Exploring Potential Therapeutic Applications of Tazarotene: Gene Regulation Mechanisms and Effects on Melanoma Cell Growth
Abstract
:1. Introduction
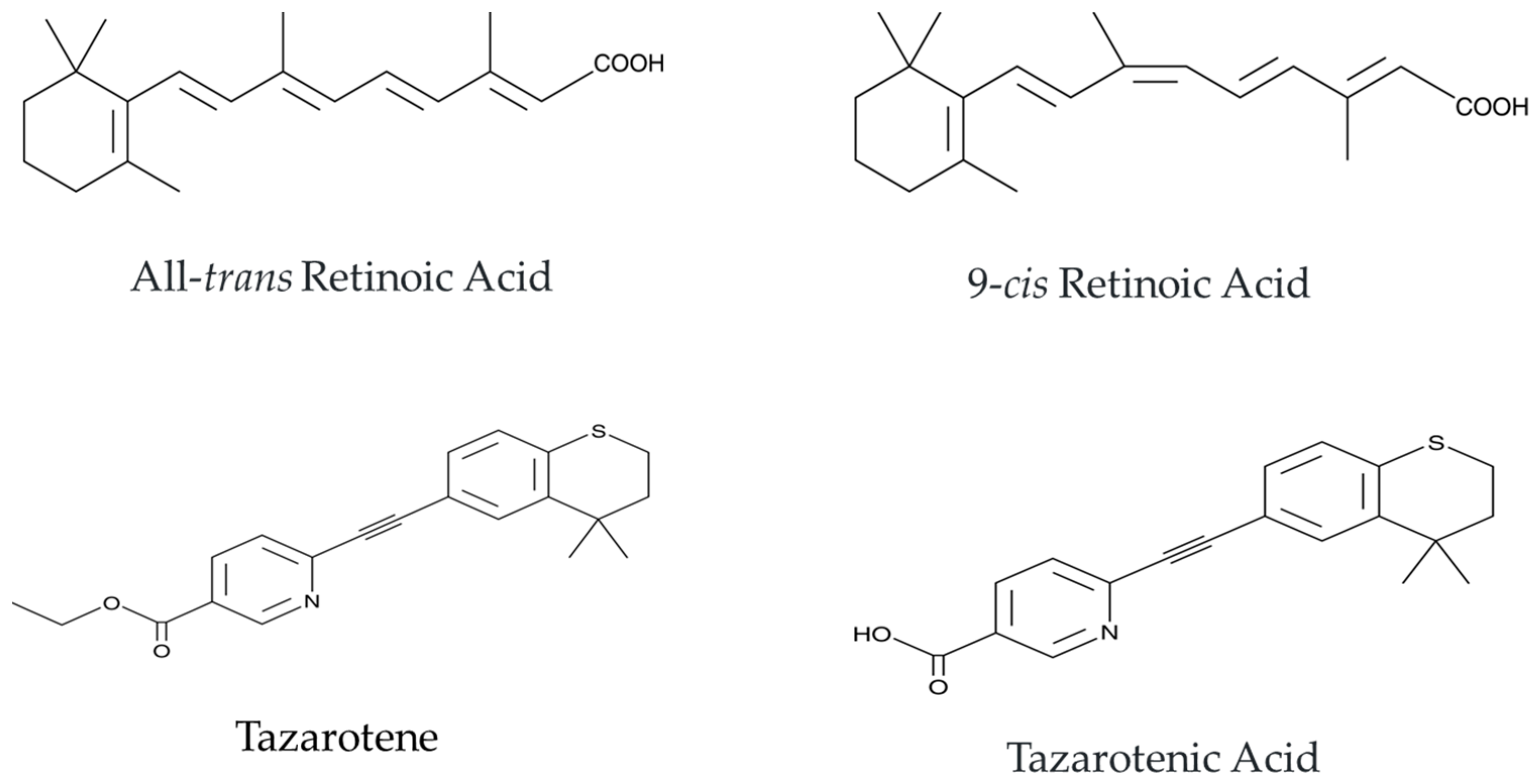
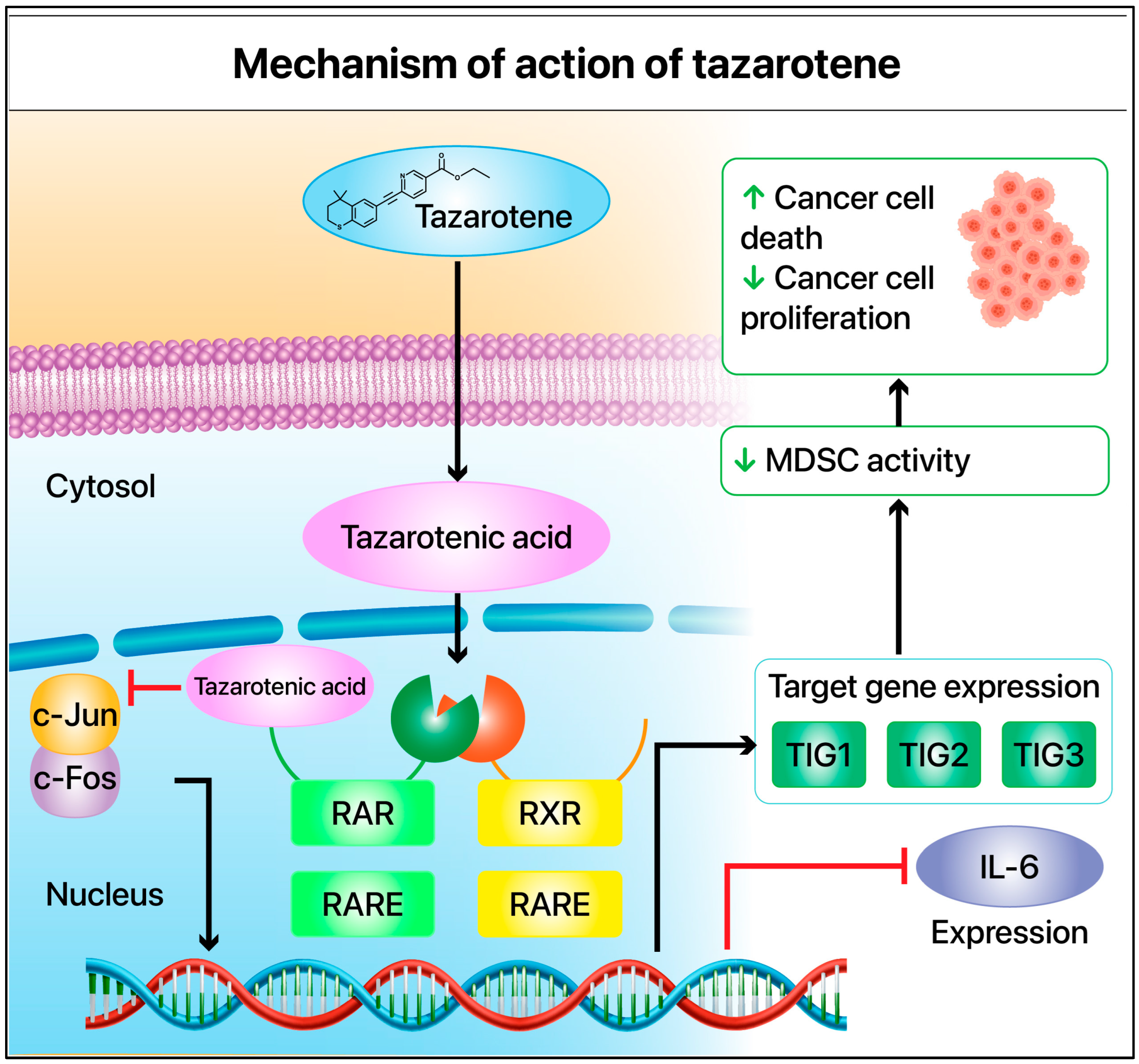
2. Tazarotene’s Mechanism of Action
3. Prevention and Clinical Management of Melanoma with RA and Tazarotene
4. Tazarotene’s Mechanism of Action in Melanoma
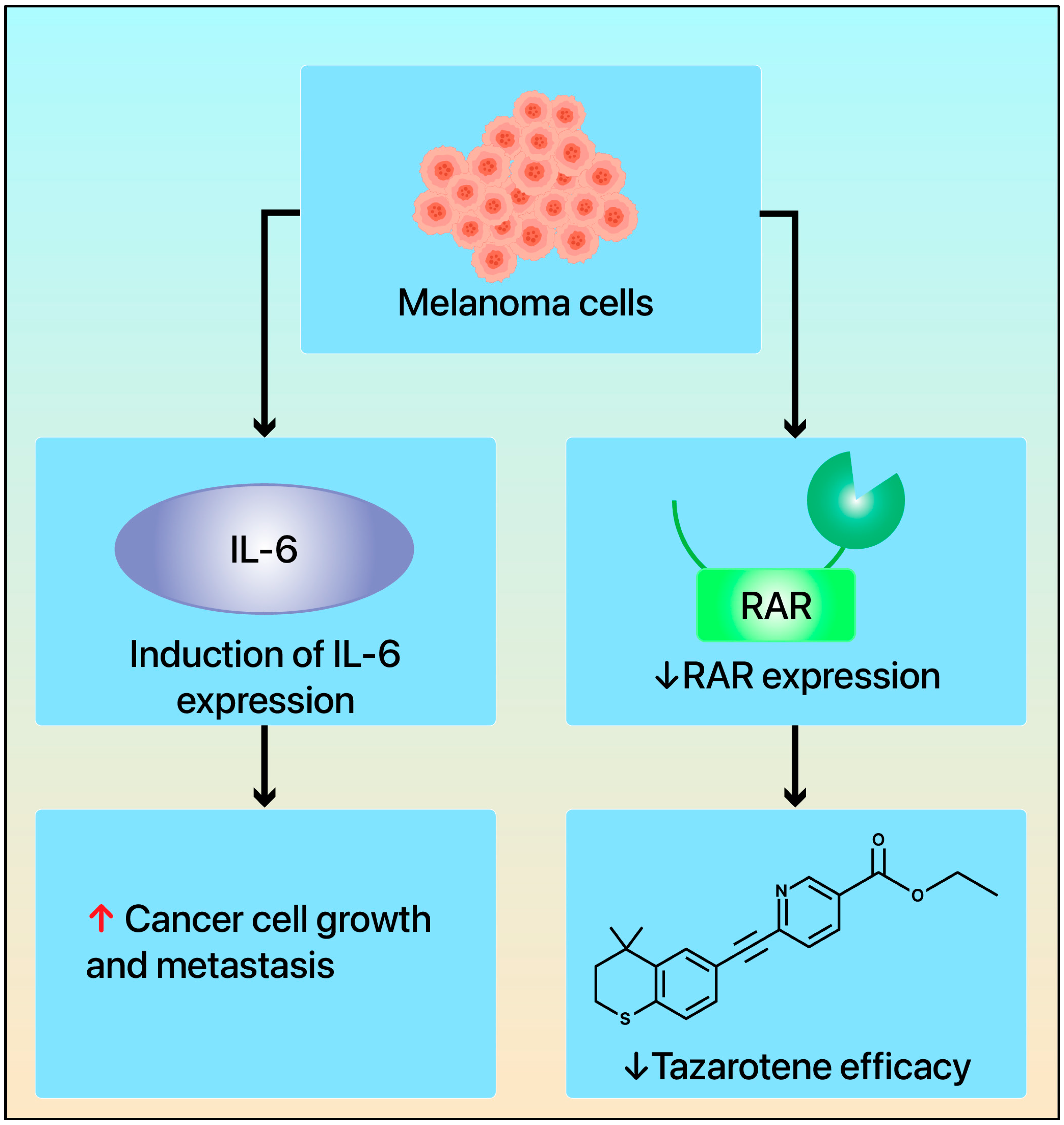
5. Mechanisms of Drug Resistance and Changes in Retinoid Receptors in Melanoma: Implications for Development and Prognosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TIG1 | tazarotene-induced gene-1 |
| RA | retinoic acid |
| ATRA | all-trans RA |
| RARs | retinoic acid receptors |
| RXRs | retinoid X receptors |
| IL-6 | interleukin-6 |
| MDSCs | myeloid-derived suppressor cells |
| LM | lentigo maligna |
| ER | endoplasmic reticulum |
| NK | natural killer |
| TAMs | tumor-associated macrophages |
| ICI | immune checkpoint inhibitor |
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [PubMed]
- Lo, S.N.; Williams, G.J.; Cust, A.E.; Ollila, D.W.; Varey, A.H.R.; Ch’ng, S.; Scolyer, R.A.; Thompson, J.F. Risk of Death Due to Melanoma and Other Causes in Patients With Thin Cutaneous Melanomas. JAMA Dermatol. 2025, 161, 167–174. [Google Scholar] [PubMed]
- Higgins, H.W., 2nd; Lee, K.C.; Galan, A.; Leffell, D.J. Melanoma in situ: Part II. Histopathology, treatment, and clinical management. J. Am. Acad. Dermatol. 2015, 73, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Karponis, D.; Stratigos, I.A.; Joshy, J.; Craig, P.J.; Mistry, K.; van Bodegraven, B.; Venables, Z.C.; Levell, N.J. Lentigo maligna: A review. Clin. Exp. Dermatol. 2024, 49, 218–225. [Google Scholar]
- Ungureanu, L.; Vasilovici, A.F.; Trufin, I.I.; Apostu, A.P.; Halmagyi, S.R. Lentigo Maligna Treatment-An Update. J. Clin. Med. 2024, 13, 2527. [Google Scholar] [CrossRef]
- Sundararajan, S.; Thida, A.M.; Yadlapati, S.; Mukkamalla, S.K.R.; Koya, S. Metastatic Melanoma; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hsieh, M.Y.; Hsu, S.K.; Liu, T.Y.; Wu, C.Y.; Chiu, C.C. Melanoma biology and treatment: A review of novel regulated cell death-based approaches. Cancer Cell Int. 2024, 24, 63. [Google Scholar]
- Friedman, E.B.; Scolyer, R.A.; Williams, G.J.; Thompson, J.F. Melanoma In Situ: A Critical Review and Re-Evaluation of Current Excision Margin Recommendations. Adv. Ther. 2021, 38, 3506–3530. [Google Scholar]
- Fan, Q.; Cohen, S.; John, B.; Riker, A.I. Melanoma in Situ Treated with Topical Imiquimod for Management of Persistently Positive Margins: A Review of Treatment Methods. Ochsner J. 2015, 15, 443–447. [Google Scholar]
- Vaienti, S.; Calzari, P.; Nazzaro, G. Topical Treatment of Melanoma In Situ, Lentigo Maligna, and Lentigo Maligna Melanoma with Imiquimod Cream: A Systematic Review of the Literature. Dermatol. Ther. 2023, 13, 2187–2215. [Google Scholar]
- Martinez-Fernandez, S.; Gonzalez-Sixto, B.; Espasandin-Arias, M.; Soto-Garcia, D.; Florez, A. Topical and Intralesional Immunotherapy for Melanoma In Situ: A Review. Cancers 2023, 15, 4468. [Google Scholar] [CrossRef]
- Alqathama, A. BRAF in malignant melanoma progression and metastasis: Potentials and challenges. Am. J. Cancer Res. 2020, 10, 1103–1114. [Google Scholar] [PubMed]
- Castellani, G.; Buccarelli, M.; Arasi, M.B.; Rossi, S.; Pisanu, M.E.; Bellenghi, M.; Lintas, C.; Tabolacci, C. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers 2023, 15, 4026. [Google Scholar] [CrossRef]
- Lee, J.; Ahmed, T.; Maurichi, A.; Di Guardo, L.; Stagno, A.M.; Warburton, L.; Taylor, A.M.; Livingstone, E.; Rehman, S.; Khattak, A.; et al. BRAF inhibitor cessation prior to disease progression in metastatic melanoma: Long-term outcomes. Eur. J. Cancer 2023, 179, 87–97. [Google Scholar] [CrossRef]
