The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms
Abstract
:1. Introduction
2. Cholesterol Level Regulation
2.1. Cholesterol Biosynthesis and Uptake
2.2. Cholesterol Excess Regulation: Cholesterol Efflux and Cholesterol Stores
3. Oxysterols Within the Brain
3.1. 27-Hydroxycholesterol
3.2. 24S-Hydroxycholesterol
3.3. 25-Hydroxycholesterol
4. Brain Cholesterol Metabolism
4.1. Role of Cholesterol in the Central Nervous System
4.2. Regulatory Mechanisms of Cholesterol Metabolism Homeostasis
4.3. Role of the Blood–Brain Barrier in Brain Cholesterol Metabolism
5. The Effects of Lipid-Lowering Therapy on Brain Cholesterol Metabolism
5.1. Statins and Central Cholesterol Regulation
5.2. Statins and Potential Effects on Cognition
5.3. Novel Agents and Their Effects on Central Cholesterol Metabolism and Cognition
5.4. Lipid-Lowering Therapies and Neurodegenerative Diseases
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bloch, K.E. Sterol, Structure and Membrane Function. Crit. Rev. Biochem. 1983, 14, 47–92. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D.; Spady, D.K. Role of Liver in the Maintenance of Cholesterol and Low Density Lipoprotein Homeostasis in Different Animal Species, Including Humans. J. Lipid Res. 1993, 34, 1637–1659. [Google Scholar] [CrossRef] [PubMed]
- Ebner, E. Critical Considerations on Statin Therapy. J. Clin. Biomed. Res. 2020, 1–2. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid Rafts: Bringing Order to Chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Van Meer, G. Lipid Sorting in Epithelial Cells. Biochemistry 1988, 27, 6197–6202. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G. The Different Hues of Lipid Rafts. Science (1979) 2002, 296, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.; Gimpl, G.; Fahrenholz, F. Regulation of Receptor Function by Cholesterol. Cell Mol. Life Sci. 2000, 57, 1577–1592. [Google Scholar] [CrossRef]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current Knowledge about Oxysterols: A Review. J. Food Sci. 2016, 81, R2299–R2308. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W. Fallacies in Modern Medicine: Statins and the Cholesterol-Heart Hypothesis. J. Am. Physicians Surg. 2015, 20, 54–56. [Google Scholar]
- Lifshitz, F.; Moses, N. Growth Failure. A Complication of Dietary Treatment of Hypercholesterolemia. Am. J. Dis. Child. 1989, 143, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Barness, L.A. Nutritional Requirements of Infants and Children with Respect to Cholesterol and Related Compounds. Am. J. Med. Genet. 1994, 50, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.; Hachey, D.L.; Insull, W.; Opekun, A.R.; Klein, P.D. Effect of Dietary Cholesterol on Cholesterol Synthesis in Breast-Fed and Formula-Fed Infants. J. Lipid Res. 1993, 34, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Thematic Review Series: Brain Lipids. Cholesterol Metabolism in the Central Nervous System during Early Development and in the Mature Animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef]
- Makover, M.E.; Shapiro, M.D.; Toth, P.P. There Is Urgent Need to Treat Atherosclerotic Cardiovascular Disease Risk Earlier, More Intensively, and with Greater Precision: A Review of Current Practice and Recommendations for Improved Effectiveness. Am. J. Prev. Cardiol. 2022, 12, 100371. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, M.; Iimuro, S.; Shinozaki, T.; Kimura, T.; Nakagawa, Y.; Ozaki, Y.; Iwata, H.; Miyauchi, K.; Daida, H.; Suwa, S.; et al. Optimal Target of LDL Cholesterol Level for Statin Treatment: Challenges to Monotonic Relationship with Cardiovascular Events. BMC Med. 2022, 20, 441. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Stattin, H.; Mednick, S. Low Cholesterol and Violent Crime. J. Psychiatr. Res. 2000, 34, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Virkkunen, M.; Penttinen, H. Serum Cholesterol in Aggressive Conduct Disorder: A Preliminary Study. Biol. Psychiatry 1984, 19, 435–439. [Google Scholar] [PubMed]
- Golomb, B.A. Cholesterol and Violence: Is There a Connection? Ann. Intern. Med. 1998, 128, 478. [Google Scholar] [CrossRef]
- Jenkins, D.C.; Hames, C.G.; Zyzanski, S.J.; Rosenman, R.H.; Friedman, M. Psychological Traits and Serum Lipids. Psychosom. Med. 1969, 31, 115–128. [Google Scholar] [CrossRef]
- Engelberg, H. Low Serum Cholesterol and Suicide. Lancet 1992, 339, 727–729. [Google Scholar] [CrossRef]
- Schatz, I.J.; Masaki, K.; Yano, K.; Chen, R.; Rodriguez, B.L.; Curb, J.D. Cholesterol and All-Cause Mortality in Elderly People from the Honolulu Heart Program: A Cohort Study. Lancet 2001, 358, 351–355. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): A Randomised Controlled Trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.O.; Wun, C.-C.; et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Alsheikh-Ali, A.A.; Maddukuri, P.V.; Han, H.; Karas, R.H. Effect of the Magnitude of Lipid Lowering on Risk of Elevated Liver Enzymes, Rhabdomyolysis, and Cancer. J. Am. Coll. Cardiol. 2007, 50, 409–418. [Google Scholar] [CrossRef]
- He, G.; Liu, X.; Liu, L.; Yu, Y.; Chen, C.; Huang, J.; Lo, K.; Huang, Y.; Feng, Y. A Nonlinear Association of Total Cholesterol with All-Cause and Cause-Specific Mortality. Nutr. Metab. 2021, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Wilson, J.D. Cholesterol Synthesis in the Squirrel Monkey: Relative Rates of Synthesis in Various Tissues and Mechanisms of Control. J. Clin. Investig. 1968, 47, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B.-L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Andronie-Cioară, F.L.; Jurcău, A.; Jurcău, M.C.; Nistor-Cseppentö, D.C.; Simion, A. Cholesterol Management in Neurology: Time for Revised Strategies? J. Pers. Med. 2022, 12, 1981. [Google Scholar] [CrossRef] [PubMed]
- Nieweg, K.; Schaller, H.; Pfrieger, F.W. Marked Differences in Cholesterol Synthesis between Neurons and Glial Cells from Postnatal Rats. J. Neurochem. 2009, 109, 125–134. [Google Scholar] [CrossRef]
- Martini, C.; Pallottini, V. Cholesterol: From Feeding to Gene Regulation. Genes. Nutr. 2007, 2, 181–193. [Google Scholar] [CrossRef]
- Reháková, R.; Cebová, M.; Matúšková, Z.; Košútová, M.; Kovácsová, M.; Pecháňová, O. Brain Cholesterol and the Role of Statins in Neuroprotection. Act. Nerv. Super. Rediviva 2016, 58, 11–17. [Google Scholar]
- Sever, N.; Yang, T.; Brown, M.S.; Goldstein, J.L.; DeBose-Boyd, R.A. Accelerated Degradation of HMG CoA Reductase Mediated by Binding of Insig-1 to Its Sterol-Sensing Domain. Mol. Cell 2003, 11, 25–33. [Google Scholar] [CrossRef]
- Theesfeld, C.L.; Pourmand, D.; Davis, T.; Garza, R.M.; Hampton, R.Y. The Sterol-Sensing Domain (SSD) Directly Mediates Signal-Regulated Endoplasmic Reticulum-Associated Degradation (ERAD) of 3-Hydroxy-3-Methylglutaryl (HMG)-CoA Reductase Isozyme Hmg2. J. Biol. Chem. 2011, 286, 26298–26307. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; DeBose-Boyd, R.A. Control of Cholesterol Synthesis through Regulated ER-Associated Degradation of HMG CoA Reductase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 185–198. [Google Scholar] [CrossRef]
- DeBose-Boyd, R.A.; Brown, M.S.; Li, W.-P.; Nohturfft, A.; Goldstein, J.L.; Espenshade, P.J. Transport-Dependent Proteolysis of SREBP. Cell 1999, 99, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Nohturfft, A.; Yabe, D.; Goldstein, J.L.; Brown, M.S.; Espenshade, P.J. Regulated Step in Cholesterol Feedback Localized to Budding of SCAP from ER Membranes. Cell 2000, 102, 315–323. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Dana, S.E.; Faust, J.R.; Beaudet, A.L.; Brown, M.S. Role of Lysosomal Acid Lipase in the Metabolism of Plasma Low Density Lipoprotein. Observations in Cultured Fibroblasts from a Patient with Cholesteryl Ester Storage Disease. J. Biol. Chem. 1975, 250, 8487–8495. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Herz, J.; Maeda, N.; Goldstein, J.L.; Brown, M.S. The Two-Receptor Model of Lipoprotein Clearance: Tests of the Hypothesis in “Knockout” Mice Lacking the Low Density Lipoprotein Receptor, Apolipoprotein E, or Both Proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 4431–4435. [Google Scholar] [CrossRef] [PubMed]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of Scavenger Receptor SR-BI as a High Density Lipoprotein Receptor. Science (1979) 1996, 271, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Landschulz, K.T.; Pathak, R.K.; Rigotti, A.; Krieger, M.; Hobbs, H.H. Regulation of Scavenger Receptor, Class B, Type I, a High Density Lipoprotein Receptor, in Liver and Steroidogenic Tissues of the Rat. J. Clin. Investig. 1996, 98, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.A.; Levin, M.C. Importance of Apolipoprotein A-I in Multiple Sclerosis. Front. Pharmacol. 2015, 6, 278. [Google Scholar] [CrossRef]
- Choroszyński, M.; Barcikowska, M.; Barczak, A. Metabolism and the Effect of Animal-Derived Oxysterols in the Diet on the Development of Alzheimer’s Disease. Ann. Nutr. Metab. 2022, 78, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic-Culf, M.; Badhwar, A. Recent Advances from Metabolomics and Lipidomics Application in Alzheimer’s Disease Inspiring Drug Discovery. Expert. Opin. Drug Discov. 2020, 15, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Suzuki, H.; Ito, H.; Korenaga, T.; Akatsu, H.; Meno, K.; Uchida, K. Serum Levels of Proteins Involved in Amyloid-β Clearance Are Related to Cognitive Decline and Neuroimaging Changes in Mild Cognitive Impairment. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pfeffer, S.R. Lysosomal Membrane Glycoproteins Bind Cholesterol and Contribute to Lysosomal Cholesterol Export. Elife 2016, 5, e21635. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Abi-Mosleh, L.; Wang, M.L.; Deisenhofer, J.; Goldstein, J.L.; Brown, M.S.; Infante, R.E. Structure of N-Terminal Domain of NPC1 Reveals Distinct Subdomains for Binding and Transfer of Cholesterol. Cell 2009, 137, 1213–1224. [Google Scholar] [CrossRef]
- Kim, W.S.; Weickert, C.S.; Garner, B. Role of ATP-binding Cassette Transporters in Brain Lipid Transport and Neurological Disease. J. Neurochem. 2008, 104, 1145–1166. [Google Scholar] [CrossRef]
- Wellington, C.L.; Walker, E.K.Y.; Suarez, A.; Kwok, A.; Bissada, N.; Singaraja, R.; Yang, Y.-Z.; Zhang, L.-H.; James, E.; Wilson, J.E.; et al. ABCA1 MRNA and Protein Distribution Patterns Predict Multiple Different Roles and Levels of Regulation. Lab. Investig. 2002, 82, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Koldamova, R.P.; Lefterov, I.M.; Ikonomovic, M.D.; Skoko, J.; Lefterov, P.I.; Isanski, B.A.; DeKosky, S.T.; Lazo, J.S. 22R-Hydroxycholesterol and 9-Cis-Retinoic Acid Induce ATP-Binding Cassette Transporter A1 Expression and Cholesterol Efflux in Brain Cells and Decrease Amyloid β Secretion. J. Biol. Chem. 2003, 278, 13244–13256. [Google Scholar] [CrossRef]
- Fukumoto, H.; Deng, A.; Irizarry, M.C.; Fitzgerald, M.L.; Rebeck, G.W. Induction of the Cholesterol Transporter ABCA1 in Central Nervous System Cells by Liver X Receptor Agonists Increases Secreted Aβ Levels. J. Biol. Chem. 2002, 277, 48508–48513. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S. Release of Cellular Cholesterol: Molecular Mechanism for Cholesterol Homeostasis in Cells and in the Body. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2000, 1529, 231–244. [Google Scholar] [CrossRef]
- Fielding, C.J.; Fielding, P.E. Cellular Cholesterol Efflux. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2001, 1533, 175–189. [Google Scholar] [CrossRef]
- Panzenboeck, U.; Balazs, Z.; Sovic, A.; Hrzenjak, A.; Levak-Frank, S.; Wintersperger, A.; Malle, E.; Sattler, W. ABCA1 and Scavenger Receptor Class B, Type I, Are Modulators of Reverse Sterol Transport at an In Vitro Blood-Brain Barrier Constituted of Porcine Brain Capillary Endothelial Cells. J. Biol. Chem. 2002, 277, 42781–42789. [Google Scholar] [CrossRef] [PubMed]
- Gosselet, F.; Candela, P.; Sevin, E.; Berezowski, V.; Cecchelli, R.; Fenart, L. Transcriptional Profiles of Receptors and Transporters Involved in Brain Cholesterol Homeostasis at the Blood–Brain Barrier: Use of an In Vitro Model. Brain Res. 2009, 1249, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Marzolo, M.P.; Bu, G. Differential Functions of Members of the Low Density Lipoprotein Receptor Family Suggested by Their Distinct Endocytosis Rates. J. Biol. Chem. 2001, 276, 18000–18006. [Google Scholar] [CrossRef]
