B Cell Dynamics and Transitional B Cells in Long COVID
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Post COVID-19 Condition (Long COVID) [Internet]. 2022. Available online: www.who.int (accessed on 27 February 2025).
- Huerne, K.; Filion, K.B.; Grad, R.; Ernst, P.; Gershon, A.S.; Eisenberg, M.J. Epidemiological and clinical perspectives of long COVID syndrome. Am. J. Med. Open 2023, 9, 100033. [Google Scholar] [CrossRef]
- Altmann, D.M.; Whettlock, E.M.; Liu, S.; Arachchillage, D.J.; Boyton, R.J. The immunology of long COVID. Nat. Rev. Immunol. 2023, 23, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.B.; Singh, V.P.P.; Chandran, R.; Venugopal, K.; Haridas, K.; Kavitha, R. COVID-19 Antibody Tests: An Overview. J. Pharm. Bioallied Sci. 2021, 13 Suppl. S1, S48–S51. [Google Scholar] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Hanley, P.; Sutter, J.A.; Goodman, N.G.; Du, Y.; Sekiguchi, D.R.; Meng, W.; Rickels, M.R.; Naji, A.; Prak, E.T. Circulating B cells in type 1 diabetics exhibit fewer maturation-associated phenotypes. Clin. Immunol. 2017, 183, 336–343. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Han, J.; Yang, M.; Zhu, J.; Jin, T. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J. Transl. Med. 2020, 18, 131. [Google Scholar] [CrossRef]
- Ueda, Y.; Liao, D.; Yang, K.; Patel, A.; Kelsoe, G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J. Immunol. 2007, 178, 3593–3601. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef]
- Sánchez-Vargas, L.A.; Kounlavouth, S.; Smith, M.L.; Anderson, K.B.; Srikiatkhachorn, A.; Ellison, D.W.; Currier, J.R.; Endy, T.P.; Mathew, A.; Rothman, A.L. Longitudinal Analysis of Memory B and T Cell Responses to Dengue Virus in a 5-Year Prospective Cohort Study in Thailand. Front. Immunol. 2019, 10, 1359. [Google Scholar] [CrossRef]
