One Giant Leap from Mouse to Man: The Microbiota–Gut–Brain Axis in Mood Disorders and Translational Challenges Moving towards Human Clinical Trials
Abstract
:1. Introduction
2. Pathways of Communication along the Microbiota–Gut–Brain Axis
2.1. The Autonomic Nervous System and the Enteric Nervous System
2.2. The Vagus Nerve
2.3. Immune Signaling
2.4. Enteroendocrine Regulation
2.5. Neurotransmitters
3. The Microbiota–Gut–Brain Axis in Stress and Related Disorders, and Opportunities by Probiotics to Relieve or Prevent Symptoms
3.1. Stress, Anxiety and Probiotics
3.2. Depression and Probiotics
4. From Preclinical to Clinical—Translational Challenges in Microbiota–Gut–Brain Axis Studies
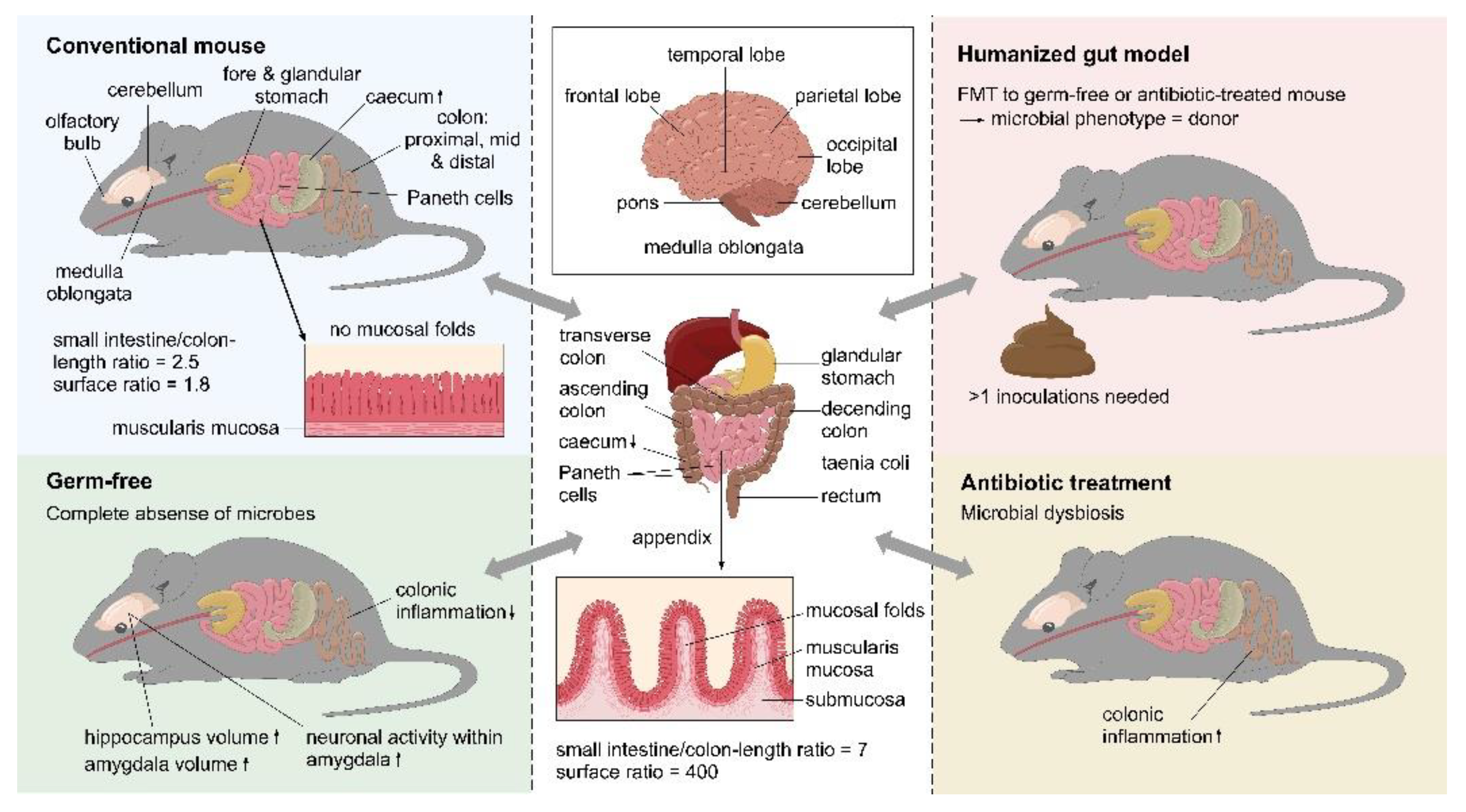
4.1. Germ-Free Models
4.2. Antibiotic Models
4.3. Humanized (Gut) Models
| Model | Changes | Effects | References | ||
|---|---|---|---|---|---|
| Germ-free | Brain physiology and function | Increase of neurogenesis in adult GF mice | Important role in learning and memory | [117] | |
| Increased hippocampal and amygdalar volume, altered dendrite and neuronal morphology within these brain regions | Structural integrity and signaling pathways within brain regions involved in stress response, anxiety behavior, and social interactions are dependent on the presence of the gut microbiota | [118,119] | |||
| Increased neuronal activity within the amygdala is associated with upregulated genes | GF mice have significantly lower BDNF mRNA expression compared to specific pathogen-free (SPF) mice | [119] | |||
| Lack of gut microbiota | Significant effect on serotonergic neurotransmission within CNS | [100] | |||
| Hippocampal concentrations of serotonin and 5-hydroxyindoleacetic acid, 5-HIAA (main metabolite of serotonin) are increased in male GF mice, and plasma concentrations of tryptophan are also increased | [100] | ||||
| Decreased expression of the serotonin receptor 1A (5HT1A) in the hippocampus | [120] | ||||
| Both increase and decrease of hippocampal BDNF mRNA expression reported in different studies | [100,120,121] | ||||
| Upregulation of genes linked to myelination and myelin plasticity in prefrontal cortex of adult GF mice | Presence of hyper-myelinated axons within prefrontal cortex | Significant impact on the future development of treatment strategies for myelination diseases, such as multiple sclerosis | [122] | ||
| Absence of gut microbiota | Microglia of GF mice are defective and display an immature phenotype and an impaired innate immune response to infection with a bacterial-associated inflammatory mediator—lipopolysaccharide (LPS) | The immune response is also defective within the periphery | [24,100,123] | ||
| Increase in BBB permeability | The CNS of GF mice is particularly vulnerable to brain damage and infection | [124] | |||
| Behavioral profiles, cognitive function and stress responses | Absence of gut microbiota | Increased pain response and visceral sensitivity | [125,126,127,128] | ||
| Impairment in sociability and social cognition, although one study has shown the opposite | i.e., the gut microbiome is essential for normal social behavior | [126,127,128,129] | |||
| Impaired short-term recognition and working memory | [130] | ||||
| Hyperactivity of the HPA axis response to stress | Varied effect of anxiety-like behavior, depending on the experimental design, species, strain, and sex | [100,101,116,120,121,126,130,131] | |||
| Antibiotic | During critical windows | Alterations or depletion of microbiota through administration of antibiotics (single/cocktail, absorbable/non-absorbable) to dams either during the periconception period, and/or during pregnancy, and/or during weaning or to the offspring in early life | Effect on neurodevelopment and behavior | [132,133,134,135] | |
| Reduced anxiety-like behavior Increased aggressive behavior Increased resilience to stress Reduction of social behavior and preference for social novelty Altered cytokine expression in the brain Altered BBB integrity | [136] | ||||
| Increased visceral hypersensitivity | [137,138] | ||||
| Reduced anxiety-like behavior, cognitive deficits, altered tryptophan metabolism and gene expression | [139] | ||||
| Expression of BDNF and its receptor in both ENS and CNS | [140] | ||||
