Liposomes as Imaging Agents of Inflammation and Oxidative Stress in Bone Implants
Abstract
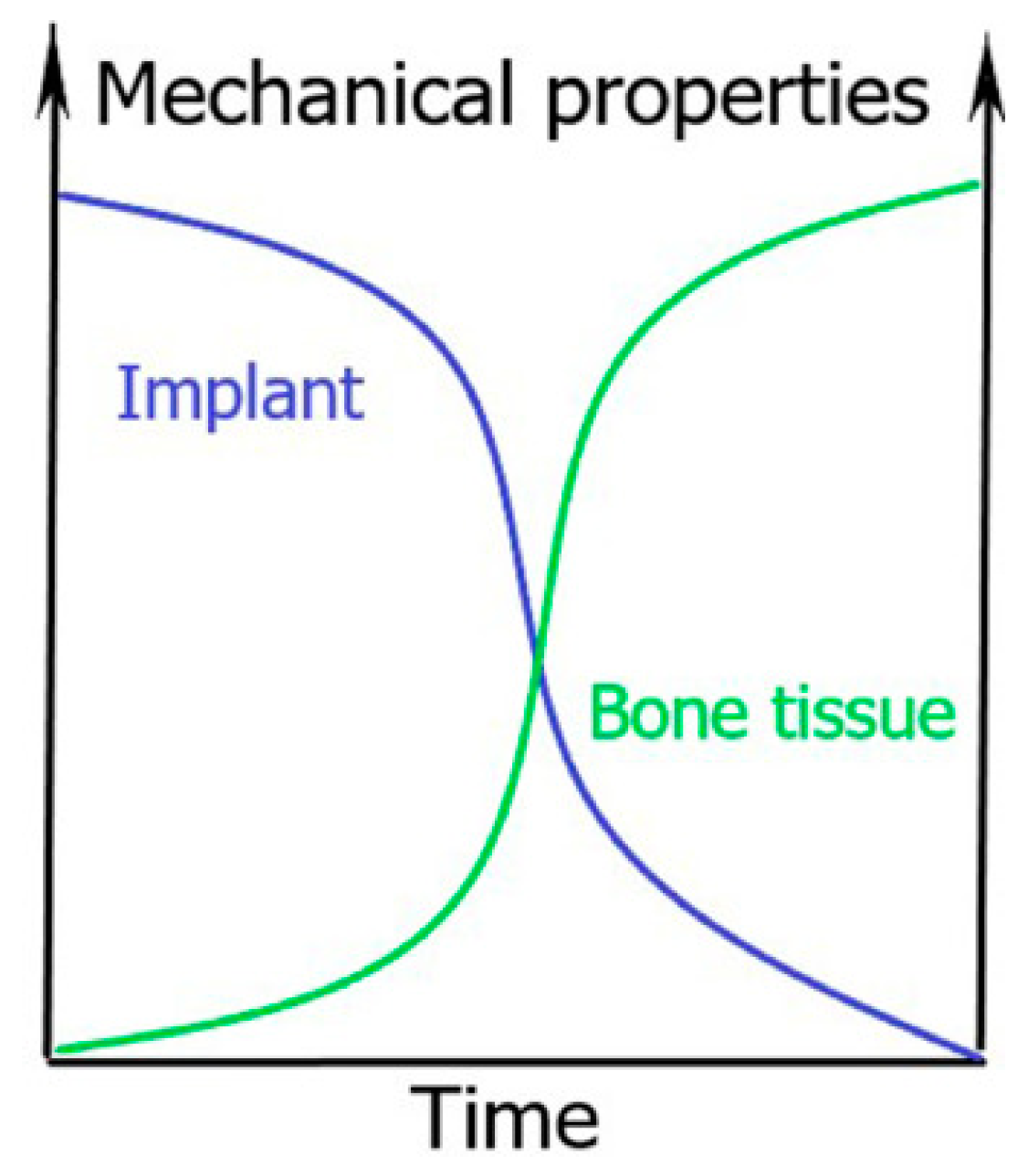
:1. General Introduction to Biodegradable Bone Implants
2. Imaging Techniques That Look at Bone Healing After Implantation
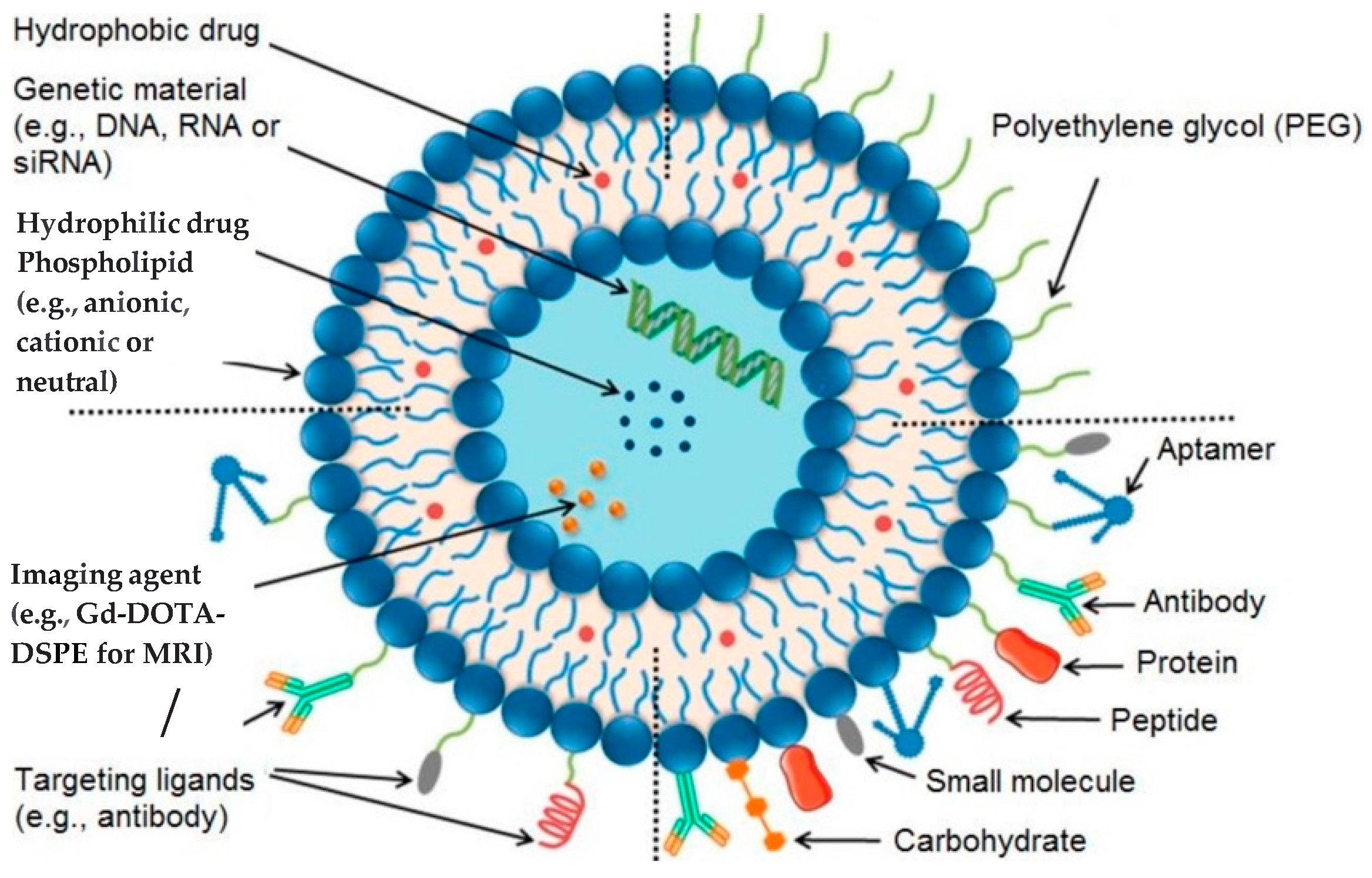
3. Nanomaterials as Imaging Agents
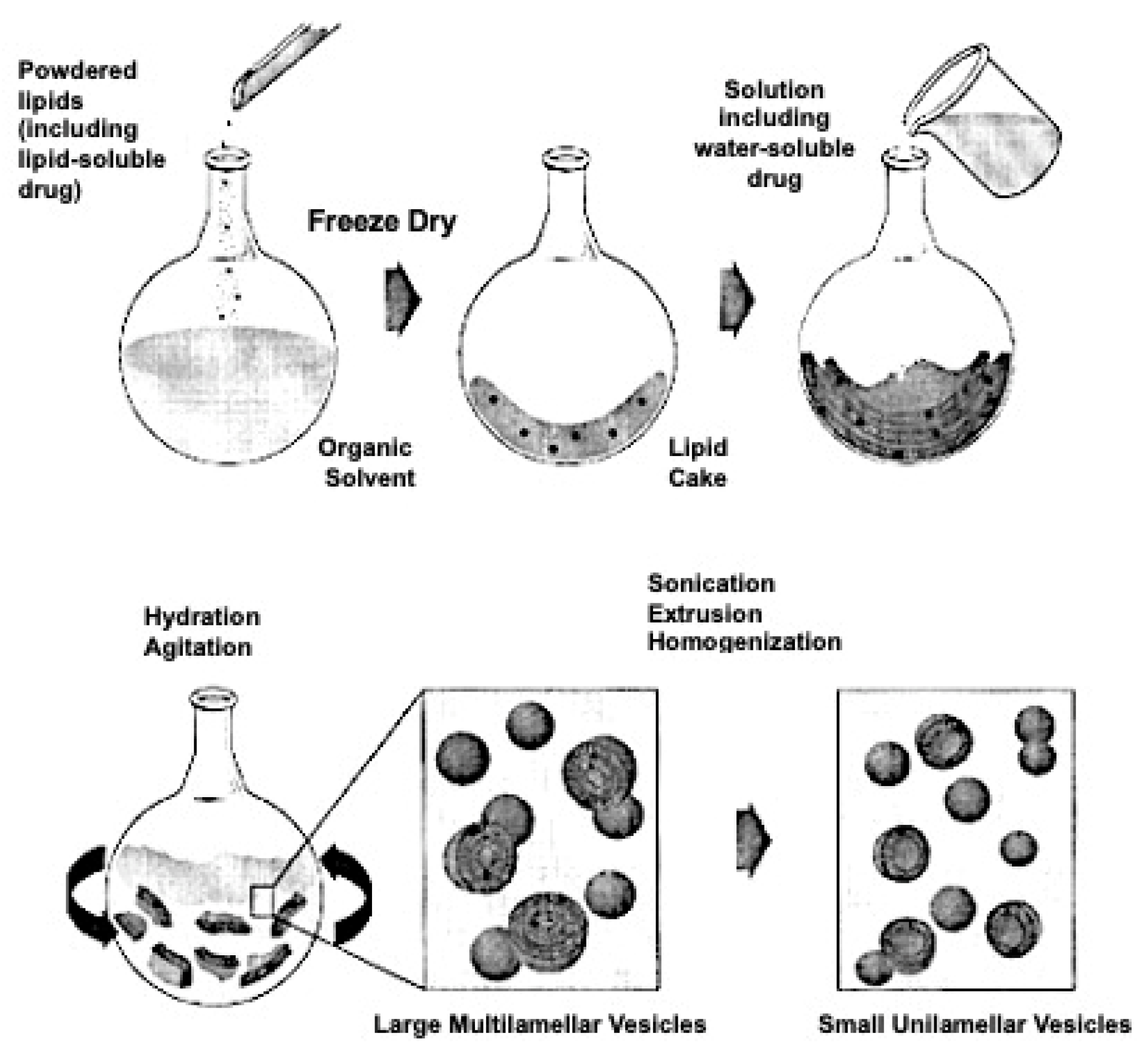
4. Liposomes and Therapeutic Strategies
| SN | Clinical Products (Approval Year) | Administration | Active Agent | Indication | Company |
|---|---|---|---|---|---|
| 1. | Doxil®(1995) | i.v. | Doxorubicin | Ovarian cancer, breast cancer, Kaposi’s sarcoma | Sequus Pharmaceuticals |
| 2. | DaunoXome®(1996) | i.v. | Daunorubicin | AIDS-related Kaposi’s sarcoma | NeXstar Pharmaceuticals |
| 3. | Depocyt®(1999) | Spinal | Cytarabine/Ara-C | Neoplastic meningitis | SkyPharma Inc. |
| 4. | Myocet®(2000) | i.v. | Doxorubicin | Combination therapy with cyclophosphamide in metastatic breast cancer | Elan Pharmaceuticals |
