front. Immunol. 2022, 13, 932303. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Futamura, M.; Kamino, H.; Nakamura, Y.; Kitamura, N.; Ohnishi, S.; Miyamoto, Y.; Ichikawa, H.; Ohta, T.; Ohki, M.; et al. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J. Hum. Genet. 2006, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Croes, L.; Beyens, M.; Fransen, E.; Ibrahim, J.; Berghe, W.V.; Suls, A.; Peeters, M.; Pauwels, P.; Van Camp, G.; de Beeck, K.O. Large-scale analysis of DFNA5 methylation reveals its potential as biomarker for breast cancer. Clin. Epigenetics 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lebron, C.; Nagpal, J.K.; Chae, Y.K.; Chang, X.; Huang, Y.; Chuang, T.; Yamashita, K.; Trink, B.; Ratovitski, E.A.; et al. Methylation of the DFNA5 increases risk of lymph node metastasis in human breast cancer. Biochem. Biophys. Res. Commun. 2008, 370, 38–43. [Google Scholar] [CrossRef]
- Yang, W.; Liu, S.; Li, Y.; Wang, Y.; Deng, Y.; Sun, W.; Huang, H.; Xie, J.; He, A.; Chen, H.; et al. Pyridoxine induces monocyte-macrophages death as specific treatment of acute myeloid leukemia. Cancer Lett. 2020, 492, 96–105. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Xu, S.; He, Y.; Lin, L.; Chen, P.; Chen, M.; Zhang, S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021, 12, 289. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170. [Google Scholar] [PubMed]
- Lu, B.; Chen, X.B.; Ying, M.D.; He, Q.J.; Cao, J.; Yang, B. The Role of Ferroptosis in Cancer Development and Treatment Response. Front. Pharmacol. 2018, 8, 992. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Weng, M.; Liu, X.; Wan, P.; Hu, Y.; Ma, M.; Zhang, Y.; Xia, H.; Lv, K. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J. Adv. Res. 2021, 37, 91–106. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Lan, Z.; Xiao, Y.; Liao, Y.; Basnet, S.; Huang, P.; Li, Y.; Yan, J.; Sheng, Y.; et al. CPEB1 Controls NRF2 Proteostasis and Ferroptosis Susceptibility in Pancreatic Cancer. Int. J. Biol. Sci. 2024, 20, 3156–3172. [Google Scholar] [CrossRef]
- Qin, J.; Li, Z.; Su, L.; Wen, X.; Tang, X.; Huang, M.; Wu, J. Expression of transferrin receptor/TFRC protein in bladder cancer cell T24 and its role in inducing iron death in bladder cancer. Int. J. Biol. Macromol. 2024, 274 Pt 1, 133323. [Google Scholar] [CrossRef]
- Mu, M.; Liang, X.; Zhao, N.; Chuan, D.; Chen, B.; Zhao, S.; Wang, G.; Fan, R.; Zou, B.; Han, B.; et al. Boosting ferroptosis and microtubule inhibition for antitumor therapy via a carrier-free supermolecule nanoreactor. J. Pharm. Anal. 2023, 13, 99–109. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef]
- Que, E.L.; Domaille, D.W.; Chang, C.J. Metals in neurobiology: Probing their chemistry and biology with molecular imaging. Chem. Rev. 2008, 108, 1517–1549. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.D.; Morehouse, L.A.; Thomas, C.E. Role of metals in oxygen radical reactions. J. Free Radic. Biol. Med. 1985, 1, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Cengiz Seval, G.; Beksac, M. The safety of bortezomib for the treatment of multiple myeloma. Expert Opin. Drug Saf. 2018, 17, 953–962. [Google Scholar] [CrossRef]
- Shen, J.; Wang, L.; Bi, J. Bioinformatics analysis and experimental validation of cuproptosis-related lncRNA LINC02154 in clear cell renal cell carcinoma. BMC Cancer 2023, 23, 160. [Google Scholar] [CrossRef]
- Luo, L.; Li, A.; Fu, S.; Du, W.; He, L.N.; Zhang, X.; Wang, Y.; Zhou, Y.; Yunpeng, Y.; Li, Z.; et al. Cuproptosis-related immune gene signature predicts clinical benefits from anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. Immunol. Res. 2023, 71, 213–228. [Google Scholar] [CrossRef]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef]
- Imamichi, Y.; Mizutani, T.; Ju, Y.; Matsumura, T.; Kawabe, S.; Kanno, M.; Yazawa, T.; Miyamoto, K. Transcriptional regulation of human ferredoxin 1 in ovarian granulosa cells. Mol. Cell. Endocrinol. 2013, 370, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef]
- Wang, D.; Tian, Z.; Zhang, P.; Zhen, L.; Meng, Q.; Sun, B.; Xu, X.; Jia, T.; Li, S. The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomed. Pharmacother. 2023, 163, 114830. [Google Scholar] [CrossRef]
- Xiong, C.; Ling, H.; Hao, Q.; Zhou, X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ. 2023, 30, 876–884. [Google Scholar] [CrossRef]
- Liu, H. Pan-cancer profiles of the cuproptosis gene set. Am. J. Cancer Res. 2022, 12, 4074–4081. [Google Scholar] [PubMed]
- Xu, J.; Hu, Z.; Cao, H.; Zhang, H.; Luo, P.; Zhang, J.; Wang, X.; Cheng, Q.; Li, J. Multi-omics pan-cancer study of cuproptosis core gene FDX1 and its role in kidney renal clear cell carcinoma. Front. Immunol. 2022, 13, 981764. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Guo, W.; Xu, J. High expression of cuproptosis-related gene FDX1 in relation to good prognosis and immune cells infiltration in colon adenocarcinoma (COAD). J. Cancer Res. Clin. Oncol. 2023, 149, 15–24. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Yang, B.; Sun, S.; Zhang, P.; Luo, Z.; Feng, T.; Cui, Z.; Zhu, T.; Li, Y.; et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 2023, 14, 6523. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yao, Q.; Li, X.; Li, X.; Zhang, W.; Qu, P. Cuproptosis-related gene SLC31A1: Prognosis values and potential biological functions in cancer. Sci. Rep. 2023, 13, 17790. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Wang, Q.; Shi, W.; Peng, L.; Jiang, Y.; Zhu, M.; Guo, J.; Peng, D.; Wang, M.; Men, L.; et al. ATF3/SPI1/SLC31A1 Signaling Promotes Cuproptosis Induced by Advanced Glycosylation End Products in Diabetic Myocardial Injury. Int. J. Mol. Sci. 2023, 24, 1667. [Google Scholar] [CrossRef]
- Chen, X.; Li, K.; Xiao, Y.; Wu, W.; Lin, H.; Qing, X.; Tian, S.; Liu, S.; Feng, S.; Wang, B.; et al. SP1/CTR1-mediated oxidative stress-induced cuproptosis in intervertebral disc degeneration. Biofactors 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Dindi, U.M.R.; Al-Ghamdi, S.; Alrudian, N.A.; Dayel, S.B.; Abuderman, A.A.; Saad Alqahtani, M.; Bahakim, N.O.; Ramesh, T.; Vilwanathan, R. Ameliorative inhibition of sirtuin 6 by imidazole derivative triggers oxidative stress-mediated apoptosis associated with Nrf2/Keap1 signaling in non-small cell lung cancer cell lines. Front. Pharmacol. 2023, 14, 1335305. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Y.; Yang, X.; Li, F.; Zheng, L.; Liu, W.; Wu, J.; Ou, R.; Zhang, G.; Hu, M.; et al. Novel histone deacetylase inhibitors derived from Magnolia officinalis significantly enhance TRAIL-induced apoptosis in non-small cell lung cancer. Pharmacol. Res. 2016, 111, 113–125. