A Transcriptomic Study on the Toxic Effects of Iodide (I−) Wet Deposition on Pepper (Capsicum annuum) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Chili Peppers
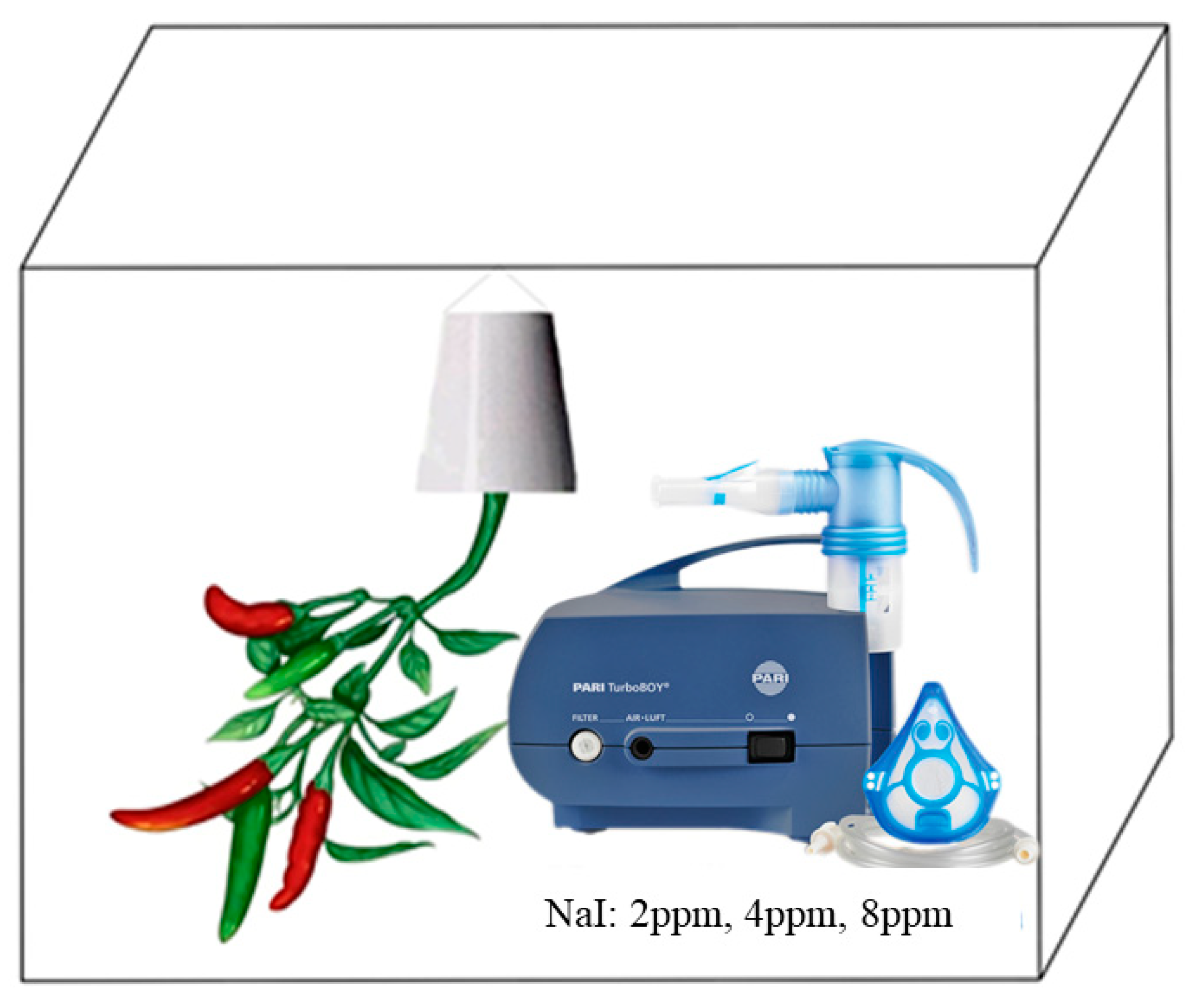
2.2. Wet Deposition Iodine Exposure Experiment
2.3. Transcriptome Sequencing
- RNA extraction (TRIzol method): Total RNA was extracted using a TRIzol kit (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, tissue samples were milled in liquid nitrogen and then fully lysed by adding TRIzol and centrifuged to remove impurities. Chloroform (CHCl3, Merck, ≥99.5%) was added for phase separation, and isopropanol (C3H8O, Merck, ≥99.9%) was used to precipitate RNA. The RNA precipitates were washed with ethanol (CH3CH2OH, Merck, 75%), dried at room temperature, and dissolved in RNase-free water, and the RNA concentration and purity were detected using a micro UV-visible spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, USA).
- mRNA purification and fragmentation: poly(A) mRNA was enriched by oligo(dT)-coupled magnetic beads (mRNA Capture Beads). mRNA was eluted with Tris buffer after two rounds of binding and washing. mRNA fragmentation was performed in a gene amplifier (ProFlex PCR, Thermo Fisher Scientific, USA) with First Strand Synthesis Buffer (Thermo Fisher Scientific, USA) and random primers.