| 5. | Mepact®(2004) | i.v. | Mifamurtide | High-grade, resectable, non-metastatic osteosarcoma | Takeda Pharmaceutical Limited |
| 6. | Marqibo®(2012) | i.v. | Vincristine | Acute lymphoblastic leukaemia | Talon Therapeutics, Inc. |
| 7. | Onivyde™ (2015) | i.v. | Irinotecan | Combination therapy with fluorouracil and leucovorin in metastatic adenocarcinoma of the pancreas | Merrimack Pharmaceuticals Inc. |
| 8. | Abelcet®(1995) | i.v. | Amphotericin B | Invasive severe fungal infections | Sigma-Tau Pharmaceuticals |
| 9. | Ambisome® (1997) | i.v. | Amphotericin B | Presumed fungal infections | Astellas Pharma |
| 10. | Amphotec® (1996) | i.v. | Amphotericin B | Severe fungal infections | Ben Venue Laboratories Inc. |
| 11. | Visudyne® (2000) | i.v. | Verteporphin | Choroidal neovascularisation | Novartis |
| 12. | DepoDur™ (2004) | Epidural | Morphine sulfate | Pain management | SkyPharma Inc. |
| 13. | Exparel®(2011) | i.v. | Bupivacaine | Pain management | Pacira Pharmaceuticals, Inc. |
| 14. | Epaxal®(1993) | i.m. | Inactivated hepatitis A virus (strain RGSB) | Hepatitis A | Crucell, Berna Biotech |
| 15. | Inflexal® V (1997) | i.m. | Inactivated hemaglutinine of Influenza virus strains A and B | Influenza | Crucell, Berna Biotech |
5. Liposomes as an Imaging Agent for Bone Implants
6. Liposomes Detect Oxidative Stress and Enhance the Bone-Healing Process
7. Liposomes for Treatment of Fracture Healing
8. Liposomes to Observe the Degradability of Implants
9. Discussion—Bioimaging
10. Conclusions
11. Search Strategy and Inclusion Criteria
- Search Terms: bone implants, biodegradable implants, orthopedic implants, magnesium-based materials, bone remodeling, bone regeneration, bone-targeting, liposomes, functionalized-liposomes, bone-targeted drug delivery, bone-targeted imaging, inflammation in bone, macrophages and implants, macrophages and bone, reactive oxygen species in bone remodeling, liposomes and bone disease, non-invasive detection, inflammatory response, tissue response, imaging agents, liposomes and macrophages, liposomes and reactive oxygen species.
