The Impact and Molecular Mechanisms of Exercise in Cancer Therapy
Abstract
1. Introduction
2. Exercise-Based Rehabilitation: A Key Pathway to Functional Recovery in Cancer Patients
3. The Role of Exercise Rehabilitation in Cancer Patients
3.1. Inflammation
3.2. Immune Function
3.3. Energy Metabolism
3.4. Hormones and Neurotransmitters
3.4.1. Insulin
3.4.2. Sex Hormone
3.4.3. Irisin
3.4.4. Dopamine
3.5. Apoptosis
3.6. Tumor Vascularity
3.7. Cancer Tissue Invasion and Metastasis
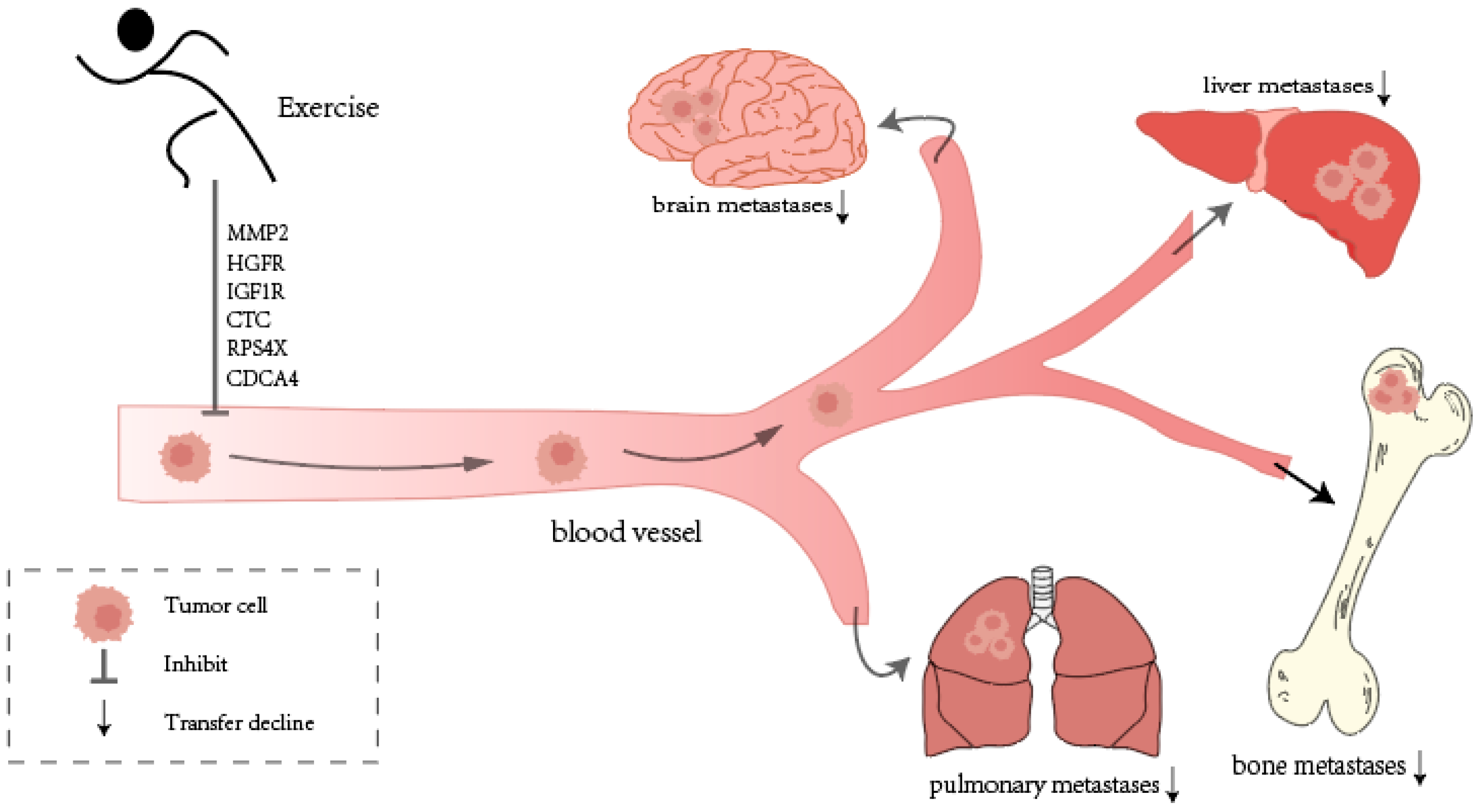
3.7.1. Exercise Inhibits Cancer Metastasis: Animal and Human Studies
3.7.2. Challenges and Solutions in Metastatic Tumors
3.8. Body Function and Composition (Human)
3.9. Cancer-Related Fatigue (Human)
3.10. Quality of Life (Human)
4. Recommended Exercise Prescription for Cancer Patients
5. The Model of Rehabilitation Training for Cancer Patients
6. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Francisco, P.M.S.B.; Friestino, J.K.O.; de Olanda Ferraz, R.; de Macedo Bacurau, A.G.; Stopa, S.R.; de Carvalho Moreira-Filho, D. Prevalence of diagnosis and types of cancer in the elderly: Data from National Health Survey 2013. Rev. Bras. Geriatr. Gerontol. 2020, 23, e200023. [Google Scholar] [CrossRef]
- Miranda, B.C.J.; Tustumi, F.; Nakamura, E.T.; Shimanoe, V.H.; Kikawa, D.; Waisberg, J. Obesity and Colorectal Cancer: A Narrative Review. Medicina 2024, 60, 1218. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.A.; Jawed, I.; Zoorob, R.J.; Salemi, J.L. Completeness of Cancer Case Ascertainment in International Cancer Registries: Exploring the Issue of Gender Disparities. Front. Oncol. 2020, 10, 1148. [Google Scholar] [CrossRef]
- Raza, S.A.; da Costa, W.L.; Thrift, A.P. Editorial: Sex differences in cancer incidence, mortality, and survival: Methodological perspectives. Front. Oncol. 2024, 14, 1441965. [Google Scholar] [CrossRef]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, P.; Lin, Y.; Guo, X.; Wang, J. Prevalence and risk factors for anxiety in patients with early- and middle-stage lung cancer: A cross-sectional study. Front. Psychol. 2024, 15, 1413591. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, L.; Motiño, O.; Barriuso, D.; de la Puente-Aldea, J.; Alvarez-Frutos, L.; Kroemer, G.; Palacios-Ramirez, R.; Senovilla, L. Obesity-associated colorectal cancer. Int. J. Mol. Sci. 2024, 25, 8836. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, J.; Wang, Q.; Liang, Y.; Li, X.; Chen, G.; Ma, L.; Liu, X.; Zhou, F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023, 13, 82. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wozniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef]
- Ni, X.; Li, Z.; Li, X.; Zhang, X.; Bai, G.; Liu, Y.; Zheng, R.; Zhang, Y.; Xu, X.; Liu, Y.; et al. Socioeconomic inequalities in cancer incidence and access to health services among children and adolescents in China: A cross-sectional study. Lancet 2022, 400, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 2022, 40, 720–737.e725. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Cheng, L. Advances in diagnosis and treatment of testicular cancer. BMJ 2022, 379, e070499. [Google Scholar] [CrossRef]
- Pan, Y.; Deng, X.; Zhuang, Y.; Li, J. Research trends around exercise rehabilitation among cancer patients: A bibliometrics and visualized knowledge graph analysis. BioMed Res. Int. 2022, 2022, 3755460. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.R.; Heesch, K.C.; Brown, W.J. Exercise and cancer rehabilitation: A systematic review. Cancer Treat. Rev. 2010, 36, 185–194. [Google Scholar] [CrossRef]
- Morrow, G.R.; Shelke, A.R.; Roscoe, J.A.; Hickok, J.T.; Mustian, K. Management of cancer-related fatigue. Cancer Investig. 2005, 23, 229–239. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise therapy and cardiovascular toxicity in cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef]
- Andrykowski, M.A.; Lykins, E.; Floyd, A. Psychological health in cancer survivors. Semin. Oncol. Nurs. 2008, 24, 193–201. [Google Scholar] [CrossRef]
- Aguilar-Aragón, M.; Perez-Archila, C.; Ferreira-Pinto, M.; Estirado, A.; Rojas, M.; Moreno, E.; Domínguez, M. A tumour protective mechanism of the brain reward system. In Proceedings of the Drosophila Spanish Meeting (2024), Sant Joan d’Alacant, Spain, 14–16 March 2024. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- McCabe, M.S.; Bhatia, S.; Oeffinger, K.C.; Reaman, G.H.; Tyne, C.; Wollins, D.S.; Hudson, M.M. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. J. Clin. Oncol. 2013, 31, 631. [Google Scholar] [CrossRef] [PubMed]
- Karloh, M.; Matias, T.S.; de Oliveira, J.M.; de Lima, F.F.; Araújo Pinheiro, D.H.; Barbosa, G.B.; Furlanetto, K.C.; Carvalho, C.R.F. Breaking barriers to rehabilitation: The role of behavior change theories in overcoming the challenge of exercise-related behavior change. Braz. J. Phys. Ther. 2023, 27, 100574. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, A.; Cromwell, R. Kinetic chain rehabilitation: A theoretical framework. Rehabil. Res. Pract. 2012, 2012, 853037. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, S.; Suarez-Cuervo, A.N.; León-Prieto, C. Exercise progressions and regressions in sports training and rehabilitation. J. Bodyw. Mov. Ther. 2024, 40, 1879–1889. [Google Scholar] [CrossRef]
- Newton, R.; Hart, N.; Clay, T. Keeping patients with cancer exercising in the age of COVID-19. JCO Oncol. Pract. 2020, 16, 656–664. [Google Scholar] [CrossRef]
- Rao, R.M.; Amritanshu, R.; Vinutha, H.; Vaishnaruby, S.; Deepashree, S.; Megha, M.; Geetha, R.; Ajaikumar, B. Role of yoga in cancer patients: Expectations, benefits, and risks: A review. Indian J. Palliat. Care 2017, 23, 225. [Google Scholar] [CrossRef]
- Hilfiker, R.; Meichtry, A.; Eicher, M.; Balfe, L.N.; Knols, R.H.; Verra, M.L.; Taeymans, J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br. J. Sports Med. 2018, 52, 651–658. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med. Sci. Sports Exerc. 2019, 51, 2391. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375. [Google Scholar] [CrossRef]
- Soong, R.Y.; Low, C.E.; Ong, V.; Sim, I.; Lee, C.; Lee, F.; Chew, L.; Yau, C.E.; Lee, A.; Chen, M.Z. Exercise Interventions for Depression, Anxiety, and Quality of Life in Older Adults With Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2457859. [Google Scholar] [CrossRef]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A systematic review of exercise systematic reviews in the cancer literature (2005-2017). PMR 2017, 9, S347–S384. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Mixon, A.C.; McLarney, M.D. Safety, precautions, and modalities in cancer rehabilitation: An updated review. Curr. Phys. Med. Rehabil. Rep. 2021, 9, 142–153. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1293–1311. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Fiorentini, C.; Mannucci, R.; Bonifazi, M.; Mondillo, S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur. J. Prev. Cardiol. 2021, 28, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Newton, R.U.; Spence, R.R.; Galvão, D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport. 2019, 22, 1175–1199. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Torres, E.; Garrido, A.; de Cabo, R.; Carretero, J.; Gómez-Cabrera, M.C. Molecular mechanisms of cancer cachexia. Role of exercise training. Mol. Asp. Med. 2024, 99, 101293. [Google Scholar] [CrossRef] [PubMed]
- Vialka-Moser, F.; Crevenna, R.; Korpan, M.; Quittan, M. Cancer rehabilitation. Particularly with aspects on physical impairment. J. Rehabil. Med. 2003, 35, 153–162. [Google Scholar]
- Crevenna, R. Cancer rehabilitation and palliative care—Two important parts of comprehensive cancer care. Support. Care Cancer 2015, 23, 3407–3408. [Google Scholar] [CrossRef]
- Crevenna, R. Physical medicine and rehabilitation—A relevant interdisciplinary speciality. Wien. Med. Wochenschr. 2016, 166, 2–3. [Google Scholar] [CrossRef][Green Version]
- Tan, G.A.; Peiris, C.L.; Dennett, A.M. Cancer survivors maintain health benefits 6 to 12 months after exercise-based rehabilitation: A systematic review and meta-analysis. J. Cancer Surviv. 2024, 18, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.J.; Covington, K.R.; Pergolotti, M.; Sharp, J.; Maynard, B.; Eagan, J.; Beasley, J. Translating research to practice using a team-based approach to cancer rehabilitation: A physical therapy and exercise-based cancer rehabilitation program reduces fatigue and improves aerobic capacity. Rehabil. Oncol. 2018, 36, 206–213. [Google Scholar] [CrossRef]
- Hasan, S.S.; Rivera, D.; Wu, X.-C.; Durbin, E.B.; Christian, J.B.; Tourassi, G. Knowledge graph-enabled cancer data analytics. IEEE J. Biomed. Health Inform. 2020, 24, 1952–1967. [Google Scholar] [CrossRef]
- Schwartz, S.A. Kuhn, consciousness, and paradigms. Explor. J. Sci. Heal. 2018, 14, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Boon, M. An engineering paradigm in the biomedical sciences: Knowledge as epistemic tool. Prog. Biophys. Mol. Biol. 2017, 129, 25–39. [Google Scholar] [CrossRef]
