Exopolysaccharides from the Green Microalga Strain Coelastrella sp. BGV—Isolation, Characterization, and Assessment of Anticancer Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Cell Lines and Culture Conditions
2.3. Algal Strain and Growth Conditions
2.4. Extraction of Extracellular Polysaccharide
2.5. Characterization of Extracellular Polysaccharide (EPS)
2.5.1. Chemical Composition Analysis
2.5.2. Determination of Molecular Weight
2.5.3. Determination of Monosaccharide Composition
2.5.4. FT-IR Spectroscopy
2.6. Anticancer Activity of Extracellular Polysaccharide/s
2.6.1. Cell Viability Assay
2.6.2. Cell Migration Assay
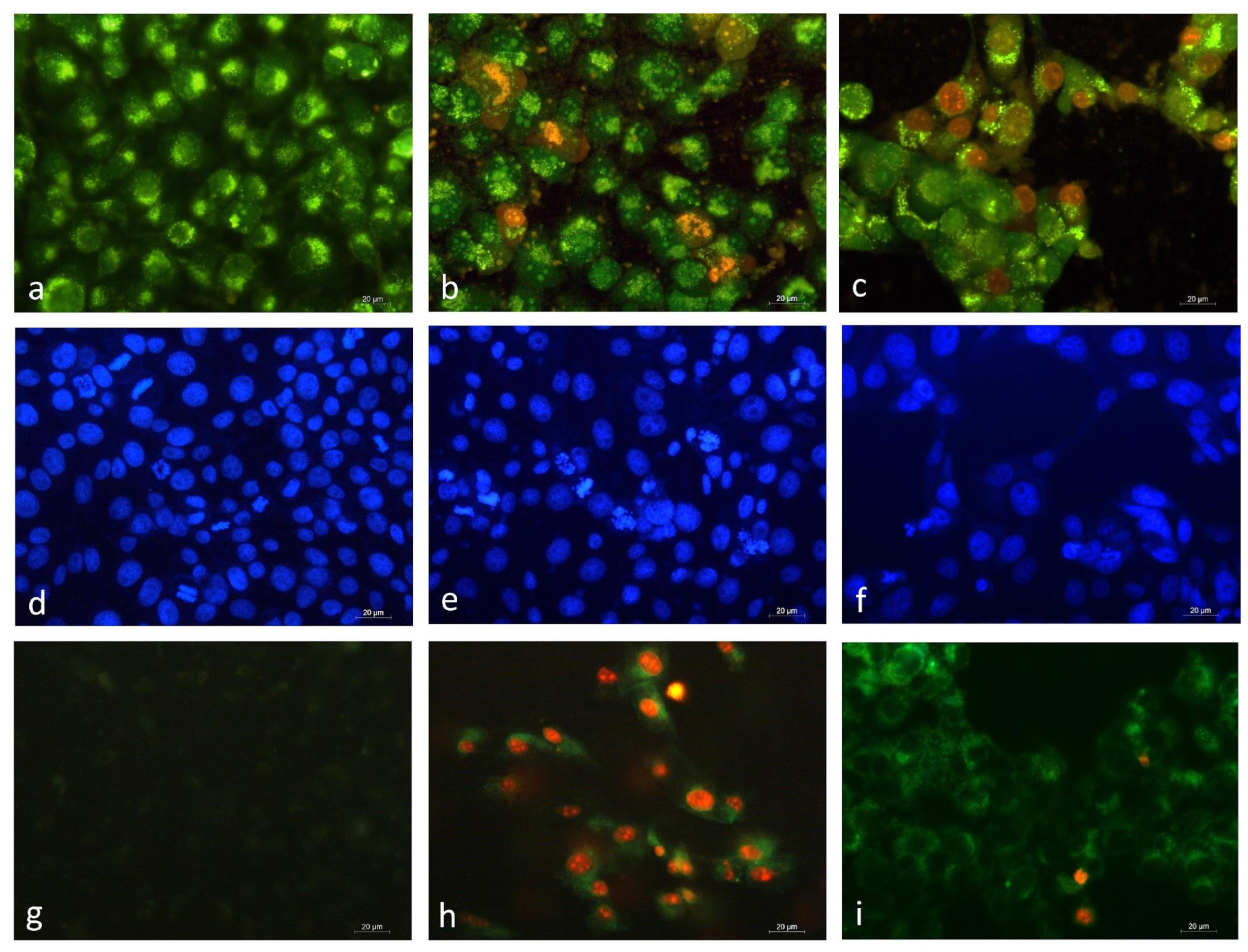
2.6.3. Fluorescence Microscopy
AO/EtBr Staining
DAPI Staining
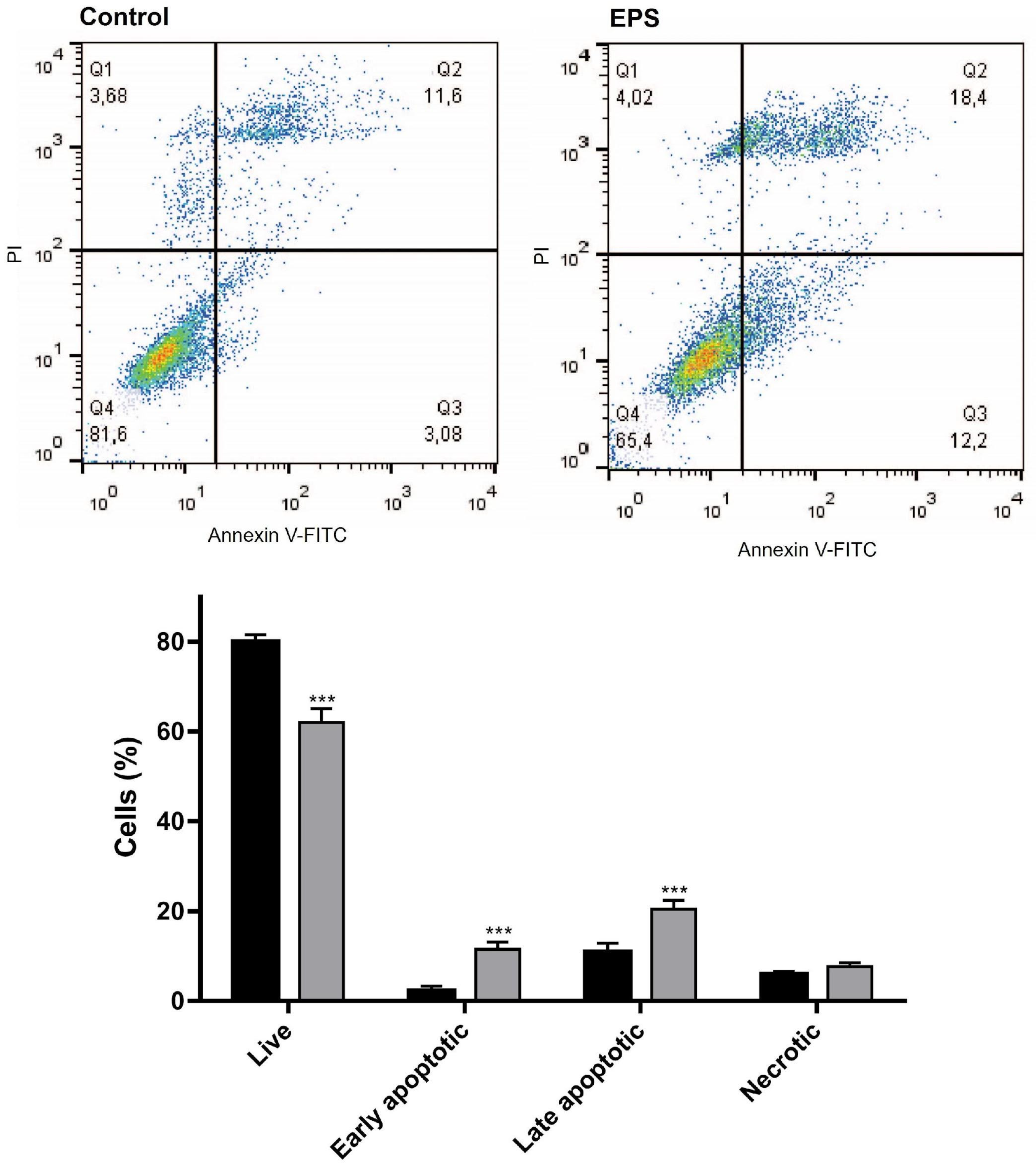
Annexin V-FITC/PI Staining
2.6.4. Flow Cytometry
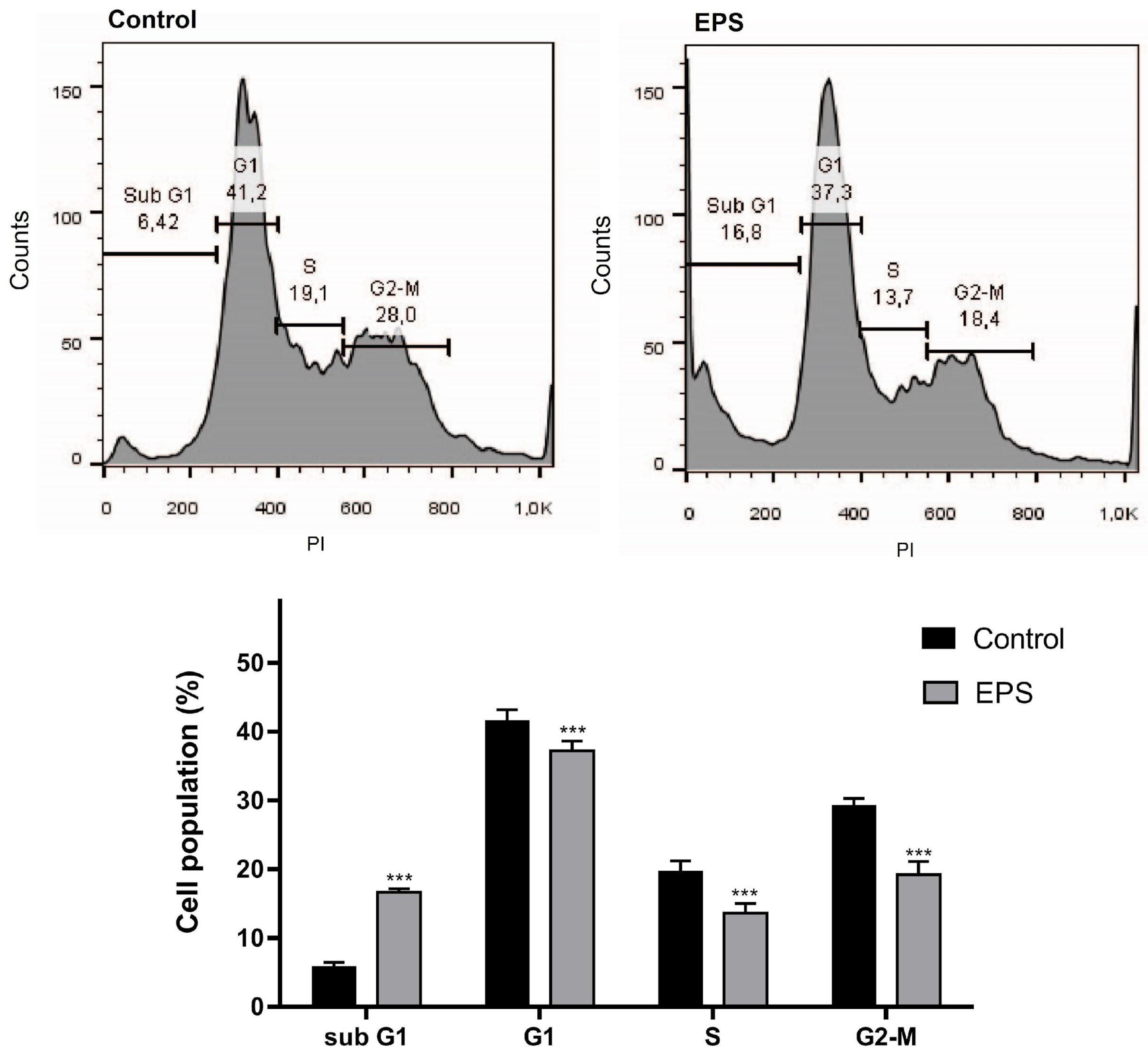
Cell Cycle Analysis
Annexin V/PI Analysis of Cancer Cell Apoptosis
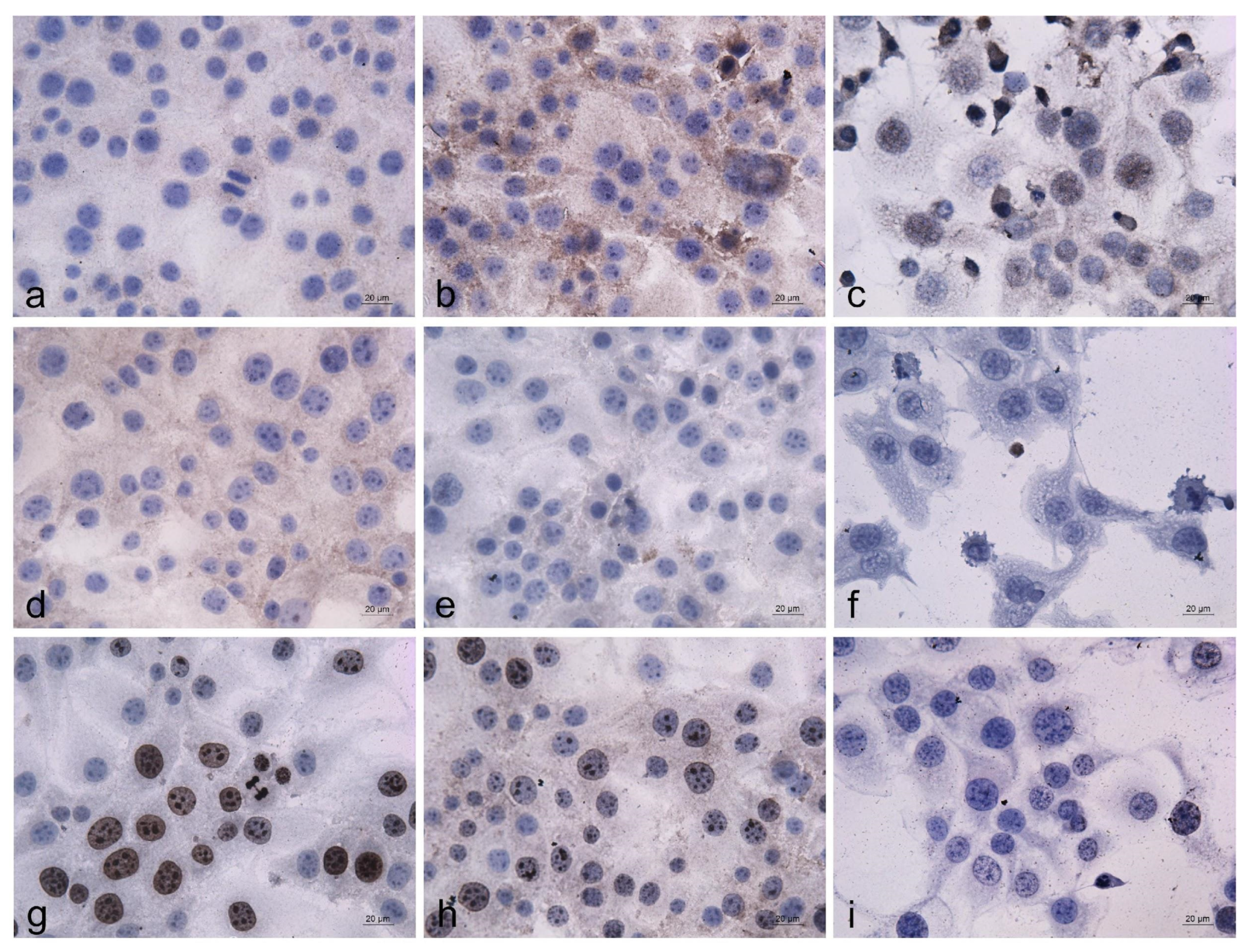
2.6.5. Immunocytochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Chemical Characterization of EPS Isolated from Coelastrella sp. BGV
3.2. Anticancer Activity of Extracellular Polysaccharide
3.2.1. Effects of EPS from Coelastrella sp. BGV on Cell Viability
3.2.2. Effects of EPS on HeLa Cancer Cell Migration
3.2.3. Fluorescent Microscopy Analysis of EPS-Induced Apoptotic Alterations in HeLa Cancer Cells
3.2.4. Flow Cytometry Analysis of the Effects of EPS on the Apoptosis and Cell Cycle Progression in HeLa Cancer Cells
Apoptosis Assay
Cell Cycle Analysis
3.2.5. Immunocytochemical Analysis of the EPS-Induced Alterations in the Expression and Intracellular Localization of the p53, bcl2, and Ki67 Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frankfurt, O.S.; Krishan, A. Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anticancer Drugs 2003, 14, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Abd El Baky, H.H.; El-Baroty, G.S. Healthy benefit of microalgal bioactive substances. J. Aquat. Sci. 2013, 1, 11–23. [Google Scholar]
