Myricetin Increases Circulating Adropin Level after Activation of Glucagon-like Peptide 1 (GLP-1) Receptor in Type-1 Diabetic Rats
Abstract
:1. Introduction
2. Results
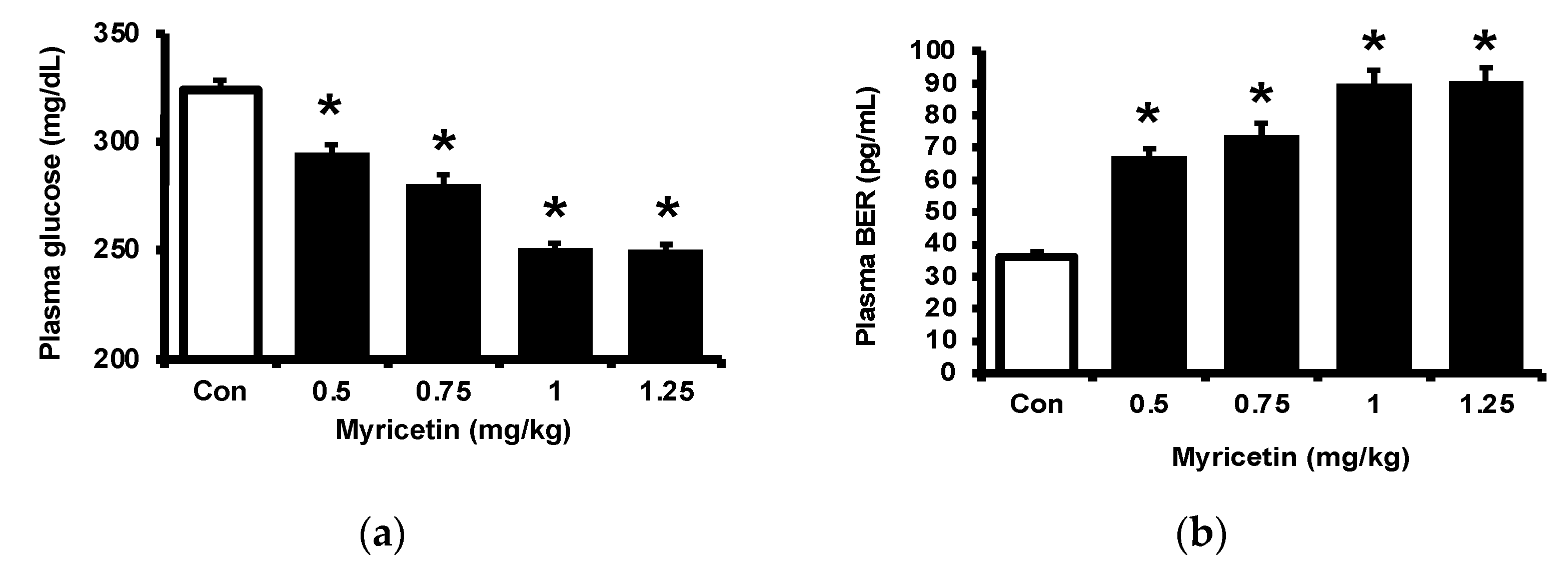
2.1. Effects of Myricetin on Plasma Glucose, Beta-Endorphin (BER), and Adropin Levels in Type-1 Diabetic Rats
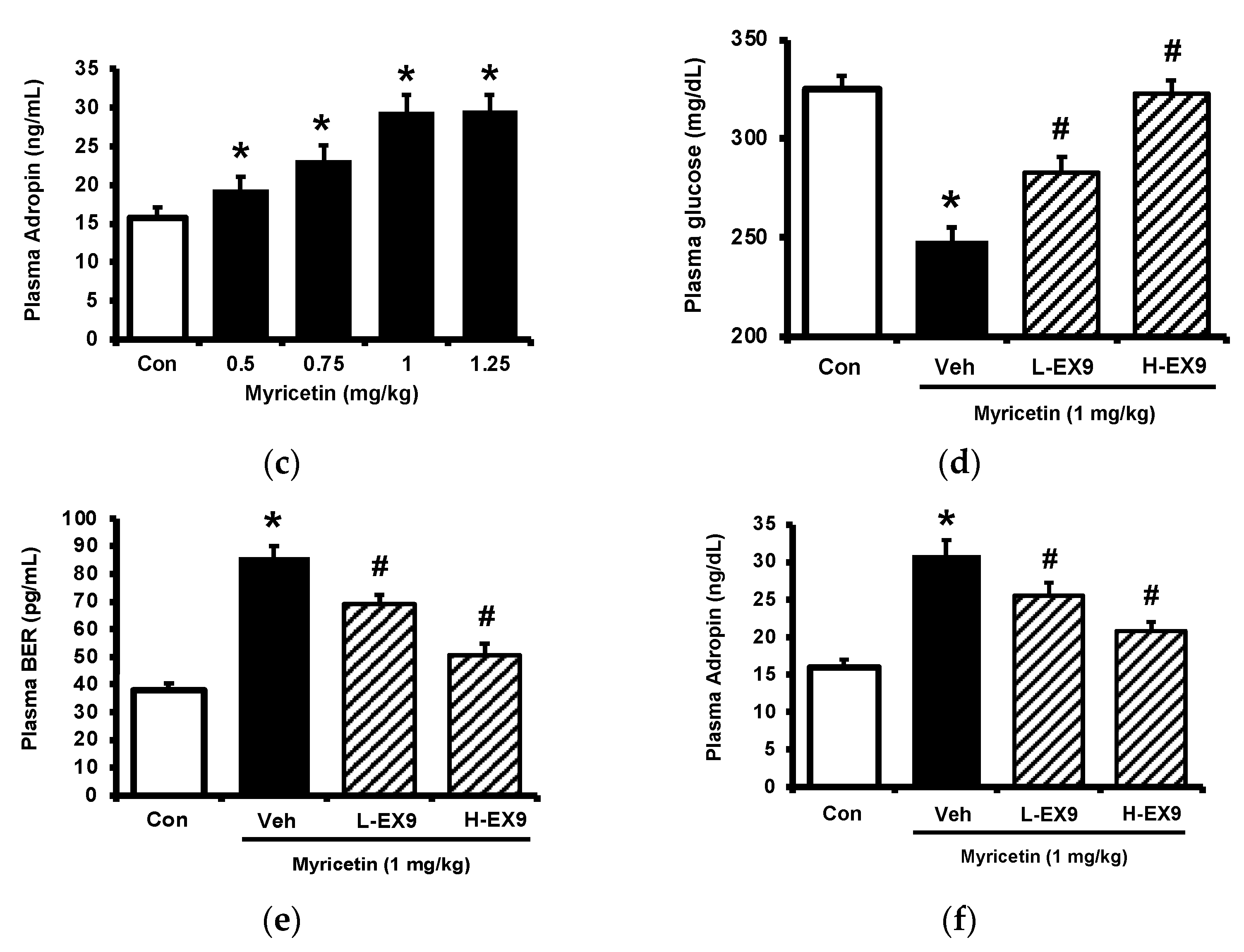
2.2. Activation of GLP-1 Receptor by Myricetin
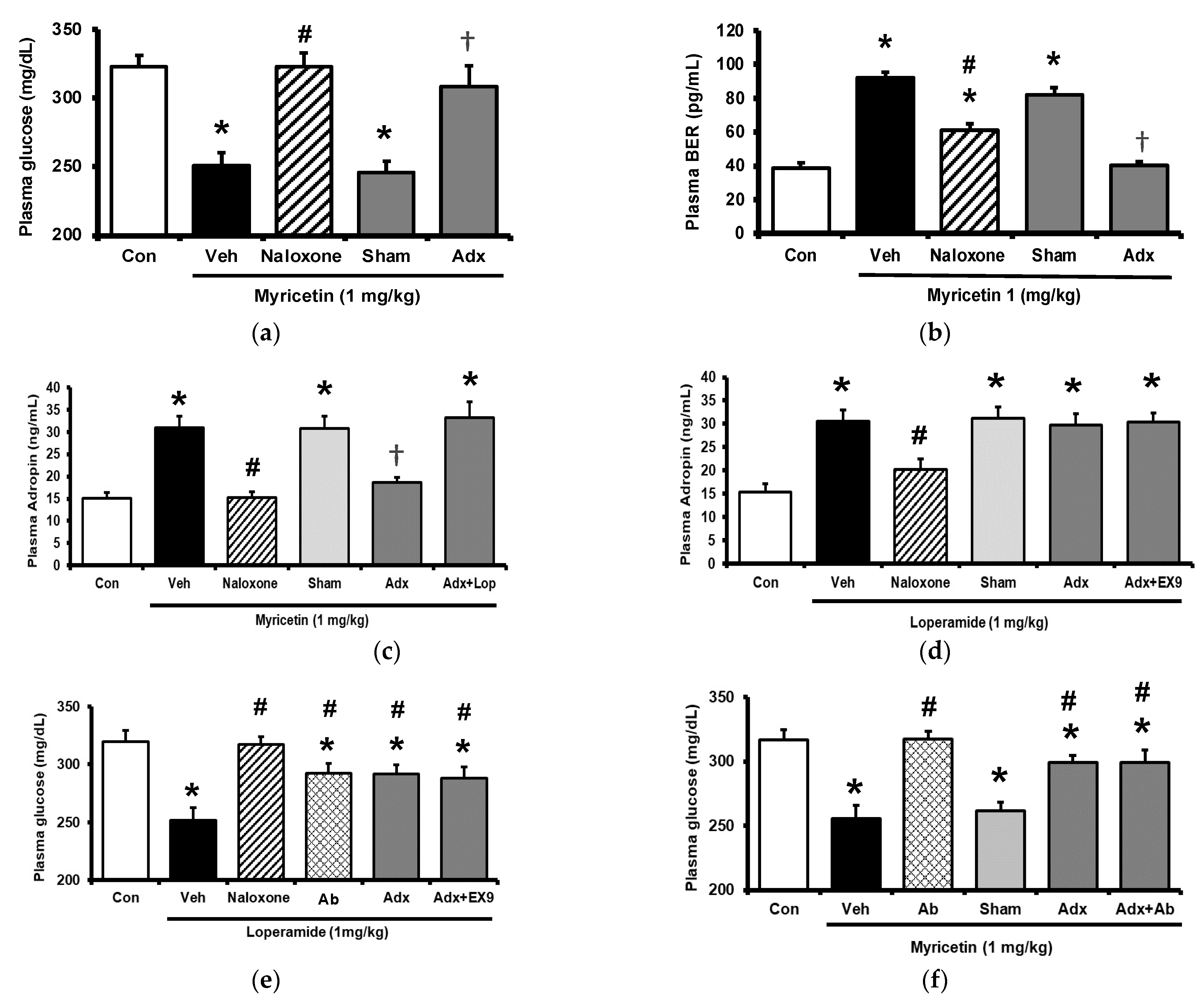
2.3. Circulating Adropin and Beta-Endorphin (BER) Levels Changed by Acute Treatment with Myricetin in Type-1 Diabetic Rats
2.4. Circulating Adropin and Beta-Endorphin (BER) Levels Changed by Chronic Treatment with Myricetin in Type-1 Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Model
4.3. Experimental Protocol
4.4. Laboratory Determinations
4.5. Adrenalectomy in Rats
4.6. Incubation of Isolated Adrenal Medulla
4.7. Incubation of Cultured Cells
4.7.1. PC-12 Cells
4.7.2. H9c2 Cells
4.7.3. HepG2 Cells
4.8. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
- forward: 5′-AGTGCGAAGAGTCCAAGCAA-3′
- reverse: 5′-TTGAGGGCAGCGTCTTTGAT-3′
- forward: 5′-GTTGTCCCGCCTCTC-3′
- reverse: 5′-CCACACACAGCGACTTCTTG-3′
- forward: 5′-CATCCAGGCTGTGTTGTCCC-3′
- reverse: 5′-CACGCACGATTTCCCTCTCA-3′
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, M.S.; Hughes, M.; Burns, J.; Lean, M.E.; Matthews, D.; Crozier, A. Survey of the Free and Conjugated Myricetin and Quercetin Content of Red Wines of Different Geographical Origins. J. Agric. Food Chem. 1998, 46, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.J.; Thompson, D.L. Effect of emulin on blood glucose in type 2 diabetics. J. Med. Food 2013, 16, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, N.; Sadashivaiah, B.; Ramaprasad, T.R.; Singh, S.A. Anti-hyperglycemic activity of myricetin, through inhibition of DPP-4 and enhanced GLP-1 levels, is attenuated by co-ingestion with lectin-rich protein. PLoS ONE 2020, 15, e0231543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, R.; Kulkarni, S.H.; Dantu, S.C.; Panigrahi, R.; Sardesai, D.M.; Malik, N.; Acharya, J.D.; Chugh, J.; Sharma, S.; Kumar, A. Myricetin protects pancreatic beta-cells from human islet amyloid polypeptide (hIAPP) induced cytotoxicity and restores islet function. Biol. Chem. 2021, 402, 179–194. [Google Scholar] [CrossRef]