- Paton, C. Of paradigms and precipitate relativism: Kuhn and the social sciences. J. Health Serv. Res. Policy 2014, 19, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Dennett, A.M.; Harding, K.E.; Reed, M.S. The challenge of timing: A qualitative study on clinician and patient perspectives about implementing exercise-based rehabilitation in an acute cancer treatment setting. Support. Care Cancer 2020, 28, 6035–6043. [Google Scholar] [CrossRef]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxid. Med. Cell Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef]
- Chow, Z.S.; Moreland, A.T.; Macpherson, H.; Teo, W.P. The Central Mechanisms of Resistance Training and Its Effects on Cognitive Function. Sports Med. 2021, 51, 2483–2506. [Google Scholar] [CrossRef]
- Schulenkorf, N.; Siefken, K. Managing sport-for-development and healthy lifestyles: The sport-for-health model. Sport Manag. Rev. 2019, 22, 96–107. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Pan, C.; Dai, L.; Wu, Y.; Deng, Y. The effect of mind-body therapies on insomnia: A systematic review and meta-analysis. Evid.-Based Complement. Altern. Med. 2019, 2019, 9359807. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M. Quantifying the association between physical activity and cardiovascular disease and diabetes: A systematic review and meta-analysis. J. Am. Heart Assoc. 2016, 5, e002495. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Flanagan, S.D.; Shurley, J.P.; Todd, J.S.; Todd, T.C. Understanding the science of resistance training: An evolutionary perspective. Sports Med. 2017, 47, 2415–2435. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.; Murrell, A.; van der Touw, T.; Smart, N. HIIT is not superior to MICT in altering blood lipids: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2019, 5, e000647. [Google Scholar] [CrossRef]
- Hou, L.; Wang, J.; Mao, M.; Zhang, Z.; Liu, D.; Gao, S.; Liang, K.; Lu, L. Effect of yoga on cancer-related fatigue in patients with breast cancer: A systematic review and meta-analysis. Medicine 2024, 103, e36468. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.B.; Cohen, L.; McCall, T.; Telles, S.; Cramer, H. The Principles and Practice of Yoga in Health Care; Jessica Kingsley Publishers: London, UK, 2024. [Google Scholar]
- Klein, P.J.; Baumgarden, J.; Schneider, R. Qigong and Tai Chi as Therapeutic Exercise: Survey of Systematic Reviews and Meta-Analyses Addressing Physical Health Conditions. Altern. Ther. Health Med. 2019, 25, 48–53. [Google Scholar]
- Fang, J.; Zhang, L.; Wu, F.; Ye, J.; Cai, S.; Lian, X. The Safety of Baduanjin Exercise: A Systematic Review. Evid. Based Complement. Altern. Med. 2021, 2021, 8867098. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Jurczyszyn, A.; Piotrowska, A.; Pilch, W.; Antosiewicz, J.; Żychowska, M. The Effect of a Six-Week Nordic Walking Training Cycle on Oxidative Damage of Macromolecules and Iron Metabolism in Older Patients with Multiple Myeloma in Remission—Randomized Clinical Trial. Int. J. Mol. Sci. 2023, 24, 15358. [Google Scholar] [CrossRef]
- Loh, E.W.; Shih, H.F.; Lin, C.K.; Huang, T.W. Effect of progressive muscle relaxation on postoperative pain, fatigue, and vital signs in patients with head and neck cancers: A randomized controlled trial. Patient Educ. Couns. 2022, 105, 2151–2157. [Google Scholar] [CrossRef]
- Cho, S.T.; Kim, K.H. Pelvic floor muscle exercise and training for coping with urinary incontinence. J. Exerc. Rehabil. 2021, 17, 379–387. [Google Scholar] [CrossRef]
- Geddes, E.L.; O’Brien, K.; Reid, W.D.; Brooks, D.; Crowe, J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: An update of a systematic review. Respir. Med. 2008, 102, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Matei, B.; Winters-Stone, K.M.; Raber, J. Examining the mechanisms behind exercise’s multifaceted impacts on body composition, cognition, and the gut microbiome in cancer survivors: Exploring the links to oxidative stress and inflammation. Antioxidants 2023, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Muenchow, M.; Wallig, M.A.; Horn, P.L.; Woods, J.A. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J. Appl. Physiol. 2004, 96, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Furrer, R.; Jauch, A.J.; Nageswara Rao, T.; Dilbaz, S.; Rhein, P.; Steurer, S.A.; Recher, M.; Skoda, R.C.; Handschin, C. Remodeling of metabolism and inflammation by exercise ameliorates tumor-associated anemia. Sci. Adv. 2021, 7, eabi4852. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Y.; He, K.; Sui, X.; Chen, J.; Wang, T.; Chen, M.; Wang, Z.; Yi, J.; Zhao, L. Voluntarily wheel running inhibits the growth of CRPC xenograft by inhibiting HMGB1 in mice. Exp. Gerontol. 2023, 174, 112118. [Google Scholar] [CrossRef]
- Veras, A.S.C.; Correia, R.R.; Batista, V.R.G.; de Almeida Tavares, M.E.; Rubira, R.J.G.; Fiais, G.A.; Giometti, I.C.; Chaves-Neto, A.H.; Teixeira, G.R. Aerobic physical exercise modifies the prostate tumoral environment. Life Sci. 2023, 332, 122097. [Google Scholar] [CrossRef]
- Bangsub, L.; Chung, W. Effects of aerobic exercise on cytokine expression in a breast cancer mouse model. Iran. J. Public Health 2020, 49, 14. [Google Scholar]
- Mader, T.; Chaillou, T.; Alves, E.S.; Jude, B.; Cheng, A.J.; Kenne, E.; Mijwel, S.; Kurzejamska, E.; Vincent, C.T.; Rundqvist, H. Exercise reduces intramuscular stress and counteracts muscle weakness in mice with breast cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 1151–1163. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Kim, A.-R.; Lee, K.-H.; Choi, S.-W. Effects of different types of exercise on inflammatory markers in cancer patients: A systematic review and Bayesian network meta-analysis. J. Sports Sci. 2025, 43, 1121–1138. [Google Scholar] [CrossRef]
- Kaushik, D.; Shah, P.K.; Mukherjee, N.; Ji, N.; Dursun, F.; Kumar, A.P.; Thompson, I.M., Jr.; Mansour, A.M.; Jha, R.; Yang, X. Effects of yoga in men with prostate cancer on quality of life and immune response: A pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2022, 25, 531–538. [Google Scholar] [CrossRef]
- Winker, M.; Stössel, S.; Neu, M.A.; Lehmann, N.; El Malki, K.; Paret, C.; Joisten, N.; Bloch, W.; Zimmer, P.; Faber, J. Exercise reduces systemic immune inflammation index (SII) in childhood cancer patients. Support. Care Cancer 2022, 30, 2905–2908. [Google Scholar] [CrossRef] [PubMed]
- Collao, N.; Sanders, O.; Caminiti, T.; Messeiller, L.; De Lisio, M. Resistance and endurance exercise training improves muscle mass and the inflammatory/fibrotic transcriptome in a rhabdomyosarcoma model. J. Cachexia Sarcopenia Muscle 2023, 14, 781–793. [Google Scholar] [CrossRef]
- Bartlett, D.B.; Hanson, E.D.; Lee, J.T.; Wagoner, C.W.; Harrell, E.P.; Sullivan, S.A.; Bates, L.C.; Alzer, M.S.; Amatuli, D.J.; Deal, A.M. The effects of 16 weeks of exercise training on neutrophil functions in breast cancer survivors. Front. Immunol. 2021, 12, 733101. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the tumor immune microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef]
- Schauer, T.; Djurhuus, S.S.; Simonsen, C.; Brasso, K.; Christensen, J.F. The effects of acute exercise and inflammation on immune function in early-stage prostate cancer. Brain Behav. Immun.-Health 2022, 25, 100508. [Google Scholar] [CrossRef]
- Berendt, M.J.; North, R.J. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J. Exp. Med. 1980, 151, 69–80. [Google Scholar] [CrossRef]
- Shimizu, J.; Yamazaki, S.; Sakaguchi, S. Induction of tumor immunity by removing CD25+ CD4+ T cells: A common basis between tumor immunity and autoimmunity. J. Immunol. 1999, 163, 5211–5218. [Google Scholar] [CrossRef]
- Chaudhary, B.; Elkord, E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: A critical systematic review of in vivo preclinical data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 2020, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zheng, Y.; Sheng, J.; Han, Y.; Yang, Y.; Pan, H.; Yao, J. CD3+ CD4-CD8-(Double-negative) T cells in inflammation, immune disorders and cancer. Front. Immunol. 2022, 13, 816005. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Gonçalves, E.; Seixas, F.; Palmeira, C.; Martins, G.; Fonseca, C.; Duarte, J.A.; Faustino-Rocha, A.I.; Colaço, B.; Pires, M.J.; Neuparth, M.J. Lifelong exercise training promotes the remodelling of the immune system and prostate signalome in a rat model of prostate carcinogenesis. GeroScience 2024, 46, 817–840. [Google Scholar] [CrossRef]
- Liguori, G.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Lavín-Pérez, A.M.; Collado-Mateo, D.; Abbasi, S.; Ferreira-Júnior, J.B.; Hekmatikar, A.H.A. Effects of exercise on immune cells with tumor-specific activity in breast cancer patients and survivors: A systematic review and meta-analysis. Support. Care Cancer 2023, 31, 507. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.N.; Chai, M.Q.; Liu, X.Y.; Wei, C.Y.; Zhang, C.C.; Sun, N.N.; Fei, Q.Z.; Peng, L.L.; Qiu, H. Exercise accelerates recruitment of CD8(+) T cell to promotes anti-tumor immunity in lung cancer via epinephrine. BMC Cancer 2024, 24, 474. [Google Scholar] [CrossRef]