| Expression of genes involved in immune function, neurotransmission, and neuroplasticity in the amygdala | Long-lasting effects on gut microbiota composition into adulthood | [141] | |||
| In mood disorders and neurodegenerative disorders | Alterations in gut microbiota | Attenuated inflammation, and β-amyloid (Aβ) and other pathologies associated with disease progression Delay disease related memory deficits | [142,143,144,145] | ||
| Depletion of gut microbiota | Decreased microglia activation, reduced | Indication that gut microbiome is important for enhancing Parkinson’s disease-like symptoms | [146] | ||
| Administration of an antibiotic cocktail from adolescence to adulthood to mice with experimental autoimmune encephalomyelitis (EAE)t | Depletion of the gut microbiota significantly delayed the onset of EAE symptoms and altered several immunological and neurobehavioral responses | [147] | |||
| Administration of antibiotics prior to exposure to chronic social defeat stress (CSDS) | No development of anhedonic-like behavior in adulthood when compared to mice administered water only | [148] | |||
| Administration of antibiotic cocktail | Depleted serotonin levels in the intestine coupled with an altered sleep/wake cycle | [149] | |||
| Humanized gut models | Generation of a humanized mouse with a gut microbiota resembling that of the human donor | GF recipient mice are already markedly altered and depending on the dose, duration, and composition of the antibiotic cocktail, antibiotic-treated recipient mice may have experienced other CNS effects or incomplete microbiome depletion | FTM from human donors with, e.g., depression, alcoholism, anorexia nervosa, IBS, and schizophrenia; rodents later presented abnormalities in behavior, indicating at least partial transfer of the clinical psychiatric phenotype | [81,82,109,110,111,112,113,114] | |
| Transplantation of the gut microbiota from patients with PD | Worsening of the motor symptoms in genetically susceptible mice compared with those in receipt of the gut microbiota from healthy controls | Highlight the gut microbiome as a significant contributing factor towards progression of PD symptoms in genetically susceptible hosts | [146] | ||
| Recipient rats developed behavioral and physiological features characteristic of the donors with depressive disorder such as increased anhedonic- and anxiety-like behaviors, as well as alterations in tryptophan metabolism and an increased inflammatory profile | [113,114] | ||||
5. Psychobiotics—Selected Case Studies of Translational Results from Preclinical to Clinical
5.1. Bifidobacterium longum 1714®
5.2. Lacticaseibacillus paracasei Lpc-37®
5.3. Lactobacillus helveticus Rosell®-52 + Bifidobacterium longum Rosell®-175
5.4. Lacticaseibacillus rhamnosus JB-1™
6. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hugon, P.; Dufour, J.-C.; Colson, P.; Fournier, P.-E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Huseyin, C.E.; Rubio, R.C.; O’Sullivan, O.; Cotter, P.D.; Scanlan, P.D. The Fungal Frontier: A Comparative Analysis of Methods Used in the Study of the Human Gut Mycobiome. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Long-Smith, C.M.; Carbia, C.; Bastiaanssen, T.F.S.; van de Wouw, M.; Wiley, N.; Strain, C.R.; Fouhy, F.; Stanton, C.; Cryan, J.F.; et al. A specific dietary fibre supplementation improves cognitive performance-an exploratory randomised, placebo-controlled, crossover study. Psychopharmacology 2021, 238, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multistrain Versus Single-strain Probiotics: Current Status and Recommendations for the Future. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Zimmermann, A.K.; Ouwehand, A.C. Contemporary meta-analysis of short-term probiotic consumption on gastrointestinal transit. World J. Gastroenterol. 2016, 22, 5122–5131. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C. Antiallergic effects of probiotics. J. Nutr. 2007, 137, 794S–797S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Chen, C.; Wen, T.; Zhao, Q. Probiotics for the Prevention of Antibiotic-associated Diarrhea in Adults: A Meta-Analysis of Randomized Placebo-Controlled Trials. J. Clin. Gastroenterol. 2020. publish ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [Green Version]