- Singh, D.V.; Upadhyay, A.K.; Singh, R.; Singh, D.P.B. Health benefits of bioactive compounds from microalgae. In Phytomedicine; Bhat, R.A., Hakeem, K.R., Dervash, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 10; pp. 291–319. [Google Scholar]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Suleria, H. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Tounsi, L.; Hentati, F.; Hlima, H.B.; Barkallah, M.; Smaoui, S.; Fendri, I.; Abdelkafi, S. Microalgae as feedstock for bioactive polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Bernaerts, T.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Mišurcová, L.B. Chemical composition of seaweeds. In Handbook of Marine Macroalgae, Biotechnology and Applied Phycology; Kim, S.-K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; Chapter 7; pp. 171–192. [Google Scholar]
- Kumar, K.S.; Kumari, S.; Singh, K.; Kushwaha, P.B. Influence of seasonal variation on chemical composition and nutritional profiles of macro-and microalgae. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Rajauria, G., Yuan, Y.V., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; Chapter 2; pp. 14–71. [Google Scholar]
- Costa Alberto Vieira, J.; Franco Lucas, B.; Gabrielle Pires Alvarenga, A.; Botelho Moreira, J.; Greque de Morais, M. Microalgae polysaccharides: An overview of production, characterization and potential applications. Polysaccharides 2021, 2, 2673–4176. [Google Scholar]
- Kraan, S.B. Algal Polysaccharides, Novel Applications and Outlook. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C., Ed.; IntechOpen: London, UK, 2012; Volume 1, Chapter 2; pp. 490–532. [Google Scholar]