- William Rebeck, G.; Reiter, J.S.; Strickland, D.K.; Hyman, B.T. Apolipoprotein E in Sporadic Alzheimer’s Disease: Allelic Variation and Receptor Interactions. Neuron 1993, 11, 575–580. [Google Scholar] [CrossRef]
- Karuna, R.; von Eckardstein, A.; Rentsch, K.M. Dopant Assisted-Atmospheric Pressure Photoionization (DA-APPI) Liquid Chromatography–Mass Spectrometry for the Quantification of 27-Hydroxycholesterol in Plasma. J. Chromatogr. B 2009, 877, 261–268. [Google Scholar] [CrossRef]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the Pathogenesis of Major Chronic Diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Ou, Z.; Ruan, X.; Gong, J. Role of Liver X Receptors in Cholesterol Efflux and Inflammatory Signaling (Review). Mol. Med. Rep. 2012, 5, 895–900. [Google Scholar] [CrossRef]
- Jacobson, M.S.; Price, M.G.; Shamoo, A.E.; Heald, F.P. Atherogenesis in White Carneau Pigeons Effects of Low-Level Cholestane-Triol Feeding. Atherosclerosis 1985, 57, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Staprans, I.; Pan, X.-M.; Rapp, J.H.; Grunfeld, C.; Feingold, K.R. Oxidized Cholesterol in the Diet Accelerates the Development of Atherosclerosis in LDL Receptor– and Apolipoprotein E–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Meynier, A.; Lherminier, J.; Demaison-Meloche, J.; Ginies, C.; Grandgirard, A.; Demaison, L. Effects of Dietary Oxysterols on Coronary Arteries in Hyperlipidaemic Hamsters. Br. J. Nutr. 2002, 87, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Staprans, I.; Pan, X.-M.; Rapp, J.H.; Feingold, K.R. Oxidized Cholesterol in the Diet Accelerates the Development of Aortic Atherosclerosis in Cholesterol-Fed Rabbits. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.K.; Mistry, J.; Fell, S.; Reis, A.; Spickett, C.M.; Polidori, M.C.; Lip, G.Y.H.; Griffiths, H.R. Oxidized LDL Lipids Increase β-Amyloid Production by SH-SY5Y Cells through Glutathione Depletion and Lipid Raft Formation. Free Radic. Biol. Med. 2014, 75, 48–59. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Béaslas, O.; Nissilä, E. Oxysterols and Their Cellular Effectors. Biomolecules 2012, 2, 76–103. [Google Scholar] [CrossRef]
- Okabe, A.; Urano, Y.; Itoh, S.; Suda, N.; Kotani, R.; Nishimura, Y.; Saito, Y.; Noguchi, N. Adaptive Responses Induced by 24S-Hydroxycholesterol through Liver X Receptor Pathway Reduce 7-Ketocholesterol-Caused Neuronal Cell Death. Redox Biol. 2014, 2, 28–35. [Google Scholar] [CrossRef]
- Babiker, A.; Diczfalusy, U. Transport of Side-Chain Oxidized Oxysterols in the Human Circulation. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1998, 1392, 333–339. [Google Scholar] [CrossRef]
- Dias, I.H.K.; Milic, I.; Lip, G.Y.H.; Devitt, A.; Polidori, M.C.; Griffiths, H.R. Simvastatin Reduces Circulating Oxysterol Levels in Men with Hypercholesterolaemia. Redox Biol. 2018, 16, 139–145. [Google Scholar] [CrossRef]
- Leoni, V.; Masterman, T.; Mousavi, F.S.; Wretlind, B.; Wahlund, L.-O.; Diczfalusy, U.; Hillert, J.; Björkhem, I. Diagnostic Use of Cerebral and Extracerebral Oxysterols. Clin. Chem. Lab. Med. (CCLM) 2004, 42, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Breuer, O.; Ahlborg, G.; Nennesmo, I.; Sidén, A.; Diczfalusy, U.; Björkhem, I. Cholesterol Homeostasis in Human Brain: Evidence for an Age-Dependent Flux of 24S-Hydroxycholesterol from the Brain into the Circulation. Proc. Natl. Acad. Sci. USA 1996, 93, 9799–9804. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.O. 24S-hydroxycholesterol: Cellular Effects and Variations in Brain Diseases. J. Neurochem. 2021, 157, 899–918. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Cholesterol Homeostasis and Function in Neurons of the Central Nervous System. Cell Mol. Life Sci. 2003, 60, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Q. Cholesterol Metabolism and Homeostasis in the Brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef]
- Guizzetti, M.; Costa, L. Cholesterol Homeostasis in the Developing Brain: A Possible New Target for Ethanol. Hum. Exp. Toxicol. 2007, 26, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Meir, K.; Kitsberg, D.; Alkalay, I.; Szafer, F.; Rosen, H.; Shpitzen, S.; Avi, L.B.; Staels, B.; Fievet, C.; Meiner, V.; et al. Human Sterol 27-Hydroxylase (CYP27) Overexpressor Transgenic Mouse Model. Evidence against 27-Hydroxycholesterol as a Critical Regulator of Cholesterol Homeostasis. J. Biol. Chem. 2002, 277, 34036–34041. [Google Scholar] [CrossRef]
- Lund, E.G.; Guileyardo, J.M.; Russell, D.W. CDNA Cloning of Cholesterol 24-Hydroxylase, a Mediator of Cholesterol Homeostasis in the Brain. Proc. Natl. Acad. Sci. USA 1999, 96, 7238–7243. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Sodero, A.O.; Vriens, J.; Ghosh, D.; Stegner, D.; Brachet, A.; Pallotto, M.; Sassoè-Pognetto, M.; Brouwers, J.F.; Helms, J.B.; Nieswandt, B.; et al. Cholesterol Loss during Glutamate-Mediated Excitotoxicity. EMBO J. 2012, 31, 1764–1773. [Google Scholar] [CrossRef]
- Wang, H.-L.; Wang, Y.-Y.; Liu, X.-G.; Kuo, S.-H.; Liu, N.; Song, Q.-Y.; Wang, M.-W. Cholesterol, 24-Hydroxycholesterol, and 27-Hydroxycholesterol as Surrogate Biomarkers in Cerebrospinal Fluid in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2016, 51, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Heverin, M.; Bogdanovic, N.; Lütjohann, D.; Bayer, T.; Pikuleva, I.; Bretillon, L.; Diczfalusy, U.; Winblad, B.; Björkhem, I. Changes in the Levels of Cerebral and Extracerebral Sterols in the Brain of Patients with Alzheimer’s Disease. J. Lipid Res. 2004, 45, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Cedazo-Minguez, A.; Leoni, V.; Meaney, S. Oxysterols and Neurodegenerative Diseases. Mol. Aspects Med. 2009, 30, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I. Crossing the Barrier: Oxysterols as Cholesterol Transporters and Metabolic Modulators in the Brain. J. Intern. Med. 2006, 260, 493–508. [Google Scholar] [CrossRef]
- Leoni, V.; Masterman, T.; Patel, P.; Meaney, S.; Diczfalusy, U.; Björkhem, I. Side Chain Oxidized Oxysterols in Cerebrospinal Fluid and the Integrity of Blood-Brain and Blood-Cerebrospinal Fluid Barriers. J. Lipid Res. 2003, 44, 793–799. [Google Scholar] [CrossRef]
- Petek, B.; Villa-Lopez, M.; Loera-Valencia, R.; Gerenu, G.; Winblad, B.; Kramberger, M.G.; Ismail, M.-A.-M.; Eriksdotter, M.; Garcia-Ptacek, S. Connecting the Brain Cholesterol and Renin-Angiotensin Systems: Potential Role of Statins and RAS-Modifying Medications in Dementia. J. Intern. Med. 2018, 284, 620–642. [Google Scholar] [CrossRef]
- Michikawa, M. Role of Cholesterol in Amyloid Cascade: Cholesterol-Dependent Modulation of Tau Phosphorylation and Mitochondrial Function. Acta Neurol. Scand. Suppl. 2006, 185, 21–26. [Google Scholar] [CrossRef]
- Dias, I.H.K.; Polidori, M.C.; Griffiths, H.R. Hypercholesterolaemia-Induced Oxidative Stress at the Blood-Brain Barrier. Biochem. Soc. Trans. 2014, 42, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Gamba, P.; Staurenghi, E.; Testa, G.; Giannelli, S.; Sottero, B.; Leonarduzzi, G. A Crosstalk Between Brain Cholesterol Oxidation and Glucose Metabolism in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 556. [Google Scholar] [CrossRef]
- Björkhem, I.; Leoni, V.; Svenningsson, P. On the Fluxes of Side-Chain Oxidized Oxysterols across Blood-Brain and Blood-CSF Barriers and Origin of These Steroids in CSF (Review). J. Steroid Biochem. Mol. Biol. 2019, 188, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Wang, T.; Liu, W.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. 27-Hydroxycholesterol Promotes the Transfer of Astrocyte-Derived Cholesterol to Neurons in Co-Cultured SH-SY5Y Cells and C6 Cells. Front. Cell Dev. Biol. 2020, 8, 580599. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-W.; Li, C.-Q.; Zhao, L.; Wang, Y.-S.; Xiao, R. NF-ΚB-Mediated Inflammatory Damage Is Differentially Affected in SH-SY5Y and C6 Cells Treated with 27-Hydroxycholesterol. Food Sci. Nutr. 2019, 7, 1685–1694. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Xu, H.; Ma, F.; Li, J.; Zhao, L.; Xu, Y. Elevated Ischaemia-Associated Lysyl Oxidase Activity in Delayed Graft Failure 6-12 Months after Renal Transplantation. Exp. Physiol. 2017, 102, 282–287. [Google Scholar] [CrossRef]
- Ismail, M.-A.-M.; Mateos, L.; Maioli, S.; Merino-Serrais, P.; Ali, Z.; Lodeiro, M.; Westman, E.; Leitersdorf, E.; Gulyás, B.; Olof-Wahlund, L.; et al. 27-Hydroxycholesterol Impairs Neuronal Glucose Uptake through an IRAP/GLUT4 System Dysregulation. J. Exp. Med. 2017, 214, 699–717. [Google Scholar] [CrossRef]
- Infante, J.; Rodríguez-Rodríguez, E.; Mateo, I.; Llorca, J.; Vázquez-Higuera, J.L.; Berciano, J.; Combarros, O. Gene–Gene Interaction between Heme Oxygenase-1 and Liver X Receptor-β and Alzheimer’s Disease Risk. Neurobiol. Aging 2010, 31, 710–714. [Google Scholar] [CrossRef]
- Khan, M.I.; Min, J.-S.; Lee, S.-O.; Yim, D.G.; Seol, K.-H.; Lee, M.; Jo, C. Cooking, Storage, and Reheating Effect on the Formation of Cholesterol Oxidation Products in Processed Meat Products. Lipids Health Dis. 2015, 14, 89. [Google Scholar] [CrossRef]
- Min, J.-S.; Lee, S.-O.; Khan, M.I.; Yim, D.G.; Seol, K.-H.; Lee, M.; Jo, C. Monitoring the Formation of Cholesterol Oxidation Products in Model Systems Using Response Surface Methodology. Lipids Health Dis. 2015, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Nunes, V.S.; Panzoldo, N.B.; Leança, C.C.; Parra, E.S.; Zago, V.S.; da Silva, E.J.; Cazita, P.M.; Nakandakare, E.R.; de Faria, E.C.; Quintão, E.C.R. Increased 27-Hydroxycholesterol Plasma Level in Men with Low High Density Lipoprotein-Cholesterol May Circumvent Their Reduced Cell Cholesterol Efflux Rate. Clin. Chim. Acta 2014, 433, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Rosano, C.; Evans, R.W.; Kuller, L.H. Brain Cholesterol Metabolism, Oxysterols, and Dementia. J. Alzheimer’s Dis. 2013, 33, 891–911. [Google Scholar] [CrossRef]
- Leoni, V. Oxysterols as Markers of Neurological Disease—A Review. Scand. J. Clin. Lab. Investig. 2009, 69, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Leoni, V.; Solomon, A.; Lövgren-Sandblom, A.; Minthon, L.; Blennow, K.; Hansson, O.; Wahlund, L.-O.; Kivipelto, M.; Björkhem, I. Diagnostic Power of 24S-Hydroxycholesterol in Cerebrospinal Fluid: Candidate Marker of Brain Health. J. Alzheimer’s Dis. 2013, 36, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Volianskis, A.; Bannister, N.; France, G.; Hanna, L.; Mercier, M.; Tidball, P.; Fang, G.; Irvine, M.W.; Costa, B.M.; et al. The NMDA Receptor as a Target for Cognitive Enhancement. Neuropharmacology 2013, 64, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Doherty, J.J.; Robichaud, A.J.; Belfort, G.M.; Chow, B.Y.; Hammond, R.S.; Crawford, D.C.; Linsenbardt, A.J.; Shu, H.-J.; Izumi, Y.; et al. The Major Brain Cholesterol Metabolite 24(S)-Hydroxycholesterol Is a Potent Allosteric Modulator of N-Methyl-d-Aspartate Receptors. J. Neurosci. 2013, 33, 17290–17300. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Izumi, Y.; Benz, A.; Zorumski, C.F.; Mennerick, S. Endogenous 24 S-Hydroxycholesterol Modulates NMDAR-Mediated Function in Hippocampal Slices. J. Neurophysiol. 2016, 115, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Nishi, T.; Kondou, S.; Kimura, H.; Mody, I. Preferential Enhancement of GluN2B-Containing Native NMDA Receptors by the Endogenous Modulator 24S-Hydroxycholesterol in Hippocampal Neurons. Neuropharmacology 2019, 148, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Outsourcing in the Brain: Do Neurons Depend on Cholesterol Delivery by Astrocytes? BioEssays 2003, 25, 72–78. [Google Scholar] [CrossRef]