- Fraussen, J.; Marquez, S.; Takata, K.; Beckers, L.; Montes Diaz, G.; Zografou, C.; Van Wijmeersch, B.; Villar, L.M.; O’Connor, K.C.; Kleinstein, S.H.; et al. Phenotypic and Ig Repertoire Analyses Indicate a Common Origin of IgD-CD27- Double Negative B Cells in Healthy Individuals and Multiple Sclerosis Patients. J. Immunol. 2019, 203, 1650–1664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korobova, Z.R.; Zueva, E.V.; Arsentieva, N.A.; Batsunov, O.K.; Liubimova, N.E.; Khamitova, I.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Savin, T.V.; Stanevich, O.V.; et al. Changes in Anti-SARS-CoV-2 IgG Subclasses over Time and in Association with Disease Severity. Viruses 2022, 14, 941. [Google Scholar] [CrossRef] [PubMed]
- Radion, E.I.; Mukhin, V.E.; Kholodova, A.V.; Vladimirov, I.S.; Alsaeva, D.Y.; Zhdanova, A.S.; Ulasova, N.Y.; Bulanova, N.V.; Makarov, V.V.; Keskinov, A.A.; et al. Functional Characteristics of Serum Anti-SARS-CoV-2 Antibodies against Delta and Omicron Variants after Vaccination with Sputnik V. Viruses 2023, 15, 1349. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Rafiullah, M.; Alkhowaiter, M.; Alotaibi, N.; Alzahrani, M.; Binkhamis, K.; Siddiqui, K.; Youssef, A.; Altalhi, H.; Almaghlouth, I.; et al. Clinical and biochemical characteristics of people experiencing post-coronavirus disease 2019-related symptoms: A prospective follow-up investigation. Front. Med. 2022, 9, 1067082. [Google Scholar] [CrossRef]
- Berger, L.; Wolf, J.; Kalbitz, S.; Kellner, N.; Lübbert, C.; Borte, S. Comparative Analysis of Lymphocyte Populations in Post-COVID-19 Condition and COVID-19 Convalescent Individuals. Diagnostics 2024, 14, 1286. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Jacka, B.; Ballouz, S.; Jackson, K.J.; Wilson, D.B.; Manandhar, B.; Klemm, V.; Tan, H.X.; Wheatley, A.; Aggarwal, A.; et al. Improvement of immune dysregulation in individuals with long COVID at 24-months following SARS-CoV-2 infection. Nat. Commun. 2024, 15, 3315. [Google Scholar] [CrossRef]
