Parasitological Assessment of Sewage Sludge Samples for Potential Agricultural Reuse in Tunisia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sludge Treatment and WWTP Characteristics
2.3. Environmental Weather Conditions
2.4. Sampling and Analyses
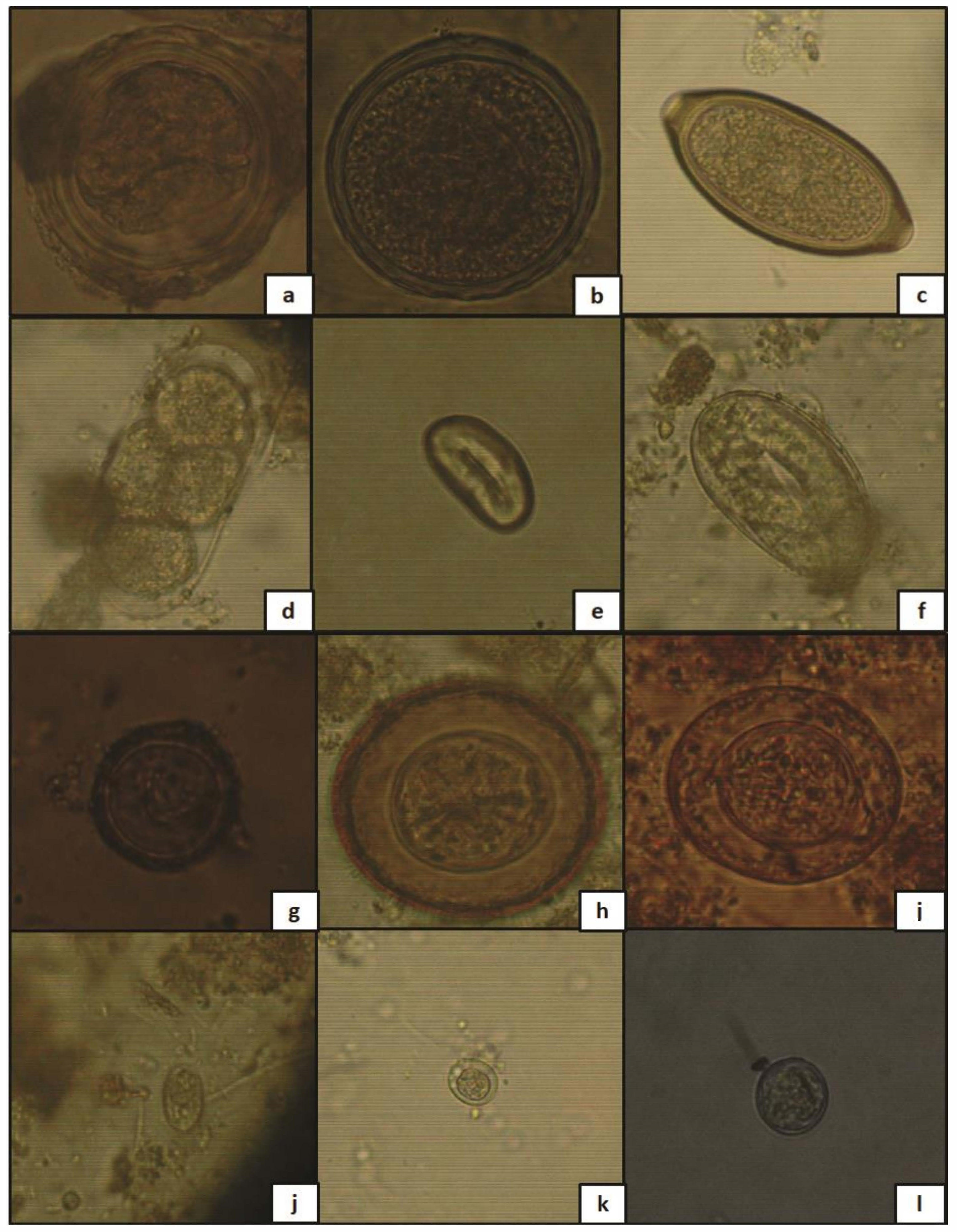
2.5. Identification and Counting of Helminth Ova and Protozoan Cysts
2.6. Statistical Analyses
3. Results and Discussion
3.1. General Parasites (Ova and Cysts) Distribution in Sludge Samples
3.2. Parasitological Results Analysis of Solar Drying Sludge in Beds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. Basic Information about Biosolids. Enforcement and Compliance History Online United States Environmental Protection Agency, USA. 2019. Available online: https://www.epa.gov/biosolids/epa-regional-and-state-contacts-biosolids (accessed on 29 October 2021).