- Brodin, P.; Lakshmikanth, T.; Kärre, K.; Höglund, P. Skewing of the NK cell repertoire by MHC class I via quantitatively controlled enrichment and contraction of specific Ly49 subsets. J. Immunol. 2012, 188, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Djurhuus, S.S.; Simonsen, C.; Toft, B.G.; Thomsen, S.N.; Wielsøe, S.; Røder, M.A.; Hasselager, T.; Østergren, P.B.; Jakobsen, H.; Pedersen, B.K. Exercise training to increase tumour natural killer-cell infiltration in men with localised prostate cancer: A randomised controlled trial. BJU Int. 2023, 131, 116–124. [Google Scholar] [CrossRef]
- Quinn, L.S.; Haugk, K.L.; Grabstein, K.H. Interleukin-15: A novel anabolic cytokine for skeletal muscle. Endocrinology 1995, 136, 3669–3672. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol.-Cell Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef] [PubMed]
- Berard, M.; Brandt, K.; Paus, S.B.; Tough, D.F. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 2003, 170, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Weisshaar, N.; Hotz-Wagenblatt, A.; Madi, A.; Ma, S.; Mieg, A.; Hering, M.; Mohr, K.; Schlimbach, T.; Borgers, H. Skeletal muscle antagonizes antiviral CD8+ T cell exhaustion. Sci. Adv. 2020, 6, eaba3458. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Jee, Y.-S. Effect of resistance exercise on acquired immunocytes in cancer survivors: A pilot study. Int. Neurourol. J. 2021, 25, S96. [Google Scholar] [CrossRef]
- Parent-Roberge, H.; Fontvieille, A.; Poirier, L.; Tai, L.-H.; Pavic, M.; Fülöp, T.; Riesco, E. Acute natural killer cells response to a continuous moderate intensity and a work-matched high intensity interval exercise session in metastatic cancer patients treated with chemotherapy. Brain Behav. Immun.-Health 2024, 40, 100825. [Google Scholar] [CrossRef]
- Ubink, A.; ten Tusscher, M.R.; van der Vliet, H.J.; Douma, J.A.; de Gruijl, T.D.; Bontkes, H.; Bonnet, P.; van Ens, D.; Hobo, W.; Dolstra, H. Exploring the Effect of a Supervised Exercise Intervention on Immune Cell Function and Tumour Infiltration in Patients with Breast Cancer Receiving Neoadjuvant Chemotherapy. medRxiv 2025. [Google Scholar] [CrossRef]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 1–21. [Google Scholar] [CrossRef]
- Dey, P.; Kimmelman, A.C.; DePinho, R.A. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 2021, 11, 1067–1081. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Aveseh, M.; Nikooie, R.; Aminaie, M. Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J. Physiol. 2015, 593, 2635–2648. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, P.; Schmidt, M.E.; Prentzell, M.T.; Berdel, B.; Wiskemann, J.; Kellner, K.H.; Debus, J.; Ulrich, C.; Opitz, C.A.; Steindorf, K. Resistance exercise reduces kynurenine pathway metabolites in breast cancer patients undergoing radiotherapy. Front. Oncol. 2019, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef]
- Shaw, R.J. Glucose metabolism and cancer. Curr. Opin. Cell Biol. 2006, 18, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic health, mitochondrial fitness, physical activity, and cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H. Cancer’s sweet tooth: The Janus effect of glucose metabolism in tumorigenesis. Lancet 2006, 367, 618–621. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Sami, N.; Norris, M.K.; Wan, J.; Kumagai, H.; Kim, S.J.; Cohen, P. Effect of aerobic and resistance exercise on the mitochondrial peptide MOTS-c in Hispanic and Non-Hispanic White breast cancer survivors. Sci. Rep. 2021, 11, 16916. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, H.; Zhou, H.; Liu, Q.; Qi, Z.; Zhang, Y.; Zhang, J. The role of mitochondria-derived peptides in cardiovascular disease: Recent updates. Biomed. Pharmacother. 2019, 117, 109075. [Google Scholar] [CrossRef]
- D’alonzo, N.J.; Qiu, L.; Sears, D.D.; Chinchilli, V.; Brown, J.C.; Sarwer, D.B.; Schmitz, K.H.; Sturgeon, K.M. WISER survivor trial: Combined effect of exercise and weight loss interventions on insulin and insulin resistance in breast cancer survivors. Nutrients 2021, 13, 3108. [Google Scholar] [CrossRef]
- Miles-Chan, J.L.; Harper, M.-E. Deconstructing interindividual variability in energy metabolism: Implications for metabolic health. Am. J. Physiol.-Endocrinol. Metab. 2023, 325, E107–E112. [Google Scholar] [CrossRef] [PubMed]
- Reho, J.J.; Nakagawa, P.; Mouradian, G.C., Jr.; Grobe, C.C.; Saravia, F.L.; Burnett, C.M.; Kwitek, A.E.; Kirby, J.R.; Segar, J.L.; Hodges, M.R. Methods for the comprehensive in vivo analysis of energy flux, fluid homeostasis, blood pressure, and ventilatory function in rodents. Front. Physiol. 2022, 13, 855054. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Sanchez-Jimenez, A.; Galiano-Castillo, N.; Diaz-Rodriguez, L.; Martin-Martin, L.; Arroyo-Morales, M. Effectiveness of Lumbopelvic Exercise in Colon Cancer Survivors: A Randomized Controlled Clinical Trial. Med. Sci. Sports Exerc. 2016, 48, 1438–1446. [Google Scholar] [CrossRef]
- Wendler, A.; Wehling, M. The translatability of animal models for clinical development: Biomarkers and disease models. Curr. Opin. Pharmacol. 2010, 10, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Diet and Exercise in Cancer Metabolism. Cancer Discov. 2022, 12, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Zhuang, X.; Tammela, T.; Schietinger, A.; Jones, L.W. Exercise and immunometabolic regulation in cancer. Nat. Metab. 2020, 2, 849–857. [Google Scholar] [CrossRef]
- Leitner, B.P.; Siebel, S.; Akingbesote, N.D.; Zhang, X.; Perry, R.J. Insulin and cancer: A tangled web. Biochem. J. 2022, 479, 583–607. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, M.; Wu, X.; Zhang, Y.; Xia, Y. Muscle-to-tumor crosstalk: The effect of exercise-induced myokine on cancer progression. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188761. [Google Scholar] [CrossRef]
- Brown, J.C.; Rickels, M.R.; Troxel, A.B.; Zemel, B.S.; Damjanov, N.; Ky, B.; Rhim, A.D.; Rustgi, A.K.; Courneya, K.S.; Schmitz, K.H. Dose–response effects of exercise on insulin among colon cancer survivors. Endocr.-Relat. Cancer 2018, 25, 11–19. [Google Scholar] [CrossRef]
- Irwin, M.L.; Varma, K.; Alvarez-Reeves, M.; Cadmus, L.; Wiley, A.; Chung, G.G.; DiPietro, L.; Mayne, S.T.; Yu, H. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: The Yale Exercise and Survivorship study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 306–313. [Google Scholar] [CrossRef]
- Onerup, A.; Thörn, S.-E.; Angenete, E.; Bock, D.; Gillheimer, E.G.; Haglind, E.; Nilsson, H. Effects of a home-based exercise program on the insulin-like growth factor axis in patients operated for colorectal cancer in Sweden: Results from the randomised controlled trial PHYSSURG-C. Growth Horm. IGF Res. 2020, 51, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hormones, E.; Group, B.C.C. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. Br. J. Cancer 2011, 105, 709. [Google Scholar]
- Lukanova, A.; Lundin, E.; Zeleniuch-Jacquotte, A.; Muti, P.; Mure, A.; Rinaldi, S.; Dossus, L.; Micheli, A.; Arslan, A.; Lenner, P.; et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: A cross-sectional study in healthy women. Eur. J. Endocrinol. 2004, 150, 161–171. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose-response meta-analysis. Cancer Manag. Res. 2018, 10, 143–151. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S. Physical exercise and sex steroid hormones in breast cancer. Hum. Mov. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Lin, L.; Cai, Q.; Li, C.; Xu, H.; Zeng, R.; Zhang, M.; Qiu, X.; Chen, S.; Zhang, X.; et al. Do testosterone and sex hormone-binding globulin affect cancer risk? A Mendelian randomization and bioinformatics study. Aging Male 2023, 26, 2261524. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Tworoger, S.S.; Ulrich, C.M.; Yasui, Y.; Irwin, M.L.; Rajan, K.B.; Sorensen, B.; Rudolph, R.E.; Bowen, D.; Stanczyk, F.Z.; et al. Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res. 2004, 64, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.S.; Lee, K.P. A systematic review of the biological mechanisms linking physical activity and breast cancer. Phys. Act. Nutr. 2020, 24, 25–31. [Google Scholar] [CrossRef]
- Swain, C.T.; Drummond, A.E.; Boing, L.; Milne, R.L.; English, D.R.; Brown, K.A.; van Roekel, E.H.; Dixon-Suen, S.C.; Lynch, M.J.; Moore, M.M. Linking physical activity to breast cancer via sex hormones, part 1: The effect of physical activity on sex steroid hormones. Cancer Epidemiol. Biomark. Prev. 2022, 31, 16–27. [Google Scholar] [CrossRef]