- Tait, C.; Sayuk, G.S. The Brain-Gut-Microbiotal Axis: A framework for understanding functional GI illness and their therapeutic interventions. Eur. J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021. [Google Scholar] [CrossRef]
- Rani, L.; Mondal, A.C. Unravelling the role of gut microbiota in Parkinson’s disease progression: Pathogenic and therapeutic implications. Neurosci. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Doifode, T.; Giridharan, V.V.; Generoso, J.S.; Bhatti, G.; Collodel, A.; Schulz, P.E.; Forlenza, O.V.; Barichello, T. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 2020, 164, 105314. [Google Scholar] [CrossRef]
- Nanthakumaran, S.; Sridharan, S.; Somagutta, M.R.; Arnold, A.A.; May, V.; Pagad, S.; Malik, B.H. The Gut-Brain Axis and Its Role in Depression. Cureus 2020, 12, e10280. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef]
- Konig, J.; Wells, J.; Cani, P.D.; Garcia-Rodenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Salles, B.I.M.; Cioffi, D.; Ferreira, S.R.G. Probiotics supplementation and insulin resistance: A systematic review. Diabetol. Metab. Syndr. 2020, 12, 98. [Google Scholar] [CrossRef]
- Milajerdi, A.; Mousavi, S.M.; Sadeghi, A.; Salari-Moghaddam, A.; Parohan, M.; Larijani, B.; Esmaillzadeh, A. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur. J. Nutr. 2020, 59, 633–649. [Google Scholar] [CrossRef]
- Xie, C.; Jones, K.L.; Rayner, C.K.; Wu, T. Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics 2020, 12, 790. [Google Scholar] [CrossRef]
- O’Malley, D. Endocrine regulation of gut function—A role for glucagon-like peptide-1 in the pathophysiology of irritable bowel syndrome. Exp. Physiol. 2019, 104, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Dickson, S.L. Enteroendocrine hormones—Central effects on behavior. Curr. Opin. Pharmacol. 2013, 13, 977–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.W.; Hsueh, S.C.; Lai, J.H.; Chen, Y.H.; Kang, S.J.; Chen, K.Y.; Hsieh, T.H.; Hoffer, B.J.; Li, Y.; Greig, N.H.; et al. Glucose-Dependent Insulinotropic Polypeptide Mitigates 6-OHDA-Induced Behavioral Impairments in Parkinsonian Rats. Int. J. Mol. Sci. 2018, 19, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Kitazawa, T.; Kaiya, H. Regulation of Gastrointestinal Motility by Motilin and Ghrelin in Vertebrates. Front. Endocrinol. 2019, 10, 278. [Google Scholar] [CrossRef]
- Koutouratsas, T.; Kalli, T.; Karamanolis, G.; Gazouli, M. Contribution of ghrelin to functional gastrointestinal disorders’ pathogenesis. World J. Gastroenterol. 2019, 25, 539–551. [Google Scholar] [CrossRef]
- Stone, L.A.; Harmatz, E.S.; Goosens, K.A. Ghrelin as a Stress Hormone: Implications for Psychiatric Illness. Biol. Psychiatry 2020, 88, 531–540. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G. Chronic stress promotes cancer development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374. [Google Scholar] [CrossRef]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, Fso23. [Google Scholar] [CrossRef] [Green Version]
- Bharwani, A.; Mian, M.F.; Foster, J.A.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology 2016, 63, 217–227. [Google Scholar] [PubMed] [Green Version]
- Golubeva, A.V.; Crampton, S.; Desbonnet, L.; Edge, D.; O’Sullivan, O.; Lomasney, K.W.; Zhdanov, A.V.; Crispie, F.; Moloney, R.D.; Borre, Y.E. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015, 60, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Partrick, K.A.; Chassaing, B.; Beach, L.Q.; McCann, K.E.; Gewirtz, A.T.; Huhman, K.L. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav. Brain Res. 2018, 345, 39. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.T.; Coe, C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 1999, 35, 146–155. [Google Scholar] [CrossRef]
- Bailey, M.T.; Lubach, G.R.; Coe, C.L. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J. Pediatric Gastroenterol. Nutr. 2004, 38, 414–421. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Jašarević, E.; Howard, C.D.; Morrison, K.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Zijlmans, M.A.; Korpela, K.; Riksen-Walraven, J.M.; de Vos, W.M.; de Weerth, C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 2015, 53, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-gut-brain axis: New therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Belgnaoui, A.; Durand, H.; Cartier, C.; Chaumaz, G.; Eutamene, H.; Ferrier, L.; Houdeau, E.; Fioramonti, J.; Bueno, L.; Theodorou, V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012, 37, 1885–1895. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Singh, D.; Singh, S.; Pinnaka, A.K.; Boparai, R.; Bishnoi, M.; Kondepudi, K.; Chopra, K. Lactobacillus plantarum MTCC 9510 supplementation protects from chronic unpredictable and sleep deprivation-induced behaviour, biochemical and selected gut microbial aberrations in mice. J. Appl. Microbiol. 2018, 125, 257–269. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.; Fahnestock, M.; Moine, D. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [Green Version]