- Abildayeva, K.; Jansen, P.J.; Hirsch-Reinshagen, V.; Bloks, V.W.; Bakker, A.H.F.; Ramaekers, F.C.S.; de Vente, J.; Groen, A.K.; Wellington, C.L.; Kuipers, F.; et al. 24(S)-Hydroxycholesterol Participates in a Liver X Receptor-Controlled Pathway in Astrocytes That Regulates Apolipoprotein E-Mediated Cholesterol Efflux. J. Biol. Chem. 2006, 281, 12799–12808. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An Oxysterol Signalling Pathway Mediated by the Nuclear Receptor LXRα. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.-L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the Nuclear Receptor LXR by Oxysterols Defines a New Hormone Response Pathway. J. Biol. Chem. 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wang, S.; Yang, N.; Wang, X.; Zhao, W.; Saed, H.S.; Daubon, T.; Huang, B.; Chen, A.; Li, G.; et al. Therapeutic Implications of Altered Cholesterol Homeostasis Mediated by Loss of CYP46A1 in Human Glioblastoma. EMBO Mol. Med. 2020, 12, e10924. [Google Scholar] [CrossRef] [PubMed]
- Czuba, E.; Steliga, A.; Lietzau, G.; Kowiański, P. Cholesterol as a Modifying Agent of the Neurovascular Unit Structure and Function under Physiological and Pathological Conditions. Metab. Brain Dis. 2017, 32, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Saint-Pol, J.; Candela, P.; Boucau, M.-C.; Fenart, L.; Gosselet, F. Oxysterols Decrease Apical-to-Basolateral Transport of Aß Peptides via an ABCB1-Mediated Process in an In Vitro Blood-Brain Barrier Model Constituted of Bovine Brain Capillary Endothelial Cells. Brain Res. 2013, 1517, 1–15. [Google Scholar] [CrossRef]
- Bogdanovic, N.; Bretillon, L.; Lund, E.G.; Diczfalusy, U.; Lannfelt, L.; Winblad, B.; Russell, D.W.; Björkhem, I. On the Turnover of Brain Cholesterol in Patients with Alzheimer’s Disease. Abnormal Induction of the Cholesterol-Catabolic Enzyme CYP46 in Glial Cells. Neurosci. Lett. 2001, 314, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W.; Halford, R.W.; Ramirez, D.M.O.; Shah, R.; Kotti, T. Cholesterol 24-Hydroxylase: An Enzyme of Cholesterol Turnover in the Brain. Annu. Rev. Biochem. 2009, 78, 1017–1040. [Google Scholar] [CrossRef]
- Sodero, A.O.; Weissmann, C.; Ledesma, M.D.; Dotti, C.G. Cellular Stress from Excitatory Neurotransmission Contributes to Cholesterol Loss in Hippocampal Neurons Aging In Vitro. Neurobiol. Aging 2011, 32, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Palomer, E.; Martín-Segura, A.; Baliyan, S.; Ahmed, T.; Balschun, D.; Venero, C.; Martin, M.G.; Dotti, C.G. Aging Triggers a Repressive Chromatin State at Bdnf Promoters in Hippocampal Neurons. Cell Rep. 2016, 16, 2889–2900. [Google Scholar] [CrossRef]
- Pérez-Cañamás, A.; Sarroca, S.; Melero-Jerez, C.; Porquet, D.; Sansa, J.; Knafo, S.; Esteban, J.A.; Sanfeliu, C.; Ledesma, M.D. A Diet Enriched with Plant Sterols Prevents the Memory Impairment Induced by Cholesterol Loss in Senescence-Accelerated Mice. Neurobiol. Aging 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in Cholesterol Metabolism as a Risk Factor for Developing Alzheimer’s Disease: Potential Novel Targets for Treatment. J. Steroid Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kotti, T.J.; Ramirez, D.M.O.; Pfeiffer, B.E.; Huber, K.M.; Russell, D.W. Brain Cholesterol Turnover Required for Geranylgeraniol Production and Learning in Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3869–3874. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Meaney, S.; Heverin, M.; Ekström, L.; Brafman, A.; Shafir, M.; Andersson, U.; Olin, M.; Eggertsen, G.; Diczfalusy, U.; et al. Studies on the Transcriptional Regulation of Cholesterol 24-Hydroxylase (CYP46A1). J. Biol. Chem. 2006, 281, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, H.; Lütjohann, D.; Tulke, A.; Björkhem, I.; Rao, M.L. The Neurotoxic Effect of 24-Hydroxycholesterol on SH-SY5Y Human Neuroblastoma Cells. Brain Res. 1999, 818, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, M.; Nunes, M.J.; Gomes, A.Q.; Gama, M.J.; Cedazo-Minguez, A.; Rodrigues, C.M.P.; Björkhem, I.; Rodrigues, E. Cholesterol 24S-Hydroxylase Overexpression Inhibits the Liver X Receptor (LXR) Pathway by Activating Small Guanosine Triphosphate-Binding Proteins (SGTPases) in Neuronal Cells. Mol. Neurobiol. 2015, 51, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Saito, Y.; Urano, Y. Diverse Functions of 24(S)-Hydroxycholesterol in the Brain. Biochem. Biophys. Res. Commun. 2014, 446, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Urano, Y.; Takabe, W.; Saito, Y. New Aspects of 24(S)-Hydroxycholesterol in Modulating Neuronal Cell Death. Free Radic. Biol. Med. 2015, 87, 366–372. [Google Scholar] [CrossRef]
- Thelen, K.M.; Falkai, P.; Bayer, T.A.; Lütjohann, D. Cholesterol Synthesis Rate in Human Hippocampus Declines with Aging. Neurosci. Lett. 2006, 403, 15–19. [Google Scholar] [CrossRef]
- Kotti, T.; Head, D.D.; McKenna, C.E.; Russell, D.W. Biphasic Requirement for Geranylgeraniol in Hippocampal Long-Term Potentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 11394–11399. [Google Scholar] [CrossRef]
- Vega, G.L.; Weiner, M.F.; Lipton, A.M.; von Bergmann, K.; Lütjohann, D.; Moore, C.; Svetlik, D. Reduction in Levels of 24S-Hydroxycholesterol by Statin Treatment in Patients With Alzheimer Disease. Arch. Neurol. 2003, 60, 510. [Google Scholar] [CrossRef] [PubMed]
- Famer, D.; Meaney, S.; Mousavi, M.; Nordberg, A.; Björkhem, I.; Crisby, M. Regulation of α- and β-Secretase Activity by Oxysterols: Cerebrosterol Stimulates Processing of APP via the α-Secretase Pathway. Biochem. Biophys. Res. Commun. 2007, 359, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, J.R.; Huls, A.; Thomasson, S.; Thompson, A.; Schommer, E.; Ghribi, O. Differential Effects of 24-Hydroxycholesterol and 27-Hydroxycholesterol on β-Amyloid Precursor Protein Levels and Processing in Human Neuroblastoma SH-SY5Y Cells. Mol. Neurodegener. 2009, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Staurenghi, E.; Giannelli, S.; Gargiulo, S.; Guglielmotto, M.; Tabaton, M.; Tamagno, E.; Gamba, P.; Leonarduzzi, G. A Silver Lining for 24-Hydroxycholesterol in Alzheimer’s Disease: The Involvement of the Neuroprotective Enzyme Sirtuin 1. Redox Biol. 2018, 17, 423–431. [Google Scholar] [CrossRef]
- Corley, J.; Shivappa, N.; Hébert, J.R.; Starr, J.M.; Deary, I.J. Associations between Dietary Inflammatory Index Scores and Inflammatory Biomarkers among Older Adults in the Lothian Birth Cohort 1936 Study. J. Nutr. Health Aging 2019, 23, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Lewis, M.; Doherty, J.J.; Shi, Y.; Cashikar, A.G.; Amelianchik, A.; Tymchuk, S.; Sullivan, P.M.; Qian, M.; Covey, D.F.; et al. 25-Hydroxycholesterol Amplifies Microglial IL-1β Production in an ApoE Isoform-Dependent Manner. J. Neuroinflamm. 2020, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.G.; Kerr, T.A.; Sakai, J.; Li, W.-P.; Russell, D.W. CDNA Cloning of Mouse and Human Cholesterol 25-Hydroxylases, Polytopic Membrane Proteins That Synthesize a Potent Oxysterol Regulator of Lipid Metabolism. J. Biol. Chem. 1998, 273, 34316–34327. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Cholesterol Feedback: From Schoenheimer’s Bottle to Scap’s MELADL. J. Lipid Res. 2009, 50, S15–S27. [Google Scholar] [CrossRef]
- Kobierski, J.; Wnętrzak, A.; Chachaj-Brekiesz, A.; Filiczkowska, A.; Petelska, A.D.; Dynarowicz-Latka, P. How the Replacement of Cholesterol by 25-Hydroxycholesterol Affects the Interactions with Sphingolipids: The Langmuir Monolayer Study Complemented with Theoretical Calculations. J. R. Soc. Interface 2021, 18, rsif.2021.0050. [Google Scholar] [CrossRef]
- Javitt, N.B.; Lee, Y.C.; Shimizu, C.; Fuda, H.; Strott, C.A. Cholesterol and Hydroxycholesterol Sulfotransferases: Identification, Distinction from Dehydroepiandrosterone Sulfotransferase, and Differential Tissue Expression. Endocrinology 2001, 142, 2978–2984. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, K.K.; Piskorska, B.; Banach, M.; Czuczwar, S.J. Neuroprotective Actions of Neurosteroids. Front. Endocrinol. 2011, 2, 50. [Google Scholar] [CrossRef]
- Vaňková, M.; Hill, M.; Velíková, M.; Včelák, J.; Vacínová, G.; Lukášová, P.; Vejražková, D.; Dvořáková, K.; Rusina, R.; Holmerová, I.; et al. Reduced Sulfotransferase SULT2A1 Activity in Patients With Alzheimer’s Disease. Physiol. Res. 2015, 64, S265–S273. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Meaney, S. Brain Cholesterol: Long Secret Life Behind a Barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Davison, A.N. Brain Sterol Metabolism. Adv. Lipid Res. 1965, 3, 171–196. [Google Scholar]
- Kabara, J.J. A Critical Review of Brain Cholesterol Metabolism. Prog. Brain Res. 1973, 40, 363–382. [Google Scholar]
- Jurevics, H.; Morell, P. Cholesterol for Synthesis of Myelin Is Made Locally, Not Imported into Brain. J. Neurochem. 1995, 64, 895–901. [Google Scholar] [CrossRef]
- Turley, S.D.; Burns, D.K.; Dietschy, J.M. Preferential Utilization of Newly Synthesized Cholesterol for Brain Growth in Neonatal Lambs. Am. J. Physiol.-Endocrinol. Metab. 1998, 274, E1099–E1105. [Google Scholar] [CrossRef]
- Quan, G.; Xie, C.; Dietschy, J.M.; Turley, S.D. Ontogenesis and Regulation of Cholesterol Metabolism in the Central Nervous System of the Mouse. Dev. Brain Res. 2003, 146, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Cholesterol Metabolism in the Brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Appelkvist, E.-L.; Kristensson, K.; Dallner, G. The Lipid Compositions of Different Regions of Rat Brain during Development and Aging. Neurobiol. Aging 1996, 17, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Cicolari, S.; Pavanello, C.; Olmastroni, E.; Puppo, M.D.; Bertolotti, M.; Mombelli, G.; Catapano, A.L.; Calabresi, L.; Magni, P. Interactions of Oxysterols with Atherosclerosis Biomarkers in Subjects with Moderate Hypercholesterolemia and Effects of a Nutraceutical Combination (Bifidobacterium Longum BB536, Red Yeast Rice Extract) (Randomized, Double-Blind, Placebo-Controlled Study). Nutrients 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Elmberger, P.O.; Edlund, C.; Kristensson, K.; Dallner, G. Rates of Cholesterol, Ubiquinone, Dolichol and Dolichyl-P Biosynthesis in Rat Brain Slices. FEBS Lett. 1990, 269, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem, I.; Heverin, M.; Leoni, V.; Meaney, S.; Diczfalusy, U. Oxysterols and Alzheimer’s Disease. Acta Neurol. Scand. 2006, 114, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Bellosta, S. Cholesterol: Its Regulation and Role in Central Nervous System Disorders. Cholesterol 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Ulusoy, E.K. Correlations between the Monocyte to High-Density Lipoprotein Cholesterol Ratio and White Matter Hyperintensities in Migraine. Neurol. Res. 2020, 42, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.M.; Ramirez, C.M.; Taylor, A.M.; Jones, R.D.; Repa, J.J.; Turley, S.D. Ontogenesis and Modulation of Intestinal Unesterified Cholesterol Sequestration in a Mouse Model of Niemann–Pick C1 Disease. Dig. Dis. Sci. 2020, 65, 158–167. [Google Scholar] [CrossRef]
- Martin, M.; Dotti, C.G.; Ledesma, M.D. Brain Cholesterol in Normal and Pathological Aging. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 934–944. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.-W. Linking Lipids to Alzheimer’s Disease: Cholesterol and Beyond. Nat. Rev. Neurosci. 2011, 12, 284–296, Erratumed in Nat. Rev. Neurosci. 2011, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.M.; Chuang, J.-C.; Turley, S.D. Measurement of Rates of Cholesterol and Fatty Acid Synthesis In Vivo Using Tritiated Water. Cholest. Homeost. Methods Protoc. 2017, 1583, 241–256. [Google Scholar]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and Cholesterol Handling in the Brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Hartmann, H.; Ling, S. Central Nervous System Cholesterol Metabolism in Health and Disease. IUBMB Life 2022, 74, 826–841. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Lovatt, D.; Sonnewald, U.; Waagepetersen, H.S.; Schousboe, A.; He, W.; Lin, J.H.-C.; Han, X.; Takano, T.; Wang, S.; Sim, F.J.; et al. The Transcriptome and Metabolic Gene Signature of Protoplasmic Astrocytes in the Adult Murine Cortex. J. Neurosci. 2007, 27, 12255–12266. [Google Scholar] [CrossRef] [PubMed]
- Henn, R.E.; Noureldein, M.H.; Elzinga, S.E.; Kim, B.; Savelieff, M.G.; Feldman, E.L. Glial-Neuron Crosstalk in Health and Disease: A Focus on Metabolism, Obesity, and Cognitive Impairment. Neurobiol. Dis. 2022, 170, 105766. [Google Scholar] [CrossRef] [PubMed]