- Monninkhof, E.M.; Velthuis, M.J.; Peeters, P.H.; Twisk, J.W.; Schuit, A.J. Effect of exercise on postmenopausal sex hormone levels and role of body fat: A randomized controlled trial. J. Clin. Oncol. 2009, 27, 4492–4499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, J.C.; Sturgeon, K.; Sarwer, D.B.; Troxel, A.B.; DeMichele, A.M.; Denlinger, C.S.; Schmitz, K.H. The effects of exercise and diet on sex steroids in breast cancer survivors. Endocr.-Relat. Cancer 2022, 29, 485–493. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Ulrich, C.; Kumai, C.; Bean, D.; Schwartz, R.; Mahloch, J.; Hastings, R.; Gralow, J.; Potter, J.D. Anthropometric and hormone effects of an eight-week exercise-diet intervention in breast cancer patients: Results of a pilot study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1998, 7, 477–481. [Google Scholar]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Sumsuzzman, D.M.; Jin, Y.; Choi, J.; Yu, J.-H.; Lee, T.H.; Hong, Y. Pathophysiological role of endogenous irisin against tumorigenesis and metastasis: Is it a potential biomarker and therapeutic? Tumor Biol. 2019, 41, 1010428319892790. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.; Park, S.; Kwon, H.J.; Lee, S.-H.; Kim, Y.; Kim, J.-H. Exercise affects high-fat diet-stimulated breast cancer metastasis through irisin secretion by altering cancer stem cell properties. Biochem. Biophys. Rep. 2024, 38, 101684. [Google Scholar] [CrossRef]
- Alizadeh Zarei, M.; Seyed Hosseini, E.; Haddad Kashani, H.; Ahmad, E.; Nikzad, H. Effects of the exercise-inducible myokine irisin on proliferation and malignant properties of ovarian cancer cells through the HIF-1 α signaling pathway. Sci. Rep. 2023, 13, 170. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Huang, C.W.; Chang, Y.H.; Lee, H.H.; Wu, J.Y.; Huang, J.X.; Chung, Y.H.; Hsu, S.T.; Chow, L.P.; Wei, K.C.; Huang, F.T. Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J. 2020, 34, 9678–9693. [Google Scholar] [CrossRef]
- Nazari, S.; Isanezhad, A.; Gharib, B.; Motlagh, A.G. Continuous moderate exercise training can increase serum level of Irisin in breast cancer patients. J. Exerc. Health Sci. 2022, 2, 1–14. [Google Scholar]
- Kim, J.-S.; Taaffe, D.R.; Galvão, D.A.; Clay, T.D.; Redfern, A.D.; Hart, N.H.; Gray, E.S.; Ryan, C.J.; Kenfield, S.A.; Saad, F. Acute effect of high-intensity interval aerobic exercise on serum myokine levels and resulting tumour-suppressive effect in trained patients with advanced prostate cancer. Prostate Cancer Prostatic Dis. 2023, 26, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Schering, L.; Buck, F.; Vlach, K.; Schober, H.C.; Drevon, C.A.; Maak, S. Irisin: Still chasing shadows. Mol. Metab. 2020, 34, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Pinkowska, A.; Podhorska-Okołów, M.; Dzięgiel, P.; Nowińska, K. The Role of Irisin in Cancer Disease. Cells 2021, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, B.; Zhang, K.; Meng, X.; Jia, Q.; Bu, Y.; Zhu, X.; Ma, D.; Ye, B.; Zhang, N. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: With reference to nervous system. Oncogene 2016, 35, 4122–4131. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Bupesh, G.; Manikandan, E.; Thanigai, A.; Magesh, S.; Kalyanaraman, R.; Maaza, M. Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J. Antimicro 2016, 2, 1000123. [Google Scholar]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Prathap, L.; Jayaraman, S. Anti Proliferative Effect of Endogenous Dopamine Replica in Human Lung Cancer Cells (A549) Via Pi3k and Akt Signalling Molecules. J. Pharm. Negat. Results 2022, 13, 1380–1386. [Google Scholar]
- Chaudhary, Z.; Singh, J.; Singh, K.D.; Fanaei, M.; Gadiparthy, M.; Ma, M.Y.; Nallagatla, T. Exercise and Breast Cancer: Exploring Dopamine, Insulin and Estrogen Pathways. University of California. eScholarship. 2024. Available online: https://escholarship.org/uc/item/0v99m295 (accessed on 20 April 2025).
- Muhammad, I.F.; Borné, Y.; Melander, O.; Orho-Melander, M.; Nilsson, J.; Söderholm, M.; Engström, G. FADD (Fas-associated protein with death domain), caspase-3, and caspase-8 and incidence of ischemic stroke. Stroke 2018, 49, 2224–2226. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Rafiei, M.M.; Soltani, R.; Kordi, M.R.; Nouri, R.; Gaeini, A.A. Gene expression of angiogenesis and apoptotic factors in female BALB/c mice with breast cancer after eight weeks of aerobic training. Iran. J. Basic Med. Sci. 2021, 24, 1196. [Google Scholar]
- Ghazizadeh Darband, S.; Saboory, E.; Sadighparvar, S.; Kaviani, M.; Mobaraki, K.; Jabbari, N.; Majidinia, M. The modulatory effects of exercise on the inflammatory and apoptotic markers in rats with 1, 2-dimethylhydrazine-induced colorectal cancer. Can. J. Physiol. Pharmacol. 2020, 98, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Park, D.; Lee, G.Y.; Curran, W.J.; Deng, X. Exercise-induced lung cancer regression: Mechanistic findings from a mouse model. Cancer 2014, 120, 3302–3310. [Google Scholar] [CrossRef]
- Sharpless, N.E.; DePinho, R.A. p53: Good cop/bad cop. Cell 2002, 110, 9–12. [Google Scholar] [CrossRef]
- Lee, J.; Savage, H.; Maegawa, S.; Ballarò, R.; Pareek, S.; Guerrouahen, B.S.; Gopalakrishnan, V.; Schadler, K. Exercise promotes pro-apoptotic ceramide signaling in a mouse melanoma model. Cancers 2022, 14, 4306. [Google Scholar] [CrossRef]
- Li, R.-Z.; Wang, X.-R.; Wang, J.; Xie, C.; Wang, X.-X.; Pan, H.-D.; Meng, W.-Y.; Liang, T.-L.; Li, J.-X.; Yan, P.-Y. The key role of sphingolipid metabolism in cancer: New therapeutic targets, diagnostic and prognostic values, and anti-tumor immunotherapy resistance. Front. Oncol. 2022, 12, 941643. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Dieterich, W.; Reljic, D.; Pilarsky, C.; Mukhopadhyay, D.; Chang, D.K.; Biankin, A.V.; Siebler, J.; Herrmann, H.J.; Neurath, M.F. Muscle-derived cytokines reduce growth, viability and migratory activity of pancreatic cancer cells. Cancers 2021, 13, 3820. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Adams, J.M. Life in the balance: How BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 2005, 17, 617–625. [Google Scholar] [CrossRef]
- Schwappacher, R.; Schink, K.; Sologub, S.; Dieterich, W.; Reljic, D.; Friedrich, O.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Physical activity and advanced cancer: Evidence of exercise-sensitive genes regulating prostate cancer cell proliferation and apoptosis. J. Physiol. 2020, 598, 3871–3889. [Google Scholar] [CrossRef]
- He, A.; Pu, Y.; Jia, C.; Wu, M.; He, H.; Xia, Y. The Influence of Exercise on Cancer Risk, the Tumor Microenvironment and the Treatment of Cancer. Sports Med. 2024, 54, 1371–1397. [Google Scholar] [CrossRef]
- Tzeng, H.T.; Huang, Y.J. Tumor Vasculature as an Emerging Pharmacological Target to Promote Anti-Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 4422. [Google Scholar] [CrossRef]
- Buzaglo, G.B.B.; Telles, G.D.; Araújo, R.B.; Junior, G.D.S.; Ruberti, O.M.; Ferreira, M.L.V.; Derchain, S.F.M.; Vechin, F.C.; Conceição, M.S. The Therapeutic Potential of Physical Exercise in Cancer: The Role of Chemokines. Int. J. Mol. Sci. 2024, 25, 13740. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Di Benedetto, M. Autocrine functions of VEGF in breast tumor cells: Adhesion, survival, migration and invasion. Cell Adh Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Santos, I.L.; Kumar, A.S.; Hausmann, F.; Meyer, M.N.; Shiferaw, S.Z.; Amoozgar, Z.; Jain, R.K.; Fukumura, D. Exercise intensity governs tumor control in mice with breast cancer. Front. Immunol. 2024, 15, 1339232. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.; Pareek, S.; Lee, J.; Ballarò, R.; Conterno Minussi, D.; Hayek, K.; Sadullozoda, M.; Lochmann, B.S.; McQuade, J.L.; LaVoy, E.C.; et al. Aerobic Exercise Alters the Melanoma Microenvironment and Modulates ERK5 S496 Phosphorylation. Cancer Immunol. Res. 2023, 11, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ruiz, A.; Fiuza-Luces, C.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Gálvez, B.G.; Martínez-Martínez, E.; Martín-Acosta, P.; Coronado, M.J.; Franco-Luzón, L.; González-Murillo, Á.; et al. Benefits of exercise and immunotherapy in a murine model of human non-small-cell lung carcinoma. Exerc. Immunol. Rev. 2020, 26, 100–115. [Google Scholar]
- Morrisson, M.J.; Bi, F.; Yang, K.; Cady, S.L.; Hartwich, T.M.; Cerchia, A.P.; Li, Z.; Kim, J.; Irwin, M.L.; Yang-Hartwich, Y. Effect of exercise on peritoneal microenvironment and progression of ovarian cancer. Am. J. Cancer Res. 2021, 11, 5045–5062. [Google Scholar]
- Patel, D.I.; Abuchowski, K.; Bedolla, R.; Rivas, P.; Musi, N.; Reddick, R.; Kumar, A.P. Nexrutine and exercise similarly prevent high grade prostate tumors in transgenic mouse model. PLoS ONE 2019, 14, e0226187. [Google Scholar] [CrossRef]
- Zylstra, J.; Whyte, G.P.; Beckmann, K.; Pate, J.; Santaolalla, A.; Gervais-Andre, L.; Russell, B.; Maisey, N.; Waters, J.; Tham, G.; et al. Exercise prehabilitation during neoadjuvant chemotherapy may enhance tumour regression in oesophageal cancer: Results from a prospective non-randomised trial. Br. J. Sports Med. 2022, 56, 402–409. [Google Scholar] [CrossRef]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef]
- Wittekind, C.; Neid, M. Cancer invasion and metastasis. Oncology 2005, 69, 14–16. [Google Scholar] [CrossRef]
