From Lineage to Longevity: A Field Guide to the Key Players in Epigenetic Contribution to Offspring Health
Abstract
1. Introduction
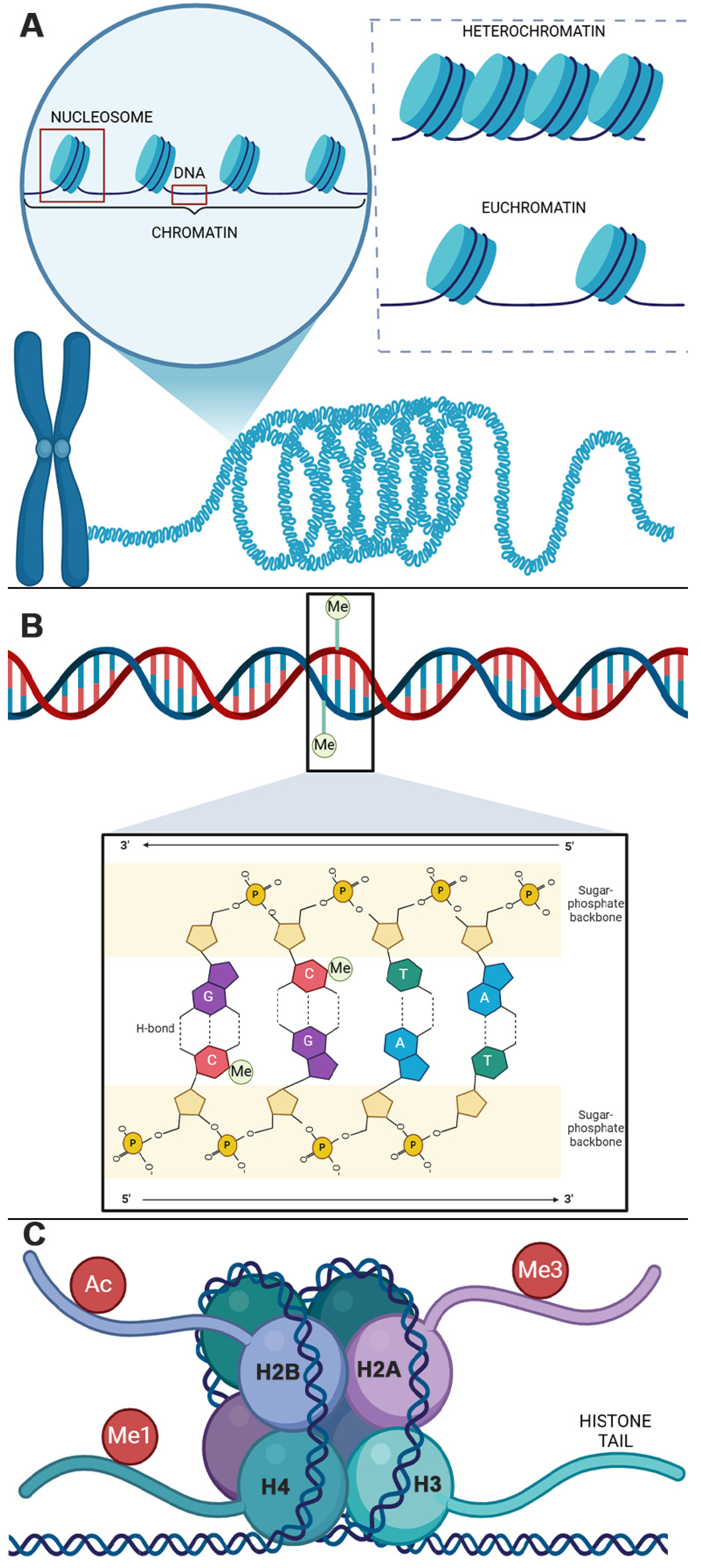
2. Epigenetic Mechanisms
2.1. DNAM
2.2. Histone Modifications
2.3. Epigenetic Regulation of Transcription
2.3.1. DNAM Modulation of Gene Expression
2.3.2. Modified Histone Effects on Gene Expression
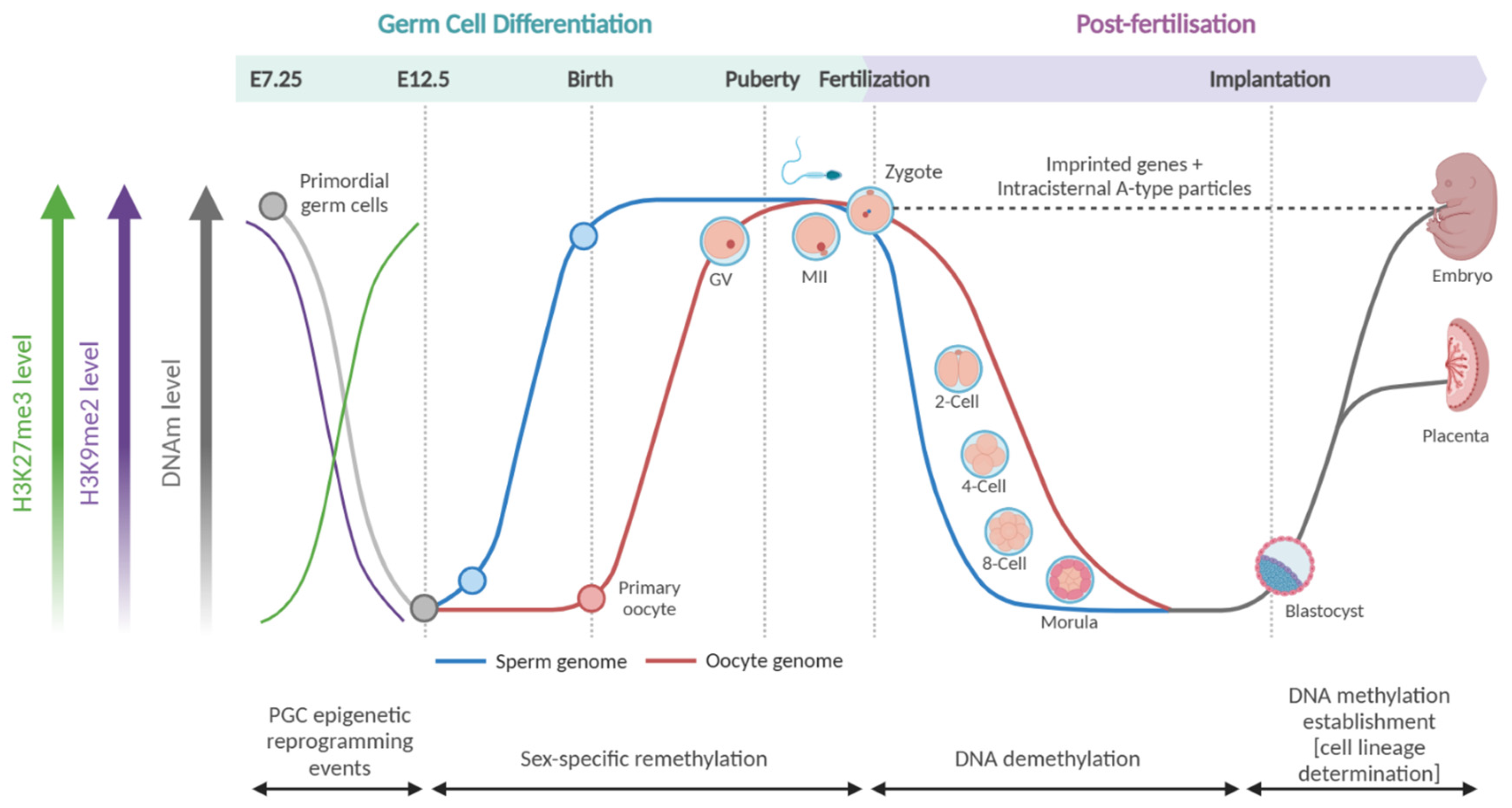
3. DNAM During Mammalian Development
3.1. Germ Cell Differentiation
3.2. Post-Fertilisation
4. Histone Modifications During Mammalian Development
4.1. Post-Fertilisation
4.2. Primordial Germ Cell Differentiation
5. Mammalian Epigenetic Transmission
5.1. Replicative Maintenance
5.2. Restorative Maintenance
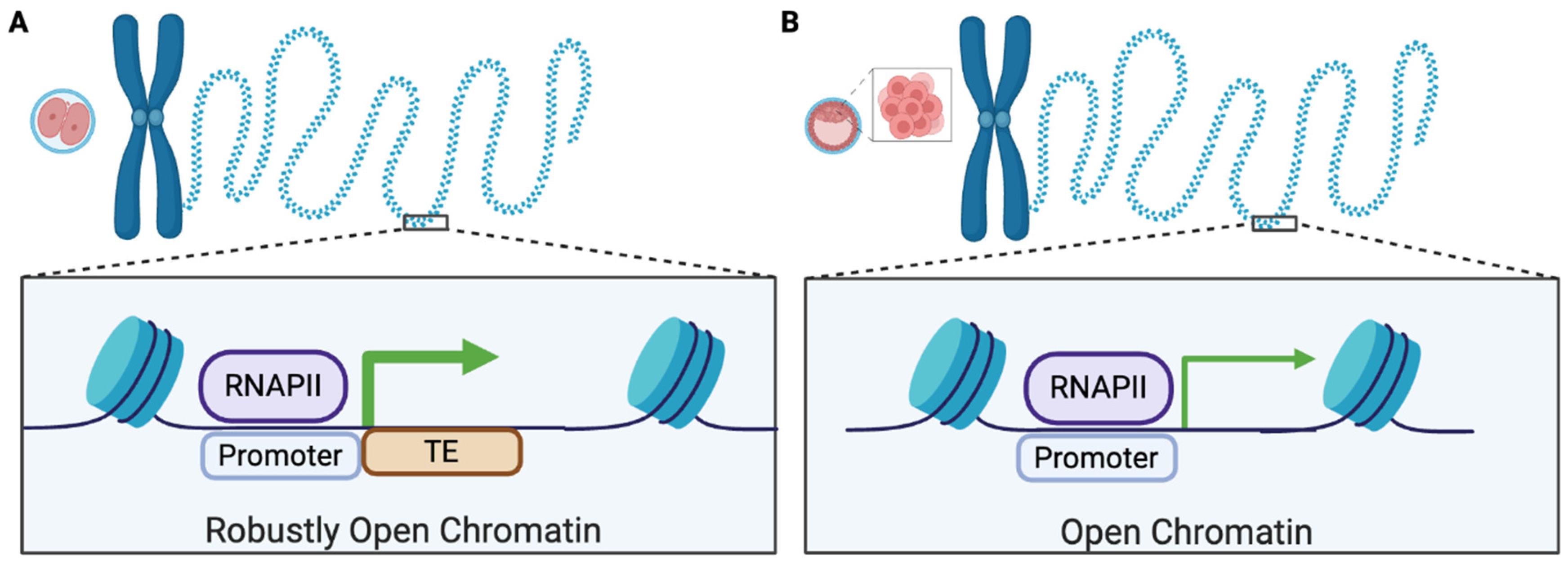
6. DNAM of TEs During Development
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5caC | 5-carboxymethylcytosine |
| 5fc | 5-formylmethylcystosine |
| 5hmC | 5-hydroxymethylcytosine |
| 5mC | 5-methylcytosine |
| BET | bromo- and extra-terminal |
| bps | base pairs |
| DNAm | DNA methylation |
| DNMT | DNA methyltransferase |
| DOHaD | Developmental Origins of Health and Disease |
| ERV | endogenous retrovirus |
| H2A | histone 2A |
| H2B | histone 2B |
| H3 | histone 3 |
| H4 | histone 4 |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylase |
| IAP | intracisternal-A particle |
| ICM | inner cell mass |
| KMT | lysine methyltransferase |
| LINE | long interspersed nuclear element |
| MZT | maternal to zygotic transcription |
| PGCs | primordial germ cells |
| PRMT | protein arginine N-methyltransferase |
| RNA Pol II | RNA polymerase II |
| SINE | short interspersed nuclear element |
| TDG | thymine DNA glycosylate |
| TE | transposable element |
| TET | ten-eleven translocation |
| UHRF1 | ubiquitin-like, containing PHD and RING finger domains, 1 |
| ZGA | zygotic genome activation |
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Hacker, K. The burden of chronic disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar]
- Countdown, N. NCD Countdown 2030: Worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 2018, 392, 1072–1088. [Google Scholar]
- Murray, C.J.L. The Global Burden of Disease Study at 30 years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Q.; Jahani, P.S.; Hurrell, B.P.; Pan, C.; Huang, P.; Gukasyan, J.; Woodward, N.C.; Eskin, E.; Gilliland, F.D.; et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Eichler, E.E.; Flint, J.; Gibson, G.; Kong, A.; Leal, S.M.; Moore, J.H.; Nadeau, J.H. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 2010, 11, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Ober, C. Lessons Learned From GWAS of Asthma. Allergy, Asthma Immunol. Res. 2019, 11, 170–187. [Google Scholar] [CrossRef]
- Weiss, S.T.; Silverman, E.K. Pro: Genome-Wide Association Studies (GWAS) in Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 631–633. [Google Scholar] [CrossRef]
- Pasipoularides, A. Linking Genes to Cardiovascular Diseases: Gene Action and Gene–Environment Interactions. J. Cardiovasc. Transl. Res. 2015, 8, 506–527. [Google Scholar] [CrossRef]
- Von Mutius, E. Gene-environment interactions in asthma. J. Allergy Clin. Immunol. 2009, 123, 3–11. [Google Scholar] [CrossRef]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2014, 6, 55–64. [Google Scholar] [CrossRef]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2019, 14, 47–50. [Google Scholar] [CrossRef]
- Waterland, R.A.; Michels, K.B. Epigenetic Epidemiology of the Developmental Origins Hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Lite, C.; Raja, G.L.; Juliet, M.; Sridhar, V.V.; Subhashree, K.D.; Kumar, P.; Chakraborty, P.; Arockiaraj, J. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ. Toxicol. Pharmacol. 2022, 89, 103779. [Google Scholar] [CrossRef]
- Deodati, A.; Inzaghi, E.; Cianfarani, S. Epigenetics and In Utero Acquired Predisposition to Metabolic Disease. Front. Genet. 2020, 10, 1270. [Google Scholar] [CrossRef]
- Oken, E.; Huh, S.Y.; Taveras, E.M.; Rich-Edwards, J.W.; Gillman, M.W. Associations of Maternal Prenatal Smoking with Child Adiposity and Blood Pressure. Obes. Res. 2005, 13, 2021–2028. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, S.; Zhao, J.; Qian, Z.; A Bassig, B.; Yang, R.; Zhang, Y.; Hu, K.; Xu, S.; Zheng, T.; et al. Maternal exposure to air pollutant PM2.5 and PM10 during pregnancy and risk of congenital heart defects. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 422–427. [Google Scholar] [CrossRef]
- Ong, T.P.; Guest, P.C. Nutritional programming effects on development of metabolic disorders in later life. In Investigations of Early Nutrition Effects on Long-Term Health: Methods and Applications; Humana Press: New York, NY, USA, 2018; pp. 3–17. [Google Scholar]
- Lu, C.; Wang, L.; Liao, H.; Li, B.; Liu, Q.; Wang, F. Impacts of intrauterine and postnatal exposure to air pollution on preschool children’s asthma: A key role in cumulative exposure. Build. Environ. 2023, 245. [Google Scholar] [CrossRef]