- Databases: PubMed, Google Scholar.
- Inclusion Criteria: Original and review papers were selected that provided information on the current issues with bone implants and possible interventions using nanotechnology, particularly liposomes. Keywords and search terms were used in searches. “And” and “or” were used for keyword searching. Full articles were saved and exported to the Mendeley Reference Manager Library. Articles from 2008 and until 2023 were included in this review.
Funding
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| PEG | Polyethylene Glycol |
| ROS | Reactive Oxygen Species |
| SM | Sphingomyelin |
| ICG | Indocyanine green |
| BMP-2 | Bone morphogenic protein 2 |
References
- Riyaz, S.; Helmholz, H.; Medina, T.P.; Medina, O.P.; Will, O.; Sun, Y.; Wiese, B.; Glüer, C.-C.; Willumeit-Römer, R. Exploring the Usability of α-MSH-SM-Liposome as an Imaging Agent to Study Biodegradable Bone Implants In Vivo. Int. J. Mol. Sci. 2023, 24, 1103. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Zeller-Plumhoff, B.; Malich, C.; Krüger, D.; Campbell, G.; Wiese, B.; Galli, S.; Wennerberg, A.; Willumeit-Römer, R.; Wieland, D.F. Analysis of the bone ultrastructure around biodegradable Mg–xGd implants using small angle X-ray scattering and X-ray diffraction. Acta Biomater. 2020, 101, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.D.; Schuster, A.; Helmholz, H.; Meyer-Rachner, A.; Willumeit-Römer, R.; Luthringer-Feyerabend, B. Inflammatory response to magnesium-based biodegradable implant materials. Acta Biomater. 2020, 101, 598–608. [Google Scholar] [CrossRef]
- Bian, D.; Deng, J.; Li, N.; Chu, X.; Liu, Y.; Li, W.; Cai, H.; Xiu, P.; Zhang, Y.; Guan, Z.; et al. In Vitro and in Vivo Studies on Biomedical Magnesium Low-Alloying with Elements Gadolinium and Zinc for Orthopedic Implant Applications. ACS Appl. Mater. Interfaces 2018, 10, 4394–4408. [Google Scholar] [CrossRef]