- Golovkin, A.; Kalinina, O.; Bezrukikh, V.; Aquino, A.; Zaikova, E.; Karonova, T.; Melnik, O.; Vasilieva, E.; Kudryavtsev, I. Imbalanced Immune Response of T-Cell and B-Cell Subsets in Patients with Moderate and Severe COVID-19. Viruses 2021, 13, 1966. [Google Scholar] [CrossRef]
- Mansourabadi, A.H.; Aghamajidi, A.; Dorfaki, M.; Keshavarz, F.; Shafeghat, Z.; Moazzeni, A.; Arab, F.L.; Rajabian, A.; Roozbehani, M.; Falak, R.; et al. B lymphocytes in COVID-19: A tale of harmony and discordance. Arch. Virol. 2023, 168, 148. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Batsunov, O.K.; Korobova, Z.R.; Khamitova, I.V.; Isakov, D.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; et al. Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents. Curr. Issues Mol. Biol. 2021, 44, 194–205. [Google Scholar] [CrossRef]
- Saitgalina, M.A.; Ostankova, Y.V.; Arsentieva, N.A.; Korobova, Z.R.; Liubimova, N.E.; Kashchenko, V.A.; Kulikov, A.N.; Pevtsov, D.E.; Stanevich, O.V.; Chernykh, E.I.; et al. Levels of TREC and KREC molecules significance determining in peripheral blood for predicting the outcome of COVID-19 disease in the acute period. Russ. J. Immunol. 2023, 26, 611–618. (In Russian) [Google Scholar]
- Saitgalina, M.A.; Ostankova, Y.V.; Arsentieva, N.A.; Korobova, Z.R.; Liubimova, N.E.; Kashchenko, V.A.; Kulikov, A.N.; Pevtsov, D.E.; Stanevich, O.V.; Chernykh, E.I.; et al. Assessment of trec and krec levels in COVID-19 patients with varying disease severity. Russ. J. Infect. Immun. 2023, 13, 873–884. (In Russian) [Google Scholar] [CrossRef]