- Sunde, R.B.; Thorsen, J.; Pedersen, C.-E.T.; Stokholm, J.; Bønnelykke, K.; Chawes, B.; Bisgaard, H. Prenatal tobacco exposure and risk of asthma and allergy outcomes in childhood. Eur. Respir. J. 2021, 59, 2100453. [Google Scholar] [CrossRef]
- Kohli, R.M.; Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Heintz, N. The Nuclear DNA Base 5-Hydroxymethylcytosine Is Present in Purkinje Neurons and the Brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef]
- Weber, A.R.; Krawczyk, C.; Robertson, A.B.; Kuśnierczyk, A.; Vågbø, C.B.; Schuermann, D.; Klungland, A.; Schär, P. Biochemical reconstitution of TET1–TDG–BER-dependent active DNA demethylation reveals a highly coordinated mechanism. Nat. Commun. 2016, 7, 10806. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Drohat, A.C. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: Potential implications for active demethylation of CpG sites. J. Biol. Chem. 2011, 286, 35334–35338. [Google Scholar] [PubMed]
- Ehrlich, M.; Ehrlich, K.C. DNA Cytosine Methylation and Hydroxymethylation at the Borders. Epigenomics 2014, 6, 563–566. [Google Scholar] [CrossRef]
- Lister, R.; Mukamel, E.A.; Nery, J.R.; Urich, M.; Puddifoot, C.A.; Johnson, N.D.; Lucero, J.; Huang, Y.; Dwork, A.J.; Schultz, M.D.; et al. Global Epigenomic Reconfiguration During Mammalian Brain Development. Science 2013, 341, 1237905. [Google Scholar] [CrossRef]
- Li, L.; Gao, Y.; Wu, Q.; Cheng, A.S.; Yip, K.Y. New guidelines for DNA methylome studies regarding 5-hydroxymethylcytosine for understanding transcriptional regulation. Genome Res. 2019, 29, 543–553. [Google Scholar] [CrossRef]
- Spruijt, C.G.; Gnerlich, F.; Smits, A.H.; Pfaffeneder, T.; Jansen, P.W.T.C.; Bauer, C.; Munzel, M.; Wagner, M.; Muller, M.; Khan, F.; et al. Dynamic readers for 5-(hydroxy) methylcytosine and its oxidized derivatives. Cell 2013, 152, 1146–1159. [Google Scholar] [CrossRef]
- Nestor, C.; Ruzov, A.; Meehan, R.R.; Dunican, D.S. Enzymatic Approaches and Bisulfite Sequencing Cannot Distinguish Between 5-Methylcytosine and 5-Hydroxymethylcytosine in DNA. BioTechniques 2010, 48, 317–319. [Google Scholar] [CrossRef]
- Bostick, M.; Kim, J.K.; Estève, P.-O.; Clark, A.; Pradhan, S.; Jacobsen, S.E. UHRF1 Plays a Role in Maintaining DNA Methylation in Mammalian Cells. Science 2007, 317, 1760–1764. [Google Scholar] [CrossRef]
- Sharif, J.; Muto, M.; Takebayashi, S.-I.; Suetake, I.; Iwamatsu, A.; Endo, T.A.; Shinga, J.; Mizutani-Koseki, Y.; Toyoda, T.; Okamura, K.; et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 2007, 450, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Liu, Y.; Upadhyay, A.K.; Chang, Y.; Howerton, S.B.; Vertino, P.M.; Zhang, X.; Cheng, X. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res. 2012, 40, 4841–4849. [Google Scholar] [CrossRef]
- Ji, D.; Lin, K.; Song, J.; Wang, Y. Effects of Tet-induced oxidation products of 5-methylcytosine on Dnmt1- and DNMT3a-mediated cytosine methylation. Mol. Biosyst. 2014, 10, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Otani, J.; Kimura, H.; Sharif, J.; Endo, T.A.; Mishima, Y.; Kawakami, T.; Koseki, H.; Shirakawa, M.; Suetake, I.; Tajima, S. Cell cycle-dependent turnover of 5-hydroxymethyl cytosine in mouse embryonic stem cells. PLoS ONE 2013, 8, e82961. [Google Scholar]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 1997, 389, 251–260. [Google Scholar]
- Uversky, V.N. Introduction to Intrinsically Disordered Proteins (IDPs). Chem. Rev. 2014, 114, 6557–6560. [Google Scholar] [CrossRef]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- A Musselman, C.; Lalonde, M.-E.; Côté, J.; Kutateladze, T.G. Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 2012, 19, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Luo, H.; Lee, S.; Jin, F.; Yang, J.S.; Montellier, E.; Buchou, T.; Cheng, Z.; Rousseaux, S.; Rajagopal, N.; et al. Identification of 67 Histone Marks and Histone Lysine Crotonylation as a New Type of Histone Modification. Cell 2011, 146, 1016–1028. [Google Scholar] [CrossRef]
- Radzisheuskaya, A.; Shliaha, P.V.; Grinev, V.V.; Shlyueva, D.; Damhofer, H.; Koche, R.; Gorshkov, V.; Kovalchuk, S.; Zhan, Y.; Rodriguez, K.L.; et al. Complex-dependent histone acetyltransferase activity of KAT8 determines its role in transcription and cellular homeostasis. Mol. Cell 2021, 81, 1749–1765.e8. [Google Scholar] [CrossRef]
- Poziello, A.; Nebbioso, A.; Stunnenberg, H.G.; Martens, J.H.; Carafa, V.; Altucci, L. Recent insights into Histone Acetyltransferase-1: Biological function and involvement in pathogenesis. Epigenetics 2020, 16, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, J.-S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Murray, K. The Occurrence of iε-N-Methyl Lysine in Histones. Biochemistry 1964, 3, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.K.; Kim, S. E-N-dimethyllysine in histones. Biochem. Biophys. Res. Commun. 1967, 27, 479–483. [Google Scholar] [CrossRef]
- Hempel, K.; Lange, H.W.; Birkofer, L. Epsilon-N-trimethyllysine, a new amino acid in histones. Naturwissenschaften 1968, 55, 37. [Google Scholar] [CrossRef]