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Usman, K.; Khan, S.; Ghulam, S.; Khan, M.U.; Khan, N.; Khan, M.A.; Khalil, S.K. Sewage sludge: An important biological resource for sustainable agriculture and its environmental implications. Am. J. Plant Sci. 2012, 3, 1708–1721. [Google Scholar] [CrossRef] [Green Version]
- Bittencourt, S. Agricultural use of sewage sludge in Paraná State, Brazil: A decade of national regulation. Recycling 2018, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- National Sanitation Utility—ONAS. Rapport D’activités Annuel: Exploitation des Stations D′épurations (Annual Activity Report: Wastewater Treatment Plants Operation); Ministry of the Environment and Sustainable Development: Tunis, Tunisia, 2014; p. 92. [Google Scholar]
- National Sanitation Utility—ONAS. Rapport D’activités Annuel: Exploitation des Stations D′épurations (Annual Activity Report: Wastewater Treatment Plants Operation); Ministry of the Environment and Sustainable Development: Tunis, Tunisia, 2017; p. 34. [Google Scholar]
- Jiménez-Cisneros, B.E. Helminth ova control in wastewater and sludge for agricultural reuse. In Water Reuse New Paradigm towards Integrated Water Resources Management: Encyclopedia of Biological, Physiological and Health Sciences, 1st ed.; Grabow, W., Ed.; EOLSS Publishers Co., Ltd. UNESCO: Abu Dhabi, United Emirates, 2006. [Google Scholar]
- Amoah, I.D.; Adegoke, A.A.; Stenström, T.A. Soil-transmitted helminth infections associated with wastewater and sludge reuse: A review of current evidence. Trop. Med. Int. Health 2018, 23, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Pharm Duc, B.; Nguyen-Viet, H.; Hattendorf, J.; Zinsstag, J.; Dac Com, P.; Odermatt, P. Risks factors for Entamoeba histolytica infection in an agricultural community in Hanam province, Vietnam. Parasites Vectors 2011, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capizzi, B.; Schwartzbrod, J. Irradiation of Ascaris ova in sludge using an electron beam accelerator. Water Res. 2001, 35, 2256–2260. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Save Use of Wastewater, Excreta and Greywater—Volume 2: Wastewater Use in Agriculture; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- French Decree of January 8th—Official Journal. Technical Requirements for the Application of Sludge on Agricultural Soils Pursuant to Decree No. 8 97-1133. 1998. Available online: https://www.legifrance.gouv.fr/eli/arrete/1998/1/8/ATEE9760538A/jo/texte (accessed on 31 January 1998). (In French)
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Regulation of the Minister for the Environment of February 6th 2015, Regarding Municipal Sewage Sludge (OJ 2015, Item 257). Available online: http://sejm.gov.pl (accessed on 18 May 2018).