- Savignac, H.M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014, 26, 1615–1627. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y.; Li, M.; Wang, W.; Liu, Z.; Xi, C.; Huang, X.; Liu, J.; Huang, J.; Tian, D. Efficacy of probiotics on stress in healthy volunteers: A systematic review and meta-analysis based on randomized controlled trials. Brain Behav. 2020, 10, e01699. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Wang, M.; Liu, J.; Ju, Y.; Zhang, Y.; Liu, T.; Li, L.; Li, Q. Efficacy of probiotics on anxiety—A meta-analysis of randomized controlled trials. Depress. Anxiety 2018, 35, 935–945. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Colica, C.; Avolio, E.; Bollero, P.; Costa de Miranda, R.; Ferraro, S.; Sinibaldi Salimei, P.; De Lorenzo, A.; Di Renzo, L. Evidences of a new psychobiotic formulation on body composition and anxiety. Mediat. Inflamm. 2017, 2017, 5650627. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol. Stress 2019, 10, 100141. [Google Scholar] [CrossRef] [PubMed]
- Marotta, A.; Sarno, E.; Del Casale, A.; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front. Psychiatry 2019, 10, 164. [Google Scholar] [CrossRef]
- Chong, H.; Yusoff, N.; Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.; Choi, S.-B.; Yusoff, M.; Wahid, N.; Abdullah, M.; Zakaria, N. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- Önning, G.; Hillman, M.; Hedin, M.; Montelius, C.; Eriksson, J.; Ahrné, S.; Jönsson, P. Intake of Lactiplantibacillus plantarum HEAL9 reduces the inflammatory markers soluble fractalkine and CD163 during acute stress: A randomized, double blind, placebo-controlled study. Physiol. Behav. 2020, 225, 113083. [Google Scholar] [CrossRef]
- Nishihira, J.; Kagami-Katsuyama, H.; Tanaka, A.; Nishimura, M.; Kobayashi, T.; Kawasaki, Y. Elevation of natural killer cell activity and alleviation of mental stress by the consumption of yogurt containing Lactobacillus gasseri SBT2055 and Bifidobacterium longum SBT2928 in a double-blind, placebo-controlled clinical trial. J. Funct. Foods 2014, 11, 261–268. [Google Scholar] [CrossRef]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; Tullberg, C.; Ahrné, S.; Hamberg, K.; Lazou Ahrén, I.; Molin, G.; Sonesson, M.; Håkansson, Å. Oral administration of Lactobacillus plantarum 299v reduces cortisol levels in human saliva during examination induced stress: A randomized, double-blind controlled trial. Int. J. Microbiol. 2016, 2016, 8469018. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.; Zhebrak, M.; Yacoub, C.; Pelletier, J.; Hawley, D. The gut-brain relationship: Investigating the effect of multispecies probiotics on anxiety in a randomized placebo-controlled trial of healthy young adults. J. Affect. Disord. 2019, 252, 271–277. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T. Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langkamp-Henken, B.; Rowe, C.C.; Ford, A.L.; Christman, M.C.; Nieves, C.; Khouri, L.; Specht, G.J.; Girard, S.-A.; Spaiser, S.J.; Dahl, W.J. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 113, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-I.; Wu, C.-C.; Tsai, P.-J.; Cheng, L.-H.; Hsu, C.-C.; Shan, I.-K.; Chan, P.-Y.; Lin, T.-W.; Ko, C.-J.; Chen, W.-L. Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study. Front. Nutr. 2021, 8, 130. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Maes, M.; Leunis, J.-C. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: Effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuroendocrinol. Lett. 2008, 29, 902. [Google Scholar] [PubMed]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S.; Abildgaard, A.; Winther, G.; Wegener, G. Altered fecal microbiota composition in the Flinders sensitive line rat model of depression. Psychopharmacology 2019, 236, 1445–1457. [Google Scholar] [CrossRef] [Green Version]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.F.; Kim, H.