- Girod, M.; Dalle, S.; Mortier, L.; Dalac, S.; Leccia, M.T.; Dutriaux, C.; Montaudie, H.; de Quatrebarbes, J.; Lesimple, T.; Brunet-Possenti, F.; et al. Non-V600E/K BRAF Mutations in Metastatic Melanoma: Molecular Description, Frequency, and Effectiveness of Targeted Therapy in a Large National Cohort. JCO Precis. Oncol. 2022, 6, e2200075. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.M.; Brasky, T.M.; White, E. Association of vitamin A and carotenoid intake with melanoma risk in a large prospective cohort. J. Investig. Dermatol. 2012, 132, 1573–1582. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chu, R.X.; Liu, H. Vitamin A intake and risk of melanoma: A meta-analysis. PLoS ONE 2014, 9, e102527. [Google Scholar] [CrossRef] [PubMed]
- Marill, J.; Idres, N.; Capron, C.C.; Nguyen, E.; Chabot, G.G. Retinoic acid metabolism and mechanism of action: A review. Curr. Drug Metab. 2003, 4, 1–10. [Google Scholar] [CrossRef]
- Lampen, A.; Meyer, S.; Arnhold, T.; Nau, H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J. Pharmacol. Exp. Ther. 2000, 295, 979–985. [Google Scholar] [CrossRef]
- Li, Y.; Wei, C.H.; Hodges, J.K.; Green, M.H.; Ross, A.C. Priming with Retinoic Acid, an Active Metabolite of Vitamin A, Increases Vitamin A Uptake in the Small Intestine of Neonatal Rats. Nutrients 2021, 13, 4275. [Google Scholar] [CrossRef]
- Le Maire, A.; Alvarez, S.; Shankaranarayanan, P.; Lera, A.R.; Bourguet, W.; Gronemeyer, H. Retinoid receptors and therapeutic applications of RAR/RXR modulators. Curr. Top. Med. Chem. 2012, 12, 505–527. [Google Scholar] [CrossRef]
- Lavudi, K.; Nuguri, S.M.; Olverson, Z.; Dhanabalan, A.K.; Patnaik, S.; Kokkanti, R.R. Targeting the retinoic acid signaling pathway as a modern precision therapy against cancers. Front. Cell Dev. Biol. 2023, 11, 1254612. [Google Scholar]
- Allenby, G.; Bocquel, M.T.; Saunders, M.; Kazmer, S.; Speck, J.; Rosenberger, M.; Lovey, A.; Kastner, P.; Grippo, J.F.; Chambon, P.; et al. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 1993, 90, 30–34. [Google Scholar] [CrossRef]
- Szymanski, L.; Skopek, R.; Palusinska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kaminski, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
- Coates, L.C.; Helliwell, P.S. Psoriatic arthritis: State of the art review. Clin. Med. 2017, 17, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Maza, A.; Montaudie, H.; Gallini, A.; Aractingi, S.; Aubin, F.; Cribier, B.; Joly, P.; Jullien, D.; Le Maitre, M.; et al. Efficacy and safety of oral retinoids in different psoriasis subtypes: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2011, 25 (Suppl. S2), 28–33. [Google Scholar] [PubMed]
- Tang, X.H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. 2011, 6, 345–364. [Google Scholar]
- Wu, S.; Zhang, D.; Zhang, Z.P.; Soprano, D.R.; Soprano, K.J. Critical role of both retinoid nuclear receptors and retinoid-X-receptors in mediating growth inhibition of ovarian cancer cells by all-trans retinoic acid. Oncogene 1998, 17, 2839–2849. [Google Scholar]
- Abu, J.; Batuwangala, M.; Herbert, K.; Symonds, P. Retinoic acid and retinoid receptors: Potential chemopreventive and therapeutic role in cervical cancer. Lancet Oncol. 2005, 6, 712–720. [Google Scholar]
- Bobal, P.; Lastovickova, M.; Bobalova, J. The Role of ATRA, Natural Ligand of Retinoic Acid Receptors, on EMT-Related Proteins in Breast Cancer: Minireview. Int. J. Mol. Sci. 2021, 22, 13345. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Chale, R.S.; Abad, A.C.; Schally, A.V. Acute promyelocytic leukemia (APL): A review of the literature. Oncotarget 2020, 11, 992–1003. [Google Scholar]