- Jones, L.W.; Antonelli, J.; Masko, E.M.; Broadwater, G.; Lascola, C.D.; Fels, D.; Dewhirst, M.W.; Dyck, J.R.; Nagendran, J.; Flores, C.T. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J. Appl. Physiol. 2012, 113, 263–272. [Google Scholar] [CrossRef]
- Murphy, E.; Davis, J.; Brown, A.S.; Carmichael, M.D.; Mayer, E.P.; Ghaffar, A. Effects of moderate exercise and oat β-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J. Appl. Physiol. 2004, 97, 955–959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Brown, J.C.; Rhim, A.D.; Manning, S.L.; Brennan, L.; Mansour, A.I.; Rustgi, A.K.; Damjanov, N.; Troxel, A.B.; Rickels, M.R.; Ky, B.; et al. Effects of exercise on circulating tumor cells among patients with resected stage I-III colon cancer. PLoS ONE 2018, 13, e0204875. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Chen, Y.; Feng, X.; Luo, Z.; Peng, Z.; Qi, B.; Qin, H.; Lin, J.; Chen, S.; Xu, L. Exercise potentially prevents colorectal cancer liver metastases by suppressing tumor epithelial cell stemness via RPS4X downregulation. Heliyon 2024, 10, e26604. [Google Scholar] [CrossRef]
- Pang, S.; Xu, Y.; Chen, J.; Li, G.; Huang, J.; Wu, X. Knockdown of cell division cycle-associated protein 4 expression inhibits proliferation of triple negative breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Lett. 2019, 17, 4393–4400. [Google Scholar] [CrossRef]
- Hayashi, R.; Goto, Y.; Ikeda, R.; Yokoyama, K.K.; Yoshida, K. CDCA4 is an E2F transcription factor family-induced nuclear factor that regulates E2F-dependent transcriptional activation and cell proliferation. J. Biol. Chem. 2006, 281, 35633–35648. [Google Scholar] [CrossRef]
- Hao, S.; Zhu, J.; Zhang, X.; Qiu, J.; Xuan, Q.; Ye, L. Comprehensive analysis of aerobic exercise-related genes identifies CDCA4 that promotes the progression of osteosarcoma. Front. Genet. 2021, 12, 637755. [Google Scholar] [CrossRef]
- Sheinboim, D.; Parikh, S.; Manich, P.; Markus, I.; Dahan, S.; Parikh, R.; Stubbs, E.; Cohen, G.; Zemser-Werner, V.; Bell, R.E. An exercise-induced metabolic shield in distant organs blocks cancer progression and metastatic dissemination. Cancer Res. 2022, 82, 4164–4178. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism insights and therapeutic intervention of tumor metastasis: Latest developments and perspectives. Signal Transduct. Target. Ther. 2024, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Joaquim, A.; Sampaio, F.; Nunes, C.; Ascensão, A.; Vilela, E.; Teixeira, M.; Oliveira, J.; Capela, A.; Amarelo, A. Exercise training benefits health-related quality of life and functional capacity during breast cancer chemotherapy: A randomized controlled trial. Med. Sci. Sports Exerc. 2024, 56, 600–611. [Google Scholar] [CrossRef]
- Wang, L.F.; Eaglehouse, Y.L.; Poppenberg, J.T.; Brufsky, J.W.; Geramita, E.M.; Zhai, S.; Davis, K.K.; Gibbs, B.B.; Metz, J.; van Londen, G. Effects of a personal trainer-led exercise intervention on physical activity, physical function, and quality of life of breast cancer survivors. Breast Cancer 2021, 28, 737–745. [Google Scholar] [CrossRef]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.U.P.; Kurian, J.R.; Lin, P.J.; Mustian, K.M. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support. Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef] [PubMed]
- Triguero-Cánovas, D.; López-Rodríguez-Arias, F.; Gómez-Martínez, M.; Sánchez-Guillén, L.; Peris-Castelló, F.; Alcaide-Quirós, M.J.; Morillas-Blasco, P.; Arroyo, A.; Ramírez, J.M. Home-based prehabilitation improves physical conditions measured by ergospirometry and 6MWT in colorectal cancer patients: A randomized controlled pilot study. Support. Care Cancer 2023, 31, 673. [Google Scholar] [CrossRef]
- Lemanne, D.; Cassileth, B.; Gubili, J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology 2013, 27, 580–585. [Google Scholar]
- Klein, I.; Kalichman, L.; Chen, N.; Susmallian, S. A pilot study evaluating the effect of early physical therapy on pain and disabilities after breast cancer surgery: Prospective randomized control trail. Breast 2021, 59, 286–293. [Google Scholar] [CrossRef]
- van der Schoot, G.G.; Ormel, H.L.; Westerink, N.-D.L.; Wempe, J.B.; Lefrandt, J.D.; May, A.M.; Vrieling, A.H.; Meijer, C.; Gietema, J.A.; Walenkamp, A.M. Physical exercise in patients with testicular cancer treated with bleomycin, etoposide and cisplatin chemotherapy: Pulmonary and vascular endothelial function—An exploratory analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 17467–17478. [Google Scholar] [CrossRef]
- Lavigne, C.; Twomey, R.; Lau, H.; Francis, G.; Culos-Reed, S.N.; Millet, G.Y. Feasibility of eccentric overloading and neuromuscular electrical stimulation to improve muscle strength and muscle mass after treatment for head and neck cancer. J. Cancer Surviv. 2020, 14, 790–805. [Google Scholar] [CrossRef]
- Shin, J.-H.; Kim, M.-Y.; Lee, J.-Y.; Jeon, Y.-J.; Kim, S.; Lee, S.; Seo, B.; Choi, Y. Effects of virtual reality-based rehabilitation on distal upper extremity function and health-related quality of life: A single-blinded, randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.; Aboelnour, N.H.; Alsharidah, A.S.; Kamel, F.H. Effect of exercise mode on physical function and quality of life in breast cancer-related lymphedema: A randomized trial. Support. Care Cancer 2022, 30, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, F.; Cui, Y.; Zhao, L.; Sun, X.; Tang, W.; Cai, P. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. Biosci. Trends 2018, 12, 220–239. [Google Scholar] [CrossRef]
- Jakobsson, S.; Taft, C.; Östlund, U.; Ahlberg, K. Performance of the Swedish version of the revised Piper fatigue scale. Eur. J. Oncol. Nurs. 2013, 17, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Siebert, S.; Kollikowski, A.; Minto, C.-A.; Byrtus, F.; Lesnik, J.; Weis, J.; Horneber, M.; Bloch, W.; Baumann, F.T.; Salchow, J. A Randomized, Controlled Pilot Study to Evaluate the Immediate Effect of Targeted Exercise Therapy on Cancer-Related Fatigue in Cancer Survivors: The FatiGO Study. Oncol. Res. Treat. 2022, 45, 639–649. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Z.; Zhang, R.; Zhang, N. Effect of exercise based on the ACSM recommendations on fatigue in patients with digestive tumors: A meta-analysis of randomized controlled trials. J. Cancer Surviv. 2025, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Lin, G.; Huang, B.; Sheng, X.; Wang, L.; Chen, L.; Qiu, X.; Wu, X.; Lin, R. The efficacy of progressive muscle relaxation training on cancer-related fatigue and quality of life in patients with cancer: A systematic review and meta-analysis of randomized controlled studies. Int. J. Nurs. Stud. 2024, 152, 104694. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, D. Effects of resistance exercise on complications, cancer-related fatigue and quality of life in nasopharyngeal carcinoma patients undergoing chemoradiotherapy: A randomised controlled trial. Eur. J. Cancer Care 2021, 30, e13355. [Google Scholar] [CrossRef]
- Hiensch, A.E.; Mijwel, S.; Bargiela, D.; Wengström, Y.; May, A.M.; Rundqvist, H. Inflammation Mediates Exercise Effects on Fatigue in Patients with Breast Cancer. Med. Sci. Sports Exerc. 2021, 53, 496–504. [Google Scholar] [CrossRef]
- Demmelmaier, I.; Brooke, H.L.; Henriksson, A.; Mazzoni, A.S.; Bjørke, A.C.H.; Igelström, H.; Ax, A.K.; Sjövall, K.; Hellbom, M.; Pingel, R. Does exercise intensity matter for fatigue during (neo-) adjuvant cancer treatment? The Phys-Can randomized clinical trial. Scand. J. Med. Sci. Sports 2021, 31, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qu, H.-Q.; Chen, F.-Y.; Li, X.-T.; Cai, L.; Chen, S.; Sun, Y.-Y. Effect of Baduanjin Qigong exercise on cancer-related fatigue in patients with colorectal cancer undergoing chemotherapy: A randomized controlled trial. Oncol. Res. Treat. 2019, 42, 431–439. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, X.; Hu, J.; Dai, L.-l.; Lv, Y.; Feng, H.; Zhang, Y.; Guo, Y.; Wang, L. Effect of Tai Chi and resistance training on cancer-related fatigue and quality of life in middle-aged and elderly cancer patients. Chin. J. Integr. Med. 2021, 27, 265–272. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, S.I.; Müller, F.; van Woezik, R.A.; Hagedoorn, M.; van der Lee, M.L. Adopting a Dyadic Approach to Treating Chronic Cancer-Related Fatigue: A Mixed Methods Study to Assess Patients’ and Partners’ Needs, Benefits, Barriers and Preferences. Eur. J. Cancer Care 2025, 2025, 8313220. [Google Scholar] [CrossRef]
- Roila, F.; Cortesi, E. Quality of life as a primary end point in oncology. Ann. Oncol. 2001, 12, S3–S6. [Google Scholar] [CrossRef]
- Sprangers, M.; Groenvold, M.; Arraras, J.I.; Franklin, J.; te Velde, A.; Muller, M.; Franzini, L.; Williams, A.; De Haes, H.; Hopwood, P. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J. Clin. Oncol. 1996, 14, 2756–2768. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Nichol, M.B.; Eton, D.; Nelson, J.B.; Mulani, P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy—Prostate: Results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009, 12, 124–129. [Google Scholar] [CrossRef]