- cDNA synthesis and library construction: reverse transcriptase was used to synthesize the first strand of cDNA and DNA polymerase I to synthesize the second strand. After magnetic beads purified double-stranded cDNA, it was sequentially subjected to end repair, addition of A-tail, and junction ligation, and uracil-containing junctions were digested using the USER enzyme.
- PCR amplification and library purification: PCR amplification of DNA from the ligated junction and purification of the amplified product by magnetic beads. Library quality was examined by a fragment analyzer (Qsep400, Bioptic, Changzhou, China) (expected fragment size: 370–470 bp) and a fluorescence quantifier (Qubit4, Thermo Fisher Scientific, USA) (concentration > 1 ng/μL).
- High-throughput sequencing: Qualified libraries were sequenced on a gene sequencer (Illumina Novaseq6000, Illumina, San Diego, CA, USA) platform.
- (1)
- Adapter Removal: Bioinformatics tools and algorithms were employed to accurately identify and remove adapter sequences from the raw data, eliminating interference from these non-target sequences in downstream analyses.
- (2)
- Quality Filtering: The remaining reads underwent quality assessment. Reads containing an excessive proportion of ambiguous bases (N > 10%) were excluded to avoid compromising the accuracy of the analysis. Additionally, a quality threshold was set, and reads were discarded if more than 50% of their bases had a quality score of Q ≤ 10, as such reads are unsuitable for further research.
2.4. Data Analysis
3. Results and Discussion
3.1. Sequencing Data Quality Control and Results Statistics
3.2. Gene Expression Analysis
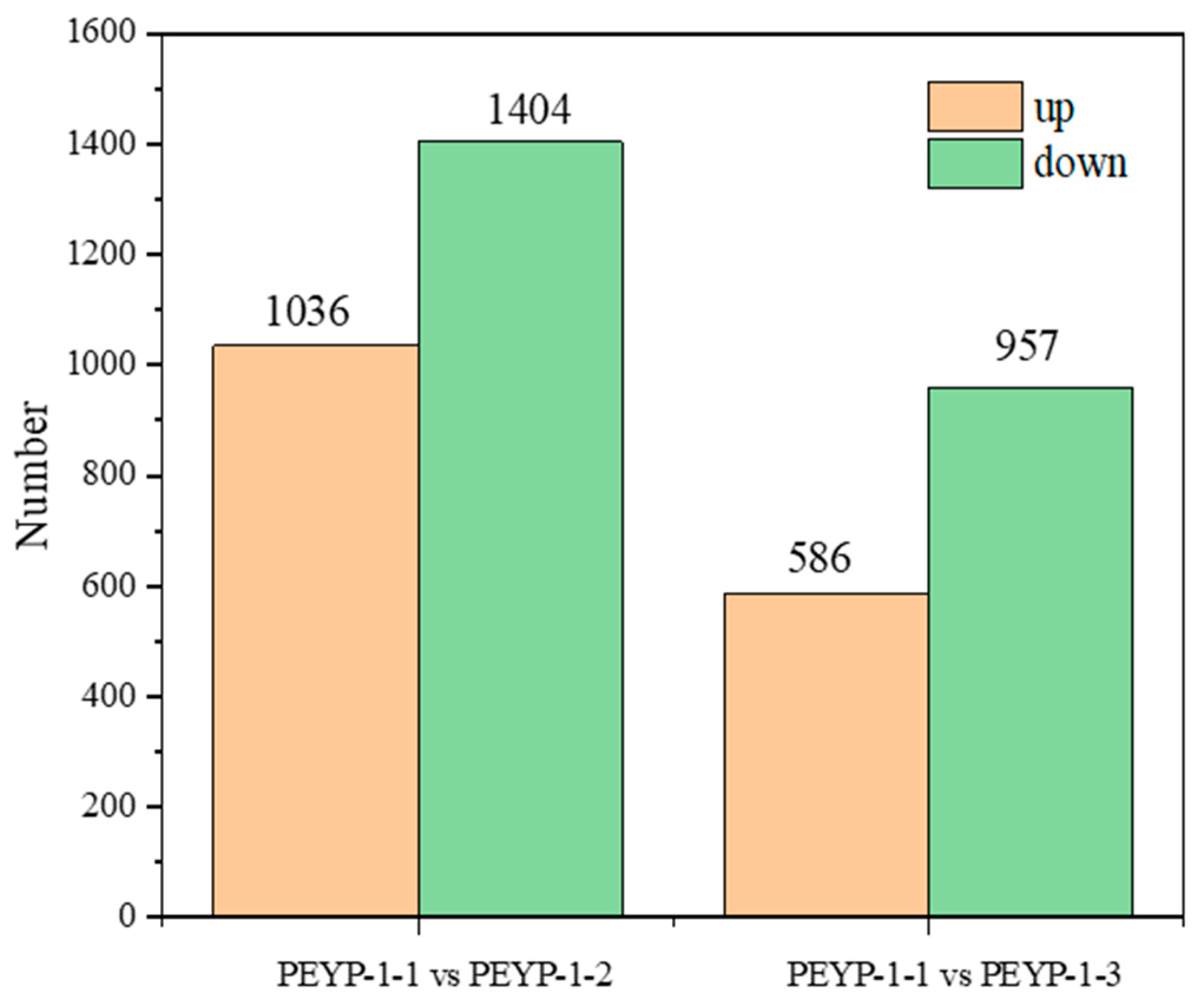
3.3. Differentially Expressed Genes Results Presentation
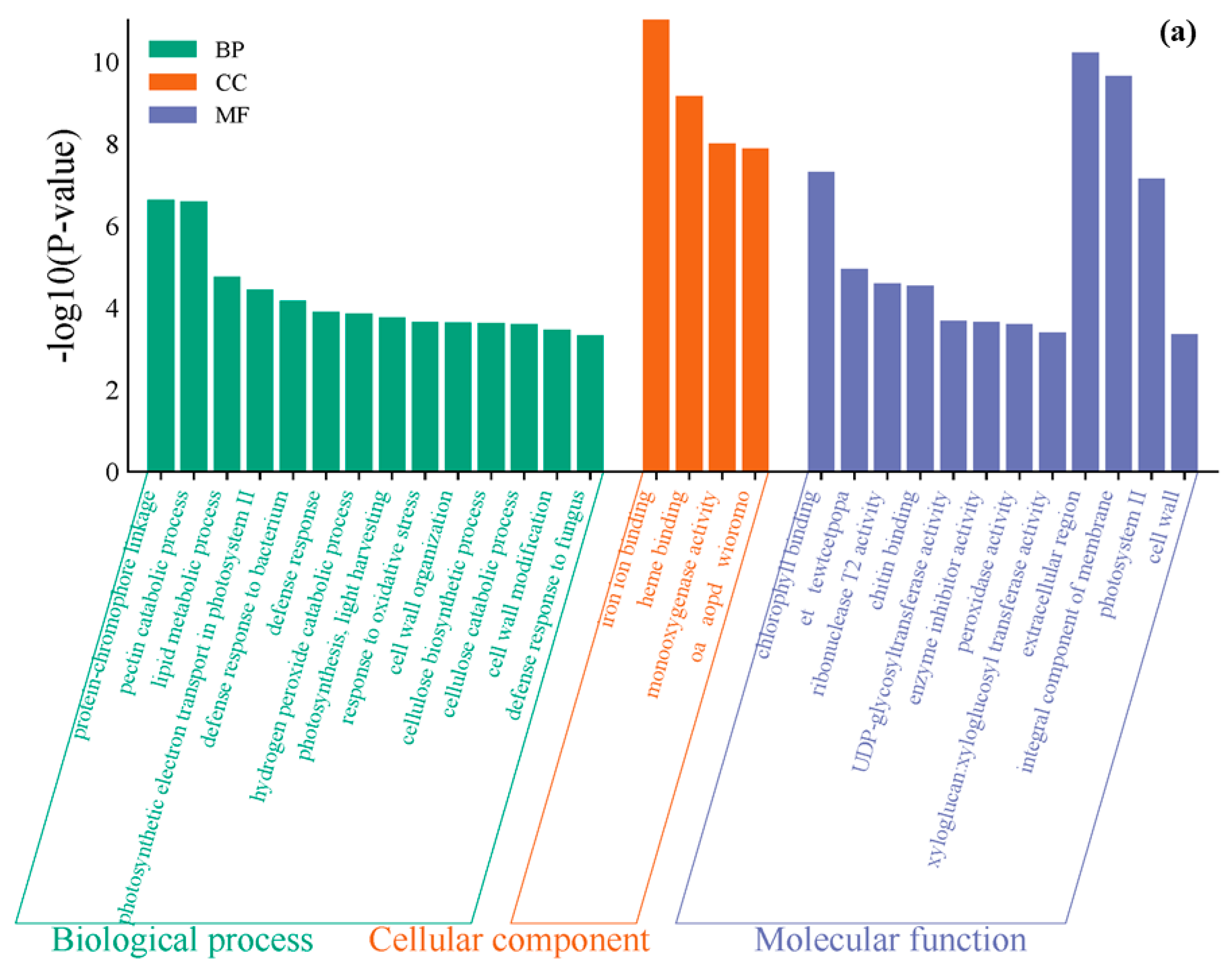
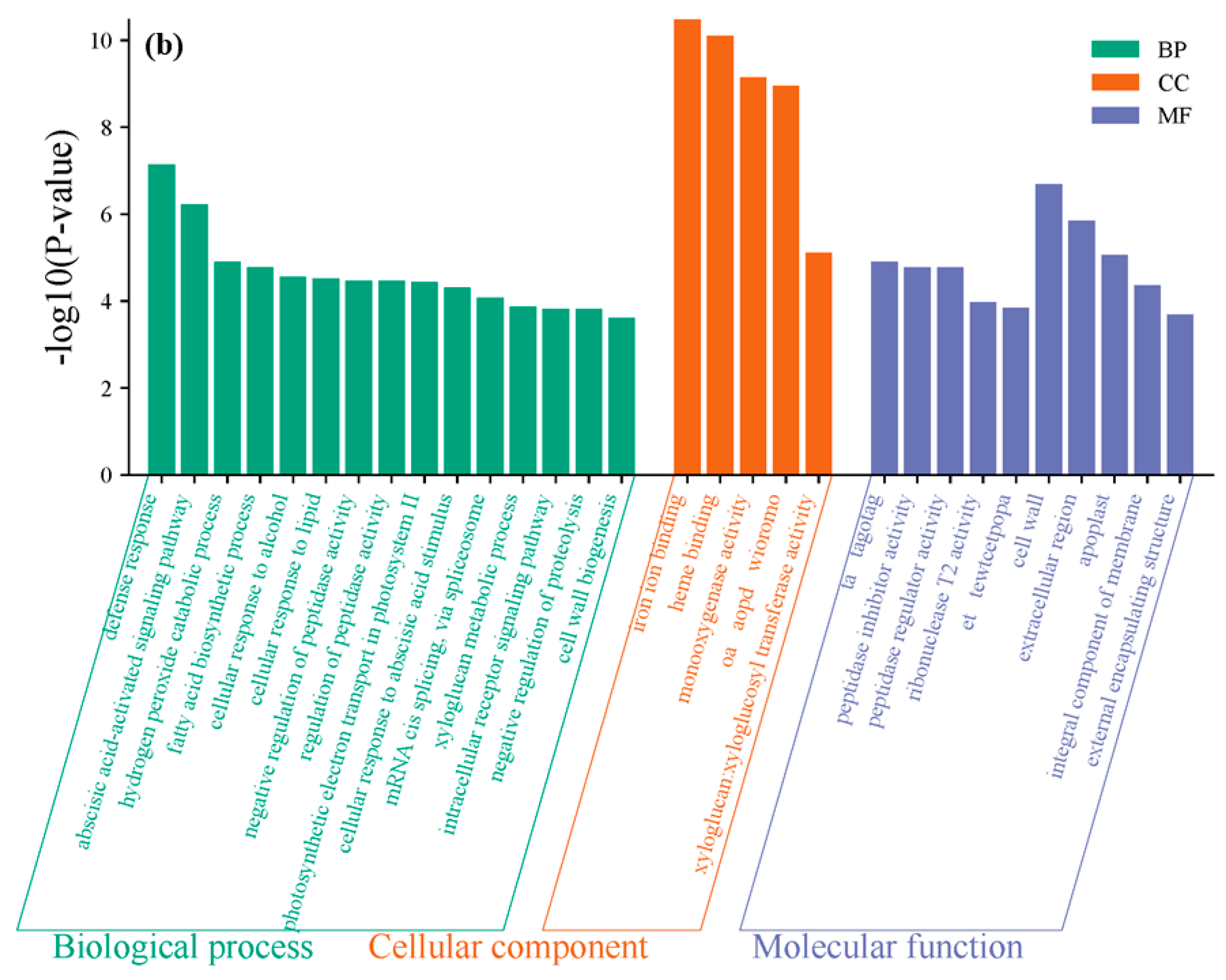