- Ghiaur, A.; Doran, C.; Gaman, M.A.; Ionescu, B.; Tatic, A.; Cirstea, M.; Stancioaica, M.C.; Hirjan, R.; Coriu, D. Acute Promyelocytic Leukemia: Review of Complications Related to All-Trans Retinoic Acid and Arsenic Trioxide Therapy. Cancers 2024, 16, 1160. [Google Scholar] [CrossRef] [PubMed]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [PubMed]
- Olson, J.M.; Ameer, M.A.; Goyal, A. Vitamin A Toxicity; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chen, G.; Weiskirchen, S.; Weiskirchen, R. Vitamin A: Too good to be bad? Front. Pharmacol. 2023, 14, 1186336. [Google Scholar]
- Lucek, R.W.; Colburn, W.A. Clinical pharmacokinetics of the retinoids. Clin. Pharmacokinet. 1985, 10, 38–62. [Google Scholar]
- Chandraratna, R.A. Tazarotene—First of a new generation of receptor-selective retinoids. Br. J. Dermatol. 1996, 135 (Suppl. S49), 18–25. [Google Scholar]
- Krueger, G.G.; Drake, L.A.; Elias, P.M.; Lowe, N.J.; Guzzo, C.; Weinstein, G.D.; Lew-Kaya, D.A.; Lue, J.C.; Sefton, J.; Chandraratna, R.A. The safety and efficacy of tazarotene gel, a topical acetylenic retinoid, in the treatment of psoriasis. Arch. Dermatol. 1998, 134, 57–60. [Google Scholar] [CrossRef]
- Marks, R. Pharmacokinetics and safety review of tazarotene. J. Am. Acad. Dermatol. 1998, 39, S134–S138. [Google Scholar]
- Fisher, G.J.; Datta, S.C.; Voorhees, J.J. Retinoic acid receptor-gamma in human epidermis preferentially traps all-trans retinoic acid as its ligand rather than 9-cis retinoic acid. J. Investig. Dermatol. 1998, 110, 297–300. [Google Scholar]
- Oda, R.M.; Shimizu, R.; Sabatine, S.; Thacher, S.; Chandraratna, R. Effects of structural changes on retinoid cytotoxicity in the CHO clonal assay. In Vitro Toxicol. 1996, 9, 173–181. [Google Scholar]
- Sebok, B.; Bonnekoh, B.; Kerenyi, M.; Gollnick, H. Tazarotene induces epidermal cell differentiation in the mouse tail test used as an animal model for psoriasis. Skin. Pharmacol. Appl. Skin. Physiol. 2000, 13, 285–291. [Google Scholar]
- Duvic, M.; Nagpal, S.; Asano, A.T.; Chandraratna, R.A. Molecular mechanisms of tazarotene action in psoriasis. J. Am. Acad. Dermatol. 1997, 37, S18–S24. [Google Scholar] [PubMed]
- Dando, T.M.; Wellington, K. Topical tazarotene: A review of its use in the treatment of plaque psoriasis. Am. J. Clin. Dermatol. 2005, 6, 255–272. [Google Scholar]
- Yen, A.; Fenning, R.; Chandraratna, R.; Walker, P.; Varvayanis, S. A retinoic acid receptor beta/gamma-selective prodrug (tazarotene) plus a retinoid X receptor ligand induces extracellular signal-regulated kinase activation, retinoblastoma hypophosphorylation, G0 arrest, and cell differentiation. Mol. Pharmacol. 2004, 66, 1727–1737. [Google Scholar] [PubMed]
- Yamanishi, D.T.; Buckmeier, J.A.; Meyskens, F.L., Jr. Expression of c-jun, jun-B, and c-fos proto-oncogenes in human primary melanocytes and metastatic melanomas. J. Investig. Dermatol. 1991, 97, 349–353. [Google Scholar] [PubMed]
- Schummer, P.; Kuphal, S.; Vardimon, L.; Bosserhoff, A.K.; Kappelmann, M. Specific c-Jun target genes in malignant melanoma. Cancer Biol. Ther. 2016, 17, 486–497. [Google Scholar]
- Duvic, M.; Asano, A.T.; Hager, C.; Mays, S. The pathogenesis of psoriasis and the mechanism of action of tazarotene. J. Am. Acad. Dermatol. 1998, 39, S129–S133. [Google Scholar]
- Zheng, Y.; Luo, S.; Wang, G.; Peng, Z.; Zeng, W.; Tan, S.; Xi, Y.; Fan, J. Downregulation of tazarotene induced gene-2 (TIG2) in skin squamous cell carcinoma. Eur. J. Dermatol. 2008, 18, 638–641. [Google Scholar]
- Duvic, M.; Helekar, B.; Schulz, C.; Cho, M.; DiSepio, D.; Hager, C.; DiMao, D.; Hazarika, P.; Jackson, B.; Breuer-McHam, J.; et al. Expression of a retinoid-inducible tumor suppressor, Tazarotene-inducible gene-3, is decreased in psoriasis and skin cancer. Clin. Cancer Res. 2000, 6, 3249–3259. [Google Scholar]