-M.; Lim, S.; Chung, M.-J.; Lim, C.-Y.; Koo, B.-S.; Kang, S.-S. Effect of probiotic administration on gut microbiota and depressive behaviors in mice. DARU J. Pharm. Sci. 2020, 28, 181–189. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.-F.; Ma, C.-L.; Wei, H.; Li, B.-M.; Luo, J. Alleviation of Anxiety/Depressive-Like Behaviors and Improvement of Cognitive Functions by Lactobacillus plantarum WLPL04 in Chronically Stressed Mice. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6613903. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology 2017, 153, 448–459.e448. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Okazaki, S.; Yamashita, S.; Miura, S.; Miki, H. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: A prospective open-label trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirbaglou, M.; Katz, J.; de Souza, R.J.; Stearns, J.C.; Motamed, M.; Ritvo, P. Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutr. Res. 2016, 36, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 2018, 228, 13–19. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Jin, H.-M.; Cui, Y.; Jung, J.M.; Park, J.-I.; Jung, E.-S.; Choi, E.-K.; Chae, S.-W. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J. Funct. Foods 2014, 10, 465–474. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [Green Version]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Dauge, V.; Naudon, L.; Rabot, S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jacan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Frohlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [Green Version]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Moloney, R.D.; Golubeva, A.V.; McVey Neufeld, K.A.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016, 339, 463–477. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.; Moloney, G.M.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 2017, 5, 102. [Google Scholar] [CrossRef] [Green Version]
- Mohle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Hu, H.; Wang, F.; Li, L.; Zhu, W.; Shen, Y.; Xiu, J.; Xu, Q. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav. Immun. 2021, 92, 102–114. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Bibbo, S.; Cammarota, G.; Gasbarrini, A. Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Hu, Y.; Li, C.; Li, N.; Zhu, S.; Tan, X.; Li, M.; Zhang, Y.; Xu, Z.; Ding, Z.; et al. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC epsilon levels in mouse. Biofactors 2020, 46, 38–54. [Google Scholar] [CrossRef]
- Hata, T.; Miyata, N.; Takakura, S.; Yoshihara, K.; Asano, Y.; Kimura-Todani, T.; Yamashita, M.; Zhang, X.T.; Watanabe, N.; Mikami, K.; et al. The Gut Microbiome Derived From Anorexia Nervosa Patients Impairs Weight Gain and Behavioral Performance in Female Mice. Endocrinology 2019, 160, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Lynch, M.D.; Lu, J.; Dang, V.T.; Deng, Y.; Jury, J.; Umeh, G.; Miranda, P.M.; Pigrau Pastor, M.; Sidani, S.; et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H.; et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 29-30, 100642. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry 2015, 78, e7–e9. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [Green Version]
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 2015, 50, 209–220. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264.e119. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. Elife 2017, 6. [Google Scholar] [CrossRef]
- Arentsen, T.; Raith, H.; Qian, Y.; Forssberg, H.; Diaz Heijtz, R. Host microbiota modulates development of social preference in mice. Microb. Ecol. Health Dis. 2015, 26, 29719. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366. [Google Scholar] [CrossRef]