- Joe, E.-H.; Choi, D.-J.; An, J.; Eun, J.-H.; Jou, I.; Park, S. Astrocytes, Microglia, and Parkinson’s Disease. Exp. Neurobiol. 2018, 27, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Varcianna, A.; Myszczynska, M.A.; Castelli, L.M.; O’Neill, B.; Kim, Y.; Talbot, J.; Nyberg, S.; Nyamali, I.; Heath, P.R.; Stopford, M.J.; et al. Micro-RNAs Secreted through Astrocyte-Derived Extracellular Vesicles Cause Neuronal Network Degeneration in C9orf72 ALS. EBioMedicine 2019, 40, 626–635. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral Blood Flow Regulation and Neurovascular Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. A Receptor-Mediated Pathway for Cholesterol Homeostasis. Science (1979) 1986, 232, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Lövgren-Sandblom, A.; Leoni, V.; Meaney, S.; Brodin, L.; Salveson, L.; Winge, K.; Pålhagen, S.; Svenningsson, P. Oxysterols and Parkinson’s Disease: Evidence That Levels of 24S-Hydroxycholesterol in Cerebrospinal Fluid Correlates with the Duration of the Disease. Neurosci. Lett. 2013, 555, 102–105. [Google Scholar] [CrossRef]
- Martín, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in Brain Disease: Sometimes Determinant and Frequently Implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef]
- Pfrieger, F.W.; Barres, B.A. Synaptic Efficacy Enhanced by Glial Cells In Vitro. Science (1979) 1997, 277, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.-C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science (1979) 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Ullian, E.M.; Sapperstein, S.K.; Christopherson, K.S.; Barres, B.A. Control of Synapse Number by Glia. Science (1979) 2001, 291, 657–661. [Google Scholar] [CrossRef]
- Li, R.; Wang, T.-J.; Lyu, P.-Y.; Liu, Y.; Chen, W.-H.; Fan, M.-Y.; Xu, J. Effects of Plasma Lipids and Statins on Cognitive Function. Chin. Med. J. 2018, 131, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.A.; Golomb, B.A. Statin-Associated Adverse Cognitive Effects: Survey Results from 171 Patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.; Slowik, A.; Gryz, E.A.; Szczudlik, A. Lower Serum Triglyceride Level Is Associated With Increased Stroke Severity. Stroke 2004, 35, e151–e152. [Google Scholar] [CrossRef] [PubMed]
- Lange, Y.; Ye, J.; Strebel, F. Movement of 25-Hydroxycholesterol from the Plasma Membrane to the Rough Endoplasmic Reticulum in Cultured Hepatoma Cells. J. Lipid Res. 1995, 36, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Meaney, S.; Bodin, K.; Diczfalusy, U.; Björkhem, I. On the Rate of Translocation In Vitro and Kinetics In Vivo of the Major Oxysterols in Human Circulation. J. Lipid Res. 2002, 43, 2130–2135. [Google Scholar] [CrossRef]
- Roheim, P.S.; Carey, M.; Forte, T.; Vega, G.L. Apolipoproteins in Human Cerebrospinal Fluid. Proc. Natl. Acad. Sci. USA 1979, 76, 4646–4649. [Google Scholar] [CrossRef]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Hui, D.; Weisgraber, K.H. Lipoproteins and Their Receptors in the Central Nervous System. Characterization of the Lipoproteins in Cerebrospinal Fluid and Identification of Apolipoprotein B,E(LDL) Receptors in the Brain. J. Biol. Chem. 1987, 262, 14352–14360. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Donarski, N.; Goetze, K.; Kreckel, M.; Stuerenburg, H.J.; Buhmann, C.; Beisiegel, U. Characterization of Four Lipoprotein Classes in Human Cerebrospinal Fluid. J. Lipid Res. 2001, 42, 1143–1151. [Google Scholar] [CrossRef]
- Fung, K.Y.; Wang, C.; Nyegaard, S.; Heit, B.; Fairn, G.D.; Lee, W.L. SR-BI Mediated Transcytosis of HDL in Brain Microvascular Endothelial Cells Is Independent of Caveolin, Clathrin, and PDZK1. Front. Physiol. 2017, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the Brain: Implications for Neurological and Psychiatric Disorders. Clin. Lipidol. 2010, 51, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer Disease: Risk, Mechanisms and Therapy. Nat. Rev. Neurol. 2013, 9, 106–118, Corrected in Nat. Rev. Neurol. 2013, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Foss, D.; Mahley, R.W. Astrocytes Synthesize Apolipoprotein E and Metabolize Apolipoprotein E-Containing Lipoproteins. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1987, 917, 148–161. [Google Scholar] [CrossRef]
- Linton, M.F.; Gish, R.; Hubl, S.T.; Bütler, E.; Esquivel, C.; Bry, W.I.; Boyles, J.K.; Wardell, M.R.; Young, S.G. Phenotypes of Apolipoprotein B and Apolipoprotein E after Liver Transplantation. J. Clin. Investig. 1991, 88, 270–281. [Google Scholar] [CrossRef]
- Budny, V.; Knöpfli, Y.; Meier, D.; Zürcher, K.; Bodenmann, C.; Peter, S.L.; Müller, T.; Tardy, M.; Cortijo, C.; Tackenberg, C. APOE4 Increases Energy Metabolism in APOE-Isogenic IPSC-Derived Neurons. Cells 2024, 13, 1207. [Google Scholar] [CrossRef]
- Dong, W.; Vuletic, S.; Albers, J.J. Differential Effects of Simvastatin and Pravastatin on Expression of Alzheimer’s Disease-Related Genes in Human Astrocytes and Neuronal Cells. J. Lipid Res. 2009, 50, 2095–2102. [Google Scholar] [CrossRef]
- Benilova, I.; Karran, E.; De Strooper, B. The Toxic Aβ Oligomer and Alzheimer’s Disease: An Emperor in Need of Clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science (1979) 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Basak, J.M.; Verghese, P.B.; Yoon, H.; Kim, J.; Holtzman, D.M. Low-Density Lipoprotein Receptor Represents an Apolipoprotein E-Independent Pathway of Aβ Uptake and Degradation by Astrocytes. J. Biol. Chem. 2012, 287, 13959–13971. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The Role of Apolipoprotein E in Alzheimer’s Disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Banks, W.A.; Reed, M.J.; Logsdon, A.F.; Rhea, E.M.; Erickson, M.A. Healthy Aging and the Blood–Brain Barrier. Nat. Aging 2021, 1, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Sienski, G.; Bonner, J.M.; Lin, Y.-T.; Seo, J.; Baru, V.; Haque, A.; Milo, B.; Akay, L.A.; Graziosi, A.; et al. PICALM Rescues Endocytic Defects Caused by the Alzheimer’s Disease Risk Factor APOE4. Cell Rep. 2020, 33, 108224. [Google Scholar] [CrossRef]
- Bryleva, E.Y.; Rogers, M.A.; Chang, C.C.Y.; Buen, F.; Harris, B.T.; Rousselet, E.; Seidah, N.G.; Oddo, S.; LaFerla, F.M.; Spencer, T.A.; et al. ACAT1 Gene Ablation Increases 24(S)-Hydroxycholesterol Content in the Brain and Ameliorates Amyloid Pathology in Mice with AD. Proc. Natl. Acad. Sci. USA 2010, 107, 3081–3086. [Google Scholar] [CrossRef]
- Sakashita, N.; Miyazaki, A.; Takeya, M.; Horiuchi, S.; Chang, C.C.Y.; Chang, T.-Y.; Takahashi, K. Localization of Human Acyl-Coenzyme A:Cholesterol Acyltransferase-1 (ACAT-1) in Macrophages and in Various Tissues. Am. J. Pathol. 2000, 156, 227–236. [Google Scholar] [CrossRef]
- Brankatschk, M.; Eaton, S. Lipoprotein Particles Cross the Blood-Brain Barrier in Drosophila. J. Neurosci. 2010, 30, 10441–10447. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E.; Johnston, R.; Lin, D.S. Metabolism of Cholesterol in the Tissues and Blood of the Chick Embryo. J. Lipid Res. 1969, 10, 388–394. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Hollander, W. Body Cholesterol Metabolism in Man. I. The Equilibration of Serum and Tissue Cholesterol. J. Clin. Investig. 1962, 41, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, F.; Laurent, B.; Plourde, M. Lipid Transport and Metabolism at the Blood-Brain Interface: Implications in Health and Disease. Front. Physiol. 2021, 12, 645646. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Furube, E.; Mannari, T.; Okuda, H.; Tatsumi, K.; Wanaka, A.; Miyata, S. Heterogeneous Vascular Permeability and Alternative Diffusion Barrier in Sensory Circumventricular Organs of Adult Mouse Brain. Cell Tissue Res. 2016, 363, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S. New Aspects in Fenestrated Capillary and Tissue Dynamics in the Sensory Circumventricular Organs of Adult Brains. Front. Neurosci. 2015, 9, 390. [Google Scholar] [CrossRef]
- Willis, C.L.; Garwood, C.J.; Ray, D.E. A Size Selective Vascular Barrier in the Rat Area Postrema Formed by Perivascular Macrophages and the Extracellular Matrix. Neuroscience 2007, 150, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.M.; Weindl, A.; Knigge, K.M. Peering through the Windows of the Brain. J. Cereb. Blood Flow Metab. 1987, 7, 663–672. [Google Scholar] [CrossRef]
- Cameron, O.G. Visceral Brain–Body Information Transfer. Neuroimage 2009, 47, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, B.; Fenart, L.; Dehouck, M.P.; Pierce, A.; Torpier, G.; Cecchelli, R. A New Function for the LDL Receptor: Transcytosis of LDL across the Blood-Brain Barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. Interactions of Lipids, Lipoproteins, and Apolipoproteins with the Blood-Brain Barrier. Pharm. Res. 2021, 38, 1469–1475. [Google Scholar] [CrossRef]
- Lütjohann, D.; Stroick, M.; Bertsch, T.; Kühl, S.; Lindenthal, B.; Thelen, K.; Andersson, U.; Björkhem, I.; von Bergmann, K.; Fassbender, K. High Doses of Simvastatin, Pravastatin, and Cholesterol Reduce Brain Cholesterol Synthesis in Guinea Pigs. Steroids 2004, 69, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Serougne, C.; Lefevre, C.; Chevallier, F. Cholesterol Transfer between Brain and Plasma in the Rat: A Model for the Turnover of Cerebral Cholesterol. Exp. Neurol. 1976, 51, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Refolo, L.M.; Pappolla, M.A.; Malester, B.; LaFrancois, J.; Bryant-Thomas, T.; Wang, R.; Tint, G.S.; Sambamurti, K.; Duff, K. Hypercholesterolemia Accelerates the Alzheimer’s Amyloid Pathology in a Transgenic Mouse Model. Neurobiol. Dis. 2000, 7, 321–331, Corrected in 2000, 9, 690–691. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, M.; Su, J.; Lu, B.; Ma, D.; Zhang, R.; Yang, L.; Wang, Q.; Ma, Y.; Fan, Y. Simvastatin Blocks Blood-Brain Barrier Disruptions Induced by Elevated Cholesterol Both In Vivo and In Vitro. Int. J. Alzheimers Dis. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, J.; Wu, Q.; Ren, Z.; Pan, L.; Tang, Z.; Jiang, Z.; Wang, G.; Liu, L. Imbalanced Cholesterol Metabolism in Alzheimer’s Disease. Clin. Chim. Acta 2016, 456, 107–114. [Google Scholar] [CrossRef]
- Lütjohann, D.; Stellaard, F.; Bölükbasi, B.; Kerksiek, A.; Parhofer, K.G.; Laufs, U. Anti-PCSK 9 Antibodies Increase the Ratios of the Brain-specific Oxysterol 24S-hydroxycholesterol to Cholesterol and to 27-hydroxycholesterol in the Serum. Br. J. Clin. Pharmacol. 2021, 87, 4252–4261. [Google Scholar] [CrossRef]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and Pharmacodynamic Alterations of 3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors: Drug–Drug Interactions and Interindividual Differences in Transporter and Metabolic Enzyme Functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Effects of Statins on Triglyceride Metabolism. Am. J. Cardiol. 1998, 81, 32B–35B. [Google Scholar] [CrossRef] [PubMed]
- Korade, Z.; Mi, Z.; Portugal, C.; Schor, N.F. Expression and P75 Neurotrophin Receptor Dependence of Cholesterol Synthetic Enzymes in Adult Mouse Brain. Neurobiol. Aging 2007, 28, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Gravelin, D. Cholesterol-Lowering Injectables: More Harm than Good? J. Am. Physicians Surg. 2015, 20, 119–121. [Google Scholar]
- Beltowski, J.; Wojcicka, G.; Jamroz-Wisniewska, A. Adverse Effects of Statins—Mechanisms and Consequences. Curr. Drug Saf. 2009, 4, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Ladea, L.; Zemba, M.; Calancea, M.I.; Călțaru, M.V.; Dragosloveanu, C.D.M.; Coroleucă, R.; Catrina, E.L.; Brezean, I.; Dinu, V. Corneal Epithelial Changes in Diabetic Patients: A Review. Int. J. Mol. Sci. 2024, 25, 3471. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Appelkvist, E.-L.; Dallner, G.; Ernster, L. Distribution and Redox State of Ubiquinones in Rat and Human Tissues. Arch. Biochem. Biophys. 1992, 295, 230–234. [Google Scholar] [CrossRef]
- Miles, M.V.; Horn, P.S.; Morrison, J.A.; Tang, P.H.; DeGrauw, T.; Pesce, A.J. Plasma Coenzyme Q10 Reference Intervals, but Not Redox Status, Are Affected by Gender and Race in Self-Reported Healthy Adults. Clin. Chim. Acta 2003, 332, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.W. Coenzyme Q 10 and Vitamin E Synergy, Electron Transfer, Antioxidation in Cell Membranes, and Interaction with Cholesterol. Eigenenergy Adelaide South Australia Australia. 2023. Available online: https://hal.science/hal-03976270/ (accessed on 16 December 2024).