- DiSepio, D.; Ghosn, C.; Eckert, R.L.; Deucher, A.; Robinson, N.; Duvic, M.; Chandraratna, R.A.; Nagpal, S. Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc. Natl. Acad. Sci. USA 1998, 95, 14811–14815. [Google Scholar]
- Nagpal, S.; Patel, S.; Asano, A.T.; Johnson, A.T.; Duvic, M.; Chandraratna, R.A. Tazarotene-induced gene 1 (TIG1), a novel retinoic acid receptor-responsive gene in skin. J. Investig. Dermatol. 1996, 106, 269–274. [Google Scholar]
- Chandraratna, R.A. Tazarotene: The first receptor-selective topical retinoid for the treatment of psoriasis. J. Am. Acad. Dermatol. 1997, 37, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wang, L.K.; Shyu, R.Y.; Tsai, F.M. The role of tazarotene-induced gene 1 in carcinogenesis: Is it a tumor suppressor gene or an oncogene? Biocell 2024, 48, 1285–1297. [Google Scholar]
- Zhang, J.; Liu, L.; Pfeifer, G.P. Methylation of the retinoid response gene TIG1 in prostate cancer correlates with methylation of the retinoic acid receptor beta gene. Oncogene 2004, 23, 2241–2249. [Google Scholar]
- Mizuiri, H.; Yoshida, K.; Toge, T.; Oue, N.; Aung, P.P.; Noguchi, T.; Yasui, W. DNA methylation of genes linked to retinoid signaling in squamous cell carcinoma of the esophagus: DNA methylation of CRBP1 and TIG1 is associated with tumor stage. Cancer Sci. 2005, 96, 571–577. [Google Scholar]
- Shutoh, M.; Oue, N.; Aung, P.P.; Noguchi, T.; Kuraoka, K.; Nakayama, H.; Kawahara, K.; Yasui, W. DNA methylation of genes linked with retinoid signaling in gastric carcinoma: Expression of the retinoid acid receptor beta, cellular retinol-binding protein 1, and tazarotene-induced gene 1 genes is associated with DNA methylation. Cancer 2005, 104, 1609–1619. [Google Scholar] [PubMed]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brezillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [PubMed]
- Jacenik, D.; Fichna, J. Chemerin in immune response and gastrointestinal pathophysiology. Clin. Chim. Acta 2020, 504, 146–153. [Google Scholar] [CrossRef]
- Huang, S.L.; Shyu, R.Y.; Yeh, M.Y.; Jiang, S.Y. Cloning and characterization of a novel retinoid-inducible gene 1(RIG1) deriving from human gastric cancer cells. Mol. Cell Endocrinol. 2000, 159, 15–24. [Google Scholar] [CrossRef]
- Hsu, T.H.; Chu, C.C.; Jiang, S.Y.; Hung, M.W.; Ni, W.C.; Lin, H.E.; Chang, T.C. Expression of the class II tumor suppressor gene RIG1 is directly regulated by p53 tumor suppressor in cancer cell lines. FEBS Lett. 2012, 586, 1287–1293. [Google Scholar] [CrossRef]
- Duvic, M.; Ni, X.; Talpur, R.; Herne, K.; Schulz, C.; Sui, D.; Ward, S.; Joseph, A.; Hazarika, P. Tazarotene-induced gene 3 is suppressed in basal cell carcinomas and reversed in vivo by tazarotene application. J. Investig. Dermatol. 2003, 121, 902–909. [Google Scholar]
- Helder, M.; Pandeya, N.; Seviiri, M.; Olsen, C.M.; Whiteman, D.C.; Law, M.H. No evidence that retinol is protective for skin cancer. medRxiv 2024. [Google Scholar] [CrossRef]
- Yin, W.; Song, Y.; Liu, Q.; Wu, Y.; He, R. Topical treatment of all-trans retinoic acid inhibits murine melanoma partly by promoting CD8(+) T-cell immunity. Immunology 2017, 152, 287–297. [Google Scholar]
- Ozbay Kurt, F.G.; Lasser, S.; Arkhypov, I.; Utikal, J.; Umansky, V. Enhancing immunotherapy response in melanoma: Myeloid-derived suppressor cells as a therapeutic target. J. Clin. Investig. 2023, 133, e170762. [Google Scholar]
- Petrova, V.; Groth, C.; Bitsch, R.; Arkhypov, I.; Simon, S.C.S.; Hetjens, S.; Muller, V.; Utikal, J.; Umansky, V. Immunosuppressive capacity of circulating MDSC predicts response to immune checkpoint inhibitors in melanoma patients. Front. Immunol. 2023, 14, 1065767. [Google Scholar]