- Taher, T.E.; Bystrom, J.; Ong, V.H.; Isenberg, D.A.; Renaudineau, Y.; Abraham, D.J.; Mageed, R.A. Intracellular B Lymphocyte Signalling and the Regulation of Humoral Immunity and Autoimmunity. Clin. Rev. Allergy Immunol. 2017, 53, 237–264. [Google Scholar] [CrossRef]
- Simon, Q.; Pers, J.O.; Cornec, D.; Le Pottier, L.; Mageed, R.A.; Hillion, S. In-depth characterization of CD24(high)CD38(high) transitional human B cells reveals different regulatory profiles. J. Allergy Clin. Immunol. 2016, 137, 1577–1584.e10. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Madhvi, M. The expanding family of regulatory B cells. Int. Immunol. 2015, 27, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Mimpen, M.; Damoiseaux, J.; van Doorn, W.; Rolf, L.; Muris, A.H.; Hupperts, R.; van Luijn, M.M.; Gerlach, O.; Smolders, J. Proportions of circulating transitional B cells associate with MRI activity in interferon beta-treated multiple sclerosis patients. J. Neuroimmunol. 2021, 358, 577664. [Google Scholar] [CrossRef]
- Sin, D.D. Is long COVID an autoimmune disease? Eur. Respir. J. 2023, 61, 2202272. [Google Scholar] [CrossRef]
- Anaya, J.M.; Herrán, M.; Beltrán, S.; Rojas, M. Is post-COVID syndrome an autoimmune disease? Expert Rev. Clin. Immunol. 2022, 18, 653–666. [Google Scholar] [CrossRef]
- Goel, N.; Goyal, N.; Spalgais, S.; Mrigpuri, P.; Varma-Basil, M.; Khanna, M.; Nagaraja, R.; Menon, B.; Kumar, R. Initial COVID-19 Severity and Long-COVID Manifestations: An Observational Analysis. Thorac. Res. Pract. 2023, 24, 22–28. [Google Scholar] [CrossRef]
- Gottlieb, M.; Yu, H.; Chen, J.; Spatz, E.S.; Gentile, N.L.; Geyer, R.E.; Santangelo, M.; Malicki, C.; Gatling, K.; Saydah, S.; et al. Differences in Long COVID severity by duration of illness, symptom evolution, and vaccination: A longitudinal cohort study from the INSPIRE group. Lancet Reg. Health Am. 2025, 44, 101026. [Google Scholar] [CrossRef]