- Liao, H.H.; Zhu, J.X.; Feng, H.; Ni, J.; Zhang, N.; Chen, S.; Liu, H.J.; Yang, Z.; Deng, W.; Tang, Q.Z. Myricetin Possesses Potential Protective Effects on Diabetic Cardiomyopathy through Inhibiting IkappaBalpha/NFkappaB and Enhancing Nrf2/HO-1. Oxid. Med. Cell Longev. 2017, 2017, 8370593. [Google Scholar] [CrossRef] [Green Version]
- Liu, I.M.; Tzeng, T.F.; Liou, S.S.; Lan, T.W. Improvement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007, 73, 1054–1060. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Yi, X.; Liu, C.; Kong, D.; Zhang, J.; Gong, M. Myricetin: A potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. FASEB J. 2017, 31, 2603–2611. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Chao, H.C.; Tsai, P.F.; Lee, S.C.; Lin, Y.S.; Wu, M.C. Effects of Myricetin-Containing Ethanol Solution on High-Fat Diet Induced Obese Rats. J. Food Sci. 2017, 82, 1947–1952. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Fang, H.; Guo, F.; Li, F.; Chen, A.; Huang, S. Flavonoids as inducers of white adipose tissue browning and thermogenesis: Signalling pathways and molecular triggers. Nutr. Metab. 2019, 16, 47. [Google Scholar] [CrossRef] [Green Version]
- Jasaszwili, M.; Billert, M.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Adropin as A Fat-Burning Hormone with Multiple Functions-Review of a Decade of Research. Molecules 2020, 25, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.A.; Havel, P.J. Adropin and insulin resistance: Integration of endocrine, circadian, and stress signals regulating glucose metabolism. Obesity 2021, 29, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Males, I.; Borovac, J.A.; Martinovic, D.; Vilovic, M. Role of Adropin in Cardiometabolic Disorders: From Pathophysiological Mechanisms to Therapeutic