- Sun, S.H.; Benner, B.; Savardekar, H.; Lapurga, G.; Good, L.; Abood, D.; Nagle, E.; Duggan, M.; Stiff, A.; DiVincenzo, M.J.; et al. Effect of Immune Checkpoint Blockade on Myeloid-Derived Suppressor Cell Populations in Patients With Melanoma. Front. Immunol. 2021, 12, 740890. [Google Scholar]
- Tobin, R.P.; Cogswell, D.T.; Cates, V.M.; Davis, D.M.; Borgers, J.S.W.; Van Gulick, R.J.; Katsnelson, E.; Couts, K.L.; Jordan, K.R.; Gao, D.; et al. Targeting MDSC Differentiation Using ATRA: A Phase I/II Clinical Trial Combining Pembrolizumab and All-Trans Retinoic Acid for Metastatic Melanoma. Clin. Cancer Res. 2023, 29, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Look, J.; Landwehr, J.; Bauer, F.; Hoffmann, A.S.; Bluethmann, H.; LeMotte, P. Marked resistance of RAR gamma-deficient mice to the toxic effects of retinoic acid. Am. J. Physiol. 1995, 269, E91–E98. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, G.; Fava, P.; Avallone, G.; Aquino, C.; Boskovic, S.; Macagno, N.; Ribero, S.; Quaglino, P. Time to next treatment and safety assessment in cutaneous-T-cell lymphomas: A retrospective analysis on patients treated with bexarotene and acitretin. Br. J. Dermatol. 2022, 187, 1019–1021. [Google Scholar] [CrossRef]
- Zhang, X.; Schlaak, M.; Fabri, M.; Mauch, C.; Kurschat, P. Successful Treatment of a Panniculitis-Like Primary Cutaneous T-Cell Lymphoma of the alpha/beta Type with Bexarotene. Case Rep. Dermatol. 2012, 4, 56–60. [Google Scholar]
- Querfeld, C.; Nagelli, L.V.; Rosen, S.T.; Kuzel, T.M.; Guitart, J. Bexarotene in the treatment of cutaneous T-cell lymphoma. Expert. Opin. Pharmacother. 2006, 7, 907–915. [Google Scholar]
- Manaka, K.; Sato, J.; Hikima, Y.; Horikoshi, H.; Taguchi, M.; Morita, A.; Suga, H.; Boki, H.; Fujimura, T.; Hirai, Y.; et al. Bexarotene-induced hypothyroidism and dyslipidemia; A nation-wide study. Endocr. J. 2024, 71, 777–787. [Google Scholar] [PubMed]
- Lalloyer, F.; Pedersen, T.A.; Gross, B.; Lestavel, S.; Yous, S.; Vallez, E.; Gustafsson, J.A.; Mandrup, S.; Fievet, C.; Staels, B.; et al. Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1488–1495. [Google Scholar] [PubMed]
- Basani, S.; Garg, A. Marked lowering of high-density lipoprotein cholesterol levels due to high dose bexarotene therapy. J. Clin. Lipidol. 2015, 9, 832–836. [Google Scholar] [PubMed]
- Shistik, G.; Prakash, A.V.; Fenske, N.A.; Glass, L.F. Treatment of locally metastatic melanoma: A novel approach. J. Drugs Dermatol. 2007, 6, 830–832. [Google Scholar]
- Connolly, K.L.; Nehal, K.S.; Busam, K.J. Lentigo maligna and lentigo maligna melanoma: Contemporary issues in diagnosis and management. Melanoma Manage. 2015, 2, 171–178. [Google Scholar]
- Naik, P.P. Diagnosis and Management of Lentigo Maligna: Clinical Presentation and Comprehensive Review. J. Skin. Cancer 2021, 2021, 7178305. [Google Scholar]
- Chimenti, S.; Carrozzo, A.M.; Citarella, L.; De Felice, C.; Peris, K. Treatment of lentigo maligna with tazarotene 0.1% gel. J. Am. Acad. Dermatol. 2004, 50, 101–103. [Google Scholar]
- Hyde, M.A.; Hadley, M.L.; Tristani-Firouzi, P.; Goldgar, D.; Bowen, G.M. A randomized trial of the off-label use of imiquimod, 5%, cream with vs without tazarotene, 0.1%, gel for the treatment of lentigo maligna, followed by conservative staged excisions. Arch. Dermatol. 2012, 148, 592–596. [Google Scholar]
- Wang, C.H.; Wang, L.K.; Wu, C.C.; Chen, M.L.; Kuo, C.Y.; Shyu, R.Y.; Tsai, F.M. TIG1 Inhibits the mTOR Signaling Pathway in Malignant Melanoma Through the VAC14 Protein. Anticancer. Res. 2023, 43, 2635–2643. [Google Scholar]
