Folliculogenesis: A Cellular Crosstalk Mechanism
Abstract
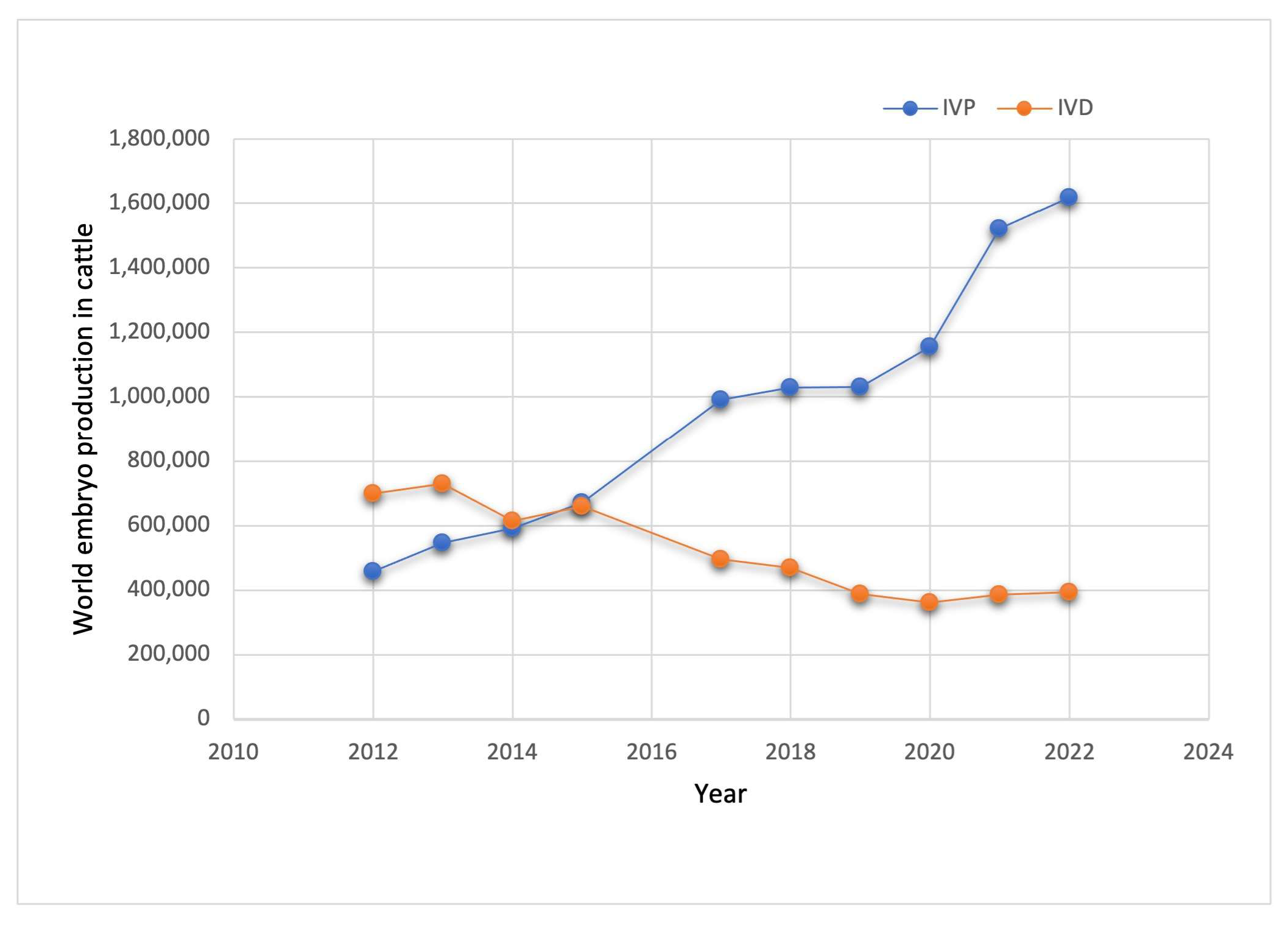
1. Introduction
2. Folliculogenesis
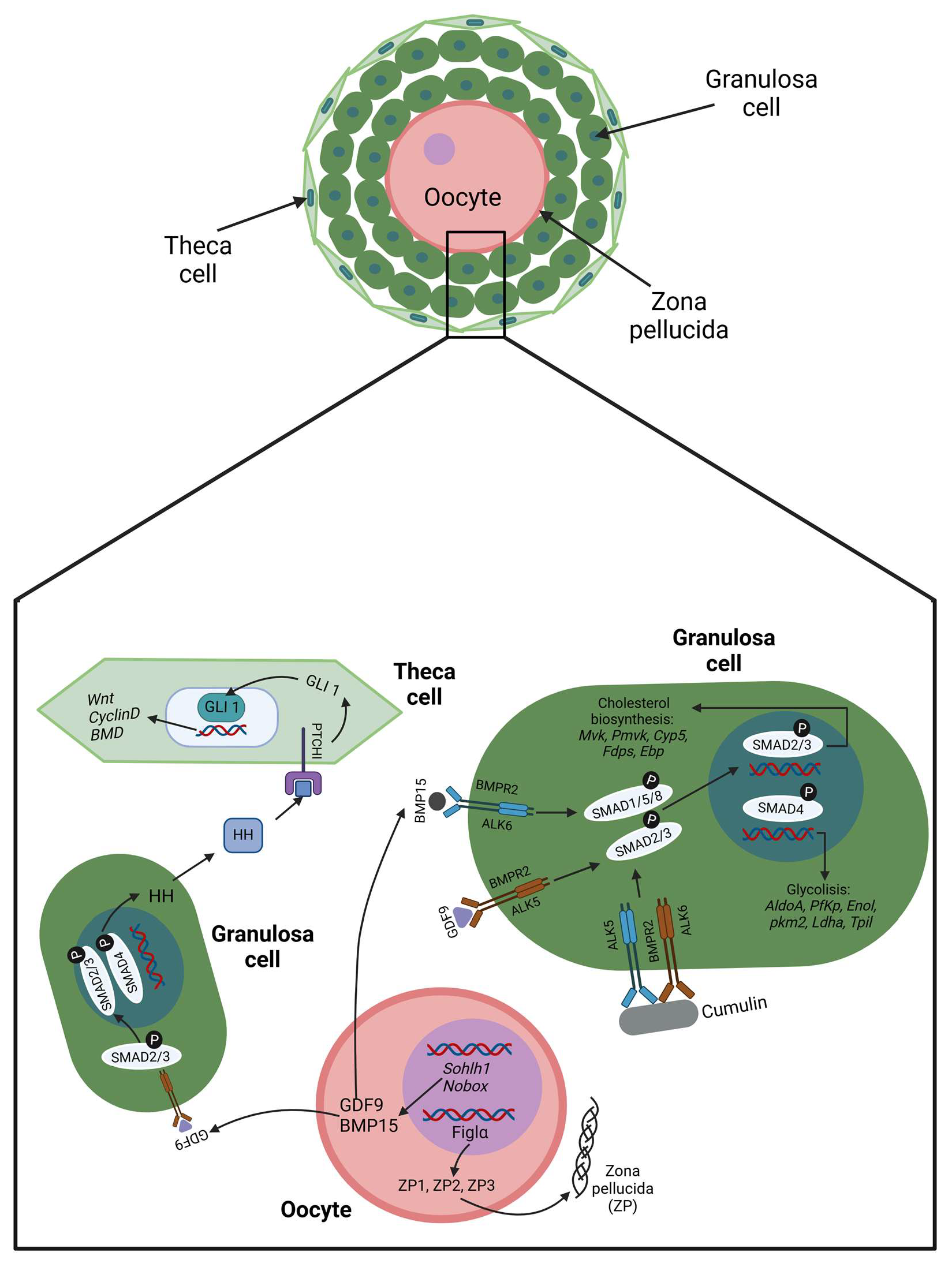
2.1. Cellular Crosstalk in the Beginning
2.2. Cellular Crosstalk at the End
3. Perspectives and Conclusions
| Characteristic | Cows | Women | References |
|---|---|---|---|
| Age at puberty | 10–12 months | 11–12 years | [77,78] |
| Length of estrous or menstrual cycle | 21 days (estrous) | 28 days (menstrual) | [72] |
| Ovulation after LH peak (hours) | 11 | 9–12 | [79,80] |
| Length of gestation period (days) | 277–290 | 271–289 | [78] |
| The average number of offspring | 1 | 1 | [61] |
| Ovary volume (cm3) | 5–6 | 6.6 | [81,82] |
| Average of oocytes at birth | 135,000 | 700,000 | [18] |
| Follicle primordial diameter (μm) | <40 | 35–40 | [14,33] |
| Follicle primary diameter (μm) | 40–80 | 50–64 | [14,33] |
| Follicle secondary diameter (μm) | 81–130 | 115–125 | [14,33] |
| Follicle tertiary (μm) | 250–500 | 150–250 | [14,33] |
| Follicle dominant diameter (mm) | 8.5–10 | 10–29 | [79,83] |
| Oocyte metaphase II diameter (μm) | 115–120 | 103–119 | [84,85] |
| Number of follicular waves | 2–3 | 2–3 | [61] |
| Number of follicles per wave | 8–41 | 4–14 | [61] |
| Genome embryonic activation | 8–16 cells | 4–8 cells | [86] |
| Blastocyst formation (days) | 7 | 5 | [86,87] |
Author Contributions
Funding
Conflicts of Interest
References
- David, L.; Bruneau, A.; Fréour, T.; Rivron, N.; Van de Velde, H. An Update on Human Pre- and Peri-Implantation Development: A Blueprint for Blastoids. Curr. Opin. Genet. Dev. 2023, 83, 102125. [Google Scholar] [CrossRef]
- Massarotti, C.; Makieva, S.; Stigliani, S. Editorial: Challenges in Fertilization and Implantation Success. Front. Cell Dev. Biol. 2023, 11, 1268635. [Google Scholar] [CrossRef]
- Hummitzsch, K.; Irving-Rodgers, H.F.; Schwartz, J.; Rodgers, R.J. Development of the Mammalian Ovary and Follicles, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128132098. [Google Scholar]
- Jones, A.S.K.; Shikanov, A. Follicle Development as an Orchestrated Signaling Network in a 3D Organoid. J. Biol. Eng. 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Rizos, D.; Rodriguez-Martinez, H.; Funahashi, H. Oocyte-Cumulus Cells Crosstalk: New Comparative Insights. Theriogenology 2023, 205, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, X.; Liu, S. Intercellular Communication in the Cumulus–Oocyte Complex during Folliculogenesis: A Review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef]