- Kananian, S.; Nemani, A.; Stangier, U. Risk and protective factors for the severity of long COVID—A network analytic perspective. J. Psychiatr. Res. 2024, 178, 291–297. [Google Scholar] [CrossRef]
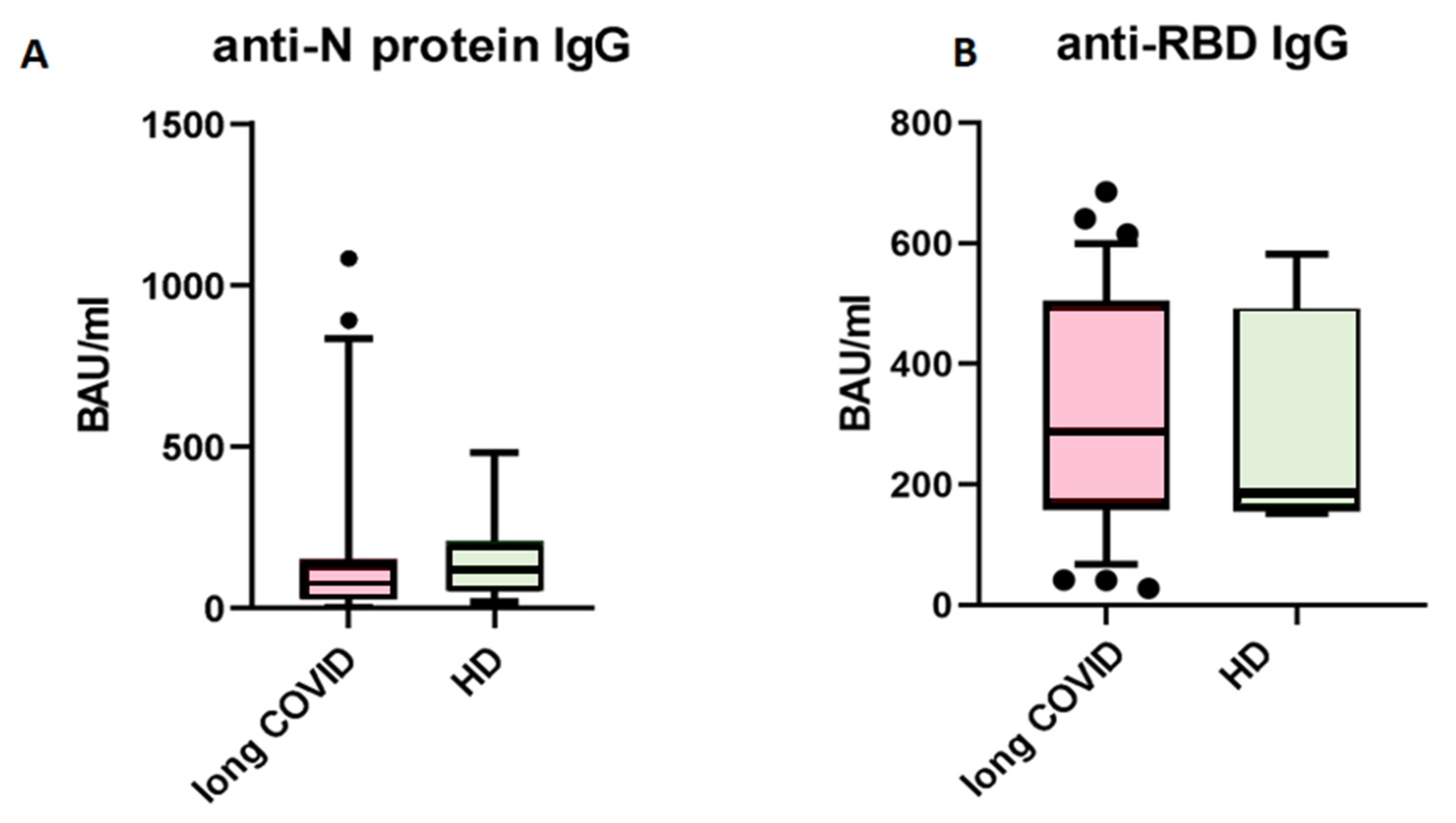
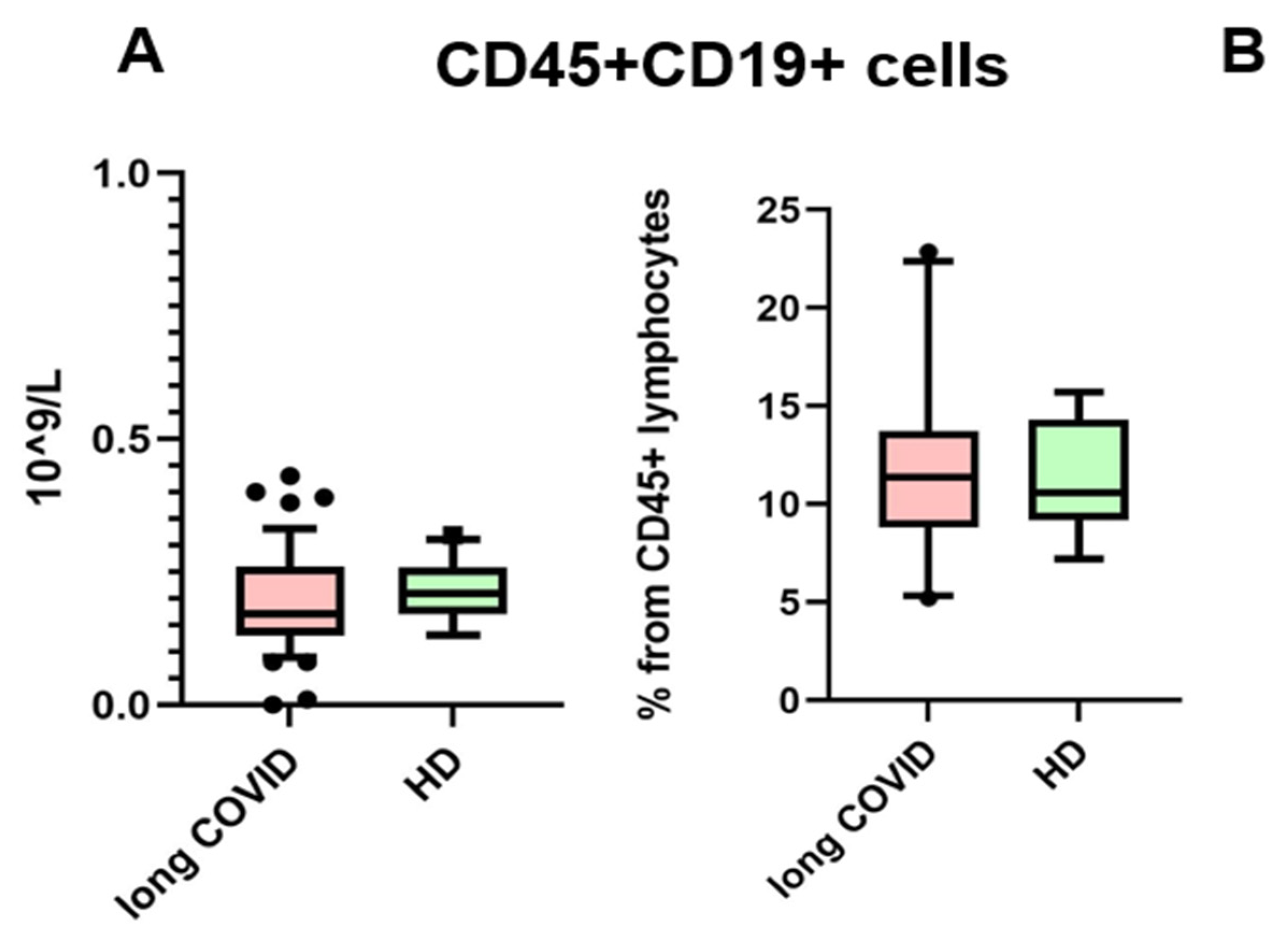
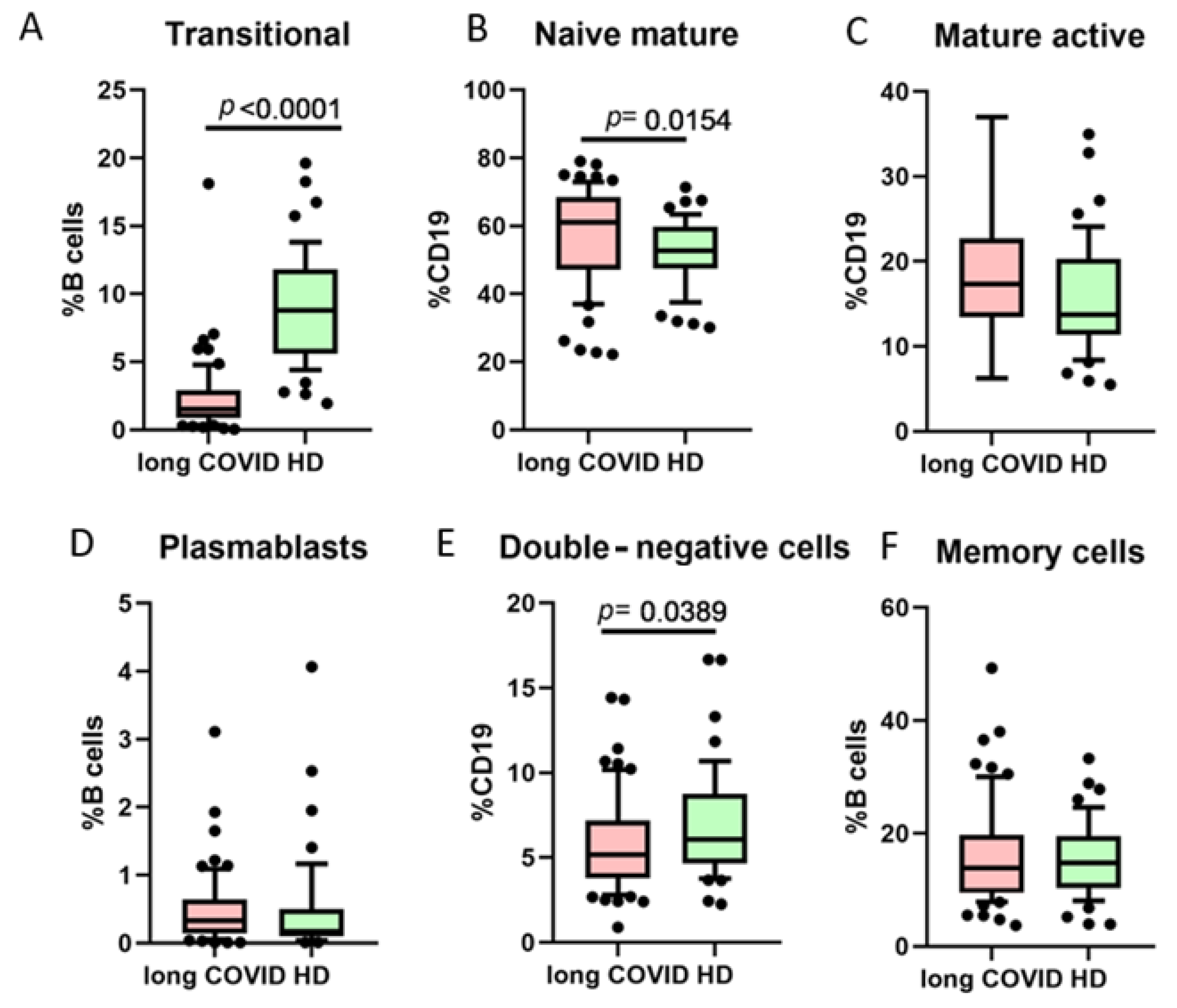


| Symptom | Number of Participants Presenting the Symptom | % of the Studied Cohort |
|---|---|---|
| Fever (>38 °C) | 53 | 84.1 |
| Shortness of breath | 25 | 39.6 |
| Lowered blood oxygen saturation (95% and less) | 4 | 6.3 |
| Lowered blood pressure below 90/60 mm Hg | 6 | 9.5% |
| Ventilatory support in the intensive care unit | 3 | 4.7 |
| CD27 vs. CD38 Phenotype | Function |
|---|---|
| CD27−CD38++ | Transitional B cells, immature bone marrow-derived regulatory subpopulation [7] |
| CD27−CD38+ | Mature naive cells in secondary lymphoid organs, initiate rapid antigen-associated B cell responses and B cell receptor diversification [8]. |
| CD27+CD38+ | Mature activated cells, early IgM memory [9]. |
| CD27+++CD38++ | Plasmablasts, responsible for long-term B-cellular immunity [10]. |
| CD27+CD38− | Resting memory B cells, providing memory reservoir [9]. |
| CD27−CD38− | Mature antigen-experienced B cells with an expression profile of developmental markers [11]. |
| Value | Long COVID (Median, Q25–Q75) | Recovery (Median, Q25–Q75) |
|---|---|---|
| B lymphocytes (% from CD45+ cells) | 13.91 (9.72–14.43) | 11.21 (8.37–14.91) |
| Transitional cells (% from B lymphocytes) | 2 (0.19–3.4) | 9.52 (6.11–12.34) |
| Naive mature cells (% from B lymphocytes) | 61.16 (47.13–68.47) | 56.32 (49.12–59.78) |
| Mature active cells (% from B lymphocytes) | 17.31 (13.42–22.73) | 12.32 (11.41–24.3) |