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, E.H.; Lee, S.H.; Lee, Y.M.; Kim, H.D.; Kim, Y.Z. Epigenetic Role of Histone 3 Lysine Methyltransferase and Demethylase in Regulating Apoptosis Predicting the Recurrence of Atypical Meningioma. J. Korean Med. Sci. 2015, 30, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Inoue, F.; Sone, K.; Kumegawa, K.; Hachijo, R.; Suzuki, E.; Tanimoto, S.; Tsuboyama, N.; Kato, K.; Toyohara, Y.; Takahashi, Y.; et al. Inhibition of protein arginine methyltransferase 6 activates interferon signaling and induces the apoptosis of endometrial cancer cells via histone modification. Int. J. Oncol. 2024, 64, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Guo, M.; Sun, G.; Zhou, K.; Pang, W.; Cao, D.; Tang, X.; Meng, X. Histone methyltransferase WHSC1 inhibits colorectal cancer cell apoptosis via targeting anti-apoptotic BCL2. Cell Death Discov. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, D.; Kirmizis, A. Depletion of histone N-terminal-acetyltransferase Naa40 induces p53-independent apoptosis in colorectal cancer cells via the mitochondrial pathway. Apoptosis 2016, 21, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Mahalanobish, S.; Dutta, S.; Saha, S.; Sil, P.C. Melatonin induced suppression of ER stress and mitochondrial dysfunction inhibited NLRP3 inflammasome activation in COPD mice. Food Chem. Toxicol. 2020, 144, 111588, Erratum in Food. Chem. Toxicol. 2024, 184, 114408. [Google Scholar] [CrossRef]
- Shahriari Felordi, M.; Alikhani, M.; Farzaneh, Z.; Alipour Choshali, M.; Ebrahimi, M.; Aboulkheyr Es, H.; Piryaei, A.; Najimi, M.; Vosough, M. (-)-Epigallocatechin-3-gallate induced apoptosis by dissociation of c-FLIP/Ku70 complex in gastric cancer cells. J. Cell. Mol. Med. 2023, 27, 2572–2582. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Tateishi, K.; Kato, H.; Fujiwara, H.; Yamamoto, K.; Kudo, Y.; Nakagawa, H.; Tanaka, Y.; Ijichi, H.; Ikenoue, T.; et al. Inhibition of histone methyltransferase G9a attenuates liver cancer initiation by sensitizing DNA-damaged hepatocytes to p53-induced apoptosis. Cell Death Dis. 2021, 12, 99. [Google Scholar] [CrossRef]
- He, C.; Liu, C.; Wang, L.; Sun, Y.; Jiang, Y.; Hao, Y. Histone methyltransferase NSD2 regulates apoptosis and chemosensitivity in osteosarcoma. Cell Death Dis. 2019, 10, 65. [Google Scholar] [CrossRef]
- Ahmed, S.G.; Abdelnabi, A.; A Maguire, C.; Doha, M.; E Sagers, J.; Lewis, R.M.; Muzikansky, A.; Giovannini, M.; Stemmer-Rachamimov, A.; Stankovic, K.M.; et al. Gene therapy with apoptosis-associated speck-like protein, a newly described schwannoma tumor suppressor, inhibits schwannoma growth in vivo. Neuro-Oncology 2019, 21, 854–866. [Google Scholar] [CrossRef]
- Miyakuni, K.; Nishida, J.; Koinuma, D.; Nagae, G.; Aburatani, H.; Miyazono, K.; Ehata, S. Genome-wide analysis of DNA methylation identifies the apoptosis-related gene UQCRH as a tumor suppressor in renal cancer. Mol. Oncol. 2022, 16, 732–749. [Google Scholar] [CrossRef]
- Costa, S.F.d.S.; Pereira, N.B.; Pereira, K.M.A.; Campos, K.; de Castro, W.H.; Diniz, M.G.; Gomes, C.C.; Gomez, R.S. DNA methylation pattern of apoptosis-related genes in ameloblastoma. Oral Dis. 2017, 23, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Benard, A.; Zeestraten, E.C.M.; Goossens-Beumer, I.J.; Putter, H.; van de Velde, C.J.H.; Hoon, D.S.B.; Kuppen, P.J.K. DNA methylation of apoptosis genes in rectal cancer predicts patient survival and tumor recurrence. Apoptosis 2014, 19, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, E.; Vallette, F.M.; Cartron, P.F. Impact of the DNA methyltransferases expression on the methylation status of apoptosis-associated genes in glioblastoma multiforme. Cell Death Dis. 2010, 1, e8. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Weisenberger, D.J.; Cheng, J.C.; Chandrasoma, S.; Siegmund, K.D.; Gonzalgo, M.L.; Toma, M.I.; Huland, H.; Yoo, C.; Tsai, Y.C.; et al. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin. Cancer Res. 2004, 10, 7457–7465. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Li, Z.; Pan, J.; Sun, X. Long noncoding RNA DUXAP8 regulates proliferation and apoptosis of ovarian cancer cells via targeting miR-590-5p. Hum. Cell 2020, 33, 1240–1251. [Google Scholar] [CrossRef]
- Lv, C.; Sun, J.; Ye, Y.; Lin, Z.; Li, H.; Liu, Y.; Mo, K.; Xu, W.; Hu, W.; Draz, E.; et al. Long noncoding RNA EIF1AX-AS1 promotes endometrial cancer cell apoptosis by affecting EIF1AX mRNA stabilization. Cancer Sci. 2022, 113, 1277–1291. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, J.; Zhu, L.; Song, R.; Zhong, M.; Guo, Y. Long noncoding RNA CEBPA-DT promotes cisplatin chemo-resistance through CEBPA/BCL2 mediated apoptosis in oral squamous cellular cancer. Int. J. Med. Sci. 2021, 18, 3728–3737. [Google Scholar] [CrossRef]
- Kitajima, H.; Maruyama, R.; Niinuma, T.; Yamamoto, E.; Takasawa, A.; Takasawa, K.; Ishiguro, K.; Tsuyada, A.; Suzuki, R.; Sudo, G.; et al. TM4SF1-AS1 inhibits apoptosis by promoting stress granule formation in cancer cells. Cell Death Dis. 2023, 14, 424. [Google Scholar] [CrossRef]
- Ci, Y.; Zhang, Y.; Zhang, X. Methylated lncRNAs suppress apoptosis of gastric cancer stem cells via the lncRNA-miRNA/protein axis. Cell. Mol. Biol. Lett. 2024, 29, 102, Erratum in Cell. Mol. Biol. Lett. 2024, 29, 51. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, H.; Zhao, G. Long Noncoding RNA SNHG6 Promotes Proliferation and Inhibits Apoptosis in Non-small Cell Lung Cancer Cells by Regulating miR-490-3p/RSF1 Axis. Cancer Biother. Radiopharm. 2020, 35, 351–361. [Google Scholar] [CrossRef]
- Lian, Z.; Tian, P.; Ma, S.; Chang, T.; Liu, R.; Feng, Q.; Li, J. Long noncoding RNA MEG3 regulates cell proliferation and apoptosis by disrupting microRNA-9-5p-mediated inhibition of NDRG1 in prostate cancer. Aging 2024, 16, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Han, Q.; Chi, C.; Zhu, Y.; Pan, J.; Dong, B.; Huang, Y.; Xia, W.; Xue, W. Upregulated KDM4B promotes prostate cancer cell proliferation by activating autophagy. J. Cell. Physiol. 2020, 235, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, S.Y.; Lim, J.; Lindroth, A.M.; Park, Y.J. EHMT2 Inhibition Induces Cell Death in Human Non-Small Cell Lung Cancer by Altering the Cholesterol Biosynthesis Pathway. Int. J. Mol. Sci. 2020, 21, 1002. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, F.; Wang, Q.; Ding, Y.; Ying, R.; Zeng, L. Inhibition of EZH2 and EGFR produces a synergistic effect on cell apoptosis by increasing autophagy in gastric cancer cells. OncoTargets Ther. 2018, 11, 8455–8463. [Google Scholar] [CrossRef]
- Wei, F.-Z.; Cao, Z.; Wang, X.; Wang, H.; Cai, M.-Y.; Li, T.; Hattori, N.; Wang, D.; Du, Y.; Song, B.; et al. Epigenetic regulation of autophagy by the methyltransferase EZH2 through an MTOR-dependent pathway. Autophagy 2015, 11, 2309–2322. [Google Scholar] [CrossRef]