- Kidder, G.M.; Vanderhyden, B.C. Bidirectional Communication between Oocytes and Follicle Cells: Ensuring Oocyte Developmental Competence. Can. J. Physiol. Pharmacol. 2010, 88, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.L.; Gilchrist, R.B.; Brown, H.M.; Thompson, J.G. Bidirectional Communication between Cumulus Cells and the Oocyte: Old Hands and New Players? Theriogenology 2016, 86, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.J. Growth of the Mammalian Oocyte: Focus on Intercellular Contact and Communication. In Human Reproductive and Prenatal Genetics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 165–187. ISBN 9780323913805. [Google Scholar]
- Marchais, M.; Gilbert, I.; Bastien, A.; Macaulay, A.; Robert, C. Mammalian Cumulus-Oocyte Complex Communication: A Dialog through Long and Short Distance Messaging. J. Assist. Reprod. Genet. 2022, 39, 1011–1025. [Google Scholar] [CrossRef]
- El-Hayek, S.; Clarke, H.J. Control of Oocyte Growth and Development by Intercellular Communication within the Follicular Niche. Results Probl. Cell Differ. 2016, 58, 191–224. [Google Scholar] [CrossRef]
- Strączyńska, P.; Papis, K.; Morawiec, E.; Czerwiński, M.; Gajewski, Z.; Olejek, A.; Bednarska-Czerwińska, A. Signaling Mechanisms and Their Regulation during In Vivo or In Vitro Maturation of Mammalian Oocytes. Reprod. Biol. Endocrinol. 2022, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Gura, M.A.; Freiman, R.N. Primordial Follicle. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–71. ISBN 9780128151457. [Google Scholar]
- Braw-Tal, R.; Yossefi, S. Studies in Vivo and in Vitro on the Initiation of Follicle Growth in the Bovine Ovary. J. Reprod. Fertil. 1997, 109, 165–171. [Google Scholar] [CrossRef]
- Reynaud, K.; Driancourt, M.A. Oocyte Attrition. Mol. Cell. Endocrinol. 2000, 163, 101–108. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, H.; Zhang, Y.; Zhang, J.V.; Wang, X.; Liu, D.; Wang, T.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; et al. Current Understandings of Core Pathways for the Activation of Mammalian Primordial Follicles. Cells 2021, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Prasasya, R.D.; Mayo, K.E. Regulation of Follicle Formation and Development by Ovarian Signaling Pathways, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128132098. [Google Scholar]
- Van Den Hurk, R.; Zhao, J. Formation of Mammalian Oocytes and Their Growth, Differentiation and Maturation within Ovarian Follicles. Theriogenology 2005, 63, 1717–1751. [Google Scholar] [CrossRef]
- Westergaard, C.G.; Byskov, A.G.; Andersen, C.Y. Morphometric Characteristics of the Primordial to Primary Follicle Transition in the Human Ovary in Relation to Age. Hum. Reprod. 2007, 22, 2225–2231. [Google Scholar] [CrossRef]
- Ford, E.A.; Beckett, E.L.; Roman, S.D.; McLaughlin, E.A.; Sutherland, J.M. Advances in Human Primordial Follicle Activation and Premature Ovarian Insufficiency. Reproduction 2020, 159, R15–R29. [Google Scholar] [CrossRef]