3.4. GO Functional Enrichment Analysis of Differentially Expressed Genes
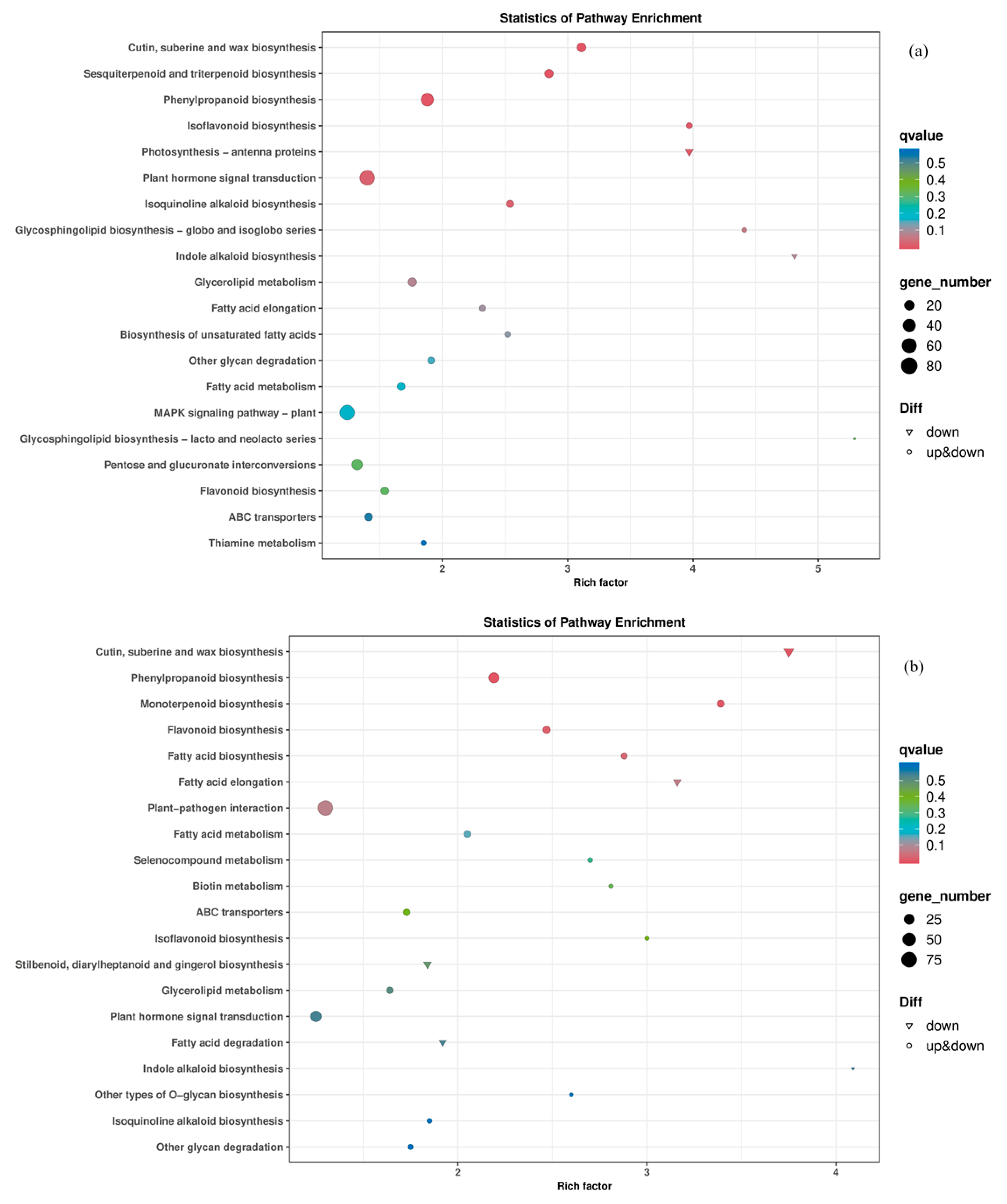
3.5. KEGG Pathway Enrichment Analysis of Differentially Expressed Genes
3.5.1. Gene Classification Based on Functional Categories
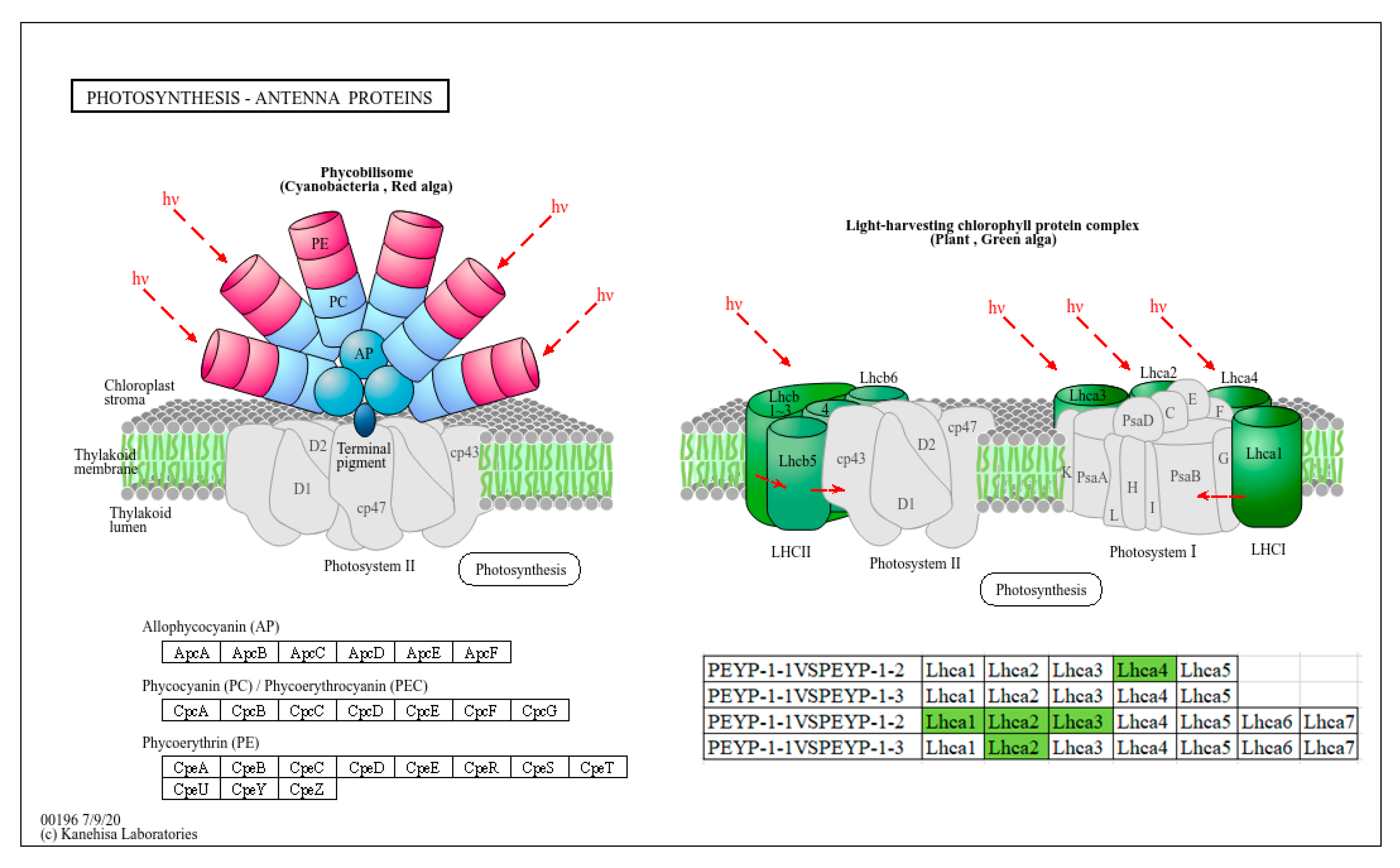
3.5.2. The Impact of I− on the Photosynthesis-Antenna Protein Pathway
3.5.3. The Impact of I− on the Biosynthesis Pathway of Cutin, Suberin, and Wax
4. Conclusions
- (1)
- In this study, 2440 and 1543 differentially expressed genes were identified in pepper leaves under 4 ppm I− and 8 ppm I− conditions, respectively.