- Tan, J.; Wang, H.-L.; Yang, J.; Liu, Q.-Q.; Li, C.-M.; Wang, Y.-Q.; Fu, L.-N.; Gao, Q.-Y.; Chen, Y.-X.; Fang, J.-Y. JMJD2B-induced amino acid alterations enhance the survival of colorectal cancer cells under glucose-deprivation via autophagy. Theranostics 2020, 10, 5763–5777. [Google Scholar] [CrossRef]
- Wang, J.; Xie, S.; Yang, J.; Xiong, H.; Jia, Y.; Zhou, Y.; Chen, Y.; Ying, X.; Chen, C.; Ye, C.; et al. The long noncoding RNA H19 promotes tamoxifen resistance in breast cancer via autophagy. J. Hematol. Oncol. 2019, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Dunwell, T.; Hesson, L.; Rauch, T.A.; Wang, L.; Clark, R.E.; Dallol, A.; Gentle, D.; Catchpoole, D.; Maher, E.R.; Pfeifer, G.P.; et al. A genome-wide screen identifies frequently methylated genes in haematological and epithelial cancers. Mol. Cancer 2010, 9, 44. [Google Scholar] [CrossRef]
- Liao, Y.-P.; Chen, L.-Y.; Huang, R.-L.; Su, P.-H.; Chan, M.W.; Chang, C.-C.; Yu, M.-H.; Wang, P.-H.; Yen, M.-S.; Nephew, K.P.; et al. Hypomethylation signature of tumor-initiating cells predicts poor prognosis of ovarian cancer patients. Hum. Mol. Genet. 2014, 23, 1894–1906. [Google Scholar] [CrossRef]
- Muhammad, J.S.; Nanjo, S.; Ando, T.; Yamashita, S.; Maekita, T.; Ushijima, T.; Tabuchi, Y.; Sugiyama, T. Autophagy impairment by Helicobacter pylori-induced methylation silencing of MAP1LC3Av1 promotes gastric carcinogenesis. Int. J. Cancer 2017, 140, 2272–2283. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, H.; Wu, S.; Zhang, Y.; Yu, Z. MicroRNA-638 induces apoptosis and autophagy in human liver cancer cells by targeting enhancer of zeste homolog 2 (EZH2). Environ. Toxicol. Pharmacol. 2021, 82, 103559. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Weng, L.; Wang, Z.; Jia, Y.; Liu, B.; Wu, S.; Cao, Y.; Sun, X.; Yin, X.; Shang, M.; et al. MiR-124 induces autophagy-related cell death in cholangiocarcinoma cells through direct targeting of the EZH2-STAT3 signaling axis. Exp. Cell Res. 2018, 366, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.T.; Shi, Y.H.; Zhou, J.; Peng, Y.F.; Liu, W.R.; Shi, G.M.; Gao, Q.; Wang, X.Y.; Song, K.; Fan, J.; et al. MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett. 2018, 412, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Z.; Liu, H.; Jiang, S.; Wang, G.; Sun, L.; Li, J.; Wang, X.; Yu, S.; Huang, J.; et al. MicroRNA-30a targets BECLIN-1 to inactivate autophagy and sensitizes gastrointestinal stromal tumor cells to imatinib. Cell Death Dis. 2020, 11, 198. [Google Scholar] [CrossRef]
- Zhang, H.; Kang, J.; Liu, L.; Chen, L.; Ren, S.; Tao, Y. MicroRNA-143 sensitizes acute myeloid leukemia cells to cytarabine via targeting ATG7- and ATG2B-dependent autophagy. Aging 2020, 12, 20111–20126. [Google Scholar] [CrossRef]
- Guan, X.; Liu, R.; Wang, B.; Xiong, R.; Cui, L.; Liao, Y.; Ruan, Y.; Fang, L.; Lu, X.; Yu, X.; et al. Inhibition of HDAC2 sensitises antitumour therapy by promoting NLRP3/GSDMD-mediated pyroptosis in colorectal cancer. Clin. Transl. Med. 2024, 14, e1692. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, Z.; Ding, L.; Du, W.; Pei, D. ALKBH4 impedes 5-FU Sensitivity through suppressing GSDME induced pyroptosis in gastric cancer. Cell Death Dis. 2024, 15, 435. [Google Scholar] [CrossRef]
- Ning, H.; Huang, S.; Lei, Y.; Zhi, R.; Yan, H.; Jin, J.; Hu, Z.; Guo, K.; Liu, J.; Yang, J.; et al. Enhancer decommissioning by MLL4 ablation elicits dsRNA-interferon signaling and GSDMD-mediated pyroptosis to potentiate anti-tumor immunity. Nat. Commun. 2022, 13, 6578. [Google Scholar] [CrossRef]
- Xia, T.; Liu, M.; Zhao, Q.; Ouyang, J.; Xu, P.; Chen, B. PRMT5 regulates cell pyroptosis by silencing CASP1 in multiple myeloma. Cell Death Dis. 2021, 12, 851. [Google Scholar] [CrossRef]
- Tan, Y.F.; Wang, M.; Chen, Z.Y.; Wang, L.; Liu, X.H. Inhibition of BRD4 prevents proliferation and epithelial-mesenchymal transition in renal cell carcinoma via NLRP3 inflammasome-induced pyroptosis. Cell Death Dis. 2020, 11, 239. [Google Scholar] [CrossRef]
- Li, W.; Huang, B.S.; Xiong, Y.Y.; Yang, L.J.; Wu, L.X. 4,5-Dimethoxycanthin-6-one is a novel LSD1 inhibitor that inhibits proliferation of glioblastoma cells and induces apoptosis and pyroptosis. Cancer Cell Int. 2022, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, J.; Xu, Z.; Yan, Y.; Hu, K. GSDMs are potential therapeutic targets and prognostic biomarkers in clear cell renal cell carcinoma. Aging 2022, 14, 2758–2774. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Mu, J.; Peng, W.; Tang, J.; Xiang, Q.; Tian, S.; Feng, Y.; He, S.; Qiu, Z.; Ren, G.; et al. DNA methylation downregulated ZDHHC1 suppresses tumor growth by altering cellular metabolism and inducing oxidative/ER stress-mediated apoptosis and pyroptosis. Theranostics 2020, 10, 9495–9511. [Google Scholar] [CrossRef]
- Khan, M.; Ai, M.; Du, K.; Song, J.; Wang, B.; Lin, J.; Ren, A.; Chen, C.; Huang, Z.; Qiu, W.; et al. Pyroptosis relates to tumor microenvironment remodeling and prognosis: A pan-cancer perspective. Front. Immunol. 2022, 13, 1062225. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, P.; Fan, H.; Liang, H.; Zhang, K.; Zhao, Y.; Guo, S.; Schrodi, S.J.; Fan, Y.; Zhang, D. Gene body hypomethylation of pyroptosis-related genes NLRP7, NLRP2, and NLRP3 facilitate non-invasive surveillance of hepatocellular carcinoma. Funct. Integr. Genom. 2023, 23, 198. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Huang, C.; Rao, D.; Sang, C.; Zhu, S.; Gu, L.; Xie, C.; Tang, Z.; Xu, X. Transcription factor Nrf2 binds to circRNAPIBF1 to regulate SOD2 in lung adenocarcinoma progression. Mol. Carcinog. 2022, 61, 1161–1176. [Google Scholar] [CrossRef]
- Rana, S.; Espinosa-Diez, C.; Ruhl, R.; Chatterjee, N.; Hudson, C.; Fraile-Bethencourt, E.; Agarwal, A.; Khou, S.; Thomas, C.R., Jr.; Anand, S. Differential regulation of microRNA-15a by radiation affects angiogenesis and tumor growth via modulation of acid sphingomyelinase. Sci. Rep. 2020, 10, 5581. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ye, F.; Xie, W.; Zhang, W.; Zeng, R.; Sheng, W.; Mi, Y.; Sheng, X. The HOXC-AS2/miR-876-5p/HKDC1 axis regulates endometrial cancer progression in a high glucose-related tumor microenvironment. Cancer Sci. 2022, 113, 2297–2310. [Google Scholar] [CrossRef]
- Dai, J.; Qu, T.; Yin, D.; Cui, Y.; Zhang, C.; Zhang, E.; Guo, R. LncRNA LINC00969 promotes acquired gefitinib resistance by epigenetically suppressing of NLRP3 at transcriptional and posttranscriptional levels to inhibit pyroptosis in lung cancer. Cell Death Dis. 2023, 14, 312. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Ji, Y.; Wang, X.; Fu, D.; Wang, W.; Shen, B. A novel pyroptosis-associated lncRNA LINC01133 promotes pancreatic adenocarcinoma development via miR-30b-5p/SIRT1 axis. Cell. Oncol. 2023, 46, 1381–1398. [Google Scholar] [CrossRef]