- McLaughlin, M.; Innell, H.L.; Anderson, R.A.; Telfer, E.E. Inhibition of Phosphatase and Tensin Homologue (PTEN) in Human Ovary in Vitro Results in Increased Activation of Primordial Follicles but Compromises Development of Growing Follicles. Mol. Hum. Reprod. 2014, 20, 736–744. [Google Scholar] [CrossRef]
- Maidarti, M.; Clarkson, Y.L.; Mclaughlin, M.; Anderson, R.A.; Telfer, E.E. Inhibition of PTEN Activates Bovine Non-Growing Follicles in Vitro but Increases DNA Damage and Reduces DNA Repair Response. Hum. Reprod. 2019, 34, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Sugiura, K.; Wigglesworth, K.; O’Brien, M.J.; Affourtit, J.P.; Pangas, S.A.; Matzuk, M.M.; Eppig, J.J. Oocyte Regulation of Metabolic Cooperativity between Mouse Cumulus Cells and Oocytes: BMP15 and GDF9 Control Cholesterol Biosynthesis in Cumulus Cells. Development 2008, 135, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Su, Y.Q.; Diaz, F.J.; Pangas, S.A.; Sharma, S.; Wigglesworth, K.; O’Brien, M.J.; Matzuk, M.M.; Shimasaki, S.; Eppig, J.J. Erratum: Oocyte-Derived BMP15 and FGFs Cooperate to Promote Glycolysis in Cumulus Cells (Development Vol. 134 (2593-2603)). Development 2008, 135, 786. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-Secreted Factors: Regulators of Cumulus Cell Function and Oocyte Quality. Hum. Reprod. Update 2008, 14, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Mottershead, D.G.; Ritter, L.J.; Gilchrist, R.B. Signalling Pathways Mediating Specific Synergistic Interactions between GDF9 and BMP15. Mol. Hum. Reprod. 2012, 18, 121–128. [Google Scholar] [CrossRef]
- Mottershead, D.G.; Sugimura, S.; Al-Musawi, S.L.; Li, J.J.; Richani, D.; White, M.A.; Martin, G.A.; Trotta, A.P.; Ritter, L.J.; Shi, J.; et al. Cumulin, an Oocyte-Secreted Heterodimer of the Transforming Growth Factor-β Family, Is a Potent Activator of Granulosa Cells and Improves Oocyte Quality. J. Biol. Chem. 2015, 290, 24007–24020. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, J.B.; Han, D.X.; Wang, H.Q.; Liu, J.B.; Xiao, Y.; Jiang, H.; Gao, Y.; Yuan, B. CiRS-187 Regulates BMPR2 Expression by Targeting MiR-187 in Bovine Cumulus Cells Treated with BMP15 and GDF9. Theriogenology 2023, 197, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gert, K.R.; Pauli, A. Species-Specific Mechanisms during Fertilization. Curr. Top. Dev. Biol. 2020, 140, 121–144. [Google Scholar] [CrossRef]
- DI Clemente, N.; Racine, C.; Pierre, A.; Taieb, J. Anti-Müllerian Hormone in Female Reproduction. Endocr. Rev. 2021, 42, 753–782. [Google Scholar] [CrossRef] [PubMed]
- Widodo, O.S.; Nishihara, S.; Pambudi, D.; Kusakabe, K.T.; Taura, Y.; Nishi, Y.; Yamato, O.; Taniguchi, M.; Takagi, M. Relationship Between Ovary Size and Anti-Müllerian Hormone Levels in Holstein–Friesian Cows. Front. Vet. Sci. 2022, 9, 828123. [Google Scholar] [CrossRef] [PubMed]