- (2)
- Under 4 ppm I− exposure, these genes were significantly enriched in categories such as protein–chromophore linkage, extracellular region function, and iron ion binding in the Gene Ontology (GO) functional enrichment analysis. Under 8 ppm I− exposure, the differentially expressed genes were significantly enriched in categories related to defense response, cell wall components, and iron ion binding.
- (3)
- Under both tested iodine exposure concentrations, Capsicum annuum leaves exhibited significant downregulation of Lhcb2—a key gene encoding the light-harvesting complex II (LHCII) protein in the photosynthesis-antenna pathway (PSII). This suppression impaired photon capture efficiency at the reaction center, thereby reducing photochemical conversion capacity and, ultimately, decreasing overall photosynthetic efficiency. Concurrently, genes involved in cutin, suberin, and wax biosynthesis (CYP86, CYP704B1, ACE, and CYP77A) were downregulated. Notably, CYP86 subfamily enzymes catalyze ω-hydroxylation of fatty acyl-CoA molecules, a critical step in cutin and suberin polymerization. As these hydrophobic polymers constitute essential epidermal barriers, their compromised synthesis likely diminished the plant’s defensive capacity against environmental stressors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riley, B.J.; Vienna, J.D.; Strachan, D.M.; McCloy, J.S.; Jerden, J.L. Materials and Processes for the Effective Capture and Immobilization of Radioiodine: A Review. J. Nucl. Mater. 2016, 470, 307–326. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Coldsnow, K.; Utgikar, V.; Sabharwall, P.; Eric Aston, D. Capture of Harmful Radioactive Contaminants from Off-Gas Stream Using Porous Solid Sorbents for Clean Environment—A Review. Chem. Eng. J. 2016, 306, 369–381. [Google Scholar] [CrossRef]
- Hou, X.; Hansen, V.; Aldahan, A.; Possnert, G.; Lind, O.C.; Lujaniene, G. A Review on Speciation of Iodine-129 in the Environmental and Biological Samples. Anal. Chim. Acta 2009, 632, 181–196. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Li, B.-Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Kiferle, C.; Martinelli, M.; Salzano, A.M.; Gonzali, S.; Beltrami, S.; Salvadori, P.A.; Hora, K.; Holwerda, H.T.; Scaloni, A.; Perata, P. Evidences for a Nutritional Role of Iodine in Plants. Front. Plant Sci. 2021, 12, 616868. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carpenter, L.J.; Leblanc, C.; Toyama, C.; Uchida, Y.; Maskrey, B.H.; Robinson, J.; Verhaeghe, E.F.; Malin, G.; Luther, G.W.; et al. In Vivo Speciation Studies and Antioxidant Properties of Bromine in Laminaria Digitata Reinforce the Significance of Iodine Accumulation for Kelps. J. Exp. Bot. 2013, 64, 2653–2664. [Google Scholar] [CrossRef][Green Version]
- Incrocci, L.; Carmassi, G.; Maggini, R.; Poli, C.; Saidov, D.; Tamburini, C.; Kiferle, C.; Perata, P.; Pardossi, A. Iodine Accumulation and Tolerance in Sweet Basil (Ocimum basilicum L.) With Green or Purple Leaves Grown in Floating System Technique. Front. Plant Sci. 2019, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef]
- Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Iodine Effects on Phenolic Metabolism in Lettuce Plants under Salt Stress. J. Agric. Food Chem. 2013, 61, 2591–2596. [Google Scholar] [CrossRef]
- Welch, R.M.; Shuman, L. Micronutrient Nutrition of Plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Watanabe, I.; Tensho, K. Further Study on Iodine Toxicity in Relation to “Reclamation Akagare” Disease of Lowland Rice. Soil Sci. Plant Nutr. 1970, 16, 192–194. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS Generated from Biotic Stress: Effects on Plants and Alleviation by Endophytic Microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Cesare, L.F.D.; Migliori, C.A.; Leteo, F.; Campanelli, G. Influence of Climatic Conditions on Nutritional and Nutraceutical Profiles of Organic-Grown Sweet Red-Peppers. Eur. Food Res. Technol. 2020, 246, 1325–1339. [Google Scholar]