- Pyssa, J. Technical and Technological Aspects of Sewage Waste Management after Amendments in Legislation in Poland. In IOP Conference Series: Earth and Environmental Science; In Proceedings of the 2nd International Conference on the Sustainable Energy and Environmental Development, Krakow, Poland, 14–17 November 2019; IOP Publishing: Bristol, UK, 2019; Volume 214, p. 012016. [Google Scholar] [CrossRef]
- Government of Bulgaria. Ordinance on the Procedure and the Way of Utilization of Sludge from the Treatment of Waste Waters through Their Use in Agriculture. Adopted by Decree of the Council of Ministers No 201 of 4.08.2016, Promulgated, SG, No. 63 of 12.08.2016. Available online: http://eea.government.bg/bg/legislation/waste/Naredba_utaiki_26.10.16.pdf (accessed on 10 September 2019). (In Bulgarian)
- Ødegaard, H.; Paulsrud, B.; Karlsson, I. Wastewater sludge as a resource: Sludge disposal strategies and corresponding treatment technologies aimed at sustainable handling a wastewater sludge. Water Sci. Technol. 2002, 46, 295–303. [Google Scholar] [CrossRef]
- Conselho Nacional do Meio Ambiente (CONAMA) (Brasil). Resolução n 8375, de 29 de agosto de 2006: Define Critérios e Procedimentos, para o Uso Agrícola de Lodos de Esgoto Gerados em Estações de Tratamento de Esgoto Sanitário e Seus Produtos Derivados, e da´ Outras Providências. Retificada pela Resolução n 8380, de 2006—Data da legislação: 29 August 2006—Publicação DOU n8 167, de 30 August 2006 (Resolution 8 375 of 29 August 2006. Defines Criteria and Procedures for the Agricultural Use of Sewage Sludge Generated in Sanitary Sewage Treatment Plants and their By-Products, and Hence Other Provisions. Amended by Resolution 8380 of 2006. Date of legislation: 29 August 2006—Publication DOU n8 167) pp. 141–146 de 29 August 2006). 2006; p. 141. Available online: http://www.mma.gov.br/port/conama/legiano/ (accessed on 13 May 2009). (In Portuguese)
- Jiménez, B.; Maya, C.; Barrios, J.A.; Navarro, I. Helminths and their role in environmental engineering. In Human Helminthiasis; Luis Rodrigo, IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Norma Oficial Mexicana NOM-004-SEMARNAT-2002 Proteccion Ambiental-Lodos y Biosólidos—Especificaciones y Limites Láximos Permisibles de Contaminantes para su Aprovechamiento y Disposicion Final (Secretariat of Environment and Natural Resources. Mexican Official Standard NOM—004—SEMARNAT-2002. Environmental Protection—Sludge and Biosolids—Specification and Maximum Permissible Limits of Contaminants for Their Supply and Final Disposal). Available online: http://tramites.semarnat.gob.mx/Doctos/DGGIMAR/Sirrep/NOM-004-SEMARNAT-2002.pdf (accessed on 11 October 2012). (In Spanish)
- US EPA. Part 503—Standards for the Use or Disposal of Sewage Sludge; United States Environmental Protection Agency: Washington, DC, USA, 1993.
- Water New Zealand. Guidelines for Beneficial Use of Organic Materials on Productive Land—Volume 2: Technical Manual; The New Zealand Water and Wastes Assciation: Wellington, New Zealand, 2017; Available online: http://www.waternz.org.nz (accessed on 20 December 2017).
- Snyman, H.G.; Herselman, J.E. Guidelines for the Utilisation and Disposal of Wastewater Sludge: Volume 2 of 5: Requirements for the Agricultural Use of Wastewater Sludge; Water Research Commission Report No. TT 262/06; Water Research Commission: Pretoria, South Africa, 2006. [Google Scholar]
- US EPA. Environmental Regulations and Technology—Control of Pathogens and Vector Attraction in Sewage Sludge (Including Domestic Septage). Under 40 CFR Part 503. Appendix I—Test Method for Detecting, Enumerating, and Determining the Viability of Ascaris ova in Sludge. United States Environmental Protection Agency, EPA/625/R–92/013, Revised July 2003, p. 166. 2003. Available online: www.epa.gov/ORD/NRMRL/pubs (accessed on 4 October 2012).
- Castellanos-Rozo, J.; Galvis-López, J.A.; Merchán- Castellanos, N.A.; Manjarres-Hernández, E.H.; Rojas, A.L. Assessment of two sludge stabilization methods in a wastewater treatment plant in Sotaquirá, Colombia. Univ. Sci. 2020, 25, 17–36. [Google Scholar] [CrossRef]
- SanPiN 2.1.7.573-96. Hygienic Requirements to Wastewater and Sewage Sludge Use for Land Irrigation and Fertilization (Approved by the Russian State Committee of Epidemiological Supervision (Goskomepidnadzor RF) on 31.10.1996 N 46). Available online: https://www.russiangost.com/p-19896-sanpin-217573-96.aspx (accessed on 12 January 2022). (In Russian).
- Jordanian Standard No. 1145/1996 Sludge: Uses of Treated Sludge in Agriculture. Specifications and Standards Organization; Specifications and Standards Organization: Amman, Jordan, 1996; p. 9.