- Granger, E.; Tal, R. Anti-Müllerian Hormone and Its Predictive Utility in Assisted Reproductive Technologies Outcomes. Clin. Obstet. Gynecol. 2019, 62, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Malo, C.; Oliván, S.; Ochoa, I.; Shikanov, A. In Vitro Growth of Human Follicles: Current and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 1510. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Mara, J.N.; Grover, A.R.; Pavone, M.E.; Duncan, F.E. Spatial Analysis of Growing Follicles in the Human Ovary to Inform Tissue Engineering Strategies. Tissue Eng. Part A 2020, 26, 733. [Google Scholar] [CrossRef]
- Pangas, S.A.; Rademaker, A.W.; Fishman, D.A.; Woodruff, T.K. Localization of the Activin Signal Transduction Components in Normal Human Ovarian Follicles: Implications for Autocrine and Paracrine Signaling in the Ovary. J. Clin. Endocrinol. Metab. 2002, 87, 2644–2657. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Allen, J.J.; Fortune, J.E. Activin Stimulates Follicle Formation and Activation and Modulates Steroidogenesis in Fetal Bovine Ovarian Tissue In Vitro1. Med. Res. Arch. 2023, 11, 1–26. [Google Scholar] [CrossRef]
- Andrade, G.M.; del Collado, M.; Meirelles, F.V.; da Silveira, J.C.; Perecin, F. Intrafollicular Barriers and Cellular Interactions during Ovarian Follicle Development. Anim. Reprod. 2019, 16, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Crozet, F.; da Silva, C.; Verlhac, M.H.; Terret, M.E. Myosin-X Is Dispensable for Spindle Morphogenesis and Positioning in the Mouse Oocyte. Development 2021, 148, dev199364. [Google Scholar] [CrossRef] [PubMed]
- Granados-Aparici, S.; Volodarsky-Perel, A.; Yang, Q.; Anam, S.; Tulandi, T.; Buckett, W.; Son, W.Y.; Younes, G.; Chung, J.T.; Jin, S.; et al. MYO10 Promotes Transzonal Projection-Dependent Germ Line-Somatic Contact during Mammalian Folliculogenesis. Biol. Reprod. 2022, 107, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, T.; Mishra, R.; Singh, R.K.; Bajpai, S. Role of Connexins in Female Reproductive System and Endometriosis. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101705. [Google Scholar] [CrossRef] [PubMed]
- Gershon, E.; Plaks, V.; Dekel, N. Gap Junctions in the Ovary: Expression, Localization and Function. Mol. Cell. Endocrinol. 2008, 282, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.H.; Miyano, T. Interaction between Growing Oocytes and Granulosa Cells In Vitro. Reprod. Med. Biol. 2020, 19, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Hsieh, M.; Musa Zamah, A.; Oh, J.S. Novel Signaling Mechanisms in the Ovary during Oocyte Maturation and Ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Pan, M.; Yin, L.; Zhang, Y.; Tang, Y.; Lu, S.; Gao, Y.; Wei, Q.; Han, B.; Ma, B. C-Type Natriuretic Peptide Pre-Treatment Improves Maturation Rate of Goat Oocytes by Maintaining Transzonal Projections, Spindle Morphology, and Mitochondrial Function. Animals 2023, 13, 3880. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Prem Kumar, K.V.; Chaube, S.K. Meiotic Cell Cycle Arrest in Mammalian Oocytes. J. Cell. Physiol. 2010, 223, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Kumar, T.R. Molecular Regulation of Follicle-Stimulating Hormone Synthesis, Secretion and Action. J. Mol. Endocrinol. 2018, 60, R131–R155. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Praveen Chakravarthi, V.; Wolfe, M.W.; Karim Rumi, M.A. ERβ Regulation of Gonadotropin Responses during Folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, N.; Li, N.; Hao, X.; Wei, K.; Xiang, X.; Xia, G.; Zhang, M. Epidermal Growth Factor Receptor Signaling-Dependent Calcium Elevation in Cumulus Cells Is Required for NPR2 Inhibition and Meiotic Resumption in Mouse Oocytes. Endocrinology 2013, 154, 3401–3409. [Google Scholar] [CrossRef]
- Neyroud, A.S.; Chiechio, R.M.; Moulin, G.; Ducarre, S.; Heichette, C.; Dupont, A.; Budzynski, M.; Even-Hernandez, P.; Faro, M.J.L.; Yefimova, M.; et al. Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. Int. J. Mol. Sci. 2022, 23, 11676. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Andrade, G.M.; Simas, R.C.; Martins-Júnior, H.A.; Eberlin, M.N.; Smith, L.C.; Perecin, F.; Meirelles, F.V. Lipid Profile of Extracellular Vesicles and Their Relationship with Bovine Oocyte Developmental Competence: New Players in Intra Follicular Cell Communication. Theriogenology 2021, 174, 1–8. [Google Scholar] [CrossRef]
- Sysoeva, A.; Akhmedova, Z.; Nepsha, O.; Makarova, N.; Silachev, D.; Shevtsova, Y.; Goryunov, K.; Karyagina, V.; Bugrova, A.; Starodubtseva, N.; et al. Characteristics of the Follicular Fluid Extracellular Vesicle Molecular Profile in Women in Different Age Groups in ART Programs. Life 2024, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, X.; Chang, X.; Yao, J.; He, Q.; Shen, Z.; Ji, Y.; Wang, K. S100-A9 Protein in Exosomes Derived from Follicular Fluid Promotes Inflammation via Activation of NF-ΚB Pathway in Polycystic Ovary Syndrome. J. Cell. Mol. Med. 2020, 24, 114–125. [Google Scholar] [CrossRef]
- Uzbekova, S.; Almiñana, C.; Labas, V.; Teixeira-Gomes, A.P.; Combes-Soia, L.; Tsikis, G.; Carvalho, A.V.; Uzbekov, R.; Singina, G. Protein Cargo of Extracellular Vesicles From Bovine Follicular Fluid and Analysis of Their Origin From Different Ovarian Cells. Front. Vet. Sci. 2020, 7, 584948. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Andrade, G.M.; del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G.; et al. Supplementation with Small-Extracellular Vesicles from Ovarian Follicular Fluid during In Vitro Production Modulates Bovine Embryo Development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Liang, L.; Racowsky, C.; Dioni, L.; Mansur, A.; Adir, M.; Bollati, V.; Baccarelli, A.A.; Hauser, R.; Machtinger, R. Extracellular MicroRNAs Profile in Human Follicular Fluid and IVF Outcomes. Sci. Rep. 2018, 8, 17036. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, C.; Pavani, K.C.; Gansemans, Y.; Azari-Dolatabad, N.; Pascottini, O.B.; Peelman, L.; Six, R.; Fan, Y.; Guan, X.; Deserranno, K.; et al. From Follicle to Blastocyst: MicroRNA-34c from Follicular Fluid-Derived Extracellular Vesicles Modulates Blastocyst Quality. J. Anim. Sci. Biotechnol. 2024, 15, 104. [Google Scholar] [CrossRef]
- Ying, W.; Yunqi, Z.; Zimeng, L.; Kangning, X.; Deji, L.; Chen, Q.; Yong, Z.; Fusheng, Q. Large Extracellular Vesicles in Bovine Follicular Fluid Inhibit the Apoptosis of Granulosa Cell and Stimulate the Production of Steroid Hormones. Theriogenology 2023, 195, 149–158. [Google Scholar] [CrossRef]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology 2018, 159, 3209–3218. [Google Scholar] [CrossRef]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9, 654028. [Google Scholar] [CrossRef]
- Adams, G.P.; Singh, J.; Baerwald, A.R. Large Animal Models for the Study of Ovarian Follicular Dynamics in Women. Theriogenology 2012, 78, 1733–1748. [Google Scholar] [CrossRef]
- Celestino, J.J.H.; Chaves, R.N.; Matos, M.H.T.; Saraiva, M.V.A.; Silva, J.R.V.; Bruno, J.B.; Maia-Júnior, J.E.; Figueiredo, J.R. Mechanisms of Atresia in Ovarian Follicles. Anim. Reprod. 2009, 6, 495–508. [Google Scholar]
- Findlay, J.K.; Dunning, K.R.; Gilchrist, R.B.; Hutt, K.J.; Russell, D.L.; Walters, K.A. Follicle Selection in Mammalian Ovaries, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128132098. [Google Scholar]
- Egbert, J.R.; Uliasz, T.F.; Shuhaibar, L.C.; Geerts, A.; Wunder, F.; Kleiman, R.J.; Humphrey, J.M.; Lampe, P.D.; Artemyev, N.O.; Rybalkin, S.D.; et al. Luteinizing Hormone Causes Phosphorylation and Activation of the CGMP Phosphodiesterase PDE5 in Rat Ovarian Follicles, Contributing, Together with PDE1 Activity, to the Resumption of Meiosis. Biol. Reprod. 2016, 94, 110. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Sun, Y.P.; Cheng, J.C. The Role of Amphiregulin in Ovarian Function and Disease. Cell. Mol. Life Sci. 2023, 80, 60. [Google Scholar] [CrossRef]
- Xi, G.; An, L.; Wang, W.; Hao, J.; Yang, Q.; Ma, L.; Lu, J.; Wang, Y.; Wang, W.; Zhao, W.; et al. The MRNA-Destabilizing Protein Tristetraprolin Targets “Meiosis Arrester” Nppc MRNA in Mammalian Preovulatory Follicles. Proc. Natl. Acad. Sci. USA 2021, 118, e2018345118. [Google Scholar] [CrossRef]