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.R.; Preston, J.E. CoQ10 Deficient Endothelial Cell Culture Model for the Investigation of CoQ10 Blood–Brain Barrier Transport. J. Clin. Med. 2020, 9, 3236. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Surcel, M.; Munteanu, A.; Scheau, C.; Savulescu-Fiedler, I.; Caruntu, C. Diabetic Neuropathy: A NRF2 Disease? J. Diabetes 2024, 16, e13524. [Google Scholar] [CrossRef] [PubMed]
- Meljon, A.; Wang, Y.; Griffiths, W.J. Oxysterols in the Brain of the Cholesterol 24-Hydroxylase Knockout Mouse. Biochem. Biophys. Res. Commun. 2014, 446, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Wanamaker, B.L.; Swiger, K.J.; Blumenthal, R.S.; Martin, S.S. Cholesterol, Statins, and Dementia: What the Cardiologist Should Know. Clin. Cardiol. 2015, 38, 243–250. [Google Scholar] [CrossRef]
- Bayorh, M.; Ganafa, A.; Eatman, D.; Walton, M.; Feuerstein, G. Simvastatin and Losartan Enhance Nitric Oxide and Reduce Oxidative Stress in Salt-Induced Hypertension. Am. J. Hypertens. 2005, 18, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, D.; Jiang, H.; Xiong, Y.; Qu, C.; Li, B.; Mahmood, A.; Zhou, D.; Chopp, M. Simvastatin-Mediated Upregulation of VEGF and BDNF, Activation of the PI3K/Akt Pathway, and Increase of Neurogenesis Are Associated with Therapeutic Improvement after Traumatic Brain Injury. J. Neurotrauma 2008, 25, 130–139. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Mailman, T.; Hariharan, M.; Karten, B. Inhibition of Neuronal Cholesterol Biosynthesis with Lovastatin Leads to Impaired Synaptic Vesicle Release Even in the Presence of Lipoproteins or Geranylgeraniol. J. Neurochem. 2011, 119, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Sodero, A.O.; Barrantes, F.J. Pleiotropic Effects of Statins on Brain Cells. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183340. [Google Scholar] [CrossRef]
- García-Román, N.; Álvarez, A.M.; Toro, M.J.; Montes, A.; Lorenzo, M.J. Lovastatin Induces Apoptosis of Spontaneously Immortalized Rat Brain Neuroblasts: Involvement of Nonsterol Isoprenoid Biosynthesis Inhibition. Mol. Cell. Neurosci. 2001, 17, 329–341. [Google Scholar] [CrossRef]
- van der Kant, R.; Langness, V.F.; Herrera, C.M.; Williams, D.A.; Fong, L.K.; Leestemaker, Y.; Steenvoorden, E.; Rynearson, K.D.; Brouwers, J.F.; Helms, J.B.; et al. Cholesterol Metabolism Is a Druggable Axis That Independently Regulates Tau and Amyloid-β in IPSC-Derived Alzheimer’s Disease Neurons. Cell Stem Cell 2019, 24, 363–375.e9. [Google Scholar] [CrossRef] [PubMed]
- Deveau, C.M.; Rodriguez, E.; Schroering, A.; Yamamoto, B.K. Serotonin Transporter Regulation by Cholesterol-Independent Lipid Signaling. Biochem. Pharmacol. 2021, 183, 114349. [Google Scholar] [CrossRef] [PubMed]
- Shepardson, N.E. Cholesterol Level and Statin Use in Alzheimer Disease. Arch. Neurol. 2011, 68, 1239. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Ramos, M.C.; Molina, P.; Esteo, C.; Vázquez, J.A.; Burgos, J.S. Statins as Neuroprotectants: A Comparative In Vitro Study of Lipophilicity, Blood-Brain-Barrier Penetration, Lowering of Brain Cholesterol, and Decrease of Neuron Cell Death. J. Alzheimer’s Dis. 2011, 23, 307–318. [Google Scholar] [CrossRef]
- Niemi, M. Transporter Pharmacogenetics and Statin Toxicity. Clin. Pharmacol. Ther. 2010, 87, 130–133. [Google Scholar] [CrossRef]
- King, D.S.; Wilburn, A.J.; Wofford, M.R.; Harrell, T.K.; Lindley, B.J.; Jones, D.W. Cognitive Impairment Associated with Atorvastatin and Simvastatin. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 1663–1667. [Google Scholar] [CrossRef]
- Ott, B.R.; Daiello, L.A.; Dahabreh, I.J.; Springate, B.A.; Bixby, K.; Murali, M.; Trikalinos, T.A. Do Statins Impair Cognition? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2015, 30, 348–358. [Google Scholar] [CrossRef]
- Locatelli, S.; Lütjohann, D.; Schmidt, H.H.-J.; Otto, C.; Beisiegel, U.; von Bergmann, K. Reduction of Plasma 24S-Hydroxycholesterol (Cerebrosterol) Levels Using High-Dosage Simvastatin in Patients With Hypercholesterolemia. Arch. Neurol. 2002, 59, 213. [Google Scholar] [CrossRef] [PubMed]
- Botti, R.E.; Triscari, J.; Pan, H.Y.; Zayat, J. Concentrations of Pravastatin and Lovastatin in Cerebrospinal Fluid in Healthy Subjects. Clin. Neuropharmacol. 1991, 14, 256–261. [Google Scholar] [CrossRef]
- Fassbender, K.; Stroick, M.; Bertsch, T.; Ragoschke, A.; Kuehl, S.; Walter, S.; Walter, J.; Brechtel, K.; Muehlhauser, F.; von Bergmann, K.; et al. Effects of Statins on Human Cerebral Cholesterol Metabolism and Secretion of Alzheimer Amyloid Peptide. Neurology 2002, 59, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Recent Progress in Vascular Aging: Mechanisms and Its Role in Age-Related Diseases. Aging Dis. 2017, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.; Sato, N.; Kurinami, H.; Takeuchi, D.; Takeda, S.; Shimamura, M.; Yamashita, T.; Uchiyama, Y.; Rakugi, H.; Morishita, R. Reduction of Brain β-Amyloid (Aβ) by Fluvastatin, a Hydroxymethylglutaryl-CoA Reductase Inhibitor, through Increase in Degradation of Amyloid Precursor Protein C-Terminal Fragments (APP-CTFs) and Aβ Clearance. J. Biol. Chem. 2010, 285, 22091–22102. [Google Scholar] [CrossRef]
- Storck, S.E.; Pietrzik, C.U. Endothelial LRP1—A Potential Target for the Treatment of Alzheimer’s Disease. Pharm. Res. 2017, 34, 2637–2651. [Google Scholar] [CrossRef]
- Schachter, M. Chemical, Pharmacokinetic and Pharmacodynamic Properties of Statins: An Update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, T.; Wang, C.; Li, G.; Zhi, W.; Yin, J.; Wan, Q.; Chen, L. Atorvastatin in Improvement of Cognitive Impairments Caused by Amyloid β in Mice: Involvement of Inflammatory Reaction. BMC Neurol. 2016, 16, 18. [Google Scholar] [CrossRef]
- Jin, H.; Chen, T.; Li, G.; Wang, C.; Zhang, B.; Cao, X.; Sha, S.; Wan, Q.; Chen, L. Dose-Dependent Neuroprotection and Neurotoxicity of Simvastatin through Reduction of Farnesyl Pyrophosphate in Mice Treated with Intracerebroventricular Injection of Aβ 1-42. J. Alzheimer’s Dis. 2016, 50, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Baytan, S.H.; Alkanat, M.; Okuyan, M.; Ekinci, M.; Gedikli, E.; Ozeren, M.; Akgun, A. Simvastatin Impairs Spatial Memory in Rats at a Specific Dose Level. Tohoku J. Exp. Med. 2008, 214, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, S.; Katsanos, A.H.; Tsivgoulis, G.; Marshall, R.S. Statins and Cerebral Hemodynamics. J. Cereb. Blood Flow Metab. 2012, 32, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, S.; Katsanos, A.H.; Kosmidou, M.; Tsivgoulis, G. Statins and Vascular Dementia: A Review. J. Alzheimer’s Dis. 2014, 42, S315–S320. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Ghorbanihaghjo, A.; Argani, H. The Balance between Induction and Inhibition of Mevalonate Pathway Regulates Cancer Suppression by Statins: A Review of Molecular Mechanisms. Chem. Biol. Interact. 2017, 273, 273–285. [Google Scholar] [CrossRef]
- Akhtar, R.S.; Ness, J.M.; Roth, K.A. Bcl-2 Family Regulation of Neuronal Development and Neurodegeneration. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1644, 189–203. [Google Scholar] [CrossRef]
- Yasuno, F.; Tanimukai, S.; Sasaki, M.; Hidaka, S.; Ikejima, C.; Yamashita, F.; Kodama, C.; Mizukami, K.; Michikawa, M.; Asada, T. Association Between Cognitive Function and Plasma Lipids of the Elderly After Controlling for Apolipoprotein E Genotype. Am. J. Geriatr. Psychiatry 2012, 20, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Wellington, C.L.; Frikke-Schmidt, R. Relation between Plasma and Brain Lipids. Curr. Opin. Lipidol. 2016, 27, 225–232. [Google Scholar] [CrossRef] [PubMed]
- McNamara, D.J. Dietary Cholesterol, Heart Disease Risk and Cognitive Dissonance. Proc. Nutr. Soc. 2014, 73, 161–166. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Nikolic, D.; Howard, G.; Howard, V.J.; Mikhailidis, D.P. Intensive LDL-Cholesterol Lowering Therapy and Neurocognitive Function. Pharmacol. Ther. 2017, 170, 181–191. [Google Scholar] [CrossRef]
- Vu, M.; Kettunen, R.; Tolppanen, A.-M.; Hartikainen, S.; Taipale, H. Statin Discontinuation in Persons with and without Alzheimer’s Disease. Eur. J. Clin. Pharmacol. 2022, 78, 1145–1153. [Google Scholar] [CrossRef]
- Olmastroni, E.; Molari, G.; De Beni, N.; Colpani, O.; Galimberti, F.; Gazzotti, M.; Zambon, A.; Catapano, A.L.; Casula, M. Statin Use and Risk of Dementia or Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Prev. Cardiol. 2022, 29, 804–814. [Google Scholar] [CrossRef]
- Rajan, K.B.; Mcaninch, E.A.; Wilson, R.S.; Dhana, A.; Evans-Lacko, S.; Evans, D.A. Statin Initiation and Risk of Incident Alzheimer Disease and Cognitive Decline in Genetically Susceptible Older Adults. Neurology 2024, 102, e209168. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Kwon, O.-H.; Chung, S. Preferred Endocytosis of Amyloid Precursor Protein from Cholesterol-Enriched Lipid Raft Microdomains. Molecules 2020, 25, 5490. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Kwon, O.-H.; Park, M.K.; Kim, T.-W.; Chung, S. Elevated Cellular Cholesterol in Familial Alzheimer’s Presenilin 1 Mutation Is Associated with Lipid Raft Localization of β-Amyloid Precursor Protein. PLoS ONE 2019, 14, e0210535. [Google Scholar] [CrossRef]
- Shie, F.-S.; Jin, L.-W.; Cook, D.G.; Leverenz, J.B.; LeBoeuf, R.C. Diet-Induced Hypercholesterolemia Enhances Brain Aβ Accumulation in Transgenic Mice. Neuroreport 2002, 13, 455–459. [Google Scholar] [CrossRef]
- Mateos, L.; Akterin, S.; Gil-Bea, F.; Spulber, S.; Rahman, A.; Björkhem, I.; Schultzberg, M.; Flores-Morales, A.; Cedazo-Mínguez, A. Activity-Regulated Cytoskeleton-Associated Protein in Rodent Brain Is Down-Regulated by High Fat Diet In Vivo and by 27-Hydroxycholesterol In Vitro. Brain Pathol. 2009, 19, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.H.; Choi, K.H.; Jang, Y.J.; Bae, S.S.; Choi, B.T.; Shin, H.K. Hypercholesterolemia Accelerates Amyloid β-Induced Cognitive Deficits. Int. J. Mol. Med. 2013, 31, 577–582. [Google Scholar] [CrossRef]
- Svobodová, H.; Kosnáč, D.; Balázsiová, Z.; Tanila, H.; Miettinen, P.O.; Sierra, A.; Vitovič, P.; Wagner, A.; Polák, Š.; Kopáni, M. Elevated age-related cortical iron, ferritin and amyloid plaques in APP(swe)/PS1(deltaE9) transgenic mouse model of Alzheimer’s disease. Physiol. Res. 2019, 68 (Suppl. 4), S445–S451. [Google Scholar] [CrossRef]
- Sparks, D.L.; Scheff, S.W.; Hunsaker, J.C.; Liu, H.; Landers, T.; Gross, D.R. Induction of Alzheimer-like β-Amyloid Immunoreactivity in the Brains of Rabbits with Dietary Cholesterol. Exp. Neurol. 1994, 126, 88–94. [Google Scholar] [CrossRef]
- Lodeiro, M.; Puerta, E.; Ismail, M.-A.-M.; Rodriguez-Rodriguez, P.; Rönnbäck, A.; Codita, A.; Parrado-Fernandez, C.; Maioli, S.; Gil-Bea, F.; Merino-Serrais, P.; et al. Aggregation of the Inflammatory S100A8 Precedes Aβ Plaque Formation in Transgenic APP Mice: Positive Feedback for S100A8 and Aβ Productions. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 72, glw073. [Google Scholar] [CrossRef]