- Byvoet, P.; Shepherd, G.; Hardin, J.; Noland, B. The distribution and turnover of labeled methyl groups in histone fractions of cultured mammalian cells. Arch. Biochem. Biophys. 1972, 148, 558–567. [Google Scholar] [CrossRef]
- Borun, T.W.; Pearson, D.; Paik, W.K. Studies of Histone Methylation during the HeLa S-3 Cell Cycle. J. Biol. Chem. 1972, 247, 4288–4298. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, H.; Ng, H.H.; Erdjument-Bromage, H.; Tempst, P.; Struhl, K.; Zhang, Y. Methylation of H3-Lysine 79 Is Mediated by a New Family of HMTases without a SET Domain. Curr. Biol. 2002, 12, 1052–1058. [Google Scholar] [CrossRef]
- Rea, S.; Eisenhaber, F.; O’Carroll, D.; Strahl, B.D.; Sun, Z.-W.; Schmid, M.; Opravil, S.; Mechtler, K.; Ponting, C.P.; Allis, C.D.; et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 2000, 406, 593–599. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Litt, M.; Qiu, Y.; Huang, S. Histone arginine methylations: Their roles in chromatin dynamics and transcriptional regulation. Biosci. Rep. 2009, 29, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat. Struct. Mol. Biol. 2005, 12, 110–112. [Google Scholar] [CrossRef]
- Swanson, R. A unifying concept for the amino acid code. Bull. Math. Biol. 1984, 46, 187–203. [Google Scholar]
- Hansen, B.K.; Gupta, R.; Baldus, L.; Lyon, D.; Narita, T.; Lammers, M.; Choudhary, C.; Weinert, B.T. Analysis of human acetylation stoichiometry defines mechanistic constraints on protein regulation. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Weinert, B.T.; Narita, T.; Satpathy, S.; Srinivasan, B.; Hansen, B.K.; Schölz, C.; Hamilton, W.B.; Zucconi, B.E.; Wang, W.W.; Liu, W.R.; et al. Time-Resolved Analysis Reveals Rapid Dynamics and Broad Scope of the CBP/p300 Acetylome. Cell 2018, 174, 231–244.e12. [Google Scholar] [CrossRef] [PubMed]
- Feller, C.; Forné, I.; Imhof, A.; Becker, P.B. Global and Specific Responses of the Histone Acetylome to Systematic Perturbation. Mol. Cell 2015, 57, 559–571. [Google Scholar] [CrossRef]
- Cai, Y.; Jin, J.; Swanson, S.K.; Cole, M.D.; Choi, S.H.; Florens, L.; Washburn, M.P.; Conaway, J.W.; Conaway, R.C. Subunit Composition and Substrate Specificity of a MOF-containing Histone Acetyltransferase Distinct from the Male-specific Lethal (MSL) Complex. J. Biol. Chem. 2010, 285, 4268–4272. [Google Scholar] [CrossRef]
- Wang, Z.; Millard, C.J.; Lin, C.-L.; E Gurnett, J.; Wu, M.; Lee, K.; Fairall, L.; Schwabe, J.W.; A Cole, P.; Brigham; et al. Diverse nucleosome Site-Selectivity among histone deacetylase complexes. eLife 2020, 9, e57663. [Google Scholar] [CrossRef]
- Baribault, C.; Ehrlich, K.C.; Ponnaluri, V.K.C.; Pradhan, S.; Lacey, M.; Ehrlich, M. Developmentally linked human DNA hypermethylation is associated with down-modulation, repression, and upregulation of transcription. Epigenetics 2018, 13, 275–289. [Google Scholar] [CrossRef]
- Shukla, S.; Kavak, E.; Gregory, M.; Imashimizu, M.; Shutinoski, B.; Kashlev, M.; Oberdoerffer, P.; Sandberg, R.; Oberdoerffer, S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 2011, 479, 74–79. [Google Scholar] [CrossRef]
- Auclair, G.; Guibert, S.; Bender, A.; Weber, M. Ontogeny of CpG island methylation and specificity of DNMT3 methyltransferases during embryonic development in the mouse. Genome Biol. 2014, 15, 545. [Google Scholar] [CrossRef]
- Borgel, J.; Guibert, S.; Li, Y.; Chiba, H.; Schübeler, D.; Sasaki, H.; Forné, T.; Weber, M. Targets and dynamics of promoter DNA methylation during early mouse development. Nat. Genet. 2010, 42, 1093–1100. [Google Scholar] [CrossRef]
- Yin, Y.; Morgunova, E.; Jolma, A.; Kaasinen, E.; Sahu, B.; Khund-Sayeed, S.; Das, P.K.; Kivioja, T.; Dave, K.; Zhong, F.; et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 2017, 356. [Google Scholar] [CrossRef]
- Chandra, S.; Baribault, C.; Lacey, M.; Ehrlich, M. Myogenic Differential Methylation: Diverse Associations with Chromatin Structure. Biology 2014, 3, 426–451. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’His, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Rapelli, S.; Krepelova, A.; Incarnato, D.; Parlato, C.; Basile, G.; Maldotti, M.; Anselmi, F.; Oliviero, S. Intragenic DNA methylation prevents spurious transcription initiation. Nature 2017, 543, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.-M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Varley, K.E.; Gertz, J.; Bowling, K.M.; Parker, S.L.; Reddy, T.E.; Pauli-Behn, F.; Cross, M.K.; Williams, B.A.; Stamatoyannopoulos, J.A.; Crawford, G.E.; et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013, 23, 555–567. [Google Scholar] [CrossRef]
- Stasevich, T.J.; Hayashi-Takanaka, Y.; Sato, Y.; Maehara, K.; Ohkawa, Y.; Sakata-Sogawa, K.; Tokunaga, M.; Nagase, T.; Nozaki, N.; McNally, J.G.; et al. Regulation of RNA polymerase II activation by histone acetylation in single living cells. Nature 2014, 516, 272–275. [Google Scholar] [CrossRef]
- Dancy, B.M.; Cole, P.A. Protein Lysine Acetylation by p300/CBP. Chem. Rev. 2015, 115, 2419–2452. [Google Scholar] [CrossRef]