- Jing, X.; Ding, Q.; Wu, Q.; Su, W.; Yu, K.; Su, Y.; Ye, B.; Gao, Q.; Sun, T.; Guo, X. Magnesium-based materials in orthopaedics: Material properties and animal models. Biomater. Transl. 2021, 2, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H.; Will, O.; Penate-Medina, T.; Humbert, J.; Damm, T.; Luthringer-Feyerabend, B.; Willumeit-Römer, R.; Glüer, C.; Penate-Medina, O. Tissue responses after implantation of biodegradable Mg alloys evaluated by multimodality 3D micro-bioimaging in vivo. J. Biomed. Mater. Res. Part A 2021, 109, 1521–1529. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Kzhyshkowska, J.; Gudima, A.; Riabov, V.; Dollinger, C.; Lavalle, P.; Vrana, N.E. Macrophage responses to implants: Prospects for personalized medicine. J. Leukoc. Biol. 2015, 98, 953–962. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Kee, P.; Bagalkot, V.; Johnson, E.; Danila, D. Noninvasive Detection of Macrophages in Atheroma Using a Radiocontrast-Loaded Phosphatidylserine-Containing Liposomal Contrast Agent for Computed Tomography. Mol. Imaging Biol. 2014, 17, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Danila, D. Aptamer functionalized liposomes for cancer treatment. Adv. Res. 2022, 23, 37–45. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Althubiti, M.A. Cancer Nanomedicine: A New Era of Successful Targeted Therapy. J. Nanomater. 2019, 2019, 4927312. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Sinder, B.P.; Pettit, A.R.; McCauley, L.K. Macrophages: Their Emerging Roles in Bone. J. Bone Miner. Res. 2015, 30, 2140–2149. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’souza, W.D.; Ii, C.B.S.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
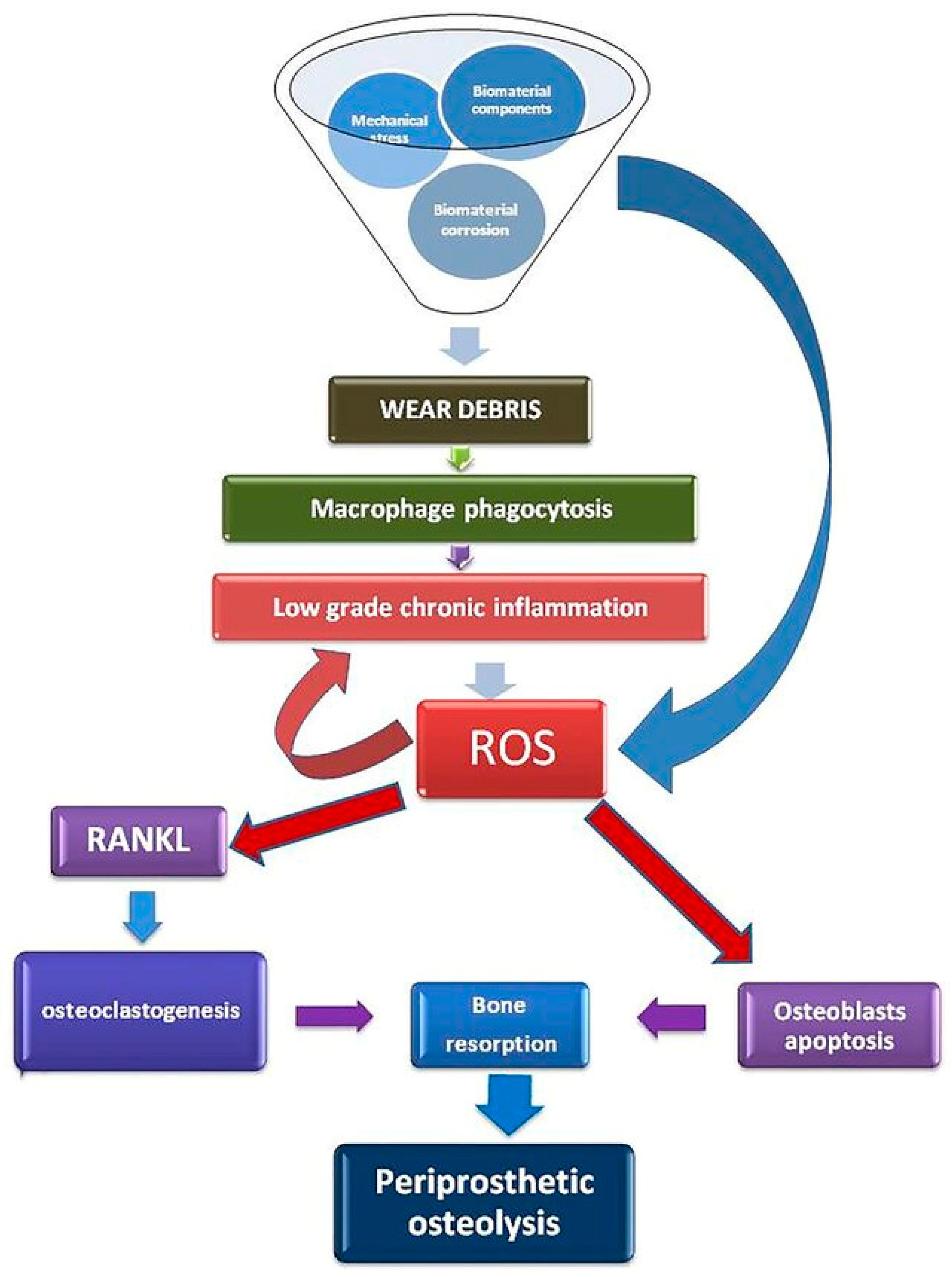