- Mabe, N.W.; Garcia, N.M.G.; Wolery, S.E.; Newcomb, R.; Meingasner, R.C.; Vilona, B.A.; Lupo, R.; Lin, C.C.; Chi, J.T.; Alvarez, J.V. G9a Promotes Breast Cancer Recurrence through Repression of a Pro-inflammatory Program. Cell Rep. 2020, 33, 108341. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, U.; Venkatesan, T.; Radhakrishnan, V.; Samuel, S.; Rathinavelu, A. Differential Mechanisms of Cell Death Induced by HDAC Inhibitor SAHA and MDM2 Inhibitor RG7388 in MCF-7 Cells. Cells 2018, 8, 8. [Google Scholar] [CrossRef]
- Tung, B.; Ma, D.; Wang, S.; Oyinlade, O.; Laterra, J.; Ying, M.; Lv, S.-Q.; Wei, S.; Xia, S. Krüppel-like factor 9 and histone deacetylase inhibitors synergistically induce cell death in glioblastoma stem-like cells. BMC Cancer 2018, 18, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, Y.; Li, K.; Mei, C.; Wang, Y.; Jiang, L.; Wang, W.; Zhang, Q.; Yang, W.; Lang, W.; et al. RIPK3 deficiency blocks R-2-hydroxyglutarate-induced necroptosis in IDH-mutated AML cells. Sci. Adv. 2024, 10, eadi1782. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, J.; Yu, L.; Zhang, Z.; Xu, F.; Jiang, L.; Zhou, X.; He, S. Regulation of RIP3 by the transcription factor Sp1 and the epigenetic regulator UHRF1 modulates cancer cell necroptosis. Cell Death Dis. 2017, 8, e3084. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhou, M.; Shang, L.; Du, Q.; Li, Y.; Xie, L.; Liu, X.; Tang, M.; Luo, X.; Fan, J.; et al. EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the RIP3 promoter. Theranostics 2019, 9, 2424–2438. [Google Scholar] [CrossRef]
- Tan, Y.; Sementino, E.; Cheung, M.; Peri, S.; Menges, C.W.; Kukuyan, A.M.; Zhang, T.; Khazak, V.; Fox, L.A.; Ross, E.A.; et al. Somatic Epigenetic Silencing of RIPK3 Inactivates Necroptosis and Contributes to Chemoresistance in Malignant Mesothelioma. Clin. Cancer Res. 2021, 27, 1200–1213. [Google Scholar] [CrossRef]
- Dupont, J.; Dedeyne, L.; Koppo, K.; Verschueren, S.; Tournoy, J.; Gielen, E. Influence of the new EWGSOP2 consensus definition on studies involving (pre)sarcopenic older persons. Comment on “Sarcopenia” by Tournadre et al. Joint Bone Spine 2019, 86, 309–314. Jt. Bone Spine 2020, 87, 277. [Google Scholar] [CrossRef]
- Lin, H.; Qu, L.; Chen, G.; Zhang, C.; Lu, L.; Chen, Y. Comprehensive analysis of necroptosis-related lncRNA signature with potential implications in tumor heterogeneity and prediction of prognosis in clear cell renal cell carcinoma. Eur. J. Med. Res. 2023, 28, 236. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Ran, Q.; Yao, Y.; Zhang, H.; Ju, J.; Yang, T.; Zhang, W.; Yu, X.; He, S. MicroRNA-381-3p Functions as a Dual Suppressor of Apoptosis and Necroptosis and Promotes Proliferation of Renal Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 290. [Google Scholar] [CrossRef]
- Xia, P.; Huang, Y.; Chen, G. A novel signature based on necroptosis-related long non-coding RNAs for predicting prognosis of patients with glioma. Front. Oncol. 2022, 12, 940220. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ma, L.; Zhang, X.; Xu, X.; Guo, S.; Wang, Y.; Qiu, S.; Cui, J.; Guo, W.; Yu, Y.; et al. Tumour cells are sensitised to ferroptosis via RB1CC1-mediated transcriptional reprogramming. Clin. Transl. Med. 2022, 12, e747. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Wang, C.; Kang, R.; Tang, D.; Liu, J. EP300 promotes ferroptosis via HSPA5 acetylation in pancreatic cancer. Sci. Rep. 2023, 13, 15004. [Google Scholar] [CrossRef]
- Zhou, L.; Jia, X.; Shang, Y.; Sun, Y.; Liu, Z.; Liu, J.; Jiang, W.; Deng, S.; Yao, Q.; Chen, J.; et al. PRMT1 inhibition promotes ferroptosis sensitivity via ACSL1 upregulation in acute myeloid leukemia. Mol. Carcinog. 2023, 62, 1119–1135. [Google Scholar] [CrossRef]
- Logie, E.; Van Puyvelde, B.; Cuypers, B.; Schepers, A.; Berghmans, H.; Verdonck, J.; Laukens, K.; Godderis, L.; Dhaenens, M.; Deforce, D.; et al. Ferroptosis Induction in Multiple Myeloma Cells Triggers DNA Methylation and Histone Modification Changes Associated with Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 12234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, X.; Xiong, X.; Xue, R.; Zang, L.; Wang, Z.; Wang, L. Novel methyltransferase G9a inhibitor induces ferroptosis in multiple myeloma through Nrf2/HO-1 pathway. Ann. Hematol. 2024, 103, 2405–2417. [Google Scholar] [CrossRef]
- Du, L.; Yang, H.; Ren, Y.; Ding, Y.; Xu, Y.; Zi, X.; Liu, H.; He, P. Inhibition of LSD1 induces ferroptosis through the ATF4-xCT pathway and shows enhanced anti-tumor effects with ferroptosis inducers in NSCLC. Cell Death Dis. 2023, 14, 716. [Google Scholar] [CrossRef]
- Wang, D.; Zu, Y.; Sun, W.; Fan, X. SETD1A-mediated Methylation of H3K4me3 Inhibits Ferroptosis in Non-small Cell Lung Cancer by Regulating the WTAPP1/WTAP Axis. Curr. Med. Chem. 2024, 31, 3217–3231. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Yu, P.; Zhang, Y.; Huang, L.; Mao, E.; Han, Y. Lysine acetyltransferase KAT2A modulates ferroptosis during colorectal cancer development. Scand. J. Gastroenterol. 2024, 59, 437–444. [Google Scholar] [CrossRef]
- Qi, Y.C.; Bai, H.; Hu, S.L.; Li, S.J.; Li, Q.Z. Coregulatory effects of multiple histone modifications in key ferroptosis-related genes for lung adenocarcinoma. Epigenomics 2024, 16, 609–633. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, P.; He, L.; Chai, X.; Zhang, Y.; Sun, S. Functional mechanism of hypoxia-like conditions mediating resistance to ferroptosis in cervical cancer cells by regulating KDM4A SUMOylation and the SLC7A11/GPX4 pathway. Environ. Toxicol. 2024, 39, 4207–4220. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhu, Y.; Mu, J.; Liu, S.; Yang, Z.; Wu, Z.; Zhao, C.; Song, X.; Ye, Y.; Gu, J.; et al. DNA methylation of RUNX3 promotes the progression of gallbladder cancer through repressing SLC7A11-mediated ferroptosis. Cell Signal. 2023, 108, 110710. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, M.; Kong, D.; Deng, J.; Zhong, Z.; Liang, J. Ferroptosis-associated DNA methylation signature predicts overall survival in patients with head and neck squamous cell carcinoma. BMC Genom. 2022, 23, 63. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Wang, F.; Xu, L.; Yan, Y.; Tong, X.; Yan, H. DNA methylation-regulated LINC02587 inhibits ferroptosis and promotes the progression of glioma cells through the CoQ-FSP1 pathway. BMC Cancer 2023, 23, 989. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Q.; Zhao, Z.; Qiu, Y.; Zhou, Y.; Wu, Z.; Jiang, C.; Wang, X.; Jiang, X. Epigenetically silenced lncRNA SNAI3-AS1 promotes ferroptosis in glioma via perturbing the m6A-dependent recognition of Nrf2 mRNA mediated by SND1. J. Exp. Clin. Cancer Res. 2023, 42, 127. [Google Scholar] [CrossRef]