- Andersson, R.; Sandelin, A. Determinants of enhancer and promoter activities of regulatory elements. Nat. Rev. Genet. 2019, 21, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Glaser, M.A.; Lansac, Y.; Maiti, P.K. Atomistic simulation of stacked nucleosome core particles: Tail bridging, the H4 tail, and effect of hydrophobic forces. J. Phys. Chem. B 2016, 120, 3048–3060. [Google Scholar] [PubMed]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Lee, K.K.; Workman, J.L. Histone acetyltransferase complexes: One size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 2007, 8, 284–295. [Google Scholar] [CrossRef]
- Jain, A.K.; Barton, M.C. Bromodomain Histone Readers and Cancer. J. Mol. Biol. 2017, 429, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- North, J.A.; Shimko, J.C.; Javaid, S.; Mooney, A.M.; Shoffner, M.A.; Rose, S.D.; Bundschuh, R.; Fishel, R.; Ottesen, J.J.; Poirier, M.G. Regulation of the nucleosome unwrapping rate controls DNA accessibility. Nucleic Acids Res. 2012, 40, 10215–10227. [Google Scholar] [CrossRef] [PubMed]
- Crump, N.T.; Hazzalin, C.A.; Bowers, E.M.; Alani, R.M.; Cole, P.A.; Mahadevan, L.C. Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. Proc. Natl. Acad. Sci. USA 2011, 108, 7814–7819. [Google Scholar]
- Rottach, A.; Frauer, C.; Pichler, G.; Bonapace, I.M.; Spada, F.; Leonhardt, H. The multi-domain protein Np95 connects DNA methylation and histone modification. Nucleic Acids Res. 2009, 38, 1796–1804. [Google Scholar] [CrossRef]
- Rothbart, S.B.; Dickson, B.M.; Ong, M.S.; Krajewski, K.; Houliston, S.; Kireev, D.B.; Arrowsmith, C.H.; Strahl, B.D. Multivalent histone engagement by the linked tandem Tudor and PHD domains of UHRF1 is required for the epigenetic inheritance of DNA methylation. Genes Dev. 2013, 27, 1288–1298. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Q.; Li, P.; Zhao, Q.; Zhang, J.; Li, J.; Koseki, H.; Wong, J. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat. Commun. 2013, 4, 1563. [Google Scholar] [CrossRef]
- Atlasi, Y.; Stunnenberg, H.G. The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 2017, 18, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Shlyueva, D.; Stampfel, G.; Stark, A. Transcriptional enhancers: From properties to genome-wide predictions. Nat. Rev. Genet. 2014, 15, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Harmston, N.; Lenhard, B. Chromatin and epigenetic features of long-range gene regulation. Nucleic Acids Res. 2013, 41, 7185–7199. [Google Scholar] [CrossRef] [PubMed]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Ernst, J.; Kellis, M. Discovery and characterization of chromatin states for systematic annotation of the human genome. Nat. Biotechnol. 2010, 28, 817–825. [Google Scholar] [CrossRef]
- Monk, M.; Boubelik, M.; Lehnert, S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 1987, 99, 371–382. [Google Scholar]
- Wang, L.; Zhang, J.; Duan, J.; Gao, X.; Zhu, W.; Lu, X.; Yang, L.; Zhang, J.; Li, G.; Ci, W. Programming and inheritance of parental DNA methylomes in mammals. Cell 2014, 157, 979–991. [Google Scholar] [PubMed]
- Zhu, P.; Guo, H.; Ren, Y.; Hou, Y.; Dong, J.; Li, R.; Lian, Y.; Fan, X.; Hu, B.; Gao, Y.; et al. Single-cell DNA methylome sequencing of human preimplantation embryos. Nat. Genet. 2017, 50, 12–19. [Google Scholar] [CrossRef]
- Grosswendt, S.; Kretzmer, H.; Smith, Z.D.; Kumar, A.S.; Hetzel, S.; Wittler, L.; Klages, S.; Timmermann, B.; Mukherji, S.; Meissner, A. Epigenetic regulator function through mouse gastrulation. Nature 2020, 584, 102–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Y.; Yin, Q.; Du, Z.; Peng, X.; Wang, Q.; Fidalgo, M.; Xia, W.; Li, Y.; Zhao, Z.-A.; et al. Dynamic epigenomic landscapes during early lineage specification in mouse embryos. Nat. Genet. 2017, 50, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Shi, J.; Gu, H.; Donaghey, J.; Clement, K.; Cacchiarelli, D.; Gnirke, A.; Michor, F.; Meissner, A. Epigenetic restriction of extraembryonic lineages mirrors the somatic transition to cancer. Nature 2017, 549, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Seisenberger, S.; Andrews, S.; Krueger, F.; Arand, J.; Walter, J.; Santos, F.; Popp, C.; Thienpont, B.; Dean, W.; Reik, W. The Dynamics of Genome-wide DNA Methylation Reprogramming in Mouse Primordial Germ Cells. Mol. Cell 2012, 48, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Hajkova, P.; Erhardt, S.; Lane, N.; Haaf, T.; El-Maarri, O.; Reik, W.; Walter, J.; Surani, M. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 2002, 117, 15–23. [Google Scholar] [CrossRef]
- Lee, J.; Inoue, K.; Ono, R.; Ogonuki, N.; Kohda, T.; Kaneko-Ishino, T.; Ogura, A.; Ishino, F. Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development 2002, 129, 1807–1817. [Google Scholar]
- Gkountela, S.; Zhang, K.X.; Shafiq, T.A.; Liao, W.-W.; Hargan-Calvopiña, J.; Chen, P.-Y.; Clark, A.T. DNA Demethylation Dynamics in the Human Prenatal Germline. Cell 2015, 161, 1425–1436. [Google Scholar] [CrossRef]
