9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diet
2.3. Study Design
2.4. Tissue Culture
2.5. βc Analysis
2.6. HDL Preparation
2.7. Cholesterol Efflux Assays
2.8. RNA Extraction and Real-Time PCR
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. β-Carotene-Enriched Diet Led to 9-cis-βc and All-Trans-βc Accumulation in Mouse Peritoneal Macrophages
3.2. 9-cis-βc Induced Cytochrome P450 26B1 (CYP26B1) Expression
3.3. 9-cis-βc Increased Macrophage Cholesterol Efflux to HDL in Vitro and ex Vivo
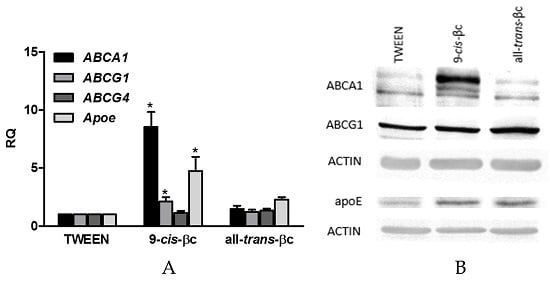
3.4. 9-cis-βc Induced mRNA and Protein Levels of Genes Involved in Macrophage Cholesterol