- Pontel, L.B.; Bueno-Costa, A.; Morellato, A.E.; Carvalho Santos, J.; Roué, G.; Esteller, M. Acute lymphoblastic leukemia necessitates GSH-dependent ferroptosis defenses to overcome FSP1-epigenetic silencing. Redox Biol. 2022, 55, 102408. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, X.; Ye, H.; Ao, M.; Xi, M.; Hou, M. LncRNA FAM83H-AS1 inhibits ferroptosis of endometrial cancer by promoting DNMT1-mediated CDO1 promoter hypermethylation. J. Biol. Chem. 2024, 300, 107680. [Google Scholar] [CrossRef]
- Liu, X.; Yang, P.; Han, L.; Zhou, Q.; Qu, Q.; Shi, X. The ncRNA-Mediated Overexpression of Ferroptosis-Related Gene EMC2 Correlates With Poor Prognosis and Tumor Immune Infiltration in Breast Cancer. Front. Oncol. 2021, 11, 777037. [Google Scholar] [CrossRef]
- Zhai, H.; Zhong, S.; Wu, R.; Mo, Z.; Zheng, S.; Xue, J.; Meng, H.; Liu, M.; Chen, X.; Zhang, G.; et al. Suppressing circIDE/miR-19b-3p/RBMS1 axis exhibits promoting-tumour activity through upregulating GPX4 to diminish ferroptosis in hepatocellular carcinoma. Epigenetics 2023, 18, 2192438. [Google Scholar] [CrossRef]
- Song, Z.; Jia, G.; Ma, P.; Cang, S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021, 276, 119399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, F.; Wang, X.; Zhu, R.; Guo, W. Cuproptosis-related gene-located DNA methylation in lower-grade glioma: Prognosis and tumor microenvironment. Cancer Biomark. 2024, 40, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kang, X.; Zhu, S.; Wang, Y.; Guo, W.; Zhu, R. Cuproptosis-related DNA methylation signature predict prognosis and immune microenvironment in cutaneous melanoma. Discov. Oncol. 2024, 15, 228. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Sun, F.; Zhu, L.; Guo, W. Exploring the Role of DNA Methylation Located in Cuproptosis-Related Genes: Implications for Prognosis and Immune Landscape in Hepatocellular Carcinoma. Front. Biosci. 2024, 29, 123. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Liu, J.; Wang, C.; Gu, R.; Wang, M.; Sun, Z.; Wei, F. Comprehensive analysis of the prognostic signature and tumor microenvironment infiltration characteristics of cuproptosis-related lncRNAs for patients with colon adenocarcinoma. Front. Oncol. 2022, 12, 1007918. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Zhang, X. A novel Cuproptosis-related LncRNA signature to predict prognosis in hepatocellular carcinoma. Sci. Rep. 2022, 12, 11325. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, H.; Xu, K.; Yuan, Q.; Aihaiti, Y.; Cai, Y.; Xu, P. A novel signature to guide osteosarcoma prognosis and immune microenvironment: Cuproptosis-related lncRNA. Front. Immunol. 2022, 13, 919231. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Liu, F.; Quan, J. A novel cuproptosis-related lncRNA signature predicts the prognosis and immune landscape in bladder cancer. Front. Immunol. 2022, 13, 1027449. [Google Scholar] [CrossRef]
- Yao, H.-F.; Xu, D.-P.; Zheng, J.-H.; Xu, Y.; Jia, Q.-Y.; Zhu, Y.-H.; He, R.-Z.; Ma, D.; Yang, M.-W.; Fu, X.-L.; et al. Analysis of cuproptosis-related lncRNA signature for predicting prognosis and tumor immune microenvironment in pancreatic cancer. Apoptosis 2023, 28, 1090–1112. [Google Scholar] [CrossRef]
- Sun, Q.; Qin, X.; Zhao, J.; Gao, T.; Xu, Y.; Chen, G.; Bai, G.; Guo, Z.; Liu, J. Cuproptosis-related LncRNA signatures as a prognostic model for head and neck squamous cell carcinoma. Apoptosis 2023, 28, 247–262. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, Z.; Xiao, G.; He, T. Prognostic signature construction and immunotherapy response analysis for Uterine Corpus Endometrial Carcinoma based on cuproptosis-related lncRNAs. Comput. Biol. Med. 2023, 159, 106905. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, S.; Zeng, L.; Ma, H.; Zhang, Y.; Yang, H.; Liu, Y.; Fang, S.; Zhao, J.; Xu, Y.; et al. An Enzyme-Engineered Nonporous Copper(I) Coordination Polymer Nanoplatform for Cuproptosis-Based Synergistic Cancer Therapy. Adv. Mater. 2022, 34, e2204733. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yang, F.; Zhang, L.; Zhao, Q.; Wang, W.; Yin, L.; Chen, D.; Wang, M.; Han, S.; Xiao, H.; et al. Cuproptosis Induced by ROS Responsive Nanoparticles with Elesclomol and Copper Combined with αPD-L1 for Enhanced Cancer Immunotherapy. Adv. Mater. 2023, 35, e2212267. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Miao, Y.; Li, Y. Cuproptosis: Advances in Stimulus-Responsive Nanomaterials for Cancer Therapy. Adv. Healthc. Mater. 2024, 13, e2400652. [Google Scholar] [CrossRef]


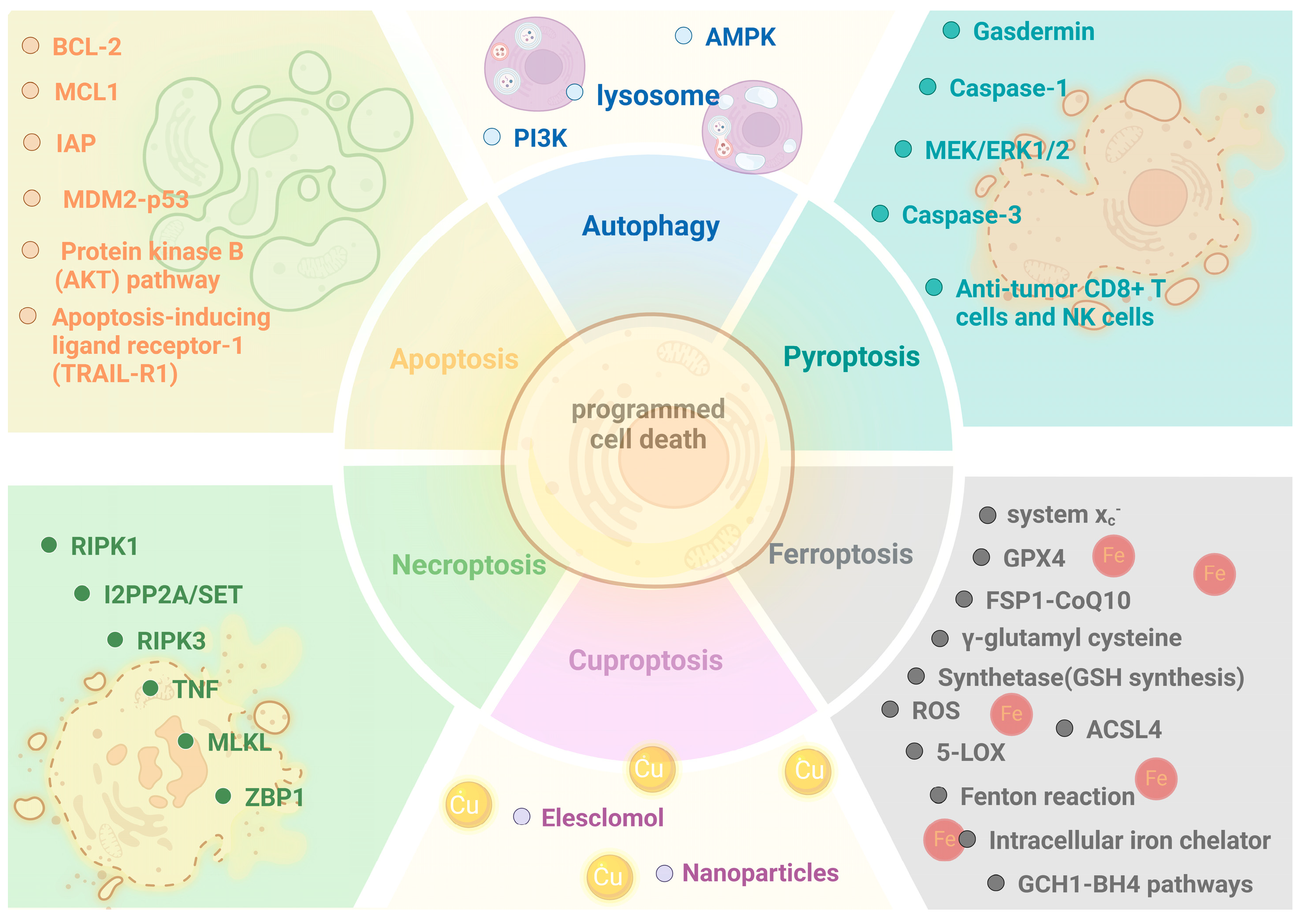
| Type | Morphology | Biochemical Characteristics | Inducement |
|---|---|---|---|
| Apoptosis | membrane blebbing; cell shrinkage; condensation of chromatin; fragmentation of DNA; absence of inflammatory response | activation of caspases | anticancer drugs; gamma and ultraviolet irradiation; deprivation of survival factors; cytokines |
| Autophagy | membrane extension; de novo formation of cytosolic vesicles; lack of chromatin condensation | caspase-independent; LC3 lipidation; formation of autophagosome; elevated autophagic flux and lysosomal activity | starvation and other stresses |
| Pyroptosis | DNA fragmentation; chromatin condensation; pore formation; cell swelling and osmotic lysis; plasma membrane rupture | inflammasome assembly; GSDM cleavage; release proinflammatory cytokines and other cellular contents | invading pathogens (pathogen-associated molecular patterns (PAMPs)); endogenous pathogens (damaged-associated molecular patterns (DAMPs)); bacteria (lipopolysaccharide (LPS)) |
| Necroptosis | cell swelling; pore formation; rupture of plasma membranes; moderate chromatin condensation | caspase-independent; RIPK1/RIPK3-mediated phosphorylation of MLKL; necrosome; infammation and immune responses | viral infection; activation of death receptors/Toll-like receptors/cytosolic nucleic acid sensors |
| Ferroptosis | loss of plasma membrane integrity; cytoplasmic swelling; swelling of cytoplasmic organelles; membrane shrinkage with increased membrane density and a reduced number of or loss of mitochondrial cristae; normal nuclear size | ROS-dependent; iron accumulation; lipid peroxidation | Erastin; intracellular iron perturbations; oxidative stress; activation of mitochondrial voltage-dependent anion channels and mitogen-activated protein kinases; up-regulation of endoplasmic reticulum stress; inhibition of cystine/glutamate antiporter |
| Cuproptosis | mitochondrial shrinkage; rupture of mitochondrial membrane | copper accumulation; aggregation of lipoylated dihydrolipoamide S-acetyltransferase (DLAT); loss of iron–sulfur cluster (Fe-S) proteins; increased level of ROS | elesclomol; lipoylated dihydrolipoamide S-acetyltransferase (DLAT) |