- Hong, C.; Weng, H.; Yan, A.; Islam, E. The Fate of Exogenous Iodine in Pot Soil Cultivated with Vegetables. Environ. Geochem. Health 2009, 31, 99–108. [Google Scholar] [CrossRef]
- Collins, C.D.; Gravett, A.E.; Bell, J.N.B. The Deposition and Translocation of Methyl Iodide by Crops. Health Phys. 2004, 87, 512–516. [Google Scholar] [CrossRef]
- Lawson, P.G.; Daum, D.; Czauderna, R.; Meuser, H.; Härtling, J.W. Soil versus Foliar Iodine Fertilization as a Biofortification Strategy for Field-Grown Vegetables. Front. Plant Sci. 2015, 6, 450. [Google Scholar] [CrossRef]
- Wójcik, P.; Wójcik, M. Preharvest Iodine Sprays at High Rates Are More Effective in Biofortification of Apples than Soil Application. Plant Soil 2021, 465, 317–334. [Google Scholar] [CrossRef]
- Fuge, R.; Johnson, C.C. Iodine and Human Health, the Role of Environmental Geochemistry and Diet, a Review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Rizwan, M.; Murtaza, G.; Zulfiqar, F.; Moosa, A.; Iqbal, R.; Ahmed, Z.; Irshad, S.; Khan, I.; Li, T.; Chen, J.; et al. Sustainable Manufacture and Application of Biochar to Improve Soil Properties and Remediate Soil Contaminated with Organic Impurities: A Systematic Review. Front. Environ. Sci. 2023, 11, 1277240. [Google Scholar] [CrossRef]
- Xu, C.; Miller, E.J.; Zhang, S.; Li, H.-P.; Ho, Y.-F.; Schwehr, K.A.; Kaplan, D.I.; Otosaka, S.; Roberts, K.A.; Brinkmeyer, R.; et al. Sequestration and Remobilization of Radioiodine (129I) by Soil Organic Matter and Possible Consequences of the Remedial Action at Savannah River Site. Environ. Sci. Technol. 2011, 45, 9975–9983. [Google Scholar] [CrossRef]
- Jung, Y.E.; Yang, J.H.; Yim, M.-S. Investigation of Bismuth-Based Metal-Organic Frameworks for Effective Capture and Immobilization of Radioiodine Gas. J. Hazard. Mater. 2024, 467, 133777. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Takahashi, H.; Suge, H. Gravimorphism in Rice and Barley: Promotion of Leaf Elongation by Vertical Inversion in Agravitropically Growing Plants. J. Plant Res. 1998, 111, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.M.; Sohrabi, R.; Paasch, B.C.; Rhodes, D.; Thireault, C.; Schulze-Lefert, P.; Tiedje, J.M.; He, S.Y. Peat-Based Gnotobiotic Plant Growth Systems for Arabidopsis Microbiome Research. Nat. Protoc. 2021, 16, 2450–2470. [Google Scholar] [CrossRef]
- Li, R.; Li, D.-W.; Liu, H.-P.; Hong, C.-L.; Song, M.-Y.; Dai, Z.-X.; Liu, J.-W.; Zhou, J.; Weng, H.-X. Enhancing Iodine Content and Fruit Quality of Pepper (Capsicum annuum L.) through Biofortification. Sci. Hortic. 2017, 214, 165–173. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Hunter, G.A.; Ferreira, G.C. Metal Ion Coordination Sites in Ferrochelatase. Coord. Chem. Rev. 2022, 460, 214464. [Google Scholar] [CrossRef]
- Lin, C.-W.; McCabe, J.W.; Russell, D.H.; Barondeau, D.P. Molecular Mechanism of ISC Iron–Sulfur Cluster Biogenesis Revealed by High-Resolution Native Mass Spectrometry. J. Am. Chem. Soc. 2020, 142, 6018–6029. [Google Scholar] [CrossRef]
- Sui, S.-F. Structure of Phycobilisomes. Annu. Rev. Biophys. 2021, 50, 53–72. [Google Scholar] [CrossRef]
- Li, P.; Weng, J.; Zhang, Q.; Yu, L.; Yao, Q.; Chang, L.; Niu, Q. Physiological and Biochemical Responses of Cucumis melo L. Chloroplasts to Low-Phosphate Stress. Front. Plant Sci. 2018, 9, 1525. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Sharma, H.; Kumar, A.; Hashem, A.; Abd_Allah, E.F.; Gupta, R.K. Unlocking the Adaptation Mechanisms of the Oleaginous Microalga Scenedesmus Sp. BHU1 under Elevated Salt Stress: A Physiochemical, Lipidomics and Transcriptomics Approach. Front. Microbiol. 2024, 15, 1475410. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-Dependent Signalling Pathways in Plants and Algae Exposed to High Light: Comparisons with Other Eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Gong, F.; Liu, J. Transcriptomic Analysis of Hydrogen Photoproduction in Chlorella Pyrenoidosa under Nitrogen Deprivation. Algal Res. 2020, 47, 101827. [Google Scholar] [CrossRef]