- Mišurcová, L.; Orsavová, J.; Ambrožová, J.V.B. Algal Polysaccharides and Health. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Chapter 4; pp. 109–144. [Google Scholar]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Park, G.T.; Go, R.E.; Lee, H.M.; Lee, G.A.; Kim, C.W.; Seo, J.W.; Hwang, K.A. Potential anti-proliferative and immunomodulatory effects of marine microalgal exopolysaccharide on various human cancer cells and lymphocytes in vitro. Mar. Biotechnol. 2017, 19, 136–146. [Google Scholar] [CrossRef]
- Netanel, L.G.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Arad, S. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef]
- Matsubara, K.; Mori, M.; Matsumoto, H.; Hori, K.; Miyazawa, K. Antiangiogenic properties of a sulfated galactan isolated from a marine green alga, Codium cylindricum. J. Appl. Phycol. 2003, 15, 87–90. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.B.; Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, structures and biological activities of polysaccharides from Chlorella: A review. Int. J. Biol. Macromol. 2020, 163, 2199–2209. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Marti-Quijal, F.J.; Barba, F.J.; Altintas, Z. Current emerging trends in antitumor activities of polysaccharides extracted by microwave and ultrasound-assisted methods. Int. J. Biol. Macromol. 2022, 202, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Toshkova-Yotova, T.; Georgieva, A.; Iliev, I.; Alexandrov, S.; Ivanova, A.; Pilarski, P.; Toshkova, R. Antitumor and antimicrobial activity of fatty acids from green microalga Coelastrella sp. BGV. South Afr. J. Bot. 2022, 151, 394–402. [Google Scholar] [CrossRef]