| Disulfidptosis | F-actin contraction and detachment from the plasma membrane; collapse of cytoskeleton proteins; cell shrinkage | disulfide stress; redox-sensitive protein disulfide binding; high expression of SLC7A11; formation of aberrant disulfide bonds between actin cytoskeleton proteins | glucose starvation; high cystine uptake; NADPH-depleting cytosolic environment |
| Cell Death Modality | Epigenetic Modification | Cancer Type | Description | Reference |
|---|---|---|---|---|
| Apoptosis | Histone modification | Non-small cell lung cancer (NSCLC) | Histone deacetylases SIRT6 impacted the modulation of antioxidant and redox signaling and apoptosis induction. | [109] |
| Magnolol and polyphenol mixture (PM) derived from Magnolia officinalis exhibited remarkable anti-tumor activities as potential inhibitors of class I HDACs and induced tumor cell apoptosis partially by epigenetically activating DR5. | [110] | |||
| Atypical meningioma (AM) | Certain apoptosis-associated factors were associated with the recurrence of AMs and regulated by histone 3 lysine methyltransferase. | [111] | ||
| Endometrial cancer | PRMT6 regulated genomic regions related to interferons and apoptosis through histone modifications. | [112] | ||
| Colorectal cancer (CRC) | WHSC1 regulated BCL2 gene through the H3K36 dimethylation level and protected colon cancer cell against apoptosis. | [113] | ||
| N-terminal acetyltransferases (NATs) Naa40 inhibited the mitochondrial caspase-9-mediated apoptotic cascade. | [114] | |||
| Gastric cancer (GC) | Anti-tumorigenic agent CIL-102 induced apoptosis in human gastric cancer by the H3K4 trimethylation of TNFR1 and TRAIL proteins. | [115] | ||
| (-)-epigallocatechin-3-gallate (EGCG) impeded cancer progression by apoptosis induction, as well as the inhibition of cell proliferation and histone deacetylase. | [116] | |||
| Hepatocellular carcinoma (HCC) | Histone methyltransferase G9a allowed DNA-damaged hepatocytes to escape p53-induced apoptosis by silencing Bcl-G (a pro-apoptotic Bcl-2 family member). | [117] | ||
| Osteosarcoma | Histone methyltransferase NSD2 promoted the transcription of genes associated with the negative regulation of apoptotic signalling pathways and regulated the expression of the apoptosis regulatory proteins BCL2 and SOX2 through the ERK and AKT pathways. | [118] | ||
| DNA methylation | Schwannoma | The promoter methylation of gene-encoding apoptosis-associated speck-like protein containing a caspase recruitment domain influences the activation of endogenous caspase-9 and caspase-3. | [119] | |
| Renal cell carcinoma (RCC) | DNMT3B silenced the gene that encoded the component of mitochondrial complex III and affected Cyt c release. | [120] | ||
| Ameloblastoma | The transcription of the apoptosis-related gene BCL2L11 was possibly regulated by promoter DNA methylation. | [121] | ||
| Rectal cancer | The methylation status of the promoter regions of key apoptosis genes correlated with apoptosis and the survival of rectal cancer patients. | [122] | ||
| Glioblastoma multiforme (GBM) | Dnmts coregulated apoptosis-associated genes and dictated whether glioma harbors the apoptosis evasion phenotype. | [123] | ||
| Bladder cancer (BCa) | The methylation of apoptosis-associated genes was significantly associated with tumor staging and grading, and methylation markers are promising tools for the noninvasive detection of bladder cancers. | [124] | ||
| Noncoding RNA | Ovarian cancer | LncRNA DUXAP8 regulated the apoptosis of ovarian cancer cells by targeting miR-590-5p. | [125] | |
| Endometrial carcinoma (EC) | LncRNA EIF1AX-AS1 markedly inhibited EC cell proliferation and promoted apoptosis. | [126] | ||
| Oral squamous cellular cancer (OSCC) | The LncRNA CEBPA-DT/CEBPA/BCL2 axis participated in the resistance to chemotherapy drug cisplatin through cell apoptosis. | [127] | ||
| GC | TM4SF1-AS1 promoted stress granule formation and inhibited apoptosis in GC cells by sequestering RACK1, an activator of the stress-responsive MAPK pathway. | [128] | ||
| The site-specific methylation of lncRNAs enhanced the stability of PSMA3-AS1 and MIR22HG to suppress apoptosis of gastric cancer stem cells via the PSMA3-AS1-miR-411-3p- or MIR22HG-miR-24-3p-SERTAD1 axis. | [129] | |||
| NSCLC | SNHG6 promoted proliferation and inhibited apoptosis in NSCLC by regulating miR-490-3p/RSF1 axis. | [130] | ||
| Prostate cancer (PCa) | LncMEG3 inhibited PCa proliferation and promoted apoptosis through the disruption of the miR-9-5p-mediated inhibition of NDRG1. | [131] | ||
| Autophagy | Histone modification | PCa | KDM4B activated autophagy by regulating the Wnt/β-catenin signaling and contributed to castration-resistant prostate cancer. | [132] |
| NSCLC | The inhibition of EHMT2, a histone methyltransferase of histone H3 lysine 9, effectively induced cell death in NSCLC cells through altering cholesterol metabolism-dependent autophagy. | [133] | ||
| GC | The inhibitor of EZH2 and EGFR exerted an effect on tumor growth inhibition through inducing autophagy. | [134] | ||
| CRC | EZH2 bonded to the promoters of the negative regulators of the MTOR and modulated subsequent MTOR pathway-related events, including the inhibition of autophagy. | [135] | ||
| JMJD2B regulated autophagy in CRC cells through LC3B as well as intracellular amino acid levels under glucose deprivation, so as to influence the survival of CRC cells. | [136] | |||
| DNA methylation | Breast caner | The H19/SAHH/DNMT3B axis regulated the methylation level of Beclin1 and then contributed to inducing autophagy. | [137] | |
| Acute lymphoblastic leukemia (ALL) | High-throughput screens uncovered the autophagy-related gene ATG16L2 was associated with a poorer prognosis in childhood ALL. | [138] | ||
| Ovarian cancer | The methylation status of ATG4A impacted the stem properties of ovarian tumor-initiating cells, and the hypomethylation of ATG4A predicted a poor prognosis for ovarian cancer patients. | [139] | ||
| GC | Methylation status influenced the expression level of MAP1LC3Av1, which is essential for autophagy as well as gastric carcinogenesis. | [140] | ||
| Noncoding RNA | Liver cancer | The high expression of miR-638 led to an increase in autophagosomes and autolysosomes through an increase and decrease in the expressions of LC3B-II and Beclin-1 proteins, respectively. | [141] | |
| Cholangiocarcinoma (CCA) | MiR-124 led to autophagic flux by the EZH2-STAT3 signaling axis. | [142] | ||
| HCC | MiR-30a directly targeted the autophagy-related protein Beclin 1 and Atg5 and mediated autophagy activity. | [143] | ||
| Gastrointestinal stromal tumors (GISTs) | MiR-30a was correlated with imatinib sensitization by the regulation of cell autophagy mediated by Beclin-1. | [144] | ||