- Shukla, V.; Han, J.-P.; Cléard, F.; Lefebvre-Legendre, L.; Gully, K.; Flis, P.; Berhin, A.; Andersen, T.G.; Salt, D.E.; Nawrath, C.; et al. Suberin Plasticity to Developmental and Exogenous Cues Is Regulated by a Set of MYB Transcription Factors. Proc. Natl. Acad. Sci. USA 2021, 118, e2101730118. [Google Scholar] [CrossRef]
- Harborne, J.B.; Boardley, M. The Widespread Occurrence in Nature of Anthocyanins as Zwitterions. Z. Für Naturforschung C 1985, 40, 305–308. [Google Scholar] [CrossRef]
- Xie, J.; Hao, X.; Shang, Y.; Chen, W. Improvement of Stability and Lipophilicity of Pelargonidin-3-Glucoside by Enzymatic Acylation with Aliphatic Dicarboxylic Acid. Food Chem. 2022, 389, 133077. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, E.A.; Guardini, Z.; Dall’Osto, L.; Bassi, R. A Paler Shade of Green: Engineering Cellular Chlorophyll Content to Enhance Photosynthesis in Crowded Environments. New Phytol. 2023, 239, 1567–1583. [Google Scholar] [CrossRef]
- Negin, B.; Hen-Avivi, S.; Almekias-Siegl, E.; Shachar, L.; Jander, G.; Aharoni, A. Tree Tobacco (Nicotiana Glauca) Cuticular Wax Composition Is Essential for Leaf Retention during Drought, Facilitating a Speedy Recovery Following Rewatering. New Phytol. 2023, 237, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, H.; Wang, J.; He, P.; Kuzyakov, Y.; Ma, M.; Ling, N. Microbial Phosphorus-cycling Genes in Soil under Global Change. Glob. Change Biol. 2024, 30, e17281. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.; Li, L.; Guo, Z.; Si, Q.; Zhu, G.; Wang, X.; Guo, W. Gb86A1-1 from Gossypium Barbadense Positively Regulates Defence against Verticillium Dahliae by Cell Wall Modification and Activation of Immune Pathways. Plant Biotechnol. J. 2020, 18, 222–238. [Google Scholar] [CrossRef]



| Sample | Clean Reads a | Clean Bases b | Q30 c (%) | GC Content d (%) |
|---|---|---|---|---|
| PEYP-1-1 | 21,837,760 | 6,528,919,648 | 94.90 | 43.25 |
| PEYP-1-2 | 23,298,372 | 6,968,424,342 | 94.47 | 43.19 |
| PEYP-1-3 | 28,962,752 | 8,655,221,162 | 94.88 | 43.31 |
| Sample | Total Reads Rate a (%) | Mapped Reads Rate b (%) | Uniq Mapped Reads Rate c (%) | Multiple Mapped Reads Rate d (%) |
|---|---|---|---|---|
| PEYP-1-1 | 100 | 92.87 | 87.20 | 5.67 |
| PEYP-1-2 | 100 | 93.68 | 88.34 | 5.34 |
| PEYP-1-3 | 100 | 93.76 | 87.87 | 5.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Ma, Z.-L.; Wang, M.; Jin, J. A Transcriptomic Study on the Toxic Effects of Iodide (I−) Wet Deposition on Pepper (Capsicum annuum) Leaves. Curr. Issues Mol. Biol. 2025, 47, 313. https://doi.org/10.3390/cimb47050313
Yu R, Ma Z-L, Wang M, Jin J. A Transcriptomic Study on the Toxic Effects of Iodide (I−) Wet Deposition on Pepper (Capsicum annuum) Leaves. Current Issues in Molecular Biology. 2025; 47(5):313. https://doi.org/10.3390/cimb47050313
Chicago/Turabian StyleYu, Rui, Zhu-Ling Ma, Min Wang, and Jie Jin. 2025. "A Transcriptomic Study on the Toxic Effects of Iodide (I−) Wet Deposition on Pepper (Capsicum annuum) Leaves" Current Issues in Molecular Biology 47, no. 5: 313. https://doi.org/10.3390/cimb47050313
APA StyleYu, R., Ma, Z.-L., Wang, M., & Jin, J. (2025). A Transcriptomic Study on the Toxic Effects of Iodide (I−) Wet Deposition on Pepper (Capsicum annuum) Leaves. Current Issues in Molecular Biology, 47(5), 313. https://doi.org/10.3390/cimb47050313