- Hargan-Calvopina, J.; Taylor, S.; Cook, H.; Hu, Z.; Lee, S.A.; Yen, M.-R.; Chiang, Y.-S.; Chen, P.-Y.; Clark, A.T. Stage-Specific Demethylation in Primordial Germ Cells Safeguards against Precocious Differentiation. Dev. Cell 2016, 39, 75–86. [Google Scholar] [CrossRef]
- Okae, H.; Chiba, H.; Hiura, H.; Hamada, H.; Sato, A.; Utsunomiya, T.; Kikuchi, H.; Yoshida, H.; Tanaka, A.; Suyama, M.; et al. Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development. PLOS Genet. 2014, 10, e1004868. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sakurai, T.; Imai, M.; Takahashi, N.; Fukuda, A.; Yayoi, O.; Sato, S.; Nakabayashi, K.; Hata, K.; Sotomaru, Y.; et al. Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks. PLOS Genet. 2012, 8, e1002440. [Google Scholar] [CrossRef]
- Shirane, K.; Toh, H.; Kobayashi, H.; Miura, F.; Chiba, H.; Ito, T.; Kono, T.; Sasaki, H. Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases. PLOS Genet. 2013, 9, e1003439. [Google Scholar] [CrossRef]
- Alkhaled, Y.; Laqqan, M.; Tierling, S.; Porto, C.L.; Amor, H.; Hammadeh, M.E. Impact of cigarette-smoking on sperm DNA methylation and its effect on sperm parameters. Andrologia 2018, 50, e12950. [Google Scholar] [CrossRef]
- Laqqan, M.; Tierling, S.; Alkhaled, Y.; Porto, C.; Solomayer, E.; Hammadeh, M. Aberrant DNA methylation patterns of human spermatozoa in current smoker males. Reprod. Toxicol. 2017, 71, 126–133. [Google Scholar] [CrossRef]
- Jenkins, T.; James, E.; Alonso, D.; Hoidal, J.; Murphy, P.; Hotaling, J.; Cairns, B.; Carrell, D.; Aston, K. Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology 2017, 5, 1089–1099. [Google Scholar] [PubMed]
- Dura, M.; Teissandier, A.; Armand, M.; Barau, J.; Lapoujade, C.; Fouchet, P.; Bonneville, L.; Schulz, M.; Weber, M.; Baudrin, L.G.; et al. DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis. Nat. Genet. 2022, 54, 469–480. [Google Scholar] [CrossRef]
- Uehara, R.; Yeung, W.K.A.; Toriyama, K.; Ohishi, H.; Kubo, N.; Toh, H.; Suetake, I.; Shirane, K.; Sasaki, H. The DNMT3A ADD domain is required for efficient de novo DNA methylation and maternal imprinting in mouse oocytes. PLOS Genet. 2023, 19, e1010855. [Google Scholar] [CrossRef]
- Greeson, K.W.; Crow, K.M.S.; Edenfield, R.C.; Easley, C.A. Inheritance of paternal lifestyles and exposures through sperm DNA methylation. Nat. Rev. Urol. 2023, 20, 356–370. [Google Scholar] [CrossRef]
- Tang, W.W.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef]
- Menezo, Y.J.; Dale, B.; Elder, K. The negative impact of the environment on methylation/epigenetic marking in gametes and embryos: A plea for action to protect the fertility of future generations. Mol. Reprod. Dev. 2019, 86, 1273–1282. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Zou, Z.; Chen, L.; Shen, C.; Xu, S.; Zhang, J.; Zhao, F.; Ge, S.; Gao, Q.; et al. Abnormal Methylation of Imprinted Genes and Cigarette Smoking: Assessment of Their Association with the Risk of Male Infertility. Reprod. Sci. 2017, 24, 114–123. [Google Scholar] [CrossRef]
- Murphy, S.K.; Itchon-Ramos, N.; Visco, Z.; Huang, Z.; Grenier, C.; Schrott, R.; Acharya, K.; Boudreau, M.-H.; Price, T.M.; Raburn, D.J. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018, 13, 1208–1221. [Google Scholar]
- Schrott, R.; Murphy, S.K.; Modliszewski, J.L.; E King, D.; Hill, B.; Itchon-Ramos, N.; Raburn, D.; Price, T.; Levin, E.D.; Vandrey, R.; et al. Refraining from use diminishes cannabis-associated epigenetic changes in human sperm. Environ. Epigenetics 2021, 7, dvab009. [Google Scholar] [CrossRef]
- Schrott, R.; Rajavel, M.; Acharya, K.; Huang, Z.; Acharya, C.; Hawkey, A.; Pippen, E.; Lyerly, H.K.; Levin, E.D.; Murphy, S.K. Sperm DNA methylation altered by THC and nicotine: Vulnerability of neurodevelopmental genes with bivalent chromatin. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Kopecka, V.; Pauciullo, A.; Rubes, J. Impact of air pollution from different sources on sperm DNA methylation. Int. J. Environ. Health Res. 2024, 34, 3503–3514. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, Q.; Lu, Y.; Li, M.; Zhou, Y.; Wu, P.; Li, J.; Pan, F.; Han, X.; Chen, M.; et al. Semen quality and sperm DNA methylation in relation to long-term exposure to air pollution in fertile men: A cross-sectional study. Environ. Pollut. 2022, 300, 118994. [Google Scholar] [CrossRef] [PubMed]
- Schrott, R.; I Feinberg, J.; Newschaffer, C.J.; Hertz-Picciotto, I.; A Croen, L.; Fallin, M.D.; E Volk, H.; Ladd-Acosta, C.; Feinberg, A.P. Exposure to air pollution is associated with DNA methylation changes in sperm. Environ. Epigenetics 2024, 10, dvae003. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, J.; Wang, J.; Zhou, Y.; Bai, S.; Tang, Q.; Li, J.; Pan, F.; Xu, Q.; Lu, C.; et al. Alterations in sperm DNA methylation may as a mediator of paternal air pollution exposure and offspring birth outcomes: Insight from a birth cohort study. Environ. Res. 2023, 244, 117941. [Google Scholar] [CrossRef]