- Mena, P.M. Legislación sobre lodos en América Latina: Un análisis comparativo. In Proceedings of the XXXI Congreso Interamericano AIDIS, Santiago de Chile, Chile, 12–15 October; 2008. [Google Scholar]
- Rizzardini, C.B.; Goi, D. Considerations about European directives and Italian regulation on sludge from municipal wastewater treatment plants: Current status and future prospective. Open Waste Manag. J. 2009, 2, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Öğlenḭ, N.; Özdemḭr, S. Pathogen reduction effects of solar drying and soil application in sewage sludge. Turk. J. Agric. For. 2010, 34, 509–515. [Google Scholar] [CrossRef]
- National Institute of Standardization and Industrial Properties—INNORPI. Fertilizers—Sludge from Urban Wastewater Treatment Works; Tunisian Standards NT 106.20; INNORPI: Tunis, Tunisia, 2002. (In French) [Google Scholar]
- Ghazy, M.; Dockhorn, T.; Dichtl, N. Sewage sludge management in Egypt: Current status and perspectives towards a sustainable agricultural use. World Acad. Sci. Eng. Technol. 2009, 33, 299–307. [Google Scholar] [CrossRef]
- Abdul Khaliq, S.J.; Ahmed, M.; Al-Wardy, M.; Al-Busaidi, A.; Choudri, B.S. Wastewater and sludge management and research in Oman: An overview. J. Air Waste Manag. Assoc. 2017, 67, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Belmeskine, H.; Ouameur, W.A.; Dilmi, N.; Aouabed, A. The vermicomposting for agricultural valorization of sludge from Algerian wastewater treatment plant: Impact on growth of snap bean Phaseolus vulgaris L. Heliyon 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Annori, A.; El Fels, L.; Ezzariai, A.; El Hayani, B.; El Mejahed, K.; El Gharous, M.; Hafidi, M. Effectiveness of helminth eggs reduction by solar drying and liming of sewage sludge. Environ. Sci. Pollut. Res. 2021, 28, 14080–14091. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Das, P.K.; Khanum, H.; Hossainey, M.R.H.; Islam, E.; Al Mahmud, H.; Islam, Md.S.; Imran, K.M.; Dey, D.; Islam, M.d.S. Time-temperature model for bacterial and parasitic annihilation from cow dung and human faecal sludge: A forthcoming bio-fertilizer. J. Bacteriol. Parasitol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Alouini, Z. Flux de la charge parasitaire dans 5 stations d’épuration en Tunisie (Fate of parasites in five wastewater treatment plants in Tunisia). Rev. Sci. L’Eau 1993, 6, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Ben Ayed, L.; Alouini, Z.; Jemli, M.; Sabbahi, S. Evaluation de la qualité parasitologique des eaux usées et des boues résiduaires en Tunisie (Evaluation of the parasite prevalence of sewage and sludge samples in Tunisia). Environ. Risques St. 2007, 6, 433–442. [Google Scholar] [CrossRef]
- Ben Ayed, L.; Schijven, J.; Alouini, Z.; Jemli, M.; Sabbahi, S. Presence of parasitic protozoa and helminth in sewage and efficiency of sewage treatment in Tunisia. Parasitol. Res. 2009, 105, 393–406. [Google Scholar] [CrossRef]
- Ben Ayed, L.; Cama, V.; Xiao, L. Parasitic contamination in wastewater and sludge samples in Tunisia using three different detection techniques. Parasitol. Res. 2010, 107, 109–116. [Google Scholar] [CrossRef]
- Sabbahi, S.; Trad, M.; Ben Ayed, L.; Marzougui, N. Occurrence of intestinal parasites in sewage samples and efficiency of wastewater treatment systems in Tunisia. Water Qual. Res. J. Can. 2018, 53, 86–101. [Google Scholar] [CrossRef]
- Alouini, Z. Devenir des œufs et kystes de parasites au cours d′un cycle d′épuration de la station Charguia à Tunis. Houille Blanche 1998, 7, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Ben Ayed, L.; Sabbahi, S.; Karanis, P. Waterborne parasites in North Africa environment. In Encyclopedia of Environmental Health; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 6, pp. 415–424. [Google Scholar] [CrossRef]
- Bouratbine, A.; Aoun, K.; Siala, E.; Chahed, M.K.; Ben Hassine, L.; Meherzi, A. Pour une meilleure estimation de la prévalence du parasitisme intestinal dans la région de Tunis (For a better estimation of intestinal parasitism prevalence in the Tunis area). Bull. Soc. Pathol. Exot. 2000, 93, 353–355. [Google Scholar]
- Siala, E.; Guidara, R.; Ben Abdallah, R.; Ben Ayed, S.; Ben Alaya, N.; Zallaga, N.; Bouratbine, A.; Aoun, K. Intestinal parasites in manipulators of foodstuffs in the Tunis region: Study of 8502 samples (1998–2008). Arch. Inst. Pasteur Tunis 2011, 88, 77–84. (In French) [Google Scholar]
- Jiménez, B.; Wang, L. Sludge Treatment and Management. In Municipal Wastewater Management in Developing Countries: Principles and Engineering; Ujang, Z., Henze, M., Eds.; IWA Publishing: London, UK, 2006; pp. 237–292. [Google Scholar]
- Paluszak, Z.; Skowron, K.; Sypuła, M.; Skowron, K.J. Microbiological evaluation of the effectiveness of sewage sludge sanitization with solar drying technology. Int. J. Photoenergy 2012, 2012, 341592. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation. 2003. Available online: http://qgis.org (accessed on 12 January 2022).