- Bai, X.L.; Li, Y.; Feng, Z.F.; Cao, F.; Wang, D.D.; Ma, J.; Yang, D.; Li, D.R.; Fang, Q.; Wang, Y.; et al. Impact of exercise on health outcomes in people with cancer: An umbrella review of systematic reviews and meta-analyses of randomised controlled trials. Br. J. Sports Med. 2025. [Google Scholar] [CrossRef]
- Sibarani, L.P.; Setijadi, A.R.; Apriningsih, H.; Harsini, H.; Raharjo, A.F.; Sutanto, Y.S. Effects of Aerobic Exercise on The Six Minutes Walking Test and Quality of Life in EGFR Mutation Non-Small Cell Lung Cancer Patients. J. Respirologi Indones. 2025, 45, 93–99. [Google Scholar] [CrossRef]
- Ohrnberger, J.; Fichera, E.; Sutton, M. The relationship between physical and mental health: A mediation analysis. Soc. Sci. Med. 2017, 195, 42–49. [Google Scholar] [CrossRef]
- Namazinia, M.; Mazlum, S.R.; Mohajer, S.; Lopez, V. Effects of laughter yoga on health-related quality of life in cancer patients undergoing chemotherapy: A randomized clinical trial. BMC Complement. Med. Ther. 2023, 23, 192. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Sze, D.M.-y.; Chan, V.W.S.; Yang, A.W.H. Effectiveness of qigong and Tai Chi for quality of life in patients with cancer: An umbrella review and meta-analysis. BMC Complement. Med. Ther. 2025, 25, 141. [Google Scholar] [CrossRef] [PubMed]
- Ax, A.-K.; Johansson, B.; Lyth, J.; Nordin, K.; Börjeson, S. Short-and long-term effect of high versus low-to-moderate intensity exercise to optimise health-related quality of life after oncological treatment—Results from the Phys-Can project. Support. Care Cancer 2022, 30, 5949–5963. [Google Scholar] [CrossRef]
- Isanejad, A.; Nazari, S.; Gharib, B.; Motlagh, A.G. Comparison of the effects of high-intensity interval and moderate-intensity continuous training on inflammatory markers, cardiorespiratory fitness, and quality of life in breast cancer patients. J. Sport Health Sci. 2023, 12, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.D.; Close, G.L.; MacLaren, D.P.; Gregson, W.; Drust, B.; Morton, J.P. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: Implications for exercise adherence. J. Sports Sci. 2011, 29, 547–553. [Google Scholar] [CrossRef]
- Morielli, A.R.; Boulé, N.G.; Usmani, N.; Tankel, K.; Joseph, K.; Severin, D.; Fairchild, A.; Nijjar, T.; Courneya, K.S. Effects of exercise during and after neoadjuvant chemoradiation on symptom burden and quality of life in rectal cancer patients: A phase II randomized controlled trial. J. Cancer Surviv. 2021, 17, 1171–1183. [Google Scholar] [CrossRef]
- Sheill, G.; Brady, L.; Hayes, B.; Baird, A.-M.; Guinan, E.; Vishwakarma, R.; Brophy, C.; Vlajnic, T.; Casey, O.; Murphy, V. ExPeCT: A randomised trial examining the impact of exercise on quality of life in men with metastatic prostate cancer. Support. Care Cancer 2023, 31, 292. [Google Scholar] [CrossRef]
- Munsie, C.; Ebert, J.; Joske, D.; Ackland, T. A randomised controlled trial investigating the ability for supervised exercise to reduce treatment-related decline in adolescent and young adult cancer patients. Support. Care Cancer 2022, 30, 8159–8171. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Gołębiewska, M.E.; Duchnik, E.; Czerniak, U.; Marchlewicz, M. Physical Activity and Cancer Incidence and Mortality: Current Evidence and Biological Mechanisms. Cancers 2025, 17, 1410. [Google Scholar] [CrossRef]
- Xu, J.; Jiao, X.; Bayat, R. Outcomes of physical exercises on initiation, progression, and treatment of breast cancer. Cell Commun. Signal. 2024, 22, 260. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Swanson, C. A runner’s high for new neurons? Potential role for endorphins in exercise effects on adult neurogenesis. Biomolecules 2021, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology: Nutrition, Energy, and Human Performance; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Reis, A.D.; Pereira, P.T.V.T.; Diniz, R.R.; de Castro Filha, J.G.L.; Dos Santos, A.M.; Ramallo, B.T.; Filho, F.A.A.; Navarro, F.; Garcia, J.B.S. Effect of exercise on pain and functional capacity in breast cancer patients. Health Qual. Life Outcomes 2018, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- White, K.R.; Lu, J.; Ibrahim, Z.; Furth, P.A. Enabling exercise prescription for survivors of cancer. Sci. Rep. 2021, 11, 9557. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription; American College of Sports Medicine: Indianapolis, IN, USA, 2018. [Google Scholar]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef]
- Gao, R.; Yu, T.; Liu, L.; Bi, J.; Zhao, H.; Tao, Y.; Li, F.; Guo, L. Exercise intervention for post-treatment colorectal cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2020, 14, 878–893. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, D. Effects of exercise on the quality of life in breast cancer patients: A systematic review of randomized controlled trials. Support. Care Cancer 2019, 27, 9–21. [Google Scholar] [CrossRef]
- Ficarra, S.; Thomas, E.; Bianco, A.; Gentile, A.; Thaller, P.; Grassadonio, F.; Papakonstantinou, S.; Schulz, T.; Olson, N.; Martin, A. Impact of exercise interventions on physical fitness in breast cancer patients and survivors: A systematic review. Breast Cancer 2022, 29, 402–418. [Google Scholar] [CrossRef]
- Ng, C.T.; Bonilla, H.M.G.; Bryce, A.H.; Singh, P.; Herrmann, J. Approaches to prevent and manage cardiovascular disease in patients receiving therapy for prostate cancer. Curr. Cardiol. Rep. 2023, 25, 889–899. [Google Scholar] [CrossRef]
- Pratiwi, D.R.; Yusniar, F.; Susanti, I.A.; Sukartini, T. Pelvic floor muscle training (PFMT) to reduce urinary incontinence post radical prostatectomy in patients with prostate cancer: A systematic review. J. Ners 2020, 15, 164–172. [Google Scholar] [CrossRef]
- Strączyńska, A.; Weber-Rajek, M.; Strojek, K.; Piekorz, Z.; Styczyńska, H.; Goch, A.; Radzimińska, A. The impact of pelvic floor muscle training on urinary incontinence in men after radical prostatectomy (RP)–a systematic review. Clin. Interv. Aging 2019, 14, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, C.; Cao, B.; Zhou, F.; Wang, J.; Ren, H.; Li, Y.; Wang, M.; Liu, Y.; Zhang, H.; et al. Safety and efficacy evaluation of personalized exercise prescription during chemotherapy for lung cancer patients. Thorac. Cancer 2024, 15, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Peddle-McIntyre, C.J.; Singh, F.; Thomas, R.; Newton, R.U.; Galvão, D.A.; Cavalheri, V. Exercise training for advanced lung cancer. Cochrane Database Syst. Rev. 2019, 2, CD012685. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.N.; Roquelaure, Y.; Evanoff, B.; Aublet-Cuvelier, A.; Porro, B. Physical activity in people diagnosed with cancer: A rapid review of recommendations and critical appraisal of international guidelines. Support. Care Cancer 2023, 31, 679. [Google Scholar] [CrossRef]
- Singh, F.; Newton, R.U.; Taaffe, D.R.; Lopez, P.; Thavaseelan, J.; Brown, M.; Ooi, E.; Nosaka, K.; Hayne, D.; Galvão, D.A. Prehabilitative versus rehabilitative exercise in prostate cancer patients undergoing prostatectomy. J. Cancer Res. Clin. Oncol. 2023, 149, 16563–16573. [Google Scholar] [CrossRef]
- Kang, D.-W.; Boulé, N.G.; Field, C.J.; Fairey, A.S.; Courneya, K.S. Effects of supervised high-intensity interval training on motivational outcomes in men with prostate cancer undergoing active surveillance: Results from a randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 126. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Cuesta-Vargas, A.I.; Lozano-Lozano, M.; Fernández-Lao, C.; Fernández-Pérez, A.; Galiano-Castillo, N. Changes in pain and muscle architecture in colon cancer survivors after a lumbopelvic exercise program: A secondary analysis of a randomized controlled trial. Pain Med. 2017, 18, 1366–1376. [Google Scholar] [CrossRef]
- Page, L.L.; Fanning, J.; Phipps, C.; Berger, A.; Reed, E.; Ehlers, D. Heart Rate Monitoring Among Breast Cancer Survivors: Quantitative Study of Device Agreement in a Community-Based Exercise Program. JMIR Cancer 2024, 10, e51210. [Google Scholar] [CrossRef]
- MacNevin, W.; Ilie, G.; Rendon, R.; Mason, R.; Spooner, J.; Chedrawe, E.; Patil, N.; Bowes, D.; Bailly, G.; Bell, D. PC-PEP, a Comprehensive Daily Six-Month Home-Based Patient Empowerment Program Leads to Weight Loss in Men with Prostate Cancer: A Secondary Analysis of a Clinical Trial. Curr. Oncol. 2024, 31, 1667–1688. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Kenfield, S.A.; Olshen, A.; Panchal, N.; Encabo, K.; Tenggara, I.; Graff, R.E.; Bang, A.S.; Shinohara, K.; Cooperberg, M.R. Effect of a home-based walking intervention on cardiopulmonary fitness and quality of life among men with prostate cancer on active surveillance: The active surveillance exercise randomized controlled trial. Eur. Urol. Oncol. 2024, 7, 519–526. [Google Scholar] [CrossRef]
- Caan, B.J.; Brown, J.C.; Lee, C.; Binder, A.M.; Weltzien, E.; Ross, M.C.; Quesenberry, C.P.; Campbell, K.L.; Cespedes Feliciano, E.M.; Castillo, A. Effect of home-based resistance training on chemotherapy relative dose intensity and tolerability in colon cancer: The FORCE randomized control trial. Cancer 2024, 130, 1858–1868. [Google Scholar] [CrossRef] [PubMed]