- Wang, C.H.; Tzeng, I.S.; Wang, L.K.; Wu, C.C.; Chen, M.L.; Kuo, C.Y.; Shyu, R.Y.; Tsai, F.M. Tazarotene-induced Gene 1 Induces Melanoma Cell Death by Triggering Endoplasmic Reticulum Stress Response. Front. Biosci. 2024, 29, 233. [Google Scholar]
- Liu, M.; Bai, R.; Zhang, G.; Liu, X.; Wang, Z.; He, K.; Gan, X.; Zhou, X.; Yin, P.; Zheng, Y.; et al. RARRES1 identified by comprehensive bioinformatic analysis and experimental validation as a promising biomarker in Skin Cutaneous Melanoma. Sci. Rep. 2024, 14, 14113. [Google Scholar]
- Pachynski, R.K.; Zabel, B.A.; Kohrt, H.E.; Tejeda, N.M.; Monnier, J.; Swanson, C.D.; Holzer, A.K.; Gentles, A.J.; Sperinde, G.V.; Edalati, A.; et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J. Exp. Med. 2012, 209, 1427–1435. [Google Scholar] [PubMed]
- Song, Y.; Yin, W.; Dan, Y.; Sheng, J.; Zeng, Y.; He, R. Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment. Immunology 2019, 157, 248–256. [Google Scholar]
- Wei, X.; Gu, X.; Ma, M.; Lou, C. Long noncoding RNA HCP5 suppresses skin cutaneous melanoma development by regulating RARRES3 gene expression via sponging miR-12. Onco Targets Ther. 2019, 12, 6323–6335. [Google Scholar] [PubMed]
- Wang, C.H.; Lu, T.J.; Wang, L.K.; Wu, C.C.; Chen, M.L.; Kuo, C.Y.; Shyu, R.Y.; Tsai, F.M. Tazarotene-induced gene 1 interacts with Polo-like kinase 2 and inhibits cell proliferation in HCT116 colorectal cancer cells. Cell Biol. Int. 2021, 45, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wang, L.K.; Wu, C.C.; Chen, M.L.; Kuo, C.Y.; Shyu, R.Y.; Tsai, F.M. Cathepsin V Mediates the Tazarotene-induced Gene 1-induced Reduction in Invasion in Colorectal Cancer Cells. Cell Biochem. Biophys. 2020, 78, 483–494. [Google Scholar]
- Aleksandrova, K.V.; Vorobev, M.L.; Suvorova, I.I. mTOR pathway occupies a central role in the emergence of latent cancer cells. Cell Death Dis. 2024, 15, 176. [Google Scholar]
- Son, B.; Lee, W.; Kim, H.; Shin, H.; Park, H.H. Targeted therapy of cancer stem cells: Inhibition of mTOR in pre-clinical and clinical research. Cell Death Dis. 2024, 15, 696. [Google Scholar]
- Marafie, S.K.; Al-Mulla, F.; Abubaker, J. mTOR: Its Critical Role in Metabolic Diseases, Cancer, and the Aging Process. Int. J. Mol. Sci. 2024, 25, 6141. [Google Scholar] [CrossRef]
- Wittamer, V.; Bondue, B.; Guillabert, A.; Vassart, G.; Parmentier, M.; Communi, D. Neutrophil-mediated maturation of chemerin: A link between innate and adaptive immunity. J. Immunol. 2005, 175, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Laffranchi, M.; Schioppa, T.; Sozio, F.; Pisera, A.; Tiberio, L.; Salvi, V.; Bosisio, D.; Musso, T.; Sozzani, S.; Del Prete, A. Chemerin in immunity. J. Leukoc. Biol. 2024, qiae181. [Google Scholar] [CrossRef] [PubMed]
- Ballet, R.; LaJevic, M.; Huskey-Mullin, N.; Roach, R.; Brulois, K.; Huang, Y.; Saeed, M.A.; Dang, H.X.; Pachynski, R.K.; Wilson, E.; et al. Chemerin triggers migration of a CD8 T cell subset with natural killer cell functions. Mol. Ther. 2023, 31, 2887–2900. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Z.; Li, Q.; Wang, L.; Chen, J.; Yang, X.; Yang, C.; Quan, Z. RARRES2 is involved in the “lock-and-key” interactions between osteosarcoma stem cells and tumor-associated macrophages. Sci. Rep. 2024, 14, 2267. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.M.; Shyu, R.Y.; Jiang, S.Y. RIG1 inhibits the Ras/mitogen-activated protein kinase pathway by suppressing the activation of Ras. Cell Signal 2006, 18, 349–358. [Google Scholar] [CrossRef]
- Tsai, F.M.; Shyu, R.Y.; Lin, S.C.; Wu, C.C.; Jiang, S.Y. Induction of apoptosis by the retinoid inducible growth regulator RIG1 depends on the NC motif in HtTA cervical cancer cells. BMC Cell Biol. 2009, 10, 15. [Google Scholar] [CrossRef]