- Galliera, E.; Massaccesi, L.; Banfi, G.; De Vecchi, E.; Ragone, V.; Romanelli, M.M.C. Effect of Oxidative Stress on Bone Remodeling in Periprosthetic Osteolysis. Clin. Rev. Bone Miner. Metab. 2021, 19, 14–23. [Google Scholar] [CrossRef]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages—Key cells in the response to wear debris from joint replacements. J. Biomed. Mater. Res. Part A 2013, 101, 3033–3045. [Google Scholar] [CrossRef]
- Rao, A.J.; Gibon, E.; Ma, T.; Yao, Z.; Smith, R.L.; Goodman, S.B. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012, 8, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The Pathology of Orthopedic Implant Failure Is Mediated by Innate Immune System Cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef]
- Jahanmard, F.; Khodaei, A.; Flapper, J.; Dogan, O.; Roohi, K.; Taheri, P.; Weinans, H.; Storm, G.; Croes, M.; Mastrobattista, E.; et al. Osteoimmunomodulatory GelMA/liposome coatings to promote bone regeneration of orthopedic implants. J. Control. Release 2023, 358, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Athanasou, N.A. The pathobiology and pathology of aseptic implant failure. Bone Jt. Res. 2016, 5, 162–168. [Google Scholar] [CrossRef]
- Powers, D.; Nosoudi, N. Liposomes; from synthesis to targeting macrophages. Biomed. Res. 2019, 30. [Google Scholar] [CrossRef]
- Michalski, M.; Zweifler, L.; Sinder, B.; Koh, A.; Yamashita, J.; Roca, H.; McCauley, L. Clodronate-Loaded Liposome Treatment Has Site-Specific Skeletal Effects. J. Dent. Res. 2019, 98, 459–467. [Google Scholar] [CrossRef]
- Dirzu, N.; Lucaciu, O.; Dirzu, D.S.; Soritau, O.; Cenariu, D.; Crisan, B.; Tefas, L.; Campian, R.S. BMP-2 Delivery through Liposomes in Bone Regeneration. Appl. Sci. 2022, 12, 1373. [Google Scholar] [CrossRef]
- Matsuo, T.; Sugita, T.; Kubo, T.; Yasunaga, Y.; Ochi, M.; Murakami, T. Injectable magnetic liposomes as a novel carrier of recombinant human BMP-2 for bone formation in a rat bone-defect model. J. Biomed. Mater. Res. Part A 2003, 66A, 747–754. [Google Scholar] [CrossRef]