- Kholmatov, S.; Madaminov, S.; Zokirjonov, D.; Sobirova, D. A comprehensive review of radiotherapy and chemotherapy-induced morphological side effects in breast cancer treatment: Strategies for management and mitigation. Sci. Innov. 2024, 3, 11–24. [Google Scholar]
- Volaklis, K.A.; Halle, M.; Tokmakidis, S.P. Exercise in the prevention and rehabilitation of breast cancer. Wien. Klin. Wochenschr. 2013, 125, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Mata, L.R.F.d.; Carvalho, E.C.d.; Gomes, C.R.G.; Silva, A.C.d.; Pereira, M.d.G. Postoperative self-efficacy and psychological morbidity in radical prostatectomy. Rev. Lat.-Am. Enferm. 2015, 23, 806–813. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Q.M.; Liu, F.P.; Mao, Q.Q. Effectiveness of preoperative pelvic floor muscle training for urinary incontinence after radical prostatectomy: A meta-analysis. BMC Urol. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; da Mata, L.R.F.; de Resende Izidoro, L.C.; de Castro Moura, C.; Araújo, B.B.A.; Pereira, M.G.; Chianca, T.C.M. Effectiveness of auricular acupuncture and pelvic floor muscle training in the management of urinary incontinence following surgical treatment for prostate cancer: A randomized clinical trial. Eur. J. Oncol. Nurs. 2024, 68, 102490. [Google Scholar] [CrossRef] [PubMed]
- Manassero, F.; Traversi, C.; Ales, V.; Pistolesi, D.; Panicucci, E.; Valent, F.; Selli, C. Contribution of early intensive prolonged pelvic floor exercises on urinary continence recovery after bladder neck-sparing radical prostatectomy: Results of a prospective controlled randomized trial. Neurourol. Urodyn. Off. J. Int. Cont. Soc. 2007, 26, 985–989. [Google Scholar] [CrossRef]
- Parekh, A.; Feng, M.; Kirages, D.; Bremner, H.; Kaswick, J.; Aboseif, S. The role of pelvic floor exercises on post-prostatectomy incontinence. J. Urol. 2003, 170, 130–133. [Google Scholar] [CrossRef]
- Pannek, J.; König, J.E. Clinical usefulness of pelvic floor reeducation for men undergoing radical prostatectomy. Urol. Int. 2005, 74, 38–43. [Google Scholar] [CrossRef]
- Yang, U.; Harikrishna, A.; Preda, V.; Chen, J. Efficacy of multidisciplinary interventions in preventing metabolic syndrome and improving body composition in prostate cancer patients treated with androgen deprivation therapy: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2023, 58, 27–49. [Google Scholar] [CrossRef]
- Bigaran, A.; Zopf, E.; Gardner, J.; La Gerche, A.; Murphy, D.G.; Howden, E.J.; Baker, M.K.; Cormie, P. The effect of exercise training on cardiometabolic health in men with prostate cancer receiving androgen deprivation therapy: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.T.; Reimer, N.; Gockeln, T.; Reike, A.; Hallek, M.; Ricci, C.; Zopf, E.M.; Schmid, D.; Taaffe, D.; Newton, R.U. Supervised pelvic floor muscle exercise is more effective than unsupervised pelvic floor muscle exercise at improving urinary incontinence in prostate cancer patients following radical prostatectomy–a systematic review and meta-analysis. Disabil. Rehabil. 2022, 44, 5374–5385. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, A.; Cantarero-Villanueva, I.; Delgado-García, G.; Molina-Barea, R.; Fernández-Lao, C.; Galiano-Castillo, N.; Arroyo-Morales, M. Physical impairments and quality of life of colorectal cancer survivors: A case–control study. Eur. J. Cancer Care 2015, 24, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Jimenez, A.; Cantarero-Villanueva, I.; Molina-Barea, R.; Fernandez-Lao, C.; Galiano-Castillo, N.; Arroyo-Morales, M. Widespread pressure pain hypersensitivity and ultrasound imaging evaluation of abdominal area after colon cancer treatment. Pain Med. 2014, 15, 233–240. [Google Scholar] [CrossRef]
- Gao, Z.; Ryu, S.; Zhou, W.; Adams, K.; Hassan, M.; Zhang, R.; Blaes, A.; Wolfson, J.; Sun, J. Effects of personalized exercise prescriptions and social media delivered through mobile health on cancer survivors’ physical activity and quality of life. J. Sport. Health Sci. 2023, 12, 705–714. [Google Scholar] [CrossRef]
- Correia, I.R.; Cardoso, V.; Cargaleiro, C.; Magalhães, J.P.; Hetherington-Rauth, M.; Rosa, G.B.; Malveiro, C.; de Matos, L.V.; Cardoso, M.J.; Sardinha, L.B. Effects of home-based exercise programs on physical fitness in cancer patients undergoing active treatment: A systematic review and meta-analysis of randomized controlled trials. J. Sci. Med. Sport 2023, 26, 222–231. [Google Scholar] [CrossRef]
- Luo, Z.; Mei, J.; Wang, X.; Wang, R.; He, Z.; Geffen, Y.; Sun, X.; Zhang, X.; Xu, J.; Wan, R. Voluntary exercise sensitizes cancer immunotherapy via the collagen inhibition-orchestrated inflammatory tumor immune microenvironment. Cell Rep. 2024, 43, 114697. [Google Scholar] [CrossRef]
- Hjorth, M.; Egan, C.L.; Telles, G.D.; Pal, M.; Gallego-Ortega, D.; Fuller, O.K.; McLennan, E.D.; Gillis, R.D.; Oh, T.G.; Muscat, G.E.O.; et al. Decorin, an exercise-induced secretory protein, is associated with improved prognosis in breast cancer patients but does not mediate anti-tumorigenic tissue crosstalk in mice. J. Sport. Health Sci. 2024, 14, 100991. [Google Scholar] [CrossRef]
- Zeng, L.; Li, W.; Chen, C.S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef]
- de Jong, M.; Maina, T. Of mice and humans: Are they the same?--Implications in cancer translational research. J. Nucl. Med. 2010, 51, 501–504. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between mouse age and human age in anti-tumor research: Significance and method establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Laher, I.; Li, S. Exercise snacks and physical fitness in sedentary populations. Sports Med. Health Sci. 2025, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Clifford, B.K.; Stamatakis, E.; Gibbs, M.T. Exercise snacks and other forms of intermittent physical activity for improving health in adults and older adults: A scoping review of epidemiological, experimental and qualitative studies. Sports Med. 2024, 54, 813–835. [Google Scholar] [CrossRef]
- Anstey, K.J. Total physical activity matters for brain health. Nat. Aging 2024, 4, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
| Exercise Types | Definition | References (Refs) |
|---|---|---|
| Aerobic exercise | Aerobic exercise refers to a type of physical activity involving continuous, rhythmic movements that elevate the heart rate and engage large muscle groups. Examples include cycling, jogging/running, swimming, and walking. | [53] |
| Resistance training | Resistance exercise refers to a form of physical activity where muscles contract to counteract an external force, with the goal of improving muscle strength, endurance, or motor performance. Common examples include weightlifting, push-ups, and pull-ups. | [54] |
| High-intensity interval training (HIIT) | HIIT refers to a type of exercise, which consists of brief bouts of vigorous (70–90% of maximal heart rate) to high-intensity (≥90% of maximal heart rate) exercise lasting from 60 s to 8 min, alternating with recovery periods ranging from 1 to 5 min. These recovery intervals can involve active recovery (40–70% of maximal heart rate) or passive recovery (complete cessation of movement). | [55] |
| Yoga | Yoga refers to a type of physical activity in which physical postures, breath control, meditation, and ethical principles are integrated to promote health and relaxation. | [56,57] |
| Traditional Chinese Exercise (TCE) | TCE refers to therapeutic physical activities in traditional Chinese medicine, emphasizing the mind–body connection, slow movements combined with deep breathing, and muscle stretching with mental concentration. Examples include Qigong, Tai Chi, and Baduanjin. | [58,59] |
| Nordic walking training | Nordic walking, also known as walking with poles, refers to a type of physical activity that is a safe option recommended for both older individuals and patients with hematologic malignancies. | [60] |
| Progressive muscle relaxation (PMR) | PMR refers to a type of mind–body relaxation technique in which specific muscle groups are contracted and relaxed to reduce muscle tension and alleviate anxiety. | [61] |
| Pelvic floor muscle training (PFMT) | PFME refers to a type of exercise aimed at improving the strength, endurance, and power of the pelvic floor muscles. It can effectively enhance pelvic floor function and prevent urinary and fecal incontinence, and is commonly used in patients with CRC and other pelvic malignancies. | [62] |
| Inspiratory muscle training (IMT) | IMT was defined as any intervention with the goal of training the inspiratory muscles. | [63] |
| Species | Exercise Mode | Specific Exercise Plan | Cancer | Finding | Refs | |
|---|---|---|---|---|---|---|
| Inflammation | Mice | Endurance training | Exercised 5 days a week for 1 h at 5° incline, velocity gradually increasing to 20 m/min over 2 weeks, 5 weeks. | LC | IL-1β ↓ | [66] |
| Aerobic exercise | Ran for 1 h per day at any time during the period. | PCa | IFN-γ ↓ TGF-β ↓ TNF-α ↓ IL-4 ↑ IL-10 ↑ | [67] | ||