| Acute myeloid leukemia (AML) | MiR-143 inhibits autophagy in cytarabine-treated AML cells by directly targeting autophagy-related proteins (ATG7 and ATG2B). | [145] | ||
| Pyroptosis | Histone modification | CRC | HDAC2 suppressed the NLRP3 transcription as well as GSDMD-mediated pyroptosis by inhibiting the formation of the H3K27ac/BRD4/p-P65 complex. | [146] |
| GC | The high expression of lysine demethylase ALKBH4 inhibited GSDME activation by inhibiting H3K4me3 histone modification and promoted the proliferation of gastric cancer cells. | [147] | ||
| Melanoma/Lung carcinoma | MLL4 ablation attenuated the expression of the RNA-induced silencing complex (RISC) and DNA methyltransferases through decommissioning enhancers/super-enhancers, which consequently led to the transcriptional reactivation of the double-stranded RNA (dsRNA) interferon response and gasdermin D (GSDMD)-mediated pyroptosis, respectively. | [148] | ||
| Multiple myeloma (MM) | PRMT5 regulates cell pyroptosis by silencing CASP1 in multiple myeloma. | [149] | ||
| RCC | BRD4 exerted an anti-tumor effect in RCC by activating the NF-κB-NLRP3-caspase-1 pyroptosis signaling pathway. | [150] | ||
| Glioblastoma | The usage of LSD1 inhibitor inhibited the proliferation of glioblastoma cells and induced their pyroptosis. | [151] | ||
| DNA methylation | Breast cancer | Breast cancer samples showed a higher DFNA5 methylation in the putative gene promoter; DFNA5 methylation showed strong potential as a detection and prognostic biomarker for breast cancer. | [75] | |
| Clear cell renal cell carcinoma (ccRCC, KIRC) | The DNA methylation levels of GSDMA/B/D/E were decreased in ccRCC patients, and the high expression of GSDME indicated a poor overall survival and relapse-free survival. | [152] | ||
| Breast cancer /CRC/GC/HCC | ZDHHC1, which is frequently silenced in cancer cells, played a role in promoting cell pyroptosis by enhancing oxidative stress and endoplasmic reticulum stress. | [153] | ||
| Uveal melanoma/Lower grade glioma/Kidney renal clear cell carcinoma | Hypomethylation led to the high expression of pyroptosis-related genes in uveal melanoma, lower grade glioma, and kidney renal clear cell carcinoma, suggesting a poor prognosis. | [154] | ||
| HCC | The hypomethylation of pyroptosis-related genes (PRGs) was associated with a poor prognosis of HCC. The gene body hypomethylation of PRGs is a promising biomarker for early HCC detection. | [155] | ||
| Noncoding RNA | Lung adenocarcinoma (LUAD) | The knockdown of circPIBF1 significantly enhanced the expression of pyroptosis-related factors and suppressed LUAD cell growth. | [156] | |
| CRC | The inhibition of miR-15a increased inflammatory cytokines, activated caspase-1 inflammasome, and increased Gasdermin D, an effector of pyroptosis. | [157] | ||
| EC | HOXC-AS2/miR-876-5p/HKDC1 signal transduction axis regulated tumor microenvironment (TME) formation by enhancing glycolysis, promoting a metabolic advantage in lactate-rich environments to further accelerate EC progression. | [158] | ||
| Lung cancer | LINC00969 interacted with EZH2 and METTL3, epigenetically repressing NLRP3 expression to suppress the activation of the NLRP3/caspase-1/GSDMD-related classical pyroptosis signalling pathways in lung cancer. | [159] | ||
| Pancreatic adenocarcinoma (PAAD) | LINC01133 functioned as a competing endogenous RNA to sequester miR-30b-5p from sponging SIRT1 mRNA to inhibit PAAD pyroptosis. | [160] | ||
| Necroptosis | Histone modification | Breast cancer | The G9a-mediated silencing of pro-necroptotic proteins was a critical step in tumor recurrence. | [161] |
| Histone deacetylase (HDAC) inhibitor hindered the progression of breast cancer by significantly up-regulating phospho-RIP3 and MLKL levels and inducing necroptosis. | [162] | |||
| Glioblastoma | HDAC inhibitor induced glioma stem cell death via both apoptosis and necroptosis pathway. | [163] | ||
| Acute myeloid leukemia (AML) | R-2HG induced RIPK1-dependent necroptosis via KDM2B inhibition in AML cells. | [164] | ||
| DNA methylation | CRC | The key player of DNA methylation, UHRF1, regulated necroptosis by methylating the promoter of RIP3. | [165] | |
| Nasopharyngeal carcinoma | EBV infection inhibited necroptosis signaling by the methylation of the RIP3 promoter. | [166] | ||
| Mesothelioma | RIPK3 functioned as a tumor suppressor in mesothelioma, and DNA methylation-mediated RIPK3 silence impeded necroptosis and contributed to cancer progression. | [167] | ||
| NSCLC | The necroptosis pathway was suppressed in lung cancer through RIP3 promoter methylation, and reactivating this pathway should be exploited for improving lung cancer chemotherapy. | [168] | ||
| Noncoding RNA | RCC | Necroptosis-related lncRNAs were selected by WGCNA; among them, RP11-133F8.2 and RP11-283G6.4 could serve as independent prognostic factors for clear cell renal cell carcinoma. | [169] | |
| miR-381-3p acted as an oncogenic miRNA through inhibiting the activation of RIPK3 and MLKL to block necroptosis. | [170] | |||
| Glioma | Necroptosis-related lncRNAs were screened based on the risk score and could be helpful to predict the prognosis of glioma patients. | [171] | ||
| Ferroptosis | Histone modification | HCC | Upon ferroptosis induction, RB1-inducible coiled-coil 1 (RB1CC1) recruited the elongator acetyltransferase complex subunit 3 (ELP3) to strengthen H4K12Ac histone modifications within enhancers linked to ferroptosis and stimulated the transcription of ferroptosis-associated genes. | [172] |
| Pancreatic ductal adenocarcinoma (PDAC) | EP300 acetyltransferase promoted ferroptosis in human PDAC cells via the acetylation of the heat shock protein family A member 5 (HSPA5); acetylated HSPA5 loses its ability to inhibit lipid peroxidation and subsequent ferroptotic cell death. | [173] | ||
| AML | PRMT1 knockout up-regulated acyl-CoA synthetase long-chain family member 1 (ACSL1), which acts as a ferroptosis promoter by increasing lipid peroxidation. | [174] | ||
| MM | Multiple myeloma cells were sensitive to ferroptosis induction and epigenetic reprogramming by RSL3, and the altered expression of histone modifications associated with DNA repair and cellular senescence. | [175] | ||
| Methyltransferase G9a inhibitor (DCG066) inhibited the proliferation and induced ferroptosis in MM cells via the Nrf2/HO-1 pathway. | [176] | |||
| NSCLC | LSD1 inhibition down-regulated the expression of ATF4 through H3K9me2, which sequentially inhibits the expression of the cystine-glutamate antiporter (xCT) and decreases glutathione (GSH) production. | [177] | ||