- Toshkova-Yotova, T.A.; Georgieva, A.; Pilarski, P.; Toshkova, R. Aqueous extracts, green microalga Coelastrella sp. BGV displays antiproliferative, proapoptotic activity in vitro against HeLa tumor cells. Compt. Rend. Acad. Bulg. Sci. 2021, 74, 696–705. [Google Scholar]
- Toshkova-Yotova, T.; Georgieva, A.; Todorova, K.; Pilarski, P.; Toshkova, R. Antitumor properties of vegetable oil extract from green microalga Coelastrella sp. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e2744. [Google Scholar] [CrossRef]
- Dimitrova, P.; Marinova, G.; Pilarski, P. Preliminary studies on the growth and biochemical composition of a promising carotenoid producing strain Coelastrella sp. Nat. Math. Sci. 2016, 6, 139–149. [Google Scholar]
- Georgiev, D.; Dilov, H.; Avramova, S. Millieu nutritif tamponne et méthode de culture intensive des microal-gues vertes. Hydrobiology 1978, 7, 14–23. [Google Scholar]
- Ivanova, J.G.; Toshkova-Yotova, T.S.; Toshkova, R.A.; Deleva, V.R.; Georgieva, A.K.; Gigova, L.G. Antioxidant and Anticancer Potential of Extracellular Polysaccharide from Porphyridium aerugineum (Rhodophyta). Fermentation 2024, 10, 259. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- McComb, E.A.; McCready, R.M. Determination of acetyl in pectin and in acetylated carbohydrate polymers. Anal. Chem. 1957, 29, 819–821. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Ognyanov, M.; Georgiev, Y.; Petkova, N.; Ivanov, I.; Vasileva, I.; Kratchanova, M. Isolation and characterization of pecticpolysaccharide fraction from in vitro suspension culture of Fumaria officinalis L. Int. J. Polym. Sci. 2018, 2018, 570503. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Wang, Q.; Wang, H.; Mei, Q. Analysis of the monosaccharide components in Angelica polysaccharides by high performance liquid chromatography. Anal. Sci. 2005, 21, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 11, 953–957. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, Y.; Zhang, L.; Cao, Y.; Yao, W.; Tang, Y.; Ding, A. A Natural triterpene derivative from Euphorbia kansui inhibits cell proliferation and induces apoptosis against rat intestinal epithelioid cell line in vitro. Int. J. Mol. Sci. 2015, 16, 18956–18975. [Google Scholar] [CrossRef]
- Yin, P.H.; Liu, X.; Qiu, Y.Y.; Cai, J.F.; Qin, J.M.; Zhu, H.R.; Li, Q. Anti-tumor activity and apoptosis-regulation mechanisms of bufalin in various cancers: New hope for cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 5339–5343. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Sandoghchian Shotorbani, S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar]
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef]
- Yalcin, I.; Hicsasmaz, Z.; Boz, B.; Bozoglu, F. Characterization of the extracellular polysaccharide from fresh-water microalgae Chlorella sp. Lebensm. Wiss. Technol. 1994, 27, 158–165. [Google Scholar] [CrossRef]
- Morineau-Thomas, O.; Jaouen, P.; Legentilhomme, P. The role of exopolysaccharides in fouling phenomenon during ultrafiltration of microalgae (Chlorella sp. and Porphyridium purpureum): Advantage of a swirling decaying flow. Bioprocess Biosyst. Eng. 2002, 25, 35–42. [Google Scholar]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Chen, F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int. J. Biol. Macromol. 2019, 134, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae polysaccharides ameliorate obesity in association with modulation of lipid metabolism and gut microbiota in high-fat-diet fed C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Deamici, K.M.; Costa, J.A.V.; Santos, L.O. Magnetic fields as triggers of microalga growth: Evaluation of its effect on Spirulina sp. Bioresour. Technol. 2016, 220, 62–67. [Google Scholar] [CrossRef]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef]