- Tian, M.; Liu, L.; Zhang, J.; Huang, Q.; Shen, H. Positive association of low-level environmental phthalate exposure with sperm motility was mediated by DNA methylation: A pilot study. Chemosphere 2019, 220, 459–467. [Google Scholar] [CrossRef]
- Wu, H.; Estill, M.S.; Shershebnev, A.; Suvorov, A.; A Krawetz, S.; Whitcomb, B.W.; Dinnie, H.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: A cross-sectional study. Hum. Reprod. 2017, 32, 2159–2169. [Google Scholar] [CrossRef]
- Oluwayiose, O.A.; Marcho, C.; Wu, H.; Houle, E.; Krawetz, S.A.; Suvorov, A.; Mager, J.; Pilsner, J.R. Paternal preconception phthalate exposure alters sperm methylome and embryonic programming. Environ. Int. 2021, 155, 106693. [Google Scholar] [CrossRef]
- Tadros, W.; Lipshitz, H.D. The maternal-to-zygotic transition: A play in two acts. Development 2009, 136, 3033–3042. [Google Scholar] [CrossRef]
- Hertig, A.T.; Rock, J.; Adams, E.C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 1956, 98, 435–493. [Google Scholar] [CrossRef] [PubMed]
- Hertig, A.T.; Rock, J.; Adams, E.C.; Mulligan, W.J. On the preimplantation stages of the human ovum: A description of four normal and four abnormal specimens ranging from the second to the fifth day of development. Contrib. Embryol. 1954, 35, 220. [Google Scholar]
- Rossant, J. Investigation of the determinative state of the mouse inner cell mass. Development 1975, 33, 991–1001. [Google Scholar] [CrossRef]
- Tarkowski, A.K.; Suwińska, A.; Czołowska, R.; Ożdżeński, W. Individual blastomeres of 16- and 32-cell mouse embryos are able to develop into foetuses and mice. Dev. Biol. 2010, 348, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Posfai, E.; Schell, J.P.; Janiszewski, A.; Rovic, I.; Murray, A.; Bradshaw, B.; Yamakawa, T.; Pardon, T.; El Bakkali, M.; Talon, I.; et al. Evaluating totipotency using criteria of increasing stringency. Nat. Cell Biol. 2021, 23, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, P.; Yan, L.; Li, R.; Hu, B.; Lian, Y.; Yan, J.; Ren, X.; Lin, S.; Li, J.; et al. The DNA methylation landscape of human early embryos. Nature 2014, 511, 606–610. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Puschendorf, M.; Terranova, R.; Boutsma, E.; Mao, X.; Isono, K.-I.; Brykczynska, U.; Kolb, C.; Otte, A.P.; Koseki, H.; Orkin, S.H.; et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat. Genet. 2008, 40, 411–420. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Chen, J.; Liu, W.; Lai, W.; Liu, B.; Li, X.; Liu, L.; Xu, S.; Dong, Q.; et al. Stella safeguards the oocyte methylome by preventing de novo methylation mediated by DNMT1. Nature 2018, 564, 136–140. [Google Scholar] [CrossRef]
- Andrews, S.; Krueger, C.; Mellado-Lopez, M.; Hemberger, M.; Dean, W.; Perez-Garcia, V.; Hanna, C.W. Mechanisms and function of de novo DNA methylation in placental development reveals an essential role for DNMT3B. Nat. Commun. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Schübeler, D. Function and information content of DNA methylation. Nature 2015, 517, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, Z.; Lifshitz, A.; Mittnenzweig, M.; Chomsky, E.; Schwartzman, O.; Ben-Kiki, O.; Zerbib, M.; Tanay, A. DNA methyltransferases 3A and 3B target specific sequences during mouse gastrulation. Nat. Struct. Mol. Biol. 2022, 29, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Moritz, L.; Hammoud, S.S. The Art of Packaging the Sperm Genome: Molecular and Structural Basis of the Histone-To-Protamine Exchange. Front. Endocrinol. 2022, 13, 895502. [Google Scholar] [CrossRef]
- Yoshida, K.; Muratani, M.; Araki, H.; Miura, F.; Suzuki, T.; Dohmae, N.; Katou, Y.; Shirahige, K.; Ito, T.; Ishii, S. Mapping of histone-binding sites in histone replacement-completed spermatozoa. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, Y. Nucleosome assembly is required for nuclear pore complex assembly in mouse zygotes. Nat. Struct. Mol. Biol. 2014, 21, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Schlissel, G.; Rine, J. The nucleosome core particle remembers its position through DNA replication and RNA transcription. Proc. Natl. Acad. Sci. USA 2019, 116, 20605–20611. [Google Scholar] [CrossRef]
- Gan, H.; Serra-Cardona, A.; Hua, X.; Zhou, H.; Labib, K.; Yu, C.; Zhang, Z. The Mcm2-Ctf4-Polα Axis Facilitates Parental Histone H3-H4 Transfer to Lagging Strands. Mol. Cell 2018, 72, 140–151.e3. [Google Scholar] [CrossRef]
- Wenger, A.; Biran, A.; Alcaraz, N.; Redó-Riveiro, A.; Sell, A.C.; Krautz, R.; Flury, V.; Reverón-Gómez, N.; Solis-Mezarino, V.; Völker-Albert, M.; et al. Symmetric inheritance of parental histones governs epigenome maintenance and embryonic stem cell identity. Nat. Genet. 2023, 55, 1567–1578. [Google Scholar] [CrossRef]
- Li, Z.; Hua, X.; Serra-Cardona, A.; Xu, X.; Gan, S.; Zhou, H.; Yang, W.-S.; Chen, C.-L.; Xu, R.-M.; Zhang, Z. DNA polymerase α interacts with H3-H4 and facilitates the transfer of parental histones to lagging strands. Sci. Adv. 2020, 6, eabb5820. [Google Scholar] [CrossRef]