| Plasmablast cells (% from B lymphocytes) | 0.33 (0.14–1.04) | 0.21 (0–1.3) |
| Memory B cells (% from B lymphocytes) | 13.88 (9.49–30.77) | 14.02 (10.12–25.81) |
| DN cells (% from B lymphocytes) | 5.15 (3.79–7.18) | 5.5 (5.15–8.44) |
| IL-4 (pg/mL) | 0.29 (0.1–1.47) | 0.29 (0.1–0.58) |
| IL-5 (pg/mL) | 46.88 (1.12–46.88) | 1.12 (1.12–29.57) |
| IL-13 (pg/mL) | 2.62 (2.1–4.01) | 0.78 (0–2.61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Butenko, A.A.; Kokoeva, A.E.; Kucherenko, N.G.; Kashchenko, V.A.; Boeva, E.V.; Norka, A.O.; et al. B Cell Dynamics and Transitional B Cells in Long COVID. Curr. Issues Mol. Biol. 2025, 47, 245. https://doi.org/10.3390/cimb47040245
Korobova ZR, Arsentieva NA, Liubimova NE, Batsunov OK, Butenko AA, Kokoeva AE, Kucherenko NG, Kashchenko VA, Boeva EV, Norka AO, et al. B Cell Dynamics and Transitional B Cells in Long COVID. Current Issues in Molecular Biology. 2025; 47(4):245. https://doi.org/10.3390/cimb47040245
Chicago/Turabian StyleKorobova, Zoia R., Natalia A. Arsentieva, Natalia E. Liubimova, Oleg K. Batsunov, Anastasia A. Butenko, Albina E. Kokoeva, Natalia G. Kucherenko, Victor A. Kashchenko, Ekaterina V. Boeva, Anna O. Norka, and et al. 2025. "B Cell Dynamics and Transitional B Cells in Long COVID" Current Issues in Molecular Biology 47, no. 4: 245. https://doi.org/10.3390/cimb47040245
APA StyleKorobova, Z. R., Arsentieva, N. A., Liubimova, N. E., Batsunov, O. K., Butenko, A. A., Kokoeva, A. E., Kucherenko, N. G., Kashchenko, V. A., Boeva, E. V., Norka, A. O., Knizhnikova, A. A., Rassokhin, V. V., Belyakov, N. A., & Totolian, A. A. (2025). B Cell Dynamics and Transitional B Cells in Long COVID. Current Issues in Molecular Biology, 47(4), 245. https://doi.org/10.3390/cimb47040245