- Trabelsi, L.; Chaieb, O.; Mnari, A.; Abid-Essafi, S.; Aleya, L. Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae Graesiella sp. BMC Complement. Altern. Med. 2016, 16, 210. [Google Scholar] [CrossRef]
- Sun, L.; Chu, J.; Sun, Z.; Chen, L. Physicochemical properties, immunomodulation and antitumor activities of polysaccharide from Pavlova viridis. Life Sci. 2016, 144, 156–161. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer protective action of polysaccharide, derived from red microalga Porphyridium cruentum—A biological background. Biotechnol. Biotechnol. Equip. 2009, 23, 783–787. [Google Scholar] [CrossRef]
- Ye, W.; Zhu, J.; Liu, Q.; Zhang, Y.; Yuan, Y.; Guo, S.; Zhang, Z. Characterization and anticancer effects of extracellular polysaccharide from DHA-producing microalga Crypthecodinium sp. SUN. Int. J. Biol. Macromol. 2023, 249, 100–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ji, M.; Liu, Y.; Zhang, M.; Zheng, C.; Wang, P. XQZ3, a Chlorella pyrenoidosa polysaccharide suppresses cancer progression by restraining mitochondrial bioenergetics via HSP90/AKT signaling pathway. Int. J. Biol. Macromol. 2024, 264, 130705. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, P. Partial characterization, the immune modulation and anticancer activities of sulfated polysaccharides from filamentous microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wan, H.; Wang, R.; Hao, D. Sulfated polysaccharides from Phaeodactylum tricornutum: Isolation, structural characteristics, and inhibiting HepG2 growth activity in vitro. PeerJ 2019, 7, e6409. [Google Scholar] [CrossRef]
- Sogawa, K.; Kodama, E.; Matsuda, M.; Shigeta, S.; Okutani, K. Marine microalgal polysaccharide induces apoptosis in human lymphoid cells. J. Mar. Biotechnol. 1998, 6, 35–38. [Google Scholar]