- European Centre for Medium-Range Weather Forecasts—ECMWF Reanalysis (ERA5). Temperature and Rain ERA5. Available online: https://www.ecmwf.int/en/forecasts/datasets/search/era5 (accessed on 12 February 2021).
- European Centre for Medium-Range Weather Forecasts—ECMWF Reanalysis—Interim (ERA-Interim). Sunshine duration. Available online: https://www.ecmwf.int/en/forecasts/dataset/ecmwf-reanalysis-interim (accessed on 12 February 2021).
- Yanko, W.A. Occurrence of Pathogens in Distribution and Marketing Municipal Sludges; Report No.: EPA/ 600/1-87/014 (NTIS: PB88-154273/AS.); National Technical Information Service: Springfield, VA, USA, 1987. [Google Scholar]
- US EPA. POTW sludge sampling and analysis guidance document. EPA/833/B-89/100, United States Environmental Protection Agency, Office of Water (4203). 1989. Available online: https://www.epa.gov/biosolids/potw-sludge-sampling-and-analysis-guidance-document (accessed on 3 December 2013).
- Jaromin-Gleń, K.; Kłapeć, T.; Łagód, G.; Karamon, J.; Malicki, J.; Skowrońska, A.; Bieganowski, A. Division of methods for counting helminths’ eggs and the problem of efficiency of these methods. Ann. Agric. Environ. Med. 2017, 24, 1–7. [Google Scholar] [CrossRef]
- Arther, R.G.; Fitzgerald, P.R.; Fox, J.C. Parasite ova in anaerobically digested sludge. J. Water Pollut. Control Fed. 1981, 53, 1333–1338. [Google Scholar]
- Pouillevet, H.; Dibakou, S.-E.; Ngoubangoye, B.; Poirotte, C.; Charpentier, M.J.E. A comparative study of four methods for the detection of nematode eggs and large protozoan cysts in Mandrill faecal material. Folia Primatol. 2017, 88, 344–357. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 16 May 2020).
- Roepstorff, A.; Nansen, P. Epidemiology, Diagnosis and Control of Helminth Parasites of Swine; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Collender, P.A.; Kirby, A.E.; Addiss, D.G.; Freeman, M.C.; Remais, J.V. Methods for quantification of soil-transmitted helminths in environmental media: Current techniques and recent advances. Trends Parasitol. 2015, 31, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, M.E.; Andersen, T.J.; Dalsgaard, A.; Olsen, A.; Thamsborg, S.M. Resuspension and settling of helminth eggs in water: Interactions with cohesive sediments. Water Res. 2012, 46, 3903–3912. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, J.; Zdybel, J.; Karamon, J.; Kochanowski, M.; Stojecki, K.; Cencek, T.; Kłapeć, T. Assessment of viability of the nematode eggs (Ascaris, Toxocara, Trichuris) in sewage sludge with the use of LIVE/DEAD bacterial viability kit. Ann. Agric. Environ. Med. 2014, 21, 35–41. [Google Scholar] [PubMed]
- Da Rocha, M.C.V.; Barés, M.E.; Braga, M.C.B. Quantification of viable helminth eggs in samples of sewage sludge. Water Res. 2016, 103, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, L.; Yang, W.; Widmer, G.; Cama, V.; Ortega, Y.; Xiao, L. Survey and genetic characterization of wastewater in Tunisia for Cryptosporidium spp., Giardia duodenalis, Enterocytozoon beneusi, Cyclospora cayetanensis and Eimeria spp. J. Water Health 2012, 10, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Arjomandzadeh, Kh.; Dalimi, A. Comparison of 12 techniques for detection of Cryptosporidium oocysts. Arch. Razi. Inst. 1994, 44/45, 31–38. [Google Scholar] [CrossRef]
- Soares, F.A.; do Nascimento Benitez, A.; dos Santos, B.M.; Loiola, S.H.N.; Rosa, S.L.; Nagata, W.B.; Inácio, S.V.; Suzuki, C.T.N.; Bresciani, K.D.S.; Falcão, A.X.; et al. A historical review of the techniques of recovery of parasites for their detection in human stools. J. Braz. Soc. Trop. Med. 2020, 53, 1–9. [Google Scholar] [CrossRef]