- Stilling, R.M.; Moloney, G.M.; Ryan, F.J.; Hoban, A.E.; Bastiaanssen, T.F.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Social interaction-induced activation of RNA splicing in the amygdala of microbiome-deficient mice. Elife 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; Macqueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013, 25, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Degroote, S.; Hunting, D.J.; Baccarelli, A.A.; Takser, L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M.L.; Crowe, S.M.; McCarthy, J.S.; Mills, J.; Mouton, J.W.; Norrby, S.R.; Paterson, D.L.; Pfaller, M.A. Kucers’ the Use of Antibiotics Sixth Edition: A Clinical Review of Antibacterial, Antifungal and Antiviral Drugs; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- O’Connor, R.; Moloney, G.M.; Fulling, C.; O’Riordan, K.J.; Fitzgerald, P.; Bastiaanssen, T.F.S.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Maternal antibiotic administration during a critical developmental window has enduring neurobehavioural effects in offspring mice. Behav Brain. Res. 2021, 404, 113156. [Google Scholar] [CrossRef]
- Cowan, C.S.M.; Dinan, T.G.; Cryan, J.F. Annual Research Review: Critical windows—the microbiota-gut-brain axis in neurocognitive development. J. Child Psychol. Psychiatry 2020, 61, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 15062. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S.; Ikeno, T.; Ito, T.; Sakurai, A.; Yamauchi, T.; Matsuzaki, H. Administration of Non-Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS ONE 2016, 11, e0138293. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Felice, V.D.; Nally, K.; Savignac, H.M.; Claesson, M.J.; Scully, P.; Woznicki, J.; Hyland, N.P.; Shanahan, F.; Quigley, E.M.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Bistoletti, M.; Caputi, V.; Baranzini, N.; Marchesi, N.; Filpa, V.; Marsilio, I.; Cerantola, S.; Terova, G.; Baj, A.; Grimaldi, A.; et al. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS ONE 2019, 14, e0212856. [Google Scholar] [CrossRef] [Green Version]
- Lach, G.; Fulling, C.; Bastiaanssen, T.F.S.; Fouhy, F.; Donovan, A.N.O.; Ventura-Silva, A.P.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry 2020, 10, 382. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Frith, M.; Sidebottom, A.; Cao, Y.; Koval, J.; Chang, E.; Sisodia, S.S. Synergistic depletion of gut microbial consortia, but not individual antibiotics, reduces amyloidosis in APPPS1-21 Alzheimer’s transgenic mice. Sci. Rep. 2020, 10, 8183. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Mezo, C.; Dokalis, N.; Mossad, O.; Staszewski, O.; Neuber, J.; Yilmaz, B.; Schnepf, D.; de Aguero, M.G.; Ganal-Vonarburg, S.C.; Macpherson, A.J.; et al. Different effects of constitutive and induced microbiota modulation on microglia in a mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeraati, M.; Enayati, M.; Kafami, L.; Shahidi, S.H.; Salari, A.A. Gut microbiota depletion from early adolescence alters adult immunological and neurobehavioral responses in a mouse model of multiple sclerosis. Neuropharmacology 2019, 157, 107685. [Google Scholar] [CrossRef]
- Wang, S.; Qu, Y.; Chang, L.; Pu, Y.; Zhang, K.; Hashimoto, K. Antibiotic-induced microbiome depletion is associated with resilience in mice after chronic social defeat stress. J. Affect. Disord. 2020, 260, 448–457. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714 Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Moloney, G.M.; Long-Smith, C.M.; Murphy, A.; Dorland, D.; Hojabri, S.F.; Ramirez, L.O.; Marin, D.C.; Bastiaanssen, T.F.; Cusack, A.-M.; Berding, K. Improvements in sleep indices during exam stress due to consumption of a Bifidobacterium longum. Brain Behav. Immun.-Health 2021, 10, 100174. [Google Scholar] [CrossRef] [PubMed]
- Stenman, L.K.; Patterson, E.; Meunier, J.; Roman, F.J.; Lehtinen, M.J. Strain specific stress-modulating effects of candidate probiotics: A systematic screening in a mouse model of chronic restraint stress. Behav. Brain Res. 2020, 379, 112376. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Griffin, S.M.; Ibarra, A.; Ellsiepen, E.; Hellhammer, J. Lacticaseibacillus paracasei Lpc-37(R) improves psychological and physiological markers of stress and anxiety in healthy adults: A randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol. Stress 2020, 13, 100277. [Google Scholar] [CrossRef]