- Sogawa, K.; Yamada, T.; Sumida, T.; Hamakawa, H.; Kuwabara, H.; Matsuda, M.; Muramatsu, Y.; Kose, H.; Matsumoto, K.; Sasaki, Y.; et al. Induction of apoptosis and inhibition of DNA topoisomerase-I in K-562 cells by a marine microalgal polysaccharide. Life Sci. 2000, 66, 227–231. [Google Scholar] [CrossRef]
- Umemura, K.; Yanase, K.; Suzuki, M.; Okutani, K.; Yamori, T.; Andoh, T. Inhibition of DNA topoisomerases I and II, and growth inhibition of human cancer cell lines by a marine microalgal polysaccharide. Biochem. Pharmacol. 2003, 66, 481–487. [Google Scholar] [CrossRef]
- Zhong, R.; Li, J.Q.; Wu, S.W.; He, X.M.; Xuan, J.C.; Long, H.; Liu, H.Q. Transcriptome analysis reveals possible antitumor mechanism of Chlorella exopolysaccharide. Gene 2022, 811, 127–146. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Uxa, S.; Castillo-Binder, P.; Kohler, R.; Stangner, K.; Müller, G.A.; Engeland, K. Ki-67 gene expression. Cell Death Differ. 2021, 28, 3357–3370. [Google Scholar] [CrossRef]
- Wei, H.; Qu, L.; Dai, S.; Li, Y.; Wang, H.; Feng, Y.; Chen, X.; Jiang, L.; Guo, M.; Li, J.; et al. Structural insight into the molecular mechanism of p53-mediated mitochondrial apoptosis. Nat. Commun. Commun. 2021, 12, 2280. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef] [PubMed]
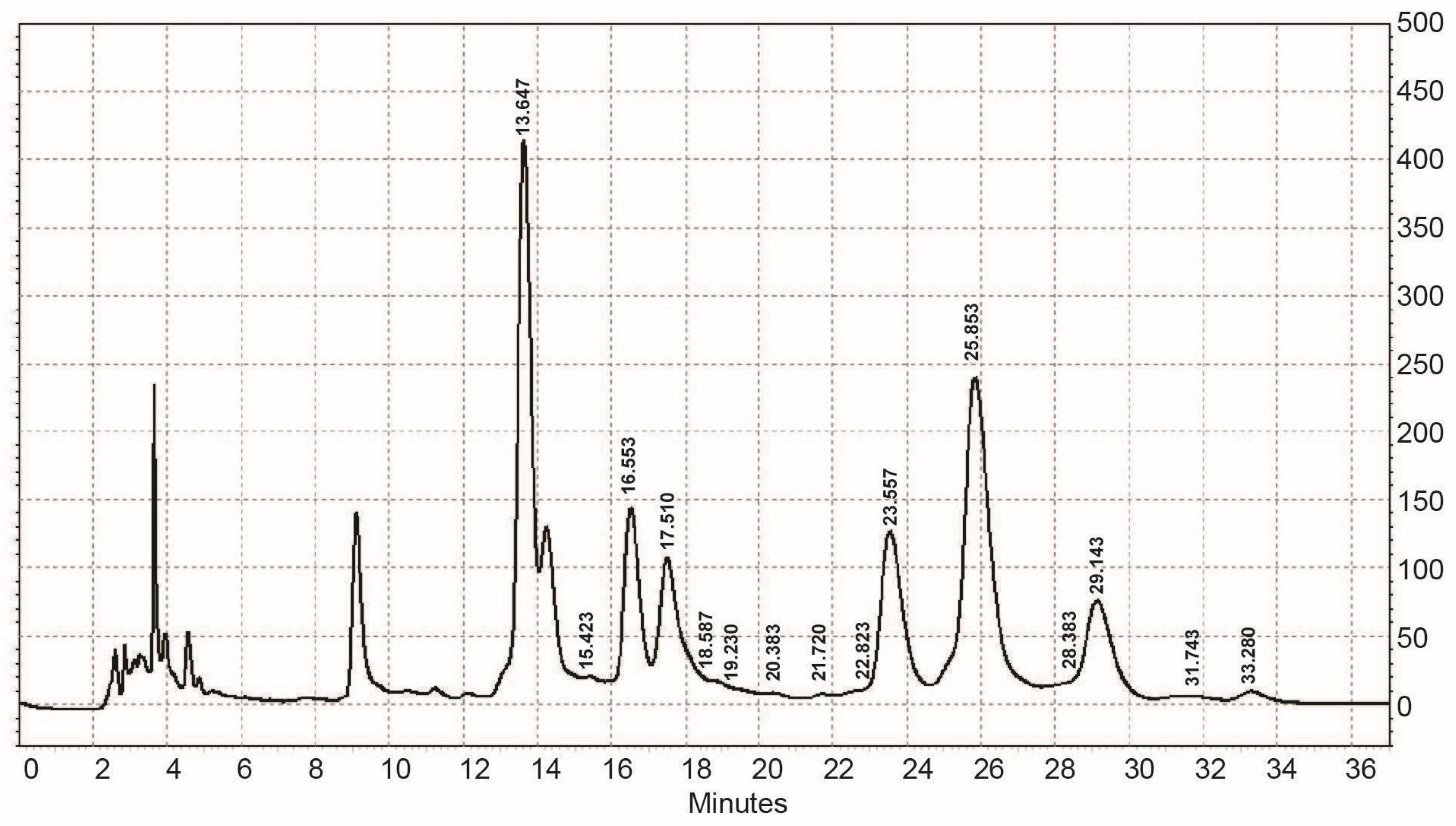
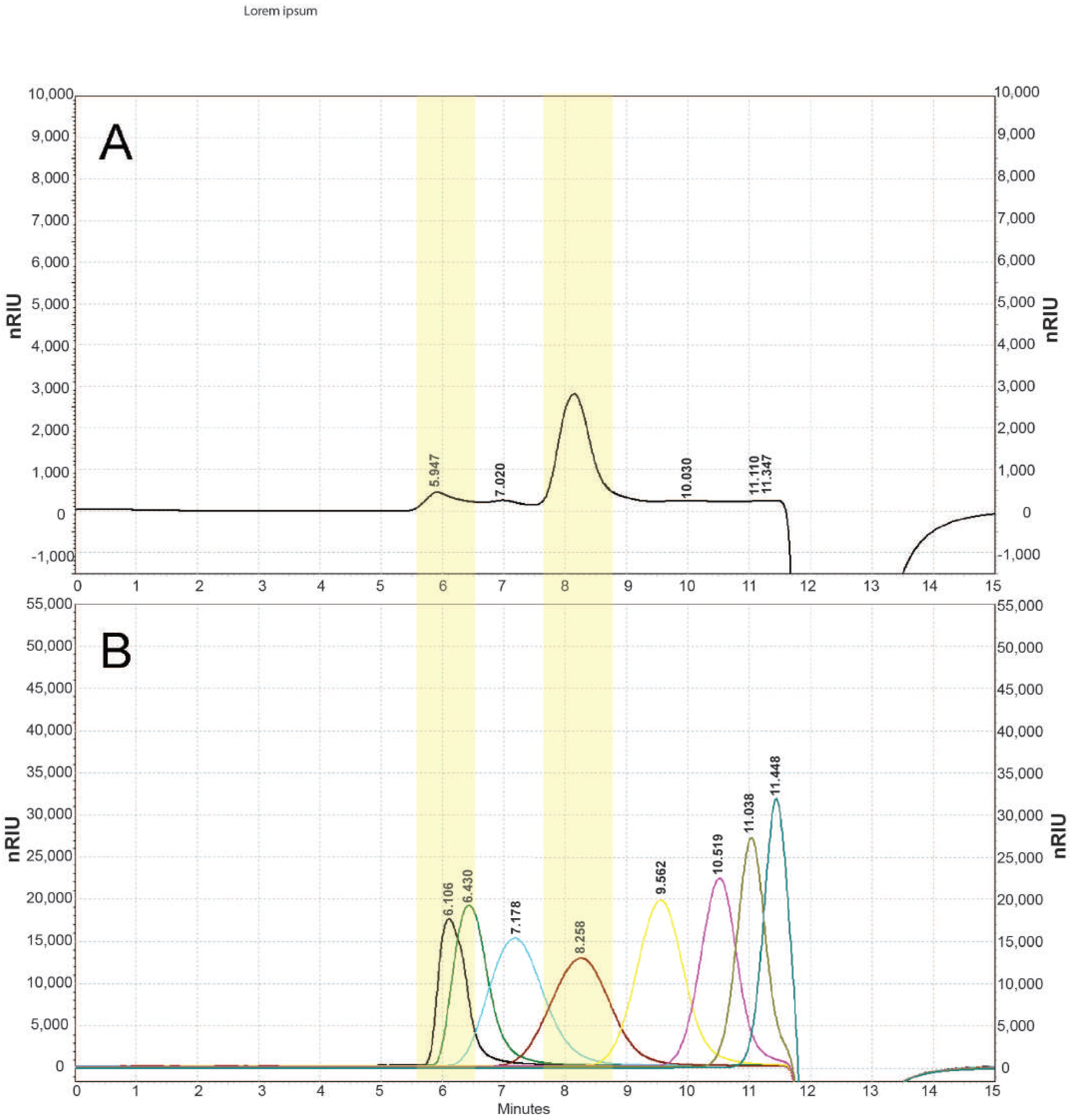

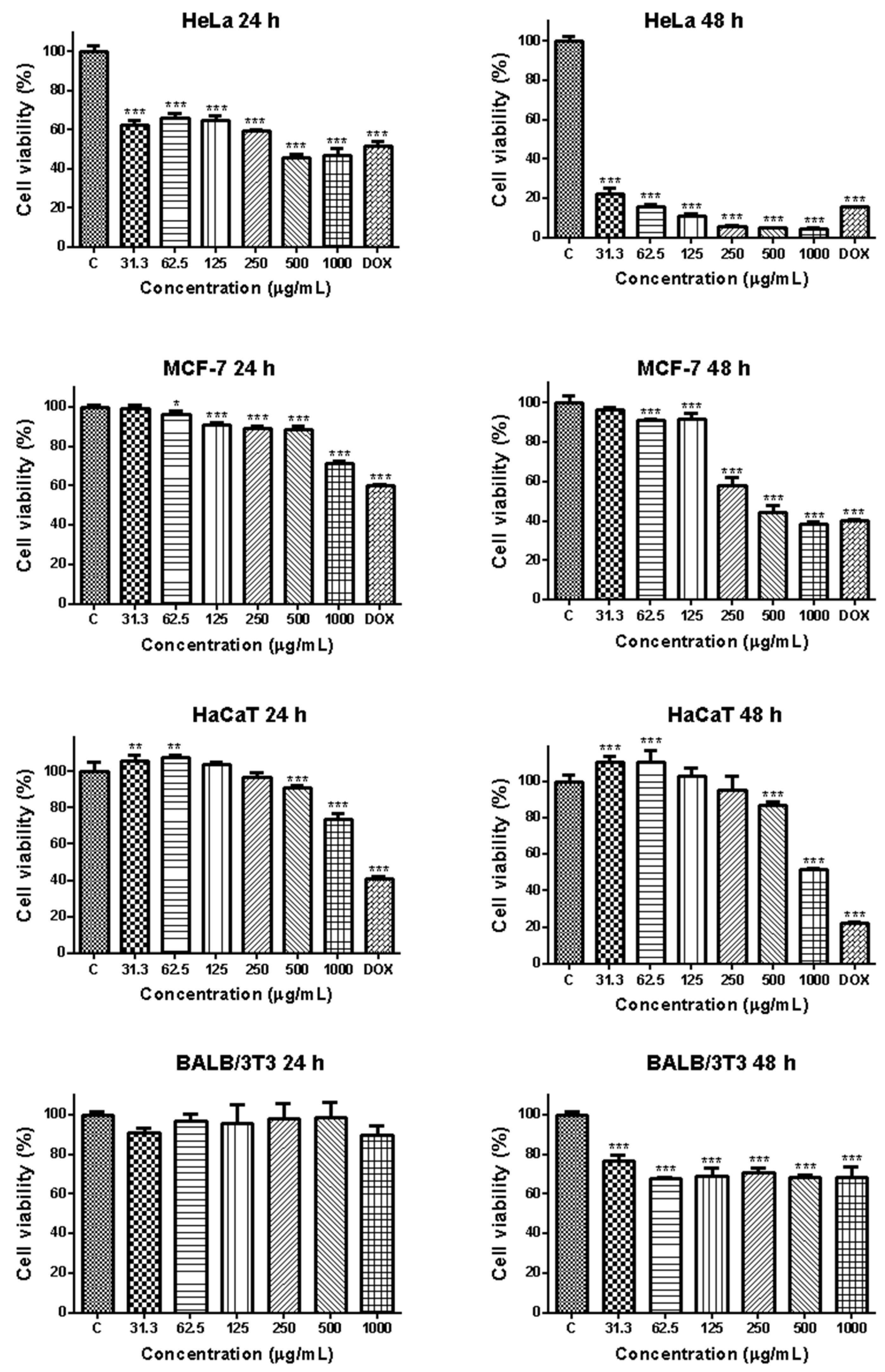
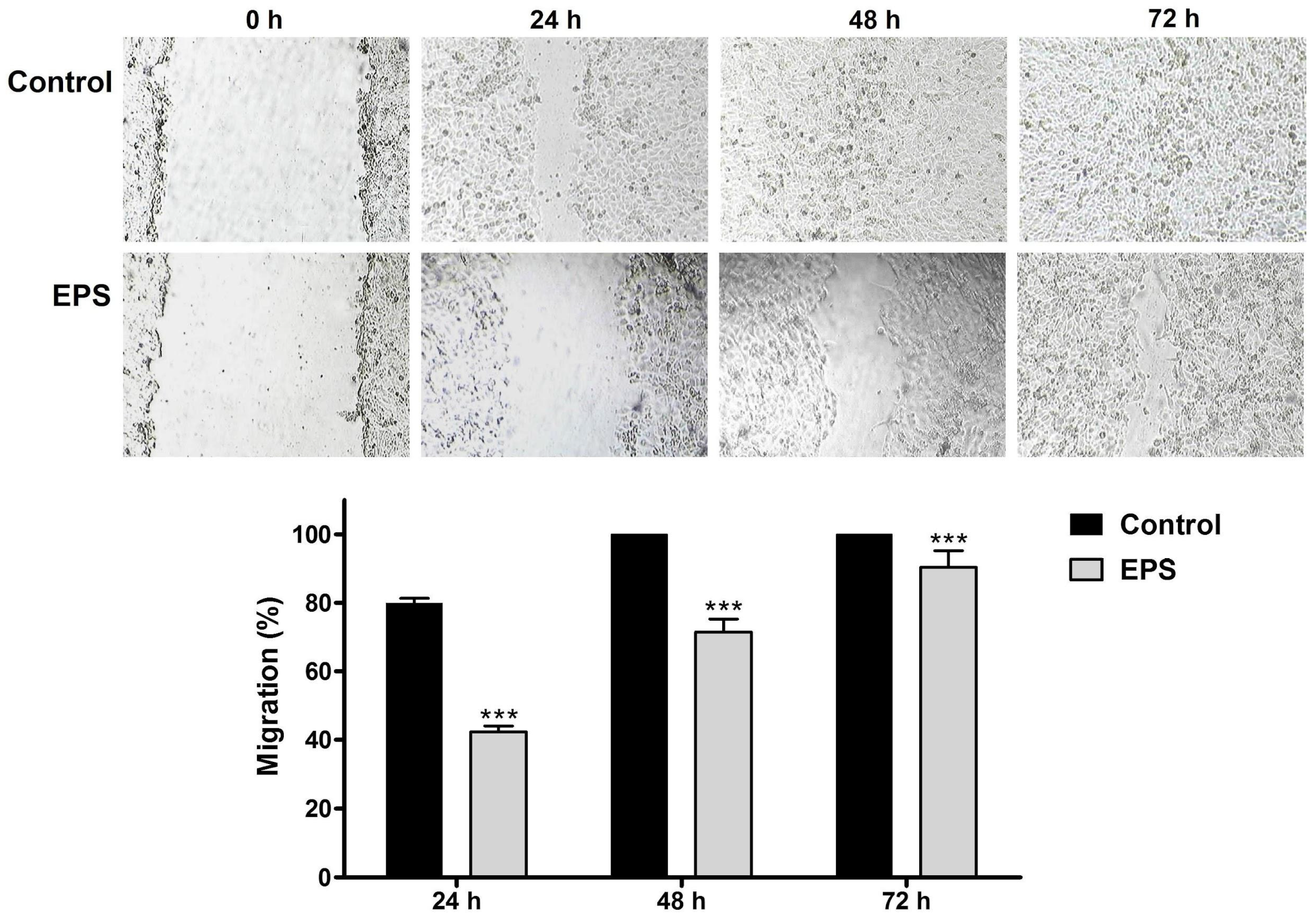
| Biochemical Composition | Concentration, w/w% |
|---|---|
| Total protein content | 7.1 |
| Neutral sugars | |
| Rhamnose (Rha) | - |
| Arabinose (Ara) | - |
| Galactose (Gal) | 1.7 |
| Glucose (Glc) | - |
| Mannose (Man) | - |
| Xylose (Xyl) | - |
| Fucose (Fuc) | 0.4 |
| Total carbohydrates (Glc equivalent) | 36.4 |