- Searcy, K.E.; Packman, A.I.; Atwill, E.R.; Harter, T. Association of Cryptosporidium parvum with suspended particles: Impact on oocyst sedimentation. Appl. Environ. Microbiol. 2005, 71, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Yashas, S.R.; Udayashankara, T.H. A mini review on prevalence of protozoan cysts in sewage sludge. Int. J. Eng. Sci. Invent. 2017, 6, 55–60. [Google Scholar]
- Cofie, O.O.; Agbottah, S.; Strauss, M.; Esseku, H.; Montangero, A.; Awuah, E.; Kone, D. Solid liquid separation of fecal sludge using drying beds in Ghana: Implications for nutrient recycling in urban agriculture. Water Res. 2006, 40, 75–82. [Google Scholar] [CrossRef]
- Al-Malack, M.H. Effect of sludge initial depth on the fate of pathogens in sand drying beds in the Eastern Province of Saudi Arabia. Int. J. Environ. Res. 2010, 4, 825–836. [Google Scholar] [CrossRef]
- Mihelcic, J.R. Sludge management: Biosolids and fecal sludge. In Global Water Pathogen Project-Part 4: Management of Risk from Excreta and Wastewater; Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, USA, 2018; pp. 1–30. [Google Scholar] [CrossRef]
- Garcia-Orenes, F.; Roldán, A.; Guerrero, C.; Mataix-Solera, J.; Navarro-Pedreňo, J.; Gómez, I.; Mataix-Beneyto, J. Effect of irrigation on the survival of total coliforms in three semiarid soils after amendment with sewage sludge. Waste Manage. 2007, 27, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Sypuła, M.; Paluszak, Z.; Ligocka, A.; Skowron, K. Effects of spring season solar drying process on sanitation indicators in sewage sludge and potential as a method for fertilizer production. Ann. Agric. Environ. Med. 2013, 20, 8–12. [Google Scholar] [PubMed]
- Phiri, J.S.; Katebe, R.C.; Mzyece, C.C.; Shaba, P.; Halwindi, H. Characterization of biosolids and evaluating the effectiveness of plastic-covered sun drying beds as a biosolids stabilization method in Luzaka, Zambia. Int. J. Recycl. Org. Waste Agric. 2014, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Quinn, B.; Smith, H.V.; Bruce, R.G.; Girdwood, R.W.A. Studies on the incidence of Toxocara and Toxascaris spp. ova in the environment. 1. A comparison of flotation procedures for recovering Toxocara spp. ova from soil. J. Hyg. 1980, 84, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.S.; Bruckner, D.A. Diagnostic Medical Parasitology, 3rd ed.; ASM Press: Washington, DC, USA, 1997; p. 937. [Google Scholar]
- Elhussein, D.M. Evaluation of Sugar Floatation Technique for Diagnosis of Gastrointestinal Parasites and Its Evaluation as Epidemiological Tool for the Study of Hymenolepis nana. Ph.D. Thesis, Department of Zoology, Faculty of Science, University of Khartoum, Khartoum, Sudan, 2005. [Google Scholar]
- El Fels, L.; El Asli, A.; Ouhdouch, Y.; Hafidi, M. Effect of co-composting on helminth eggs removal. Environ. Eng. Manag. J. 2018, 17, 459–465. [Google Scholar] [CrossRef]
- Khadra, A.; Ezzariai, A.; Kouisni, L.; Hafidi, M. Helminth eggs inactivation efficiency by sludge co-composting under arid climates. Int. J. Environ. Health Res. 2019, 31, 530–537. [Google Scholar] [CrossRef]
- Seginer, I.; Bux, M. Modeling solar drying rate of wastewater sludge. Dry. Technol. 2006, 24, 1353–1363. [Google Scholar] [CrossRef]
- Shanahan, E.F.; Raiko, A.; Tindale, N.W.; Thomas, M.P.; Walpole, R.; Kurtböke, D.İ. Evaluation of pathogen removal in a solar sludge drying facility using microbial indicators. Int. J. Environ. Res. Public Health 2010, 7, 565–582. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Environmental Regulations and Technology Control of Pathogens and Vector Attraction in Sewage Sludge; EPA/625/R-92/013; United States Environmental Protection Agency: Cincinnati, OH, USA, 1992.