- Wang, F.; Tang, Y.; Cai, Y.; Yang, R.; Wang, Z.; Wang, X.; Yang, Q.; Wang, W.; Tian, J.; An, L. Intrafollicular Retinoic Acid Signaling Is Important for Luteinizing Hormone-Induced Oocyte Meiotic Resumption. Genes 2023, 14, 946. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kiyosu, C.; Akiyama, K.; Kunieda, T. CNP/NPR2 Signaling Maintains Oocyte Meiotic Arrest in Early Antral Follicles and Is Suppressed by EGFR-Mediated Signaling in Preovulatory Follicles. Mol. Reprod. Dev. 2012, 79, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wen, D.; Yin, L.; Tang, Y.; Lu, S.; Gao, Y.; Pan, M.H.; Han, B.; Ma, B. Estrogen Influences the Transzonal Projection Assembly of Cumulus-Oocyte Complexes through G Protein-Coupled Estrogen Receptor during Goat Follicle Development. Mol. Reprod. Dev. 2024, 91, e23763. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.K. Sir Robert Edwards: From the Science of Human Conception to the Reality of IVF Birth. Reprod. BioMedicine Online 2024, 49, 104478. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.F.; Jeff Huang, C.C. Bovine Models for Human Ovarian Diseases. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2022; Volume 189, pp. 101–154. ISBN 9780323994415. [Google Scholar]
- Sirard, M.A. The Ovarian Follicle of Cows as a Model for Human. In Animal Models and Human Reproduction; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 127–144. [Google Scholar] [CrossRef]
- Lonergan, P.; Rizos, D.; Guitiérrez-Adán, A.; Boland, M.P. Effect of Culture Environment on Embryo Quality and Gene Expression—Experience Form Animal Studies. Reprod. Biomed. Online 2003, 7, 657–663. [Google Scholar] [CrossRef]
- Simopoulou, M.; Sfakianoudis, K.; Rapani, A.; Giannelou, P.; Anifandis, G.; Bolaris, S.; Pantou, A.; Lambropoulou, M.; Pappas, A.; Deligeoroglou, E.; et al. Considerations Regarding Embryo Culture Conditions: From Media to Epigenetics. In Vivo 2018, 32, 451. [Google Scholar] [CrossRef]
- de Avila Ferronato, G.; dos Santos, C.M.; Rosa, P.M.d.S.; Bridi, A.; Perecin, F.; Meirelles, F.V.; Sangalli, J.R.; da Silveira, J.C. Bovine In Vitro Oocyte Maturation and Embryo Culture in Liquid Marbles 3D Culture System. PLoS ONE 2023, 18, e0284809. [Google Scholar] [CrossRef]
- Fortune, J.E.; Yang, M.Y.; Allen, J.J.; Herrick, S.L. Triennial Reproduction Symposium: The Ovarian Follicular Reserve in Cattle: What Regulates Its Formation and Size? J. Anim. Sci. 2013, 91, 3041–3050. [Google Scholar] [CrossRef][Green Version]
- Day, M.L.; Nogueira, G.P. Management of Age at Puberty in Beef Heifers to Optimize Efficiency of Beef Production. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Mossa, F.; Walsh, S.W.; Evans, A.C.O.; Jimenez-Krassel, F.; Ireland, J.J. Early Developmental Programming of the Ovarian Reserve, Ovarian Function, and Fertility. In Animal Models and Human Reproduction; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 91–108. [Google Scholar] [CrossRef]
- Cain, J.W.; Lefevre, C.; Ross, A.; Johnson, G.A. Hormones and Reproductive Cycles in Ungulates. In Hormones and Reproduction of Vertebrates; Academic Press: Cambridge, MA, USA, 2024; pp. 365–375. [Google Scholar] [CrossRef]