- Hackett, J.A.; Sengupta, R.; Zylicz, J.J.; Murakami, K.; Lee, C.; Down, T.A.; Surani, M.A. Germline DNA Demethylation Dynamics and Imprint Erasure Through 5-Hydroxymethylcytosine. Science 2013, 339, 448–452. [Google Scholar] [CrossRef]
- Gruhn, W.H.; Tang, W.W.; Dietmann, S.; Alves-Lopes, J.P.; Penfold, C.A.; Wong, F.C.K.; Ramakrishna, N.B.; Surani, M.A. Epigenetic resetting in the human germ line entails histone modification remodeling. Sci. Adv. 2023, 9, eade1257. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.M.; Goyal, P.; Maksakova, I.A.; Bilenky, M.; Leung, D.; Tang, J.X.; Shinkai, Y.; Mager, D.L.; Jones, S.; Hirst, M.; et al. DNA Methylation and SETDB1/H3K9me3 Regulate Predominantly Distinct Sets of Genes, Retroelements, and Chimeric Transcripts in mESCs. Cell Stem Cell 2011, 8, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Dietmann, S.; Irie, N.; Leitch, H.G.; Floros, V.I.; Bradshaw, C.R.; Hackett, J.A.; Chinnery, P.F.; Surani, M.A. A Unique Gene Regulatory Network Resets the Human Germline Epigenome for Development. Cell 2015, 161, 1453–1467. [Google Scholar] [CrossRef]
- Zhao, R.; Nakamura, T.; Fu, Y.; Lazar, Z.; Spector, D.L. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat. Cell Biol. 2011, 13, 1295–1304. [Google Scholar] [CrossRef]
- Reverón-Gómez, N.; González-Aguilera, C.; Stewart-Morgan, K.R.; Petryk, N.; Flury, V.; Graziano, S.; Johansen, J.V.; Jakobsen, J.S.; Alabert, C.; Groth, A. Accurate Recycling of Parental Histones Reproduces the Histone Modification Landscape during DNA Replication. Mol. Cell 2018, 72, 239–249.e5. [Google Scholar] [CrossRef] [PubMed]
- Petryk, N.; Dalby, M.; Wenger, A.; Stromme, C.B.; Strandsby, A.; Andersson, R.; Groth, A. MCM2 promotes symmetric inheritance of modified histones during DNA replication. Science 2018, 361, 1389–1392. [Google Scholar] [CrossRef]
- Escobar, T.M.; Oksuz, O.; Saldaña-Meyer, R.; Descostes, N.; Bonasio, R.; Reinberg, D. Active and Repressed Chromatin Domains Exhibit Distinct Nucleosome Segregation during DNA Replication. Cell 2019, 179, 953–963.e11. [Google Scholar] [CrossRef] [PubMed]
- Alabert, C.; Loos, C.; Voelker-Albert, M.; Graziano, S.; Forné, I.; Reveron-Gomez, N.; Schuh, L.; Hasenauer, J.; Marr, C.; Imhof, A.; et al. Domain Model Explains Propagation Dynamics and Stability of Histone H3K27 and H3K36 Methylation Landscapes. Cell Rep. 2020, 30, 1223–1234.e8. [Google Scholar] [CrossRef]
- Pelham-Webb, B.; Polyzos, A.; Wojenski, L.; Kloetgen, A.; Li, J.; Di Giammartino, D.C.; Sakellaropoulos, T.; Tsirigos, A.; Core, L.; Apostolou, E. H3K27ac bookmarking promotes rapid post-mitotic activation of the pluripotent stem cell program without impacting 3D chromatin reorganization. Mol. Cell 2021, 81, 1732–1748.e8. [Google Scholar] [CrossRef]
- Aravin, A.A.; Sachidanandam, R.; Girard, A.; Fejes-Toth, K.; Hannon, G.J. Developmentally Regulated piRNA Clusters Implicate MILI in Transposon Control. Science 2007, 316, 744–747. [Google Scholar] [CrossRef]
- Kuramochi-Miyagawa, S.; Watanabe, T.; Gotoh, K.; Totoki, Y.; Toyoda, A.; Ikawa, M.; Asada, N.; Kojima, K.; Yamaguchi, Y.; Ijiri, T.W.; et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008, 22, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Kremsky, I.; Corces, V.G. Protection from DNA re-methylation by transcription factors in primordial germ cells and pre-implantation embryos can explain trans-generational epigenetic inheritance. Genome Biol. 2020, 21, 1–31. [Google Scholar] [CrossRef]
- Festuccia, N.; Gonzalez, I.; Owens, N.; Navarro, P. Mitotic bookmarking in development and stem cells. Development 2017, 144, 3633–3645. [Google Scholar] [CrossRef]
- Soufi, A.; Dalton, S. Cycling through developmental decisions: How cell cycle dynamics control pluripotency, differentiation and reprogramming. Development 2016, 143, 4301–4311. [Google Scholar]
- Rakyan, V.K.; E Blewitt, M.; Druker, R.; I Preis, J.; Whitelaw, E. Metastable epialleles in mammals. Trends Genet. 2002, 18, 348–351. [Google Scholar] [CrossRef]
- Wolff, G.L. Influence of maternal phenotype on metabolic differentiation of agouti locus mutants in the mouse. Genetics 1978, 88, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Duhl, D.M.J.; Vrieling, H.; Miller, K.A.; Wolff, G.L.; Barsh, G.S. Neomorphic agouti mutations in obese yellow mice. Nat. Genet. 1994, 8, 59–65. [Google Scholar] [CrossRef]
- Belyaev, D.K.; Ruvinsky, A.O.; Borodin, P.M. Inheritance of alternative states of the fused gene in mice. J. Hered. 1981, 72, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Dickies, M.M. A NEW VIABLE YELLOW MUTATION IN THE HOUSE MOUSE. J. Hered. 1962, 53, 84–86. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Fullston, T.; Teague, E.M.C.O.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F 2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.J.; Fonseca, M.A.; Campbell, K.E.; Moyce, B.L.; Cole, L.K.; Hatch, G.M.; Doucette, C.A.; Klein, J.; Aliani, M.; Dolinsky, V.W. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J. Physiol. 2015, 593, 3181–3197. [Google Scholar] [CrossRef]