| Aerobic exercise | Ran for 30 min, at a speed of 18 m/min for 5 days per 12 weeks | BC | TNF-α ↓ IL-6 ↓ CRP ↓ | [69] | ||
| Voluntary wheel running | 10% incline, speed from 6 m/min to 33 m/min, for 4 weeks. | BC | TNF-α ↓ | [70] | ||
| Rat | Aerobic exercise | For 8 weeks, 5 sessions per week, at 59% of maximum capacity. | PCa | TNF-α ↓ NF-κB ↓ | [68] | |
| Human | Yoga | 60 min of yoga twice weekly for 6 weeks. | PCa | inflammation ↓ | [72] | |
| Walking test | 6–8-week; 45–60 min | Child hood cancer | SII ↓ | [73] | ||
| Community-based and Acute exercise | 40–60 min, 10–30 min of aerobic exercise, and 30 min of resistance training. | BC | BCS ↑ IL-8 ↑ | [75] | ||
| Immune Function | Mice | / | / | BC | CD8 T cells ↑ CXCR3 ↑ | [87] |
| Human | Yoga | 60 min, twice weekly for 6 weeks | PCa | CD4+ and CD8+ T-cells ↑ | [72] | |
| Resistance exercises | 4 days a week for 12 weeks | OC | CD4+ and CD8+ T-cells ↑ | [97] | ||
| Acute exercise | A watt-max test and four high-intensity intervals | PCa | NK ↑ NKT-like ↑ CD8 T cell ↑ Granzyme-B ↑ | [78] | ||
| HIIT | 4–6 cycles | PCa | NK-cell ↑ | [92] | ||
| Resistance exercise | 12-week; twice a week | BC | KYN levels ↓ KYN/TRP ratio ↓ | [105] | ||
| Aerobic and resistance exercises | six-week | BC | NK-cell ↑ | [99] | ||
| Energy Metabolism | Human | Nordic Walking Trainings | 6 weeks, 60 min/day (5 min warm-up, 45 min main part, 10 min cool down). | MM | Fe ↓ | [60] |
| Dopamine | Mice | 8 min/day, 9 weeks | Liver cancer | DR2 ↑ | [147] | |
| Sex hormone | Human | Aerobic exercise | Thrice-weekly; 8-week | BC | Free estradiol ↓ Estrone sulfate ↓ Total testosterone ↓ Androstenedione ↓ Dehydroepiandrosterone ↓ | [136] |
| Irisin | Mice | Wheel running | 3 weeks, 6.1 km/mouse/day | Glioblastoma | Irisin ↑ | [142] |
| Apoptosis | Mice | Aerobic exercise | At 12 m/min for 45 min for 5 consecutive days; with 2 days of rest per week for 2 weeks | Mel | MDM2 ↓ CerS6 ↑ Sphk1 ↓ C16-ceramide ↑ The total ceramide concentration ↓ 30.4% | [158] |
| Aerobic exercise | 1 h per day for 21 days | PCa | Bax ↑ Caspase-9 ↑ Caspase-3 ↑ Bcl-2 ↓ | [67] | ||
| Human | Resistance exercise | 20 min/session, 2 sessions per week, for 12 weeks | PCa; CRC | HT29 cells ↓ 10.6% LNCaP cells ↓ 7.7% PC3 cells ↓ 7.4% | [162] | |
| Cancer Tissue Invasion and Metastasis | Mice | Voluntary wheel running | Voluntarily use wheels 24 h a day, 8 weeks | PCa | CXCR4 ↑ twofold IGFR-1 ↓ MMP2 ↓ | [175] |
| Swimming | 8 min/day, 9 week | Liver cancer | Weight ↓ 19.4% Lung metastases ratio ↓ 34.3% DA ↑ 68.3% | [147] | ||
| Body Function and Composition (Human) | Human | Family exercise | Flexibility exercises, wall push-ups, and upper body resistance exercises three times a week for 30 weeks. | BC | The 2 min step test ↑ The back scratch test ↑ The timed bicep curls test ↑ | [188] |
| Aerobic and resistance exercises | 11-week, with 5 sessions per week at an intensity of 3–5/10 on the RPE scale. | HNC | 6MWT ↑ 37 m. Physical Component Score ↑ 10.5% Fatigue values ↑ 33.78% | [189] | ||
| Therapeutic exercises and Home exercise | Three times a day, five repetitions of each exercise, for six months. | BC | Pain values (NPRS) ↓ The flexion ROM ↑ The extension ROM ↑ | [192] | ||
| VR training and Resistance exercises (RE) training | 8 weeks, 5 times a week, with 50% to 60% of the maximum number of repetitions of game based training. | BC | VR Training Group: VAS scores ↓ DASH scores ↓ Shoulder flexion ↑, Abduction ↑ Eternal rotation ↑ RE Training Group: Shoulder flexion ↑ Abduction ↑ External rotation ↑ Handgrip strength ↑ | [196] | ||
| Home-based prehabilitation Aerobic exercises and Endurance exercises | Daily aerobic exercises and 3 weekly sessions of each lasting 30 min, 6 weeks | CRC | Postoperative complications ↓ 6MWT ↑ + 78.9 m Ergospirometry ↑ 1.5METs | [190] | ||
| Eccentric overloaded strength training and Neuromuscular electrical stimulation | 2 sets of 8 repetitions of unilateral squats, 12 weeks. | HNC | MIVC ↑ Cross-sectional area ↑ Cycling exercise time ↑ | [194] | ||
| Cancer-Related Fatigue (Human) | Human | RT-HIIT AT-HIIT | 60 min exercise sessions twice weekly for 16 weeks. | BC | IL-6 ↑ CD8a ↑ | [204] |
| / | 60 min each session; twice a week for 12 weeks. | NC | General fatigue ↓ Physical fatigue ↓ Emotional fatigue ↓ Mental fatigue ↓ QOL ↑ Physical function ↑ Social function ↑ Role function ↑ | [203] | ||
| Endurance and Strength Training | 3 times a week, lasting 30 min, for a period of 4 weeks | BC, CRC PCa, Lymphoma | General fatigue ↓ physical fatigue ↓ | [199] | ||
| Resistance training and home-based endurance training | 6-month, resistance training twice a week, 150 min of moderate to low-intensity walking or cycling per week. | BC, CRC PCa | Physical fatigue ↓ Cardiorespiratory fitness ↑ Leg strength ↑ | [205] | ||
| Baduanjin Qigong | At least 5 times a week, for 20–40 min each time, 24 weeks | I-III CRC | The KPS scores ↑ The PSQI scores ↓ | [206] | ||
| Tai Chi and Resistance Training | 12-week (3 days per week), 40 min per day | LC, BC, Gastric cancer | GAD-7 scores ↑ PHQ-9 scores ↑ the PSQI scores ↓ the QLQ-CCC ↑ | [207] | ||
| Aerobic and resistance exercises | Aerobic: 150 min/week Resistance: Two times a week | Cancer | CRF ↓ | [201] | ||
| QOL (Human) | Human | Aerobic and resistance training | Three times a week | BC | QoL ↑ QLQ-C30 ↑ | [187] |
| Yoga | 60 min each time, twice weekly for 6 weeks | PC | QoL ↑ FACT-physical well-being ↑ FACT-Social wellbeing ↑ | [72] | ||
| Laughter yoga | Four weeks, four times a week, 20–30 min each time | Cancer | Emotional function ↑ Role function ↑ QoL ↑ | [215] | ||
| Aerobic, Strength, and Flexibility Exercise | Twice a week, 60 min each time, 10 weeks | Cancer | the ADS total score ↓ Anxiety score ↓ QOL ↑ | [222] | ||
| HIIT and MICT | Three times a week, 12 weeks | BC | The physical well-being ↑ Social well-being ↑ Emotional well-being scores ↑ Functional well-being subscale scores ↑ FACT-G scores ↑ Functional well-being subscale scores ↑ | [218] | ||
| Walks | 15–20 min, 8 weeks | LC | QOL ↑ | [213] | ||
| Qigong and Tai Chi | / | Cancer | QoL ↑ physical functioning ↑ sleep quality ↑ Mental health ↑ Anxiety ↓ | [216] |
| Exercise Types | Suitable Population | Applicable Phase | Exercise Intensity | Frequency/Duration | Period | Refs |
|---|---|---|---|---|---|---|
| Aerobic exercises | Adult cancer patients | Postoperative/Late stage patients | Low | 5–10 min Accumulate at least 20 min | / | [37] |
| Aerobic and resistance exercises | Adult cancer patients | / | Moderate | Aerobic: 150 min/week Resistance: Two times a week | As defined by the patient | [230] |
| Aerobic and resistance exercises | Adult cancer patients | / | High | Aerobic: >75 min/week Resistance: two times a week | As defined by the patient | [230] |
| Aerobic exercises | CRC patients | After post-primary cancer treatment (surgery, chemotherapy, radiotherapy) | Moderate | >150 min/week; Three to seven times a week | ≥12 weeks | [231,232] |
| Aerobic and resistance exercises | BC Patients | Within five years of treatment/after treatment completion | Moderate | 50 min each time; Three times a week | 12~26 weeks | [233,234] |
| Aerobic, resistance, and impact exercises | PCa patients | patients with locally advanced and metastatic PCa | Moderate-to-high | Aerobic: ≥5 days/week; 20–30 min/session Impact: ≥3 days/week; 15–20 min/session Resistance: 2–3 days/week; 30–45 min/session | / | [235] |
| Pelvic floor muscle training (PFMT) | PCa patients | After radical prostatectomy | High | Perform contractions and relaxation for 5–10 s in three positions: standing, sitting, and lying down, A total of 10–45 min in three groups. | 12~18 weeks | [236,237] |
| Aerobic and resistance exercises | LC patients | During chemotherapy | Moderate-to-high | Brisk walking for 30 min per day; 5 days a week; Two resistance training sessions per week, each lasting 20 min | 12 weeks | [238] |
| Inspiratory muscle training (IMT) | LC patients | Advanced lung cancer | Moderate | 15~20 min each time; Twice a day | >4 weeks | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Ma, Y.; Shi, L.; Liu, T.; Dong, Y.; Jin, Q. The Impact and Molecular Mechanisms of Exercise in Cancer Therapy. Curr. Issues Mol. Biol. 2025, 47, 374. https://doi.org/10.3390/cimb47050374
Sun Y, Ma Y, Shi L, Liu T, Dong Y, Jin Q. The Impact and Molecular Mechanisms of Exercise in Cancer Therapy. Current Issues in Molecular Biology. 2025; 47(5):374. https://doi.org/10.3390/cimb47050374
Chicago/Turabian StyleSun, Yingjie, Yixiao Ma, Lei Shi, Tong Liu, Yahong Dong, and Qiguan Jin. 2025. "The Impact and Molecular Mechanisms of Exercise in Cancer Therapy" Current Issues in Molecular Biology 47, no. 5: 374. https://doi.org/10.3390/cimb47050374
APA StyleSun, Y., Ma, Y., Shi, L., Liu, T., Dong, Y., & Jin, Q. (2025). The Impact and Molecular Mechanisms of Exercise in Cancer Therapy. Current Issues in Molecular Biology, 47(5), 374. https://doi.org/10.3390/cimb47050374