| Country | Intestinal Parasites | Reference |
|---|---|---|
| WHO | <1 viable helminth egg/g DM | [11] |
| France | <3 viable helminth egg/10 g DM | [12] |
| Poland | 0 live egg of intestinal parasites (Ascaris spp., Trichuris spp., Toxocara spp.)/kg DM | [13,14,15,16] |
| Lithuania | Helminth egg and larvae, 0 units/kg | [13,14] |
| Luxembourg | No eggs of worm likely be contagious | [13,14] |
| Bulgaria | Viable helminth egg and larvae, 1 unit/kg DM | [13,14,17] |
| Austria (Carinthia) | No helminth egg (Applied to all classes) | [14] |
| Austria (Lower Austria) | No helminth egg | [14] |
| Austria (Steiermark) | No helminth egg | [14] |
| Norway | 0 helminth egg/g TS (Dry weight basis) | [18] |
| Brazil | 0.25 viable helminth egg/g DM (Class A) 10 viable helminth eggs/g DM (Class B) | [19] |
| Chile | 0.25 helminth egg/g DM (Class A) | [20] |
| Mexico | <1 viable helminth egg/g DM (Class A) <10 helminth eggs/g DM (Class B) | [21] |
| New Zealand | <1 Helminth egg/4 g TS (Class A) | [22,23] |
| South Africa | 1 viable helminth egg/g DM (Class A) 4 viable helminth eggs/g DM (Class B) >4 viable helminth eggs/g DM (Class C) | [24] |
| United States | <1 viable helminth egg/4 g TS (dry weight basis) | [25] |
| Colombia | <1 viable helminth egg/4 g DM (Class A) <10 viable helminth eggs/4 g DM (Class B) | [14,26] |
| Russia | <1 Viable eggs of geohelminths (roundworms, whipworms, hookworms)/1 dm3 DM <1 Viable eggs of biohelminths (oncospheres of teniid, eggs of fascioli)/1 dm3 DM <1 viable cysts of intestinal pathogenic protozoa (cysts of Giardia, Balantidium, Cryptosporidium oocysts/1 dm3 DM | [27] |
| Jordan | <1 worm live ova/4 g DM (Sludge treated to the second level) | [28] |
| Design and Performance | Characteristics of Dried Sludge | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | District | Capacity EI | Flow Rate (m3/Day) | kg BOD5/Day | Secondary Wastewater Treatment | Treatment Efficiency (%) | Treatment Type | Volume (m3) | Dry Matter (%) | Coordinates | ||
| BOD5 | COD | TSS | ||||||||||
| WWTP1 | DT | 51,000 | 2800 | 1704 | OD | 96 | 93 | 91 | T+DB | 431 | 77 | 36°51′47.62″ N; 9°57′10.60″ E |
| WWTP2 | NW | 17,968 | 1280 | 719 | OD | 91 | 86 | 82 | AD+T+DB | 292 | 75 | 36°27′50.46″ N; 9°16′8.57″ E |
| WWTP3 | NW | 18,874 | 1180 | 720 | OD | 93 | 90 | 91 | AD+T+DB | 180 | 64 | 36°34′0.18″ N; 9°26′35.41″ E |
| WWTP4 | NW | 51,000 | 4530 | 2450 | OD | 80 | 76 | 68 | T+DB | 750 | 80–90 | 36°7′11.09″ N; 9°23′0.75″ E |
| WWTP5 | NW | 95,000 | 8500 | 4000 | LLAS | 91 | 90 | 91 | T+DB | 986 | 74–90 | 36°8′19.63″ N; 8°41′6.07″ E |
| WWTP6 | C | 236,000 | 20,000 | 9000 | OD | 94 | 93 | 94 | T+DB | 5768 | 49–65 | 35°43′46.59″ N; 10°6′53.86″ E |