- Nowak, R.A. Estrous and Menstrual Cycles. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 114–120. ISBN 9780128151457. [Google Scholar]
- Pavlik, E.J.; Depriest, P.D.; Gallion, H.H.; Ueland, F.R.; Reedy, M.B.; Kryscio, R.J.; Van Nagell, J.R. Ovarian Volume Related to Age. Gynecol. Oncol. 2000, 77, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.H.; Duarte, K.M.R.; Carvalho, J.B.P.; Rocha, C.C.; Junior, G.A.A.; Trevisol, E.; Melo, A.J.F.; Pugliesi, G. Ovarian Morphology and Follicular Dynamics Associated with Ovarian Aging in Bos Indicus Beef Cows. Anim. Reprod. Sci. 2023, 254, 107279. [Google Scholar] [CrossRef]
- Pierson, R.A. Human Folliculogenesis Revisited: The Menstrual Cycle Visualized by Ultrasonography. In The Ovary; Elsevier: Amsterdam, The Netherlands, 2019; pp. 51–69. [Google Scholar]
- Kitasaka, H.; Konuma, Y.; Tokoro, M.; Fukunaga, N.; Asada, Y. Oocyte Cytoplasmic Diameter of ≥130 Μm Can Be Used to Determine Human Giant Oocytes. F S Sci. 2022, 3, 10–17. [Google Scholar] [CrossRef]
- Otoi, T.; Yamamoto, K.; Koyama, N.; Tachikawa, S.; Suzuki, T. Bovine Oocyte Diameter in Relation to Developmental Competence. Theriogenology 1997, 48, 769–774. [Google Scholar] [CrossRef]
- Vajta, G.; Rienzi, L.; Cobo, A.; Yovich, J. Embryo Culture: Can We Perform Better than Nature? Reprod. Biomed. Online 2010, 20, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Hyttel, P.; Sinowatz, F.; Vejlsted, M.; Betteridge, K. Essentials of Domestic Animal Embryology; Elsevier: Amsterdam, The Netherlands, 2014; p. 471. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Galindo, B.V.; Sánchez-Ramírez, B.; González-Trevizo, C.L.; Castro-Valenzuela, B.; Varela-Rodríguez, L.; Burrola-Barraza, M.E. Folliculogenesis: A Cellular Crosstalk Mechanism. Curr. Issues Mol. Biol. 2025, 47, 113. https://doi.org/10.3390/cimb47020113
Orozco-Galindo BV, Sánchez-Ramírez B, González-Trevizo CL, Castro-Valenzuela B, Varela-Rodríguez L, Burrola-Barraza ME. Folliculogenesis: A Cellular Crosstalk Mechanism. Current Issues in Molecular Biology. 2025; 47(2):113. https://doi.org/10.3390/cimb47020113
Chicago/Turabian StyleOrozco-Galindo, Bianca Viviana, Blanca Sánchez-Ramírez, Cynthia Lizeth González-Trevizo, Beatriz Castro-Valenzuela, Luis Varela-Rodríguez, and M. Eduviges Burrola-Barraza. 2025. "Folliculogenesis: A Cellular Crosstalk Mechanism" Current Issues in Molecular Biology 47, no. 2: 113. https://doi.org/10.3390/cimb47020113
APA StyleOrozco-Galindo, B. V., Sánchez-Ramírez, B., González-Trevizo, C. L., Castro-Valenzuela, B., Varela-Rodríguez, L., & Burrola-Barraza, M. E. (2025). Folliculogenesis: A Cellular Crosstalk Mechanism. Current Issues in Molecular Biology, 47(2), 113. https://doi.org/10.3390/cimb47020113