- Rahman, A.; Akterin, S.; Flores-Morales, A.; Crisby, M.; Kivipelto, M.; Schultzberg, M.; Cedazo-Mínguez, A. High Cholesterol Diet Induces Tau Hyperphosphorylation in Apolipoprotein E Deficient Mice. FEBS Lett. 2005, 579, 6411–6416. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.H. Diversification of Gamma-Secretase Activity versus Beta-Secretase Inhibition by Cholesterol Depletion. Neurobiol. Aging 2000, 21, 278. [Google Scholar] [CrossRef]
- He, Q.; Li, Q.; Zhao, J.; Wu, T.; Ji, L.; Huang, G.; Ma, F. Relationship between Plasma Lipids and Mild Cognitive Impairment in the Elderly Chinese: A Case-Control Study. Lipids Health Dis. 2016, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yin, Z.; Zhu, P.; Luo, J.; Shi, X.; Gao, X. Blood Cholesterol in Late-Life and Cognitive Decline: A Longitudinal Study of the Chinese Elderly. Mol. Neurodegener. 2017, 12, 24. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Seripa, D.; Imbimbo, B.P.; Sancarlo, D.; D’Onofrio, G.; Addante, F.; Paris, F.; Pilotto, A.; Solfrizzi, V. Metabolic Syndrome, Mild Cognitive Impairment and Dementia. Curr. Alzheimer Res. 2011, 8, 492–509. [Google Scholar] [CrossRef]
- Power, M.C.; Rawlings, A.; Sharrett, A.R.; Bandeen-Roche, K.; Coresh, J.; Ballantyne, C.M.; Pokharel, Y.; Michos, E.D.; Penman, A.; Alonso, A.; et al. Association of Midlife Lipids with 20-Year Cognitive Change: A Cohort Study. Alzheimers Dement. 2018, 14, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Gamba, P.; Testa, G.; Gargiulo, S.; Staurenghi, E.; Poli, G.; Leonarduzzi, G. Oxidized Cholesterol as the Driving Force behind the Development of Alzheimer’s Disease. Front. Aging Neurosci. 2015, 7, 119. [Google Scholar] [CrossRef]
- Schultz, B.G.; Patten, D.K.; Berlau, D.J. The Role of Statins in Both Cognitive Impairment and Protection against Dementia: A Tale of Two Mechanisms. Transl. Neurodegener. 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-B.; Yin, Z.X.; Chei, C.-L.; Brasher, M.S.; Zhang, J.; Kraus, V.B.; Qian, F.; Shi, X.; Matchar, D.B.; Zeng, Y. Serum Cholesterol Levels within the High Normal Range Are Associated with Better Cognitive Performance among Chinese Elderly. J. Nutr. Health Aging 2016, 20, 280–287. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; Chen, E.S.; Smith, M.C.; Bertolucci, P.H.F. Longitudinal Lipid Profile Variations and Clinical Change in Alzheimer’s Disease Dementia. Neurosci. Lett. 2017, 646, 36–42. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.-X.; Manly, J.; Schupf, N.; Mayeux, R.; Luchsinger, J.A. Plasma Lipid Levels in the Elderly Are Not Associated with the Risk of Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2008, 25, 232–237. [Google Scholar] [CrossRef]
- Kivipelto, M.; Solomon, A. Cholesterol as a Risk Factor for Alzheimer’s Disease—Epidemiological Evidence. Acta Neurol. Scand. 2006, 114, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Zandi, P.P.; Sjögren, M.; Gustafson, D.; Östling, S.; Steen, B.; Skoog, I. High Total Cholesterol Levels in Late Life Associated with a Reduced Risk of Dementia. Neurology 2005, 64, 1689–1695. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Panza, F.; D’Introno, A.; Colacicco, A.M.; Capurso, C.; Basile, A.M.; Capurso, A. Lipoprotein(a), Apolipoprotein E Genotype, and Risk of Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2002, 72, 732–736. [Google Scholar] [CrossRef]
- Partonen, T.; Haukka, J.; Virtamo, J.; Taylor, P.R.; Lönnqvist, J. Association of Low Serum Total Cholesterol with Major Depression and Suicide. Br. J. Psychiatry 1999, 175, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Repo-Tiihonen, E.; Halonen, P.; Tiihonen, J.; Virkkunen, M. Total Serum Cholesterol Level, Violent Criminal Offences, Suicidal Behavior, Mortality and the Appearance of Conduct Disorder in Finnish Male Criminal Offenders with Antisocial Personality Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Vicario, A.; Del Sueldo, M.; Fernández, R.A.; Enders, J.; Zilberman, J.; Cerezo, G.H. Cognition and Vascular Risk Factors: An Epidemiological Study. Int. J. Hypertens. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Cibickova, L.; Radomir, H.; Stanislav, M.; Norbert, C.; Helena, Z.; Daniel, J.; Alena, T.; Eva, B.; Vladimir, P. The Influence of Simvastatin, Atorvastatin and High-Cholesterol Diet on Acetylcholinesterase Activity, Amyloid Beta and Cholesterol Synthesis in Rat Brain. Steroids 2009, 74, 13–19, Corrected in Steroids 2009, 74, 721. [Google Scholar] [CrossRef]
- Wolozin, B. Decreased Prevalence of Alzheimer Disease Associated With 3-Hydroxy-3-Methyglutaryl Coenzyme A Reductase Inhibitors. Arch. Neurol. 2000, 57, 1439. [Google Scholar] [CrossRef] [PubMed]
- Jick, H.; Zornberg, G.; Jick, S.; Seshadri, S.; Drachman, D. Statins and the Risk of Dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.B.; Lin, V.W.; Boudreau, D.; Devine, E.B. Statins in the Prevention of Dementia and Alzheimer’s Disease: A Meta-analysis of Observational Studies and an Assessment of Confounding. Pharmacoepidemiol. Drug Saf. 2013, 22, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Green, R.C.; McNagny, S.E.; Jayakumar, P.; Cupples, L.A.; Benke, K.; Farrer, L.A. Statin Use and the Risk of Alzheimer’s Disease: The MIRAGE Study. Alzheimer’s Dement. 2006, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-K.; Yang, Y.-H.; Lin, T.-T.; Tsai, C.-T.; Hwang, J.-J.; Lin, J.-L.; Chen, P.-C.; Chiang, F.-T.; Lin, L.-Y. Statin Use Reduces the Risk of Dementia in Elderly Patients: A Nationwide Data Survey and Propensity Analysis. J. Intern. Med. 2015, 277, 343–352. [Google Scholar] [CrossRef]
- Zhu, X.-C.; Dai, W.-Z.; Ma, T. Overview the Effect of Statin Therapy on Dementia Risk, Cognitive Changes and Its Pathologic Change: A Systematic Review and Meta-Analysis. Ann. Transl. Med. 2018, 6, 435. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Choi, E.-A.; Kim, Y.-S.; Kim, Y.; You, H.-S.; Han, Y.-E.; Kim, H.-S.; Bae, Y.-J.; Kim, J.; Kang, H.-T. Statin Exposure and the Risk of Dementia in Individuals with Hypercholesterolaemia. J. Intern. Med. 2020, 288, 689–698. [Google Scholar] [CrossRef]
- Mora, S.; Ridker, P.M. Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER)—Can C-Reactive Protein Be Used to Target Statin Therapy in Primary Prevention? Am. J. Cardiol. 2006, 97, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Coats, A. MRC/BHF Heart Protection Study of Cholesterol Lowering with Simvastatin in 20 536 High-Risk Individuals: A Randomised Placebocontrolled Trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Appleton, J.P.; Scutt, P.; Sprigg, N.; Bath, P.M. Hypercholesterolaemia and Vascular Dementia. Clin. Sci. 2017, 131, 1561–1578. [Google Scholar] [CrossRef]
- Ancelin, M.-L.; Carrière, I.; Barberger-Gateau, P.; Auriacombe, S.; Rouaud, O.; Fourlanos, S.; Berr, C.; Dupuy, A.-M.; Ritchie, K. Lipid Lowering Agents, Cognitive Decline, and Dementia: The Three-City Study. J. Alzheimers Dis. 2012, 30, 629–637. [Google Scholar] [CrossRef]
- Alsehli, A.M.; Olivo, G.; Clemensson, L.E.; Williams, M.J.; Schiöth, H.B. The Cognitive Effects of Statins Are Modified by Age. Sci. Rep. 2020, 10, 6187. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.S.; Bishara, D.; Perera, G.; Molokhia, M.; Rajendran, L.; Stewart, R.J. Benefits and Harms of Statins in People with Dementia: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2020, 68, 650–658. [Google Scholar] [CrossRef]
- Dagliati, A.; Peek, N.; Brinton, R.D.; Geifman, N. Sex and APOE Genotype Differences Related to Statin Use in the Aging Population. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12156. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hyman, D.; Ayyala, S.; Bakhshi, A.; Kim, S.H.; Anoruo, N.; Weinstock, J.; Balogun, A.; D’Souza, M.; Filatova, N.; et al. Cognitive Function Assessment in Patients on Moderate- or High-Intensity Statin Therapy. J. Clin. Med. Res. 2020, 12, 255–265. [Google Scholar] [CrossRef]
- Ghosh, A.; Roy, A.; Matras, J.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Simvastatin Inhibits the Activation of P21ras and Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease. J. Neurosci. 2009, 29, 13543–13556. [Google Scholar] [CrossRef] [PubMed]
- FDA—US Food and Drug Administration. FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol-Lowering Statin Drugs; US Food and Drug Administration: Rockville, MD, USA, 2012.
- Posvar, E.L.; Radulovic, L.L.; Cilla, D.D.; Whitfield, L.R.; Sedman, A.J. Tolerance and Pharmacokinetics of Single-Dose Atorvastatin, a Potent Inhibitor of HMG-CoA Reductase, in Healthy Subjects. J. Clin. Pharmacol. 1996, 36, 728–731. [Google Scholar] [CrossRef]
- Rojas-Fernandez, C.H.; Cameron, J.-C.F. Is Statin-Associated Cognitive Impairment Clinically Relevant? A Narrative Review and Clinical Recommendations. Ann. Pharmacother. 2012, 46, 549–557. [Google Scholar] [CrossRef]
- Wagstaff, L.R.; Mitton, M.W.; Arvik, B.M.; Doraiswamy, P.M. Statin-Associated Memory Loss: Analysis of 60 Case Reports and Review of the Literature. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef]
- Swiger, K.J.; Manalac, R.J.; Blumenthal, R.S.; Blaha, M.J.; Martin, S.S. Statins and Cognition: A Systematic Review and Meta-Analysis of Short- and Long-Term Cognitive Effects. Mayo Clin. Proc. 2013, 88, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Schoen, M.; French, B.; Umscheid, C.A.; Mitchell, M.D.; Arnold, S.E.; Heidenreich, P.A.; Rader, D.J.; deGoma, E.M. Statins and Cognitive Function. Ann. Intern. Med. 2013, 159, 688. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Li, R.; Cheng, O. Statins for Treating Alzheimer’s Disease: Truly Ineffective? Eur. Neurol. 2015, 73, 360–366. [Google Scholar] [CrossRef]
- Seo, W.-K.; Hosseini, M.B.; Bang, O.Y.; Liebeskind, D.S. Recent Updates in Dyslipidemia Management: Perspectives in Stroke-Specific Situation. Precis. Future Med. 2020, 4, 9–20. [Google Scholar] [CrossRef]
- Schulz, R.; Schlüter, K.-D.; Laufs, U. Molecular and Cellular Function of the Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9). Basic. Res. Cardiol. 2015, 110, 4. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Wickham, L.; Marcinkiewicz, J.; Jasmin, S.B.; Stifani, S.; Basak, A.; Prat, A.; Chrétien, M. The Secretory Proprotein Convertase Neural Apoptosis-Regulated Convertase 1 (NARC-1): Liver Regeneration and Neuronal Differentiation. Proc. Natl. Acad. Sci. USA 2003, 100, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Lagace, T.A.; Curtis, D.E.; Garuti, R.; McNutt, M.C.; Park, S.W.; Prather, H.B.; Anderson, N.N.; Ho, Y.K.; Hammer, R.E.; Horton, J.D. Secreted PCSK9 Decreases the Number of LDL Receptors in Hepatocytes and Inlivers of Parabiotic Mice. J. Clin. Investig. 2006, 116, 2995–3005. [Google Scholar] [CrossRef]