- Sheppard, A.J.; Barfield, A.M.; Barton, S.; Dong, Y. Understanding Reactive Oxygen Species in Bone Regeneration: A Glance at Potential Therapeutics and Bioengineering Applications. Front. Bioeng. Biotechnol. 2022, 10, 836764. [Google Scholar] [CrossRef]
- Reis, J.; Ramos, A. In Sickness and in Health: The Oxygen Reactive Species and the Bone. Front. Bioeng. Biotechnol. 2021, 9, 745911. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, H.; Wu, X.; Weng, W.; Wang, X.; Su, J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front. Bioeng. Biotechnol. 2022, 9, 820468. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.S.; Vaezi, Z.; Shojaedin-Givi, B.; Naderi-Manesh, H. Chemiluminescent liposomes as a theranostic carrier for detection of tumor cells under oxidative stress. Anal. Chim. Acta 2019, 1059, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Manea, S.-A.; Vlad, M.-L.; Rebleanu, D.; Lazar, A.-G.; Fenyo, I.M.; Calin, M.; Simionescu, M.; Manea, A. Detection of Vascular Reactive Oxygen Species in Experimental Atherosclerosis by High-Resolution Near-Infrared Fluorescence Imaging Using VCAM-1-Targeted Liposomes Entrapping a Fluorogenic Redox-Sensitive Probe. Oxidative Med. Cell. Longev. 2021, 2021, 6685612. [Google Scholar] [CrossRef]
- Sadzuka, Y.; Nakagawa, K.; Yoshioka, H.; Sonobe, T. A liposomal formulation study of 2,7-dichlorodihydrofluorescein for detection of reactive oxygen species. Int. J. Pharm. 2008, 356, 300–305. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, H.; Gao, X.; Zheng, X.; Chen, H.; Li, H.; Peng, J.; Liang, W.; Wang, W.; Qiu, Z.; et al. Bone-Targeting Liposome-Encapsulated Salvianic Acid A Improves Nonunion Healing Through the Regulation of HDAC3-Mediated Endochondral Ossification. Drug Des. Dev. Ther. 2020, 14, 3519–3533. [Google Scholar] [CrossRef]