- Scharadin, T.M.; Jiang, H.; Jans, R.; Rorke, E.A.; Eckert, R.L. TIG3 tumor suppressor-dependent organelle redistribution and apoptosis in skin cancer cells. PLoS ONE 2011, 6, e23230. [Google Scholar] [CrossRef]
- Lotz, K.; Kellner, T.; Heitmann, M.; Nazarenko, I.; Noske, A.; Malek, A.; Gontarewicz, A.; Schafer, R.; Sers, C. Suppression of the TIG3 tumor suppressor gene in human ovarian carcinomas is mediated via mitogen-activated kinase-dependent and -independent mechanisms. Int. J. Cancer 2005, 116, 894–902. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Samudrala Venkatesiah, S.; Rao, R.S.; Banavar, S.R.; Patil, S.; Augustine, D.; Haragannavar, V.C. Immunohistochemical expression of Tazarotene-induced Gene 3 in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2021, 50, 403–409. [Google Scholar] [CrossRef]
- Shyu, R.Y.; Jiang, S.Y.; Chou, J.M.; Shih, Y.L.; Lee, M.S.; Yu, J.C.; Chao, P.C.; Hsu, Y.J.; Jao, S.W. RARRES3 expression positively correlated to tumour differentiation in tissues of colorectal adenocarcinoma. Br. J. Cancer 2003, 89, 146–151. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, T.; Liao, D.; Wu, X.; Zhong, Y.; Liu, S.; Yang, H.; Nie, Y. The antitumor effect of TIG3 in liver cancer cells is involved in ERK1/2 inhibition. Tumour Biol. 2016, 37, 11311–11320. [Google Scholar] [PubMed]
- Raskova, M.; Lacina, L.; Kejik, Z.; Venhauerova, A.; Skalickova, M.; Kolar, M.; Jakubek, M.; Rosel, D.; Smetana, K., Jr.; Brabek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef]
- Delyon, J.; Lebbe, C. IL-6 blockade in cancer patients treated with immune checkpoint blockade: A win-win strategy. Cancer Cell 2022, 40, 450–451. [Google Scholar]
- Armstrong, C.A.; Murray, N.; Kennedy, M.; Koppula, S.V.; Tara, D.; Ansel, J.C. Melanoma-derived interleukin 6 inhibits in vivo melanoma growth. J. Investig. Dermatol. 1994, 102, 278–284. [Google Scholar]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar]
- Linnskog, R.; Jonsson, G.; Axelsson, L.; Prasad, C.P.; Andersson, T. Interleukin-6 drives melanoma cell motility through p38alpha-MAPK-dependent up-regulation of WNT5A expression. Mol. Oncol. 2014, 8, 1365–1378. [Google Scholar] [PubMed]
- Hoejberg, L.; Bastholt, L.; Johansen, J.S.; Christensen, I.J.; Gehl, J.; Schmidt, H. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res. 2012, 22, 287–293. [Google Scholar] [PubMed]
- Wang, Y.; Ramachandran, V.; Sui, D.; Xu, K.; Haydu, L.E.; Fang, S.; McQuade, J.L.; Fisher, S.B.; Lucci, A.; Keung, E.Z.; et al. Evaluation of Plasma IL-6 in Patients with Melanoma as a Prognostic and Checkpoint Immunotherapy Predictive Biomarker. J. Investig. Dermatol. 2022, 142, 2046–2049. [Google Scholar]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000842. [Google Scholar]
- Soler, M.F.; Abaurrea, A.; Azcoaga, P.; Araujo, A.M.; Caffarel, M.M. New perspectives in cancer immunotherapy: Targeting IL-6 cytokine family. J. Immunother. Cancer 2023, 11, e007530. [Google Scholar]
- Zitnik, R.J.; Kotloff, R.M.; Latifpour, J.; Zheng, T.; Whiting, N.L.; Schwalb, J.; Elias, J.A. Retinoic acid inhibition of IL-1-induced IL-6 production by human lung fibroblasts. J. Immunol. 1994, 152, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Kirchmeyer, M.; Koufany, M.; Sebillaud, S.; Netter, P.; Jouzeau, J.Y.; Bianchi, A. All-trans retinoic acid suppresses interleukin-6 expression in interleukin-1-stimulated synovial fibroblasts by inhibition of ERK1/2 pathway independently of RAR activation. Arthritis Res. Ther. 2008, 10, R141. [Google Scholar] [CrossRef]