Target. Biomedicines 2021, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Kuo, F.Y.; Cheng, K.C.; Li, Y.; Cheng, J.T.; Tsai, C.C. Promotion of Adropin Expression by Hyperglycemia Is Associated with STAT3 Activation in Diabetic Rats. Diabetes Metab. Syndr. Obes. 2020, 13, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, D.; Fujiwara, Y.; Komohara, Y.; Mizuta, H.; Takeya, M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem. Biophys. Res. Commun. 2012, 425, 304–308. [Google Scholar] [CrossRef]
- Liu, I.M.; Liou, S.S.; Lan, T.W.; Hsu, F.L.; Cheng, J.T. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2005, 71, 617–621. [Google Scholar] [CrossRef]
- Liu, I.M.; Liou, S.S.; Cheng, J.T. Mediation of beta-endorphin by myricetin to lower plasma glucose in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2006, 104, 199–206. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Xu, G.; Kaneto, H.; Laybutt, D.R.; Duvivier-Kali, V.F.; Trivedi, N.; Suzuma, K.; King, G.L.; Weir, G.C.; Bonner-Weir, S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: Possible contribution to impaired incretin effects in diabetes. Diabetes 2007, 56, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Chang, W.T.; Kuo, F.Y.; Chen, Z.C.; Li, Y.; Cheng, J.T. TGR5 activation ameliorates hyperglycemia-induced cardiac hypertrophy in H9c2 cells. Sci. Rep. 2019, 9, 3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, D.Y.; Park, S.H.; Marquez, J.; Kwak, H.B.; Kim, T.N.; Bae, J.H.; Koh, J.H.; Han, J. Hepatokines as a Molecular Transducer of Exercise. J. Clin. Med. 2021, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Kocaman, N.; Citil, C.; Kendir, Y. Expression of adropin in rat brain, cerebellum, kidneys, heart, liver, and pancreas in streptozotocin-induced diabetes. Mol. Cell Biochem. 2013, 380, 73–81. [Google Scholar] [CrossRef]
- Mierzwicka, A.; Bolanowski, M. New peptides players in metabolic disorders. Postepy. Hig. Med. Dosw. 2016, 70, 881–886. [Google Scholar] [CrossRef]