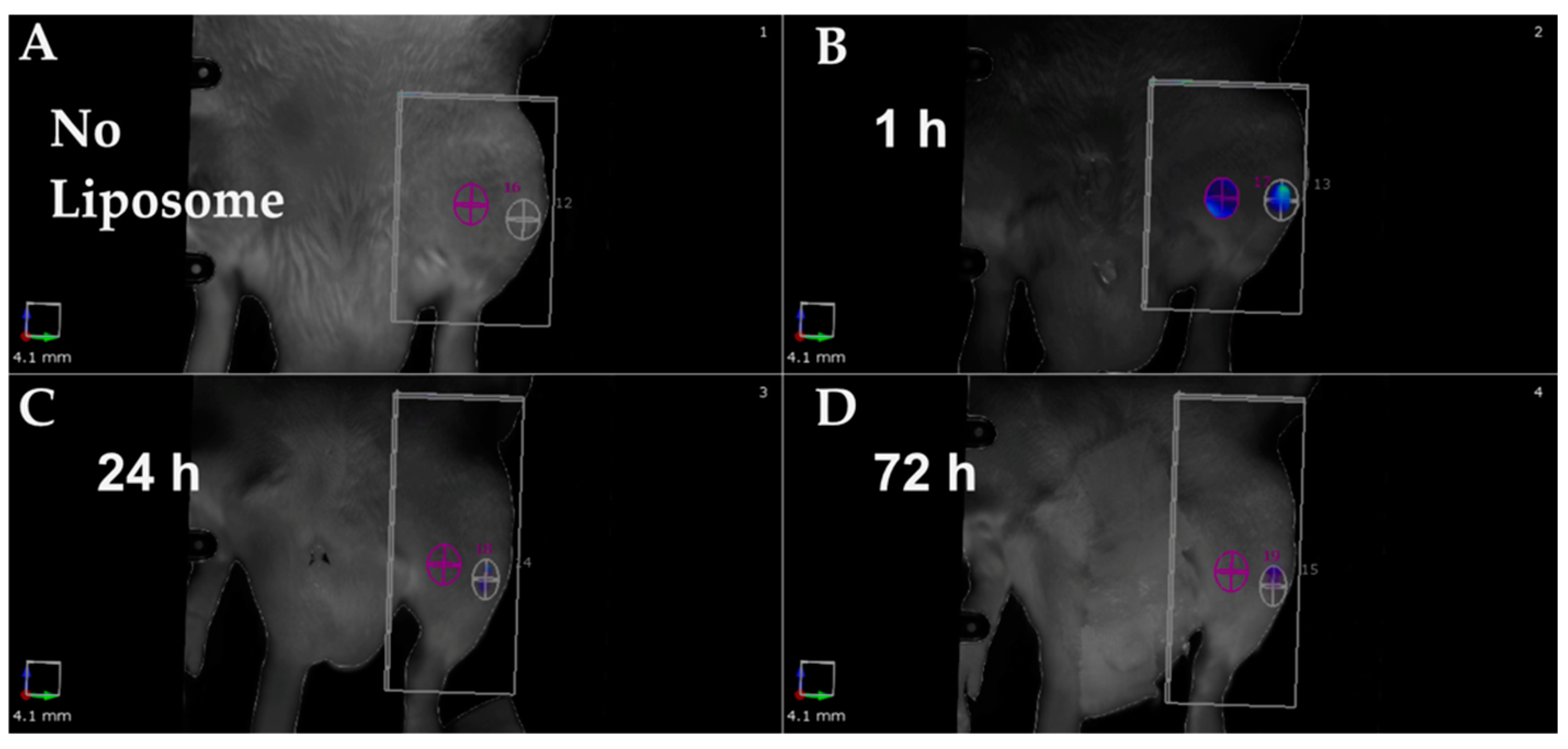

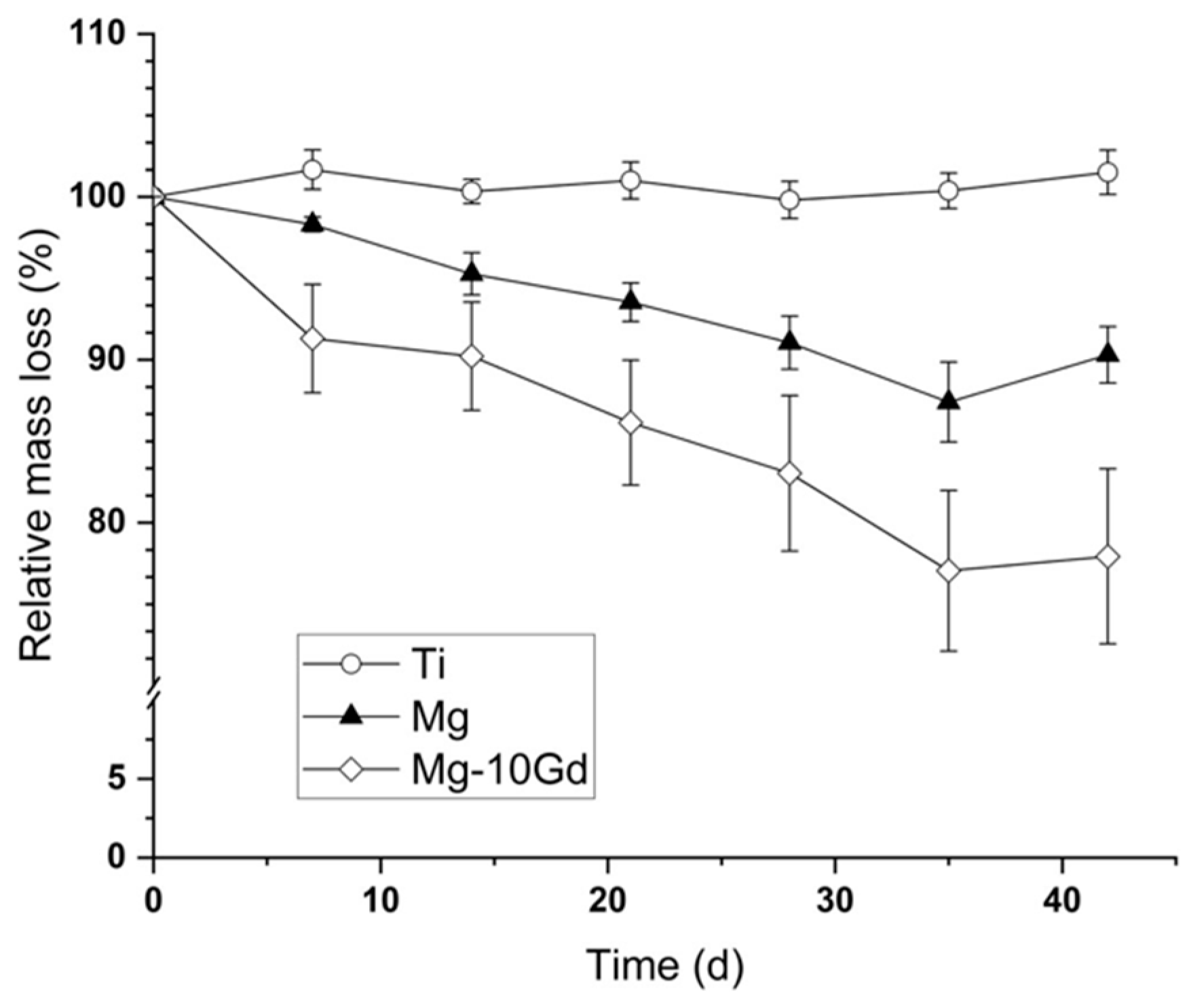
| Advantages | Disadvantages |
|---|---|
| Good bioresorbability | High hydrogen release |
| Low density comparable with cortical bone (1.738 to 1.84 g/cm3) | In vitro degradation rate (407 mm per year) |
| Good strength-to-weight ratio | Fast degradation kinetics (implant degradation should occur concomitantly with bone remodeling) |
| Good biocompatibility | |
| High damping capacity | |
| Elastic modulus (41–45 GPa) is close to that of cortical bone | Low elastic modulus (implant must support loading without deformation) |
| Less stress shielding | High corrosion rate, thereby creating bio-incompatible environment in the surrounding tissues |
| Fracture toughness is greater than of ceramic biomaterials | Gas embolism |
| High machinability and dimensional accuracy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danila, D.; Pardo, P.S.; Misra, R.D.K.; Boriek, A.M. Liposomes as Imaging Agents of Inflammation and Oxidative Stress in Bone Implants. Curr. Issues Mol. Biol. 2025, 47, 295. https://doi.org/10.3390/cimb47050295
Danila D, Pardo PS, Misra RDK, Boriek AM. Liposomes as Imaging Agents of Inflammation and Oxidative Stress in Bone Implants. Current Issues in Molecular Biology. 2025; 47(5):295. https://doi.org/10.3390/cimb47050295
Chicago/Turabian StyleDanila, Delia, Patricia S. Pardo, R. Devesh Kumar Misra, and Aladin M. Boriek. 2025. "Liposomes as Imaging Agents of Inflammation and Oxidative Stress in Bone Implants" Current Issues in Molecular Biology 47, no. 5: 295. https://doi.org/10.3390/cimb47050295
APA StyleDanila, D., Pardo, P. S., Misra, R. D. K., & Boriek, A. M. (2025). Liposomes as Imaging Agents of Inflammation and Oxidative Stress in Bone Implants. Current Issues in Molecular Biology, 47(5), 295. https://doi.org/10.3390/cimb47050295