- Arseneault-Breard, J.; Rondeau, I.; Gilbert, K.; Girard, S.A.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br. J. Nutr. 2012, 107, 1793–1799. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, K.; Arseneault-Breard, J.; Flores Monaco, F.; Beaudoin, A.; Bah, T.M.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Attenuation of post-myocardial infarction depression in rats by n-3 fatty acids or probiotics starting after the onset of reperfusion. Br. J. Nutr. 2013, 109, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Malick, M.; Gilbert, K.; Daniel, J.; Arseneault-Breard, J.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Vagotomy prevents the effect of probiotics on caspase activity in a model of postmyocardial infarction depression. Neurogastroenterol. Motil. 2015, 27, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Lalonde, R.; Violle, N.; Javelot, H.; Desor, D.; Nejdi, A.; Bisson, J.F.; Rougeot, C.; Pichelin, M.; Cazaubiel, M.; et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011, 105, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Theodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, G.; Dargahi, L.; Peymani, A.; Mirzanejad, Y.; Alizadeh, S.A.; Naserpour, T.; Nassiri-Asl, M. The Effects of Probiotic Formulation Pretreatment (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) on a Lipopolysaccharide Rat Model. J. Am. Coll. Nutr. 2019, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S.; Awwad, H.M.; Eskelund, A.R.; Treccani, G.; Geisel, J.; Wegener, G.; Obeid, R. Probiotics Affect One-Carbon Metabolites and Catecholamines in a Genetic Rat Model of Depression. Mol. Nutr. Food Res. 2018, 62, e1701070. [Google Scholar] [CrossRef]
- Partrick, K.A.; Rosenhauer, A.M.; Auger, J.; Arnold, A.R.; Ronczkowski, N.M.; Jackson, L.M.; Lord, M.N.; Abdulla, S.M.; Chassaing, B.; Huhman, K.L. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 2021, 11, 3763. [Google Scholar] [CrossRef] [PubMed]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Foster, J.A.; Soares, C.N.; Milev, R.V. The Effects of Probiotics on Symptoms of Depression: Protocol for a Double-Blind Randomized Placebo-Controlled Trial. Neuropsychobiology 2020, 79, 108–116. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results From an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef]
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 2006, 55, 191–196. [Google Scholar] [CrossRef]
- Ma, X.; Mao, Y.K.; Wang, B.; Huizinga, J.D.; Bienenstock, J.; Kunze, W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G868–G875. [Google Scholar] [CrossRef] [Green Version]
- Kunze, W.A.; Mao, Y.K.; Wang, B.; Huizinga, J.D.; Ma, X.; Forsythe, P.; Bienenstock, J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mao, Y.K.; Diorio, C.; Pasyk, M.; Wu, R.Y.; Bienenstock, J.; Kunze, W.A. Luminal administration ex vivo of a live Lactobacillus species moderates mouse jejunal motility within minutes. FASEB J. 2010, 24, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Pasyk, M.; Wang, B.; Forsythe, P.; Bienenstock, J.; Mao, Y.K.; Sharma, P.; Stanisz, A.M.; Kunze, W.A. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol. Motil. 2013, 25, e205–e214. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Wu, R.Y.; Wong, A.; Stanisz, A.M.; Yan, R.; Min, K.K.; Pasyk, M.; McVey Neufeld, K.A.; Karamat, M.I.; Foster, J.A.; et al. Lactobacillus rhamnosus strain JB-1 reverses restraint stress-induced gut dysmotility. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Perez-Burgos, A.; Wang, B.; Mao, Y.K.; Mistry, B.; McVey Neufeld, K.A.; Bienenstock, J.; Kunze, W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G211–G220. [Google Scholar] [CrossRef] [Green Version]
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Marin, I.A.; Goertz, J.E.; Ren, T.; Rich, S.S.; Onengut-Gumuscu, S.; Farber, E.; Wu, M.; Overall, C.C.; Kipnis, J.; Gaultier, A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017, 7, 43859. [Google Scholar] [CrossRef]
- Kochalska, K.; Oakden, W.; Slowik, T.; Chudzik, A.; Pankowska, A.; Lazorczyk, A.; Koziol, P.; Andres-Mach, M.; Pietura, R.; Rola, R.; et al. Dietary supplementation with Lactobacillus rhamnosus JB-1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr. Res. 2020, 82, 44–57. [Google Scholar] [CrossRef]
- Liu, Y.; Steinhausen, K.; Bharwani, A.; Mian, M.F.; McVey Neufeld, K.A.; Forsythe, P. Increased persistence of avoidance behaviour and social deficits with L.rhamnosus JB-1 or selective serotonin reuptake inhibitor treatment following social defeat. Sci. Rep. 2020, 10, 13485. [Google Scholar] [CrossRef] [PubMed]