- Lovren, F.; Pan, Y.; Quan, A.; Singh, K.K.; Shukla, P.C.; Gupta, M.; Al-Omran, M.; Teoh, H.; Verma, S. Adropin is a novel regulator of endothelial function. Circulation 2010, 122, S185–S192. [Google Scholar] [CrossRef] [Green Version]
- Goetze, J.P.; Albrethsen, J. Adropin: A new regulatory peptide in cardiovascular endocrinology. Regul. Pept. 2014, 190–191, 41–42. [Google Scholar] [CrossRef]
- Aydin, S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef]
- Ticinovic Kurir, T.; Milicevic, T.; Novak, A.; Vilovic, M.; Bozic, J. Adropin—Potential Link in Cardiovascular Protection for Obese Male Type 2 Diabetes Mellitus Patients Treated with Liraglutide. Acta Clin. Croat. 2020, 59, 344–350. [Google Scholar] [CrossRef]
- Liu, I.M.; Cheng, J.T. Mediation of Endogenous beta-Endorphin in the Plasma Glucose-Lowering Action of Herbal Products Observed in Type 1-Like Diabetic Rats. Evid.-Based Complement. Alternat. Med. 2011, 2011, 987876. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.C.; Asakawa, A.; Li, Y.X.; Liu, I.M.; Amitani, H.; Cheng, J.T.; Inui, A. Opioid mu-receptors as new target for insulin resistance. Pharmacol. Ther. 2013, 139, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Cozzolino, D.; Salvatore, T.; Ceriello, A.; Torella, R. Dual effect of beta-endorphin on insulin secretion in man. Horm. Metab. Res. 1987, 19, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.A.; Khan, S.; Smith, M.E. Evidence for a hormonal action of beta-endorphin to increase glucose uptake in resting and contracting skeletal muscle. J. Endocrinol. 1997, 155, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Hope, P.; Pyle, D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.A.; Mells, J.; Dunham, R.M.; Grakoui, A.; Handy, J.; Saxena, N.K.; Anania, F.A. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 2010, 51, 1584–1592. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Saxena, N.K.; Lin, S.; Gupta, N.A.; Anania, F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006, 43, 173–181. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Yang, J.; Xiao, W.; Le, Y.; Yu, F.; Gu, L.; Lang, S.; Tian, Q.; Jin, T.; et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine 2019, 41, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Bifari, F.; Manfrini, R.; Dei Cas, M.; Berra, C.; Siano, M.; Zuin, M.; Paroni, R.; Folli, F. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Pharmacol. Res. 2018, 137, 219–229. [Google Scholar] [CrossRef]
- Jin, T.; Weng, J. Hepatic functions of GLP-1 and its based drugs: Current disputes and perspectives. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E620–E627. [Google Scholar] [CrossRef]
- Li, Y.X.; Cheng, K.C.; Asakawa, A.; Kato, I.; Sato, Y.; Amitani, H.; Kawamura, N.; Cheng, J.T.; Inui, A. Role of musclin in the pathogenesis of hypertension in rat. PLoS ONE 2013, 8, e72004. [Google Scholar] [CrossRef] [Green Version]
- Celik, A.; Balin, M.; Kobat, M.A.; Erdem, K.; Baydas, A.; Bulut, M.; Altas, Y.; Aydin, S.; Aydin, S. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc. Ther. 2013, 31, 174–178. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Cheng, K.-C.; Liu, I.-M.; Niu, H.-S. Myricetin Increases Circulating Adropin Level after Activation of Glucagon-like Peptide 1 (GLP-1) Receptor in Type-1 Diabetic Rats. Pharmaceuticals 2022, 15, 173. https://doi.org/10.3390/ph15020173
Li Y-X, Cheng K-C, Liu I-M, Niu H-S. Myricetin Increases Circulating Adropin Level after Activation of Glucagon-like Peptide 1 (GLP-1) Receptor in Type-1 Diabetic Rats. Pharmaceuticals. 2022; 15(2):173. https://doi.org/10.3390/ph15020173
Chicago/Turabian StyleLi, Ying-Xiao, Kai-Chun Cheng, I-Min Liu, and Ho-Shan Niu. 2022. "Myricetin Increases Circulating Adropin Level after Activation of Glucagon-like Peptide 1 (GLP-1) Receptor in Type-1 Diabetic Rats" Pharmaceuticals 15, no. 2: 173. https://doi.org/10.3390/ph15020173
APA StyleLi, Y.-X., Cheng, K.-C., Liu, I.-M., & Niu, H.-S. (2022). Myricetin Increases Circulating Adropin Level after Activation of Glucagon-like Peptide 1 (GLP-1) Receptor in Type-1 Diabetic Rats. Pharmaceuticals, 15(2), 173. https://doi.org/10.3390/ph15020173