- Papotti, B.; Adorni, M.P.; Marchi, C.; Zimetti, F.; Ronda, N.; Panighel, G.; Lupo, M.G.; Vilella, A.; Giuliani, D.; Ferri, N.; et al. PCSK9 Affects Astrocyte Cholesterol Metabolism and Reduces Neuron Cholesterol Supplying In Vitro: Potential Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12192. [Google Scholar] [CrossRef] [PubMed]
- Witte, M.E.; Geurts, J.J.G.; de Vries, H.E.; van der Valk, P.; van Horssen, J. Mitochondrial Dysfunction: A Potential Link between Neuroinflammation and Neurodegeneration? Mitochondrion 2010, 10, 411–418. [Google Scholar] [CrossRef]
- Yadav, R.; Weng, H.-R. EZH2 Regulates Spinal Neuroinflammation in Rats with Neuropathic Pain. Neuroscience 2017, 349, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Crisby, M.; Rahman, S.M.A.; Sylvén, C.; Winblad, B.; Schultzberg, M. Effects of High Cholesterol Diet on Gliosis in Apolipoprotein E Knockout Mice. Neurosci. Lett. 2004, 369, 87–92. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, E.M.; Lohoff, F.W. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in the Brain and Relevance for Neuropsychiatric Disorders. Front. Neurosci. 2020, 14, 609. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Ashish, K.; Hajra, A.; Qureshi, A.; Ghosh, R.K. Cardiovascular Outcomes of PCSK9 Inhibitors: With Special Emphasis on Its Effect beyond LDL-Cholesterol Lowering. J. Lipids 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ruscica, M.; Ferri, N.; Bernini, F.; Zimetti, F. Proprotein Convertase Subtilisin/Kexin Type 9, Brain Cholesterol Homeostasis and Potential Implication for Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 120. [Google Scholar] [CrossRef]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.G.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr–/– Mice: Impact on Cognitive Function. J. Alzheimer’s Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, H.; Bigot, E.; Pichelin, M.; Guyomarch, B.; Boutoleau-Bretonnière, C.; Le May, C.; Derkinderen, P.; Cariou, B. PCSK9 Concentrations in Cerebrospinal Fluid Are Not Specifically Increased in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1519–1525. [Google Scholar] [CrossRef]
- Zimetti, F.; Caffarra, P.; Ronda, N.; Favari, E.; Adorni, M.P.; Zanotti, I.; Bernini, F.; Barocco, F.; Spallazzi, M.; Galimberti, D.; et al. Increased PCSK9 Cerebrospinal Fluid Concentrations in Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 315–320. [Google Scholar] [CrossRef]
- Mazura, A.D.; Ohler, A.; Storck, S.E.; Kurtyka, M.; Scharfenberg, F.; Weggen, S.; Becker-Pauly, C.; Pietrzik, C.U. PCSK9 Acts as a Key Regulator of Aβ Clearance across the Blood–Brain Barrier. Cell. Mol. Life Sci. 2022, 79, 212. [Google Scholar] [CrossRef]
- Vilella, A.; Bodria, M.; Papotti, B.; Zanotti, I.; Zimetti, F.; Remaggi, G.; Elviri, L.; Potì, F.; Ferri, N.; Lupo, M.G.; et al. PCSK9 Ablation Attenuates Aβ Pathology, Neuroinflammation and Cognitive Dysfunctions in 5XFAD Mice. Brain Behav. Immun. 2024, 115, 517–534. [Google Scholar] [CrossRef]
- Mahley, R.W. Central Nervous System Lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-S.; Bai, X.-Q.; Gao, Y.; Wu, Q.; Ren, Z.; Li, Q.; Pan, L.-H.; He, N.-Y.; Peng, J.; Tang, Z.-H. PCSK9 Promotes OxLDL-Induced PC12 Cell Apoptosis Through the Bcl-2/Bax-Caspase 9/3 Signaling Pathway. J. Alzheimer’s Dis. 2017, 57, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Mayer, G.; Benjannet, S.; Bergeron, E.; Marcinkiewicz, J.; Nassoury, N.; Mayer, H.; Nimpf, J.; Prat, A.; Seidah, N.G. The Proprotein Convertase PCSK9 Induces the Degradation of Low Density Lipoprotein Receptor (LDLR) and Its Closest Family Members VLDLR and ApoER2. J. Biol. Chem. 2008, 283, 2363–2372. [Google Scholar] [CrossRef]
- Badimon, L.; Luquero, A.; Crespo, J.; Peña, E.; Borrell-Pages, M. PCSK9 and LRP5 in Macrophage Lipid Internalization and Inflammation. Cardiovasc. Res. 2021, 117, 2054–2068. [Google Scholar] [CrossRef] [PubMed]
- Jaén, R.I.; Povo-Retana, A.; Rosales-Mendoza, C.; Capillas-Herrero, P.; Sánchez-García, S.; Martín-Sanz, P.; Mojena, M.; Prieto, P.; Boscá, L. Functional Crosstalk between PCSK9 Internalization and Pro-Inflammatory Activation in Human Macrophages: Role of Reactive Oxygen Species Release. Int. J. Mol. Sci. 2022, 23, 9114. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 Induces a Pro-Inflammatory Response in Macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.-T.; Li, T.-H.; Wang, Z.; Wei, D.-H.; Liu, L.-S.; Zheng, X.-L.; et al. New Role of PCSK9 in Atherosclerotic Inflammation Promotion Involving the TLR4/NF-ΚB Pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Poirier, A.; Bélanger, S.; Labonté, A.; Auld, D.; Poirier, J. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Alzheimer’s Disease: A Genetic and Proteomic Multi-Cohort Study. PLoS ONE 2019, 14, e0220254. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Favero, C.; Ceresa, A.; Vigna, L.; Conti, D.M.; Pesatori, A.C.; Racagni, G.; Corsini, A.; Ferri, N.; Sirtori, C.R.; et al. Depression and Cardiovascular Risk—Association among Beck Depression Inventory, PCSK9 Levels and Insulin Resistance. Cardiovasc. Diabetol. 2020, 19, 187. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Ray, K.K.; Colhoun, H.M.; Szarek, M.; Baccara-Dinet, M.; Bhatt, D.L.; Bittner, V.A.; Budaj, A.J.; Diaz, R.; Goodman, S.G.; Hanotin, C.; et al. Effects of Alirocumab on Cardiovascular and Metabolic Outcomes after Acute Coronary Syndrome in Patients with or without Diabetes: A Prespecified Analysis of the ODYSSEY OUTCOMES Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2019, 7, 618–628, Corrected in Lancet Diabetes Endocrinol. 2019, 7, E21. [Google Scholar] [CrossRef]
- Goodman, S.G.; Steg, P.G.; Poulouin, Y.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Garon, G.; Harrington, R.A.; Jukema, J.W.; Manvelian, G.; et al. Long-Term Efficacy, Safety, and Tolerability of Alirocumab in 8242 Patients Eligible for 3 to 5 Years of Placebo-Controlled Observation in the ODYSSEY OUTCOMES Trial. J. Am. Hear. Assoc. 2023, 12, e029216, Corrected in J. Am. Hear. Assoc. 2023, 12, e027745. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Robinson, J.G.; Cannon, C.P.; Lorenzato, C.; Pordy, R.; Chaudhari, U.; Colhoun, H.M. Efficacy and Safety of the Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor Alirocumab among High Cardiovascular Risk Patients on Maximally Tolerated Statin Therapy: The ODYSSEY COMBO I Study. Am. Heart J. 2015, 169, 906–915.e13. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.S.F.; Campos, A.M.; Sposito, A.C. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors and Incident Type 2 Diabetes: A Systematic Review and Meta-Analysis With Over 96,000 Patient-Years. Diabetes Care 2018, 41, 364–367. [Google Scholar] [CrossRef] [PubMed]
- di Mauro, G.; Zinzi, A.; Scavone, C.; Mascolo, A.; Gaio, M.; Sportiello, L.; Ferrajolo, C.; Rafaniello, C.; Rossi, F.; Capuano, A. PCSK9 Inhibitors and Neurocognitive Adverse Drug Reactions: Analysis of Individual Case Safety Reports from the Eudravigilance Database. Drug Saf. 2021, 44, 337–349. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From Cholesterol Metabolites to Key Mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Katsuki, S.; Matoba, T.; Nakano, Y.; Takase, S.; Nakashiro, S.; Yamamoto, M.; Mukai, Y.; Inoue, S.; Oi, K.; et al. Association of Serum Oxysterols with Cholesterol Metabolism Markers and Clinical Factors in Patients with Coronary Artery Disease: A Covariance Structure Analysis. Nutrients 2023, 15, 2997. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.W.; Davis, H.R.; Zhu, L.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science (1979) 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, Y.; Takada, T.; Tanaka, Y.; Ogata, Y.; Toyoda, Y.; Ito, S.M.; Kitani, M.; Oshida, N.; Okada, K.; Shoda, J.; et al. Hepatic Niemann-Pick C1-Like 1 Exacerbates Non-Alcoholic Fatty Liver Disease by Re-Absorbing Specific Biliary Oxysterols. Biomed. Pharmacother. 2022, 156, 113877. [Google Scholar] [CrossRef]
- Fieldhouse, J.L.P.; Doorduijn, A.S.; de Leeuw, F.A.; Verhaar, B.J.H.; Koene, T.; Wesselman, L.M.P.; de van der Schueren, M.A.E.; Visser, M.; van de Rest, O.; Scheltens, P.; et al. A Suboptimal Diet Is Associated with Poorer Cognition: The NUDAD Project. Nutrients 2020, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.; Mayeux, R.; Luchsinger, J.A. Mediterranean Diet and Risk for Alzheimer’s Disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Zhou, F.; Deng, W.; Ding, D.; Zhao, Q.; Liang, X.; Wang, F.; Luo, J.; Zheng, L.; Guo, Q.; Hong, Z. High Low-Density Lipoprotein Cholesterol Inversely Relates to Dementia in Community-Dwelling Older Adults: The Shanghai Aging Study. Front. Neurol. 2018, 9, 952. [Google Scholar] [CrossRef]
- Liu, H.; Zou, L.; Zhou, R.; Zhang, M.; Gu, S.; Zheng, J.; Hukportie, D.N.; Wu, K.; Huang, Z.; Yuan, Z.; et al. Long-Term Increase in Cholesterol Is Associated With Better Cognitive Function: Evidence From a Longitudinal Study. Front. Aging Neurosci. 2021, 13, 691423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; An, Y.; Ma, W.; Feng, L.; Wang, C.; Lu, Y.; Xiao, R. High-cholesterol Diet Results in Elevated Amyloid-β and Oxysterols in Rats. Mol. Med. Rep. 2017, 17, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Staurenghi, E.; Zerbinati, C.; Gargiulo, S.; Iuliano, L.; Giaccone, G.; Fantò, F.; Poli, G.; Leonarduzzi, G.; Gamba, P. Changes in Brain Oxysterols at Different Stages of Alzheimer’s Disease: Their Involvement in Neuroinflammation. Redox Biol. 2016, 10, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Berardi, S.; Scarpona, S.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Gut Microbiota Manipulation through Probiotics Oral Administration Restores Glucose Homeostasis in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2020, 87, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Parent, M.; Prakash, S. Cholesterol-Lowering Efficacy of a Microencapsulated Bile Salt Hydrolase-Active Lactobacillus Reuteri NCIMB 30242 Yoghurt Formulation in Hypercholesterolaemic Adults. Br. J. Nutr. 2012, 107, 1505–1513. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Gong, C.; Cuccioloni, M.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Microbiota Modulation as Preventative and Therapeutic Approach in Alzheimer’s Disease. FEBS J. 2021, 288, 2836–2855. [Google Scholar] [CrossRef]
- Bonfili, L.; Cuccioloni, M.; Gong, C.; Cecarini, V.; Spina, M.; Zheng, Y.; Angeletti, M.; Eleuteri, A.M. Gut Microbiota Modulation in Alzheimer’s Disease: Focus on Lipid Metabolism. Clin. Nutr. 2022, 41, 698–708. [Google Scholar] [CrossRef]