| WWTP7 | SE | 19,500 | 1395 | 700 | OD | 97 | 94 | 97 | T+DB | - | 90 | 36°40′29.71″ N; 10°32′58.68″ E |
| WWTP8 | E | 17,000 | 1500 | 600 | OD | 95 | 92 | 95 | T+DB | 98 | 50–80 | 35°43′13.54″ N; 10°40′26.87″ E |
| WWTP9 | SE | 526,800 | 49,500 | 21,600 | OD | 79 | 71 | 72 | T+DB | 2790 | 84 | 34°50′2.13″ N; 10°51′15.70″ E |
| WWTP10 | E | 10,000 | 780 | 400 | OD | 87 | 81 | 86 | DB | 362 | 86 | 34°31′3.80″ N; 10°29′34.66″ E |
| Helmith Eggs | Protozoan Cysts | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nematodes | Cestodes | Flagellates | Amoebas | |||||||||||
| Parasite | Asc. spp. | Toxo. spp. | E. v. | Trich.spp. | H. W | S. s. | Strg. spp. | Tris. spp. | Tae eggs | H. d. | H. n. | G. spp. | E. h. | Entamoeba coli |
| Min | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.33 | 1.32 |
| 1st. Qu. | 0.00 | 0.00 | 0.00 | 0.00 | - | - | 0.33 | 0.00 | 0.00 | - | - | 1.65 | 1.32 | 3.99 |
| Medium | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.66 | 0.00 | 0.33 | 0.00 | 0.33 | 2.31 | 1.66 | 4.65 |
| Mean * | 0.28 | ≅0.00 ** | 0.14 | ≅0.00 ** | 0.08 | 0.32 | 0.6 | 0.15 | 0.31 | ≅0.00 ** | 0.25 | 2.25 | 1.79 | 4.74 |
| 3rd. Qu. | 0.33 | 0.00 | 0.33 | 0.00 | 0.00 | 0.33 | 0.66 | 0.33 | 0.33 | 0.00 | 0.33 | 2.67 | 2.32 | 5.64 |
| Max. | 1.78 | 0.33 | 1.33 | 0.33 | 0.66 | 1.10 | 3.30 | 0.66 | 1.98 | 0.33 | 0.89 | 4.65 | 5.78 | 10.7 |
| Std. Dev. | 0.34 | 0.05 | 0.21 | 0.03 | 0.15 | 0.27 | 0.42 | 0.20 | 0.31 | 0.04 | 0.23 | 0.81 | 0.82 | 1.23 |
| C.V. | 1.21 | 5.0 | 1.50 | - | 1.87 | 0.84 | 0.7 | 1.33 | 1.03 | - | 0.92 | 0.36 | 0.46 | 0.26 |
| Sum | 32.4 | 0.82 | 14.4 | 0.33 | 8.52 | 35.9 | 70.14 | 16.5 | 37.76 | 0.66 | 29.1 | 260.6 | 210.1 | 538.1 |
| % positive | 56.9 | 2.6 | 37.9 | 0.9 | 28.45 | 72.4 | 91.4 | 44.0 | 74.1 | 1.72 | 67.2 | 100.0 | 100.0 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbahi, S.; Ben Ayed, L.; Trad, M.; Berndtsson, R.; Karanis, P. Parasitological Assessment of Sewage Sludge Samples for Potential Agricultural Reuse in Tunisia. Int. J. Environ. Res. Public Health 2022, 19, 1657. https://doi.org/10.3390/ijerph19031657
Sabbahi S, Ben Ayed L, Trad M, Berndtsson R, Karanis P. Parasitological Assessment of Sewage Sludge Samples for Potential Agricultural Reuse in Tunisia. International Journal of Environmental Research and Public Health. 2022; 19(3):1657. https://doi.org/10.3390/ijerph19031657
Chicago/Turabian StyleSabbahi, Sonia, Layla Ben Ayed, Monia Trad, Ronny Berndtsson, and Panagiotis Karanis. 2022. "Parasitological Assessment of Sewage Sludge Samples for Potential Agricultural Reuse in Tunisia" International Journal of Environmental Research and Public Health 19, no. 3: 1657. https://doi.org/10.3390/ijerph19031657