| SETD1A amplified WTAP expression through WTAPP1 up-regulation by mediating H3K4me3 modification in the WTAPP1 promoter region, thus promoting NSCLC cell proliferation and migration and inhibiting ferroptosis. | [178] | |||
| CRC | Lysine acetyltransferase 2 A (KAT2A) modulated the histone acetylation of GPX4 to regulate the proliferation, metastasis, and ferroptosis of CRC cells. | [179] | ||
| LUAD | Multiple histone modifications had the coregulatory mechanisms of key ferroptosis-related genes in LUAD. | [180] | ||
| Cervical cancer (CC) | Hypoxia-like conditions enhanced the SUMOylation of KDM4A at the K471 locus specifically, repressed H3K9me3 levels, and up-regulated SLC7A11/GPX4 to enhance CC cell ferroptosis resistance. | [181] | ||
| DNA methylation | GC | DNA methylation in the encoding gene of ELOVL5 and FADS1 rendered cells resistant to ferroptosis. | [182] | |
| Gall bladder cancer (GBC) | The down-regulation of RUNX3 was mediated by DNA methylation, which promoted the pathogenesis of gall bladder cancer through attenuating SLC7A11-mediated ferroptosis. | [183] | ||
| HNSCC | A novel ferroptosis-related 16-DNA methylation signature that could be applied as an alternative tool to predict prognosis outcome in patients with HNSCC. | [184] | ||
| Glioma | The methylation of LINC02587 could inhibit cellular proliferative, migrative, and invasive properties and induce ferroptosis within gliomas through the CoQ-FSP1 pathway. | [185] | ||
| The DNA methylation level of lncRNA SNAI3-AS1 promoter reduced its expression and impeded the anti-tumor activity of erastin through ferroptosis. | [186] | |||
| ALL | In ALL samples, the promoter of the gene coding for FSP1 was hypermethylated, silencing the expression of FSP1 and creating a selective dependency on GSH-centered anti-ferroptosis defenses. | [187] | ||
| Noncoding RNA | EC | LncRNA FAM83H-AS1 inhibited ferroptosis in EC by recruiting DNMT1 to increase CDO1 promoter methylation level and inhibit its expression. | [188] | |
| Breast cancer | The LINC00665-miR-410-3p axis was identified as the most potential upstream ncRNA-related pathway of ferroptosis-related gene EMC2 in breast cancer. | [189] | ||
| HCC | The CircIDE/miR-19b-3p/RBMS1 axis influenced HCC cell growth by regulating the expression of glutathione peroxidase 4 (GPX4) and ferroptosis. | [190] | ||
| NSCLC | MiR-4443 regulated the expression of FSP1 in an m6A-dependent manner. | [191] | ||
| Cuproptosis | DNA methylation | Glioma | Cuproptosis-related gene-located DNA-methylation sites linked to patient prognosis/immune microenvironment were established. | [192] |
| Cutaneous melanoma | [193] | |||
| HCC | [194] | |||
| Noncoding RNA | Colon adenocarcinoma (COAD) | Cuproptosis-related lncRNA signature was screened to predict patient prognosis and immune landscape in cancer. | [195] | |
| HCC | [196] | |||
| Osteosarcoma | [197] | |||
| Bladder cancer (BLCA) | [198] | |||
| Pancreatic cancer (PC) | [199] | |||
| HNSCC | [200] | |||
| Uterine corpus endometrial carcinoma (UCEC) | [201] |
| Types | Drug/Small Compound | Target | Indications |
|---|---|---|---|
| Apoptosis | Venetoclax | BCL-2 | Chronic myeloid leukaemia (CML), Chronic lymphocytic leukemia (CLL), AML, ALL |
| Navitoclax | CLL | ||
| ABT-737 | AML, Pca | ||
| APG-1252 | NSCLC, GC, HCC | ||
| Lisaftoclax (APG-2575) | AML, WM, MM, CLL/SLL, Follicular lymphoma (FL) | ||
| BCL-201 | FL, Mantle cell lymphoma (MCL) | ||
| AZD4320 | Lymphoma, MM | ||
| Sonrotoclax (BGB-11417) | CLL/SLL, AML, Non-Hodgkin’s Lymphoma, Multiple myeloma | ||
| AMG176 | MCL1 | AML, MM, CLL | |
| MIK665 | HCC, MM, Lymphoma | ||
| Xevinapant | IAP | Squamous cell carcinoma of the head and neck (SCCHN), PDAC, CRC | |
| AT-406 | CC, CRC | ||
| Tollinapant | Advanced malignant solid tumor, T-cell lymphoma | ||
| APG-1387 | Pca, Hepatitis B | ||
| LCL161 | HNSCC, MM, ALL | ||
| APG-115 | MDM2-p53 | AML, GC | |
| Idasanutlin | AML, Neuroblastoma | ||
| Nelfinavir | Protein kinase B (AKT) pathway | CC | |
| Mapatumumab | Apoptosis-inducing ligand receptor-1 (TRAIL-R1) | CC | |
| Autophagy | Chloroquine (CQ) | Lysosome | CRC, Breast cancer |
| HCQ | Breast cancer | ||
| SBI-0206965 | AMPK | LUAD, Glioblastoma | |
| 3MA | PI3K | GC, Melanoma, Glioma | |
| Pyroptosis | Disulfiram | GSDMD | NSCLC, Liver cancer, Breast cancer, Pca, PC |
| Gambogic Acid | CNPY3 | Pca | |
| Metformin | GSDME | Breast cancer, Colon cancer | |
| Docosahexaenoic acid | Caspase-1,GSDMD | Triple-negative breast cancer | |
| Simvastatin | Caspase-1 | NSCLC | |
| Mirdametinib, Vemurafenib | MEK/ERK1/2 | Melanoma | |
| Paclitaxel | Caspase-3/GSDME | Lung cancer | |
| Doxorubicin | Anti-tumor CD8+ T cells and NK cells | HNSCC | |
| Tetraarsenic hexoxide | Caspase-3/GSDME | Triple-negative breast cancer | |
| Antibody Targeting Gasdermin-B | GSDMB | Breast cancer | |
| Dimethyl fumarate (DMF) | GSDMD | Breast cancer | |
| Necroptosis | Necrostatin-1 | RIPK1 | CRC |
| FTY720 | I2PP2A/SET | Lung cancer | |
| Chloroquine (CQ) | RIPK3 | CRC | |
| Emodin | TNF/RIPK1/RIPK3 | Glioma | |
| Ophiopogonin D’ | RIPK1 | Pca | |
| Tanshinol A | MLKL | Lung cancer | |
| CBL0137 | ZBP1 | Breast cancer, CRC, Melanoma | |
| Ferroptosis | Erastin | VDAC2/VDCA3 | Breast cancer, Melanoma, CC |
| RSL3 | GPX4 | CRC | |
| Deferoxamine | Fenton reaction | Breast cancer, HCC | |
| β-mercaptoethanol | System xc- | NSCLC | |
| Ciclopirox | Intracellular iron chelator | GC | |
| Sorafenib | System xc- | HCC | |
| Cisplatin | GSH levels, GPXs | CRC | |
| Apatinib | GSH levels, GPX4 | GC | |
| AS-252424 (AS) | ACSL4 | Kidney ischemia/reperfusion injury, Acute liver injury (ALI) | |
| BRD4770 | System Xc--GPX4, FSP1-CoQ10, GCH1-BH4 pathways | Aortic dissection (AD) | |
| Sulfasalazine | System xc- | Rheumatoid arthritis, Brain cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saqirile; Deng, Y.; Li, K.; Yan, W.; Li, K.; Wang, C. Gene Expression Regulation and the Signal Transduction of Programmed Cell Death. Curr. Issues Mol. Biol. 2024, 46, 10264-10298. https://doi.org/10.3390/cimb46090612
Saqirile, Deng Y, Li K, Yan W, Li K, Wang C. Gene Expression Regulation and the Signal Transduction of Programmed Cell Death. Current Issues in Molecular Biology. 2024; 46(9):10264-10298. https://doi.org/10.3390/cimb46090612
Chicago/Turabian StyleSaqirile, Yuxin Deng, Kexin Li, Wenxin Yan, Ke Li, and Changshan Wang. 2024. "Gene Expression Regulation and the Signal Transduction of Programmed Cell Death" Current Issues in Molecular Biology 46, no. 9: 10264-10298. https://doi.org/10.3390/cimb46090612
APA StyleSaqirile, Deng, Y., Li, K., Yan, W., Li, K., & Wang, C. (2024). Gene Expression Regulation and the Signal Transduction of Programmed Cell Death. Current Issues in Molecular Biology, 46(9), 10264-10298. https://doi.org/10.3390/cimb46090612