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Zelcer, N.; Khanlou, N.; Clare, R.; Jiang, Q.; Reed-Geaghan, E.G.; Landreth, G.E.; Vinters, H.V.; Tontonoz, P. Attenuation of Neuroinflammation and Alzheimer’s Disease Pathology by Liver x Receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 10601–10606. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, T.G.; Sleph, P.; Ostrowski, J.; Lupisella, J.; Ryan, C.S.; Liu, X.; Fernando, G.; Grimm, D.; Shipkova, P.; Zhang, R.; et al. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 2016, 24, 223–233. [Google Scholar] [CrossRef]
- Muse, E.D.; Yu, S.; Edillor, C.R.; Tao, J.; Spann, N.J.; Troutman, T.D.; Seidman, J.S.; Henke, A.; Roland, J.T.; Ozeki, K.A.; et al. Cell-Specific Discrimination of Desmosterol and Desmosterol Mimetics Confers Selective Regulation of LXR and SREBP in Macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E4680–E4689. [Google Scholar] [CrossRef] [PubMed]
- Baria, D.; Shah, U.; Egbuna, C.; Mtewa, A. Secondary Metabolites and Toxins of Microbial Origin for the Treatment of Diseases. Research Gate. In Poisonous Plants and Phytochemicals in Drug Discovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 225–248. [Google Scholar]
- Li, R.; Liu, R.; Chen, L.; Wang, G.; Qin, L.; Yu, Z.; Wan, Z. Microbiota from Exercise Mice Counteracts High-Fat High-Cholesterol Diet-Induced Cognitive Impairment in C57BL/6 Mice. Oxid. Med. Cell. Longev. 2023, 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New Perspectives in Fermented Dairy Products and Their Health Relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Chen, H.; Meng, L.; Shen, L. Multiple Roles of Short-Chain Fatty Acids in Alzheimer Disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now? Vasc. Health Risk Manage 2023, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Rouvinen-Watt, K. The Role of Food Peptides in Lipid Metabolism during Dyslipidemia and Associated Health Conditions. Int. J. Mol. Sci. 2015, 16, 9303–9313. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, V.M.; Vorobyeva, I.S.; Kochetkova, A.A.; Mazo, V.K.; Zorin, S.N.; Khaider, K.; Sha-Rafetdinov, K.K. Specialize Hypocholesterolemic Foods: Ingredients, Technology, Effects. Foods Raw Mater. 2020, 8, 20–29. [Google Scholar] [CrossRef]
- Knodel, L.C.; Talbert, R.L. Adverse Effects of Hypolipidaemic Drugs. Med. Toxicol. 1987, 2, 10–32. [Google Scholar] [CrossRef]
- Silva, I.M.V.; Machado, F.; Moreno, M.J.; Nunes, C.; Coimbra, M.A.; Coreta-Gomes, F. Polysaccharide Structures and Their Hypocholesterolemic Potential. Molecules 2021, 26, 4559. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, E.M.; Spence, J.D.; Jordan, J.; Wetmore, S.; Freeman, D.J.; Piché, L.A.; Serratore, P. HDL-Cholesterol-Raising Effect of Orange Juice in Subjects with Hypercholesterolemia. Am. J. Clin. Nutr. 2000, 72, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L.J.; Borradaile, N.M.; de Dreu, L.E.; Huff, M.W. Secretion of Hepatocyte ApoB Is Inhibited by the Flavonoids, Naringenin and Hesperetin, via Reduced Activity and Expression of ACAT2 and MTP. J. Lipid Res. 2001, 42, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules 2021, 26, 5718. [Google Scholar] [CrossRef]
- Bok, S.-H.; Lee, S.-H.; Park, Y.-B.; Bae, K.-H.; Son, K.-H.; Jeong, T.-S.; Choi, M.-S. Plasma and Hepatic Cholesterol and Hepatic Activities of 3-Hydroxy-3-Methyl-Glutaryl-CoA Reductase and Acyl CoA: Cholesterol Transferase Are Lower in Rats Fed Citrus Peel Extract or a Mixture of Citrus Bioflavonoids. J. Nutr. 1999, 129, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Mayer, G. The Biology of PCSK9 from the Endoplasmic Reticulum to Lysosomes: New and Emerging Therapeutics to Control Low-Density Lipoprotein Cholesterol. Drug Des. Dev. Ther. 2013, 7, 1135–1148. [Google Scholar] [CrossRef]
- Mollace, R.; Macrì, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Fini, M.; Volterrani, M.; Mollace, V. Comparative Effect of Bergamot Polyphenolic Fraction and Red Yeast Rice Extract in Rats Fed a Hyperlipidemic Diet: Role of Antioxidant Properties and PCSK9 Expression. Nutrients 2022, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Mondello, M.R.; Monforte, M.T.; Sdrafkakis, V.; Dugo, P.; Crupi, M.L.; Trovato, A. Hypolipidemic Effects of Citrus Bergamia Risso et Poiteau Juice in Rats Fed a Hypercholesterolemic Diet. J. Agric. Food Chem. 2007, 55, 10671–10677. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Giné-González, J.; Alisente, S.; Bea, A.M.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Jarauta, E.; Cenarro, A.; Civeira, F.; et al. Effect of Bergamot on Lipid Profile in Humans: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3133–3143. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
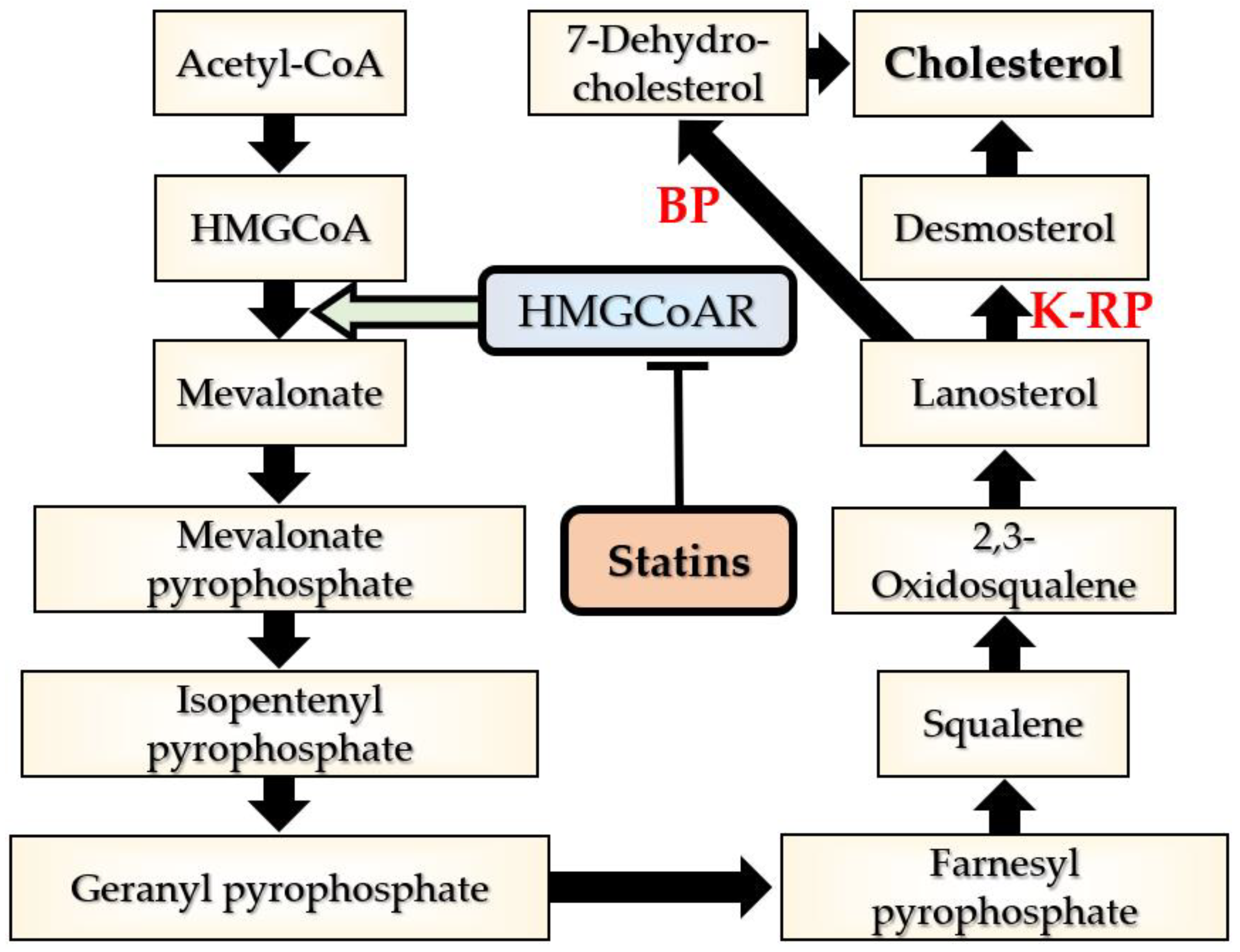

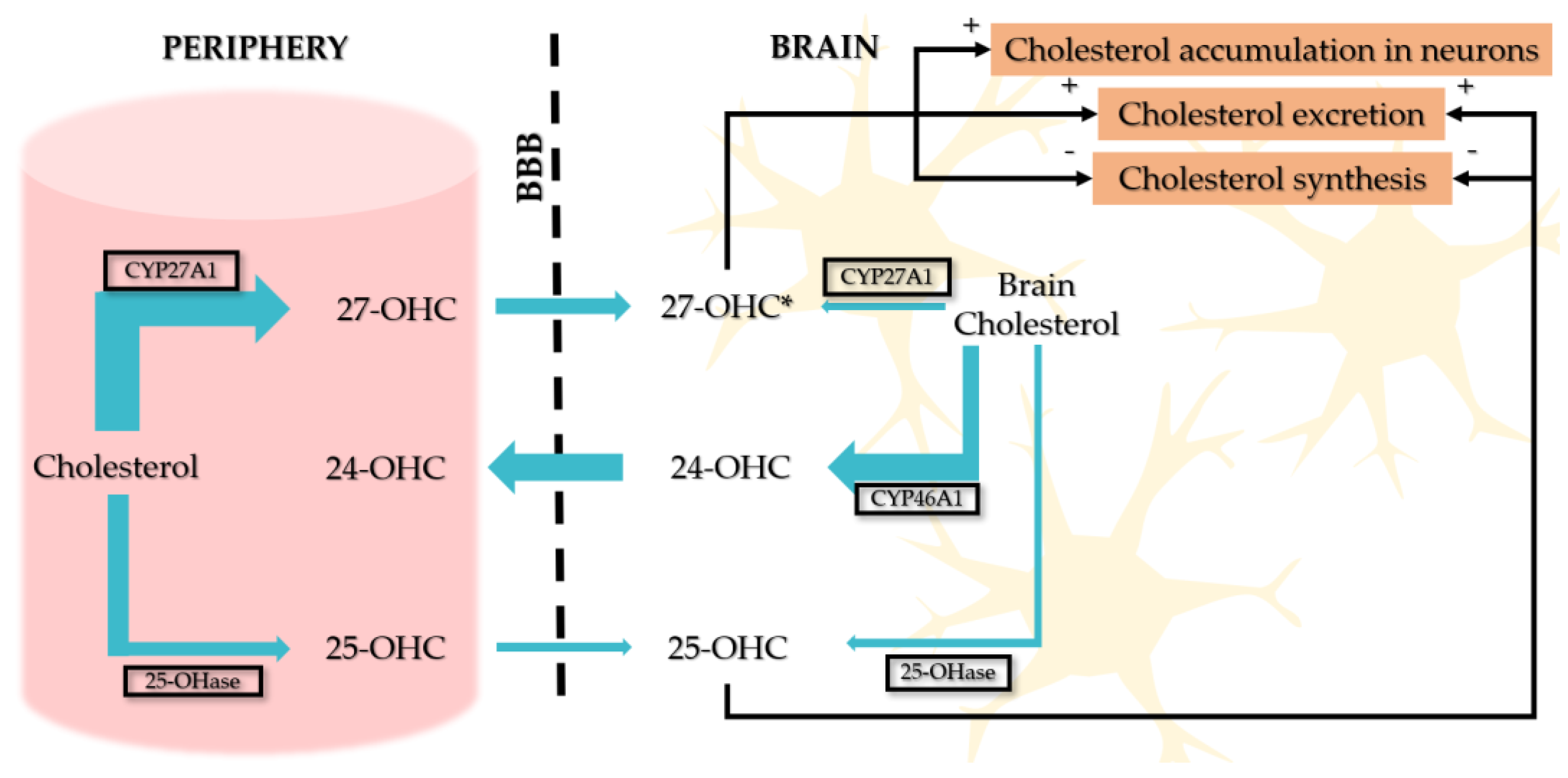
| Lipid-Lowering Therapy | Average LDL-C Reduction | Indication | Mechanism of Action | Adverse Effects |
|---|---|---|---|---|
| Moderate-intensity statins | 30% (monotherapy) | First-line treatment for lipid-lowering therapy and ASCVD risk reduction | ↓ Hepatic cholesterol production by blocking HMGCoAR → ↑ LDLr expression → higher uptake of LDLs from the bloodstream | Myopathy Rhabdomyolysis Hepatotoxicity Diabetes mellitus Hemorrhagic stroke Proteinuria |
| High-intensity statins | 50% (monotherapy) | Recommended up to the highest tolerated dose to reach the goals set for the specific level of risk | ||
| Ezetimibe | 65% (in combination with high-intensity statins) 85% (in combination with high-intensity statins and PCSK9 inhibitors) | Second-line therapy in association with statins when the therapeutic goal is not achieved at the maximal tolerated statin dose or when statins cannot be prescribed | Blocks intestinal absorption of dietary and biliary cholesterol → ↑ LDLr expression → ↑ LDL clearance from the bloodstream | Gastrointestinal adverse effects |
| Bile acid sequestrants | 18–25% (monotherapy) 10–25% further decrease in LDL-C in combination with statins 18% further decrease in LDL-C when added to statins and ezetimibe | Second-line therapy in association with statins when the therapeutic goal is not achieved at the maximal tolerated statin dose or in cases of statin intolerance in combination with ezetimibe. Third-line therapy as an addition to statin plus ezetimibe therapy when the therapeutic goal is not achieved | Prevent the reabsorbtion of both the drug and cholesterol in the blood by binding the bile acids in the intestinum → liver synthesizes more bile acids from hepatic cholesterol → ↑ demand for cholesterol and a ↑ LDL-R expression | Gastrointestinal adverse effects Increased circulating TG levels (contraindicated in baseline TG > 400 mg/dL) Affects the absorption of many drugs and fat-soluble vitamins |
| PCSK9 inhibitors | 60% (monotherapy) 75% (in combination with high-intensity statin) 85% (in combination with high-intensity statin plus ezetimibe) | Third-line therapy for the following:
| PCSK9 binds to the LDL-R and promotes its degradation → ↑ LDL concentration in the plasma. PCSK9 inhibitors increase LDL-R expression by reduction in the plasma levels of PCSK9 → ↑ clearance of LDLs → decrease in LDL-C levels PCSK9 inhibitors also decrease Lp(a) levels | Itching, erythema, swelling, pain at the site of injection Allergic reactions—flu-like symptoms Increased risk of new, onset diabetes mellitus or neurocognitive dysfunction have been suspected but not demonstrated Occurrence of antidrug antibodies—very rare |
| Inclisiran | No data | Investigational | Stimulates the catalytic breakdown of PCSK9 mRNA in hepatocytes → reduction in hepatic synthesis of PCSK9 → increase in LDL-R expression → increased clearance of LDLs → decrease in LDL-C levels | Injection-site adverse reactions |
| Bempedroic acid | 21.4% (monotherapy) 18% (in combination with statins) 38% (in combination with ezetimibe) | Reducing the risk of myocardial infarction and coronary revascularization in adults at risk and unable to take statins therapy. Adjunct to diet in combination with other LDL-C lowering therapies or alone when concomitant LDL-C lowering therapy is not possible | Inhibits ACLY which catalyzes the formation of acetyl-CoA → decreased cholesterol synthesis in liver → increased LDL-R expression → decrease in LDL-C levels | Hyperuricemia Tendon rupture Renal toxicity Cholelithiasis Benign prostatic hyperplasia Combination with simvastatin or pravastatin causes an increase in the concentrations of these drugs and, therefore, may increase the risk of myopathy |
| Mipomersen | No data | Adjunct to lipid-lowering medications and diet for the treatment of statin-intolerant patients with severe HoFH | Inhibits of ApoB 100 production in the liver by binding with ApoB mRNA and preventing its translation → lowering LDL and VLDL levels | Adverse reactions at the injection site Liver toxicity |
| Lomitapide | 30–50% (dose-dependent) | Indicated as once daily oral treatment for lowering LDL-C levels in adults with HoFH | Inhibits MTP → hinders the production of VLDLs in the liver and chylomicrons in the intestine | Gastrointestinal adverse effects Reduced absorption of fat-soluble vitamins and essential fatty acids Hepatotoxicity |
| Fibrates | 50% reduction in the TG levels <20% reduction in the LDL-C levels | Indicated for patients with elevated TG levels and low HDL-C levels | Agonists of PPARs, acting via transcription factors regulating various steps in lipid and lipoprotein metabolisms | Renal dysfunction Liver disease Gallbladder disease Increased risk of pancreatitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savulescu-Fiedler, I.; Dorobantu-Lungu, L.-R.; Dragosloveanu, S.; Benea, S.N.; Dragosloveanu, C.D.M.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Scheau, C. The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms. Curr. Issues Mol. Biol. 2025, 47, 115. https://doi.org/10.3390/cimb47020115
Savulescu-Fiedler I, Dorobantu-Lungu L-R, Dragosloveanu S, Benea SN, Dragosloveanu CDM, Caruntu A, Scheau A-E, Caruntu C, Scheau C. The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms. Current Issues in Molecular Biology. 2025; 47(2):115. https://doi.org/10.3390/cimb47020115
Chicago/Turabian StyleSavulescu-Fiedler, Ilinca, Luiza-Roxana Dorobantu-Lungu, Serban Dragosloveanu, Serban Nicolae Benea, Christiana Diana Maria Dragosloveanu, Ana Caruntu, Andreea-Elena Scheau, Constantin Caruntu, and Cristian Scheau. 2025. "The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms" Current Issues in Molecular Biology 47, no. 2: 115. https://doi.org/10.3390/cimb47020115
APA StyleSavulescu-Fiedler, I., Dorobantu-Lungu, L.-R., Dragosloveanu, S., Benea, S. N., Dragosloveanu, C. D. M., Caruntu, A., Scheau, A.-E., Caruntu, C., & Scheau, C. (2025). The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms. Current Issues in Molecular Biology, 47(2), 115. https://doi.org/10.3390/cimb47020115