| Total uronic acids content | 4.5 |
| Degree of methoxylation, mol% | 0.74 |
| Acetyl groups content | 0.0 |
| Rare sugars test (+ positive, − negative) | +++ |
| Cell Line | 24 h | 48 h |
|---|---|---|
| HeLa | 643.11 | <31.3 |
| MCF-7 | >1000 | 486.40 |
| HaCaT | >1000 | >1000 |
| BALB/3T3 | >1000 | >1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toshkova-Yotova, T.; Sulikovska, I.; Djeliova, V.; Petrova, Z.; Ognyanov, M.; Denev, P.; Toshkova, R.; Georgieva, A. Exopolysaccharides from the Green Microalga Strain Coelastrella sp. BGV—Isolation, Characterization, and Assessment of Anticancer Potential. Curr. Issues Mol. Biol. 2024, 46, 10312-10334. https://doi.org/10.3390/cimb46090614
Toshkova-Yotova T, Sulikovska I, Djeliova V, Petrova Z, Ognyanov M, Denev P, Toshkova R, Georgieva A. Exopolysaccharides from the Green Microalga Strain Coelastrella sp. BGV—Isolation, Characterization, and Assessment of Anticancer Potential. Current Issues in Molecular Biology. 2024; 46(9):10312-10334. https://doi.org/10.3390/cimb46090614
Chicago/Turabian StyleToshkova-Yotova, Tanya, Inna Sulikovska, Vera Djeliova, Zdravka Petrova, Manol Ognyanov, Petko Denev, Reneta Toshkova, and Ani Georgieva. 2024. "Exopolysaccharides from the Green Microalga Strain Coelastrella sp. BGV—Isolation, Characterization, and Assessment of Anticancer Potential" Current Issues in Molecular Biology 46, no. 9: 10312-10334. https://doi.org/10.3390/cimb46090614
APA StyleToshkova-Yotova, T., Sulikovska, I., Djeliova, V., Petrova, Z., Ognyanov, M., Denev, P., Toshkova, R., & Georgieva, A. (2024). Exopolysaccharides from the Green Microalga Strain Coelastrella sp. BGV—Isolation, Characterization, and Assessment of Anticancer Potential. Current Issues in Molecular Biology, 46(9), 10312-10334. https://doi.org/10.3390/cimb46090614