- DiSepio, D.; Malhotra, M.; Chandraratna, R.A.; Nagpal, S. Retinoic acid receptor-nuclear factor-interleukin 6 antagonism. A novel mechanism of retinoid-dependent inhibition of a keratinocyte hyperproliferative differentiation marker. J. Biol. Chem. 1997, 272, 25555–25559. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yan, W.; Wang, C.; Liu, W.; Lin, X.; Zou, Z.; Sun, W.; Chen, Y. BRAF Inhibitor Resistance in Melanoma: Mechanisms and Alternative Therapeutic Strategies. Curr. Treat. Options Oncol. 2022, 23, 1503–1521. [Google Scholar] [PubMed]
- Sullivan, R.J.; Flaherty, K.T. Resistance to BRAF-targeted therapy in melanoma. Eur. J. Cancer 2013, 49, 1297–1304. [Google Scholar]
- Luebker, S.A.; Koepsell, S.A. Diverse Mechanisms of BRAF Inhibitor Resistance in Melanoma Identified in Clinical and Preclinical Studies. Front. Oncol. 2019, 9, 268. [Google Scholar] [CrossRef]
- Frazao, A.; Rethacker, L.; Jeudy, G.; Colombo, M.; Pasmant, E.; Avril, M.F.; Toubert, A.; Moins-Teisserenc, H.; Roelens, M.; Dalac, S.; et al. BRAF inhibitor resistance of melanoma cells triggers increased susceptibility to natural killer cell-mediated lysis. J. Immunother. Cancer 2020, 8, e000275. [Google Scholar]
- Hartman, M.L.; Sztiller-Sikorska, M.; Gajos-Michniewicz, A.; Czyz, M. Dissecting Mechanisms of Melanoma Resistance to BRAF and MEK Inhibitors Revealed Genetic and Non-Genetic Patient- and Drug-Specific Alterations and Remarkable Phenotypic Plasticity. Cells 2020, 9, 142. [Google Scholar] [CrossRef]
- Chakravarti, N.; Lotan, R.; Diwan, A.H.; Warneke, C.L.; Johnson, M.M.; Prieto, V.G. Decreased expression of retinoid receptors in melanoma: Entailment in tumorigenesis and prognosis. Clin. Cancer Res. 2007, 13, 4817–4824. [Google Scholar]
- Tanghetti, E.A.; Kircik, L.H.; Green, L.J.; Guenin, E.; Harris, S.; Martin, G.; Pillai, R. A Phase 2, Multicenter, Double-Blind, Randomized, Vehicle-Controlled Clinical Study to Compare the Safety and Efficacy of a Novel Tazarotene 0.045% Lotion and Tazarotene 0.1% Cream in the Treatment of Moderate-to-Severe Acne Vulgaris. J. Drugs Dermatol. 2019, 18, 542. [Google Scholar]
- Afra, T.P.; Razmi, T.M.; Narang, T.; Dogra, S.; Kumar, A. Topical Tazarotene Gel, 0.1%, as a Novel Treatment Approach for Atrophic Postacne Scars: A Randomized Active-Controlled Clinical Trial. JAMA Facial Plast. Surg. 2019, 21, 125–132. [Google Scholar] [PubMed]
- Han, G.; Wu, J.J.; Del Rosso, J.Q. Use of Topical Tazarotene for the Treatment of Acne Vulgaris in Pregnancy: A Literature Review. J. Clin. Aesthet. Dermatol. 2020, 13, E59–E65. [Google Scholar] [PubMed]
| Gene | Function | References |
|---|---|---|
| TIG1 | Regulation of mTOR signaling and ER stress response | [82,83,84] |
| TIG2 | Recruitment of NK and CD8+ T cells | [85,86] |
| TIG3 | Inducing of cell differentiation and death | [50,87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Wang, L.-K.; Tsai, F.-M. Exploring Potential Therapeutic Applications of Tazarotene: Gene Regulation Mechanisms and Effects on Melanoma Cell Growth. Curr. Issues Mol. Biol. 2025, 47, 237. https://doi.org/10.3390/cimb47040237
Wang C-H, Wang L-K, Tsai F-M. Exploring Potential Therapeutic Applications of Tazarotene: Gene Regulation Mechanisms and Effects on Melanoma Cell Growth. Current Issues in Molecular Biology. 2025; 47(4):237. https://doi.org/10.3390/cimb47040237
Chicago/Turabian StyleWang, Chun-Hua, Lu-Kai Wang, and Fu-Ming Tsai. 2025. "Exploring Potential Therapeutic Applications of Tazarotene: Gene Regulation Mechanisms and Effects on Melanoma Cell Growth" Current Issues in Molecular Biology 47, no. 4: 237. https://doi.org/10.3390/cimb47040237
APA StyleWang, C.-H., Wang, L.-K., & Tsai, F.-M. (2025). Exploring Potential Therapeutic Applications of Tazarotene: Gene Regulation Mechanisms and Effects on Melanoma Cell Growth. Current Issues in Molecular Biology, 47(4), 237. https://doi.org/10.3390/cimb47040237