- Kayyal, M.; Javkar, T.; Firoz Mian, M.; Binyamin, D.; Koren, O.; McVey Neufeld, K.A.; Forsythe, P. Sex dependent effects of post-natal penicillin on brain, behavior and immune regulation are prevented by concurrent probiotic treatment. Sci. Rep. 2020, 10, 10318. [Google Scholar] [CrossRef] [PubMed]
- McVey Neufeld, K.A.; Kay, S.; Bienenstock, J. Mouse Strain Affects Behavioral and Neuroendocrine Stress Responses Following Administration of Probiotic Lactobacillus rhamnosus JB-1 or Traditional Antidepressant Fluoxetine. Front. Neurosci. 2018, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
| Neurotransmitter | Endogenous Production | Exogenous Production | Function | Remarks | References |
|---|---|---|---|---|---|
| Nitric oxide (NO) | Enteric inhibitory neurons | Enterobacteria, some lactobacilli and bifidobacteria, and some oral anaerobes | Gut motility, brain development, memory, and anti-anxiety | Enteric-produced NO does not play a role in anxiety | [38,39,40] |
| γ-aminobutyric acid (GABA) | GABA-ergic neurons | Some lactic acid bacteria and bifidobacteria | Neuroprotection, anti-diabetic, antioxidant, anti-inflammatory, anti-allergic, hepatoprotection, renoprotection, anti-depression, and anti-insomnia | Does not cross blood–brain barrier | [41,42,43] |
| Norepinephrine | Enteric nerve cells | E. coli, Bacillus, Saccharomyces spp., S. marcescens and P. vulgaris | Anti-inflammatory, anti-stress, and anti-anxiety | Does not cross blood–brain barrier | [44,45,46,47,48,49] |
| Dopamine | Central nervous system, various other tissues | Bacillus spp. | Locomotion, learning, working memory, cognition, and emotion | Does not cross blood–brain barrier | [46,49,50] |
| Acetylcholine | Cholinergic neurons | L. plantarum | Cognitive function and intestinal motility | Does not cross blood–brain barrier | [51,52,53] |
| Serotonin (5-hydroxytryptamine; 5-HT) | Serotonergic neurons mainly in the gut | Candida, E. coli, Lc. lactis, L. plantarum, S. thermophilus, M. morganii, K. pneumoniae, H. alvei and Enterococcus spp. | Regulation of mood, appetite, sleep, and cognitive function | Does not cross blood–brain barrier | [49,54,55,56,57] |
| Melatonin | Enterochromaffin cells in the gut | - | Regulation of circadian rhythm | Intestinal microbiota may be involved in breakdown | [57,58,59] |
| Indole | - | Actinobacteria, Firmicutes, Bacteroidetes, Proteobacteria, Fusobacteria, Clostridium, Burkholderia, Streptomyces, Pseudomonas and Bacillus | May influence emotional behavior | Crosses blood–brain barrier | [57,60] |
| Kynurenine and/kynurenic acid | Central nervous system, various other tissues | B. infantis | Associated with depression and schizophrenia | Increased kynurenic acid: kynurenine is neuroprotective. Both can cross blood–brain barrier | [57,61,62] |
| Quinolinic acid | Epithelial cells and intestinal immune cells | - | Associated with depression | Neurotoxic. Does not cross blood–brain barrier. May be blocked by L. helveticus and B. longum | [57,61,63,64] |
| Histamine | Mast cells and other immune cells | Certain lactic acid bacteria fermented foods | Mediates arousal, attention, and reactivity | Does not cross blood–brain barrier | [56,65,66,67] |
| Short chain fatty acids (SCFA) * | Muscle tissue | Most anaerobes in the gut | Regulate inflammation, appetite, depression, and gut motility | Crosses blood–brain barrier | [68,69,70,71,72,73,74,75,76,77,78,79,80,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forssten, S.D.; Ouwehand, A.C.; Griffin, S.M.; Patterson, E. One Giant Leap from Mouse to Man: The Microbiota–Gut–Brain Axis in Mood Disorders and Translational Challenges Moving towards Human Clinical Trials. Nutrients 2022, 14, 568. https://doi.org/10.3390/nu14030568
Forssten SD, Ouwehand AC, Griffin SM, Patterson E. One Giant Leap from Mouse to Man: The Microbiota–Gut–Brain Axis in Mood Disorders and Translational Challenges Moving towards Human Clinical Trials. Nutrients. 2022; 14(3):568. https://doi.org/10.3390/nu14030568
Chicago/Turabian StyleForssten, Sofia D., Arthur C. Ouwehand, Síle M. Griffin, and Elaine Patterson. 2022. "One Giant Leap from Mouse to Man: The Microbiota–Gut–Brain Axis in Mood Disorders and Translational Challenges Moving towards Human Clinical Trials" Nutrients 14, no. 3: 568. https://doi.org/10.3390/nu14030568