- Landon, M.B.; Rice, M.M.; Varner, M.W.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Biggio, J.J.R.; Thorp, J.M.; Chien, E.K.; et al. Mild Gestational Diabetes Mellitus and Long-Term Child Health. Diabetes Care 2014, 38, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Quilter, C.R.; Cooper, W.N.; Cliffe, K.M.; Skinner, B.M.; Prentice, P.M.; Nelson, L.; Bauer, J.; Ong, K.K.; Constância, M.; Lowe, W.L.; et al. Impact on offspring methylation patterns of maternal gestational diabetes mellitus and intrauterine growth restraint suggest common genes and pathways linked to subsequent type 2 diabetes risk. FASEB J. 2014, 28, 4868–4879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alba-Linares, J.J.; Pérez, R.F.; Tejedor, J.R.; Bastante-Rodríguez, D.; Ponce, F.; Carbonell, N.G.; Zafra, R.G.; Fernández, A.F.; Fraga, M.F.; Lurbe, E. Maternal obesity and gestational diabetes reprogram the methylome of offspring beyond birth by inducing epigenetic signatures in metabolic and developmental pathways. Cardiovasc. Diabetol. 2023, 22, 1–18. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Yeung, E.; Biedrzycki, R.J.; Herrera, L.C.G.; Issarapu, P.; Dou, J.; Marques, I.F.; Mansuri, S.R.; Page, C.M.; Harbs, J.; Khodasevich, D.; et al. Maternal age is related to offspring DNA methylation: A meta-analysis of results from the PACE consortium. Aging Cell 2024, 23, e14194. [Google Scholar] [CrossRef]
- Cardenas, A.; Faleschini, S.; Cortes Hidalgo, A.; Rifas-Shiman, S.L.; Baccarelli, A.A.; DeMeo, D.L.; Litonjua, A.A.; Neumann, A.; Felix, J.F.; Jaddoe, V.W.V.; et al. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: Epigenome-wide associations at birth and persistence into early childhood. Clin. Epigenet. 2019, 11, 56. [Google Scholar] [CrossRef]
- Bogdanović, O.; Smits, A.H.; Mustienes, E.d.l.C.; Tena, J.J.; Ford, E.; Williams, R.; Senanayake, U.; Schultz, M.D.; Hontelez, S.; van Kruijsbergen, I.; et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 2016, 48, 417–426. [Google Scholar] [CrossRef]
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 2011, 479, 365–371. [Google Scholar] [CrossRef]
- Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Generational comparisons (F1 versus F3) of vinclozolin induced epigenetic transgenerational inheritance of sperm differential DNA methylation regions (epimutations) using MeDIP-Seq. Environ. Epigenetics 2017, 3, dvx016. [Google Scholar] [CrossRef]
- Macfarlan, T.S.; Gifford, W.D.; Driscoll, S.; Lettieri, K.; Rowe, H.M.; Bonanomi, D.; Firth, A.; Singer, O.; Trono, D.; Pfaff, S.L. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 2012, 487, 57–63. [Google Scholar] [CrossRef]
- Wu, J.; Huang, B.; Chen, H.; Yin, Q.; Liu, Y.; Xiang, Y.; Zhang, B.; Liu, B.; Wang, Q.; Xia, W.; et al. The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 2016, 534, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Ma, H.; Liu, D. Endogenous Retroviruses Function as Gene Expression Regulatory Elements During Mammalian Pre-implantation Embryo Development. Int. J. Mol. Sci. 2019, 20, 790. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hong, C.; Zhang, B.; Lowdon, R.F.; Xing, X.; Li, D.; Zhou, X.; Lee, H.J.; Maire, C.L.; Ligon, K.L.; et al. DNA hypomethylation within specific transposable element families associates with tissue-specific enhancer landscape. Nat. Genet. 2013, 45, 836–841. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Maleki, M.; Vargoorani, M.E.; Amiri, V. A review of epigenetic changes in asthma: Methylation and acetylation. Clin. Epigenetics 2021, 13, 1–23. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lin, Z.-J.; Li, C.-C.; Lin, X.; Shan, S.-K.; Guo, B.; Zheng, M.-H.; Li, F.; Yuan, L.-Q.; Li, Z.-H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 1–27. [Google Scholar] [CrossRef]
- Raghubeer, S. The influence of epigenetics and inflammation on cardiometabolic risks. Semin. Cell Dev. Biol. 2023, 154, 175–184. [Google Scholar] [CrossRef]
- Kucher, A.; Nazarenko, M. Epigenetics of Cardiomyopathy: Histone Modifications and DNA Methylation. Russ. J. Genet. 2023, 59, 226–241. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakarya, R. From Lineage to Longevity: A Field Guide to the Key Players in Epigenetic Contribution to Offspring Health. Curr. Issues Mol. Biol. 2025, 47, 323. https://doi.org/10.3390/cimb47050323
Zakarya R. From Lineage to Longevity: A Field Guide to the Key Players in Epigenetic Contribution to Offspring Health. Current Issues in Molecular Biology. 2025; 47(5):323. https://doi.org/10.3390/cimb47050323
Chicago/Turabian StyleZakarya, Razia. 2025. "From Lineage to Longevity: A Field Guide to the Key Players in Epigenetic Contribution to Offspring Health" Current Issues in Molecular Biology 47, no. 5: 323. https://doi.org/10.3390/cimb47050323
APA StyleZakarya, R. (2025). From Lineage to Longevity: A Field Guide to the Key Players in Epigenetic Contribution to Offspring Health. Current Issues in Molecular Biology, 47(5), 323. https://doi.org/10.3390/cimb47050323


_Kim.png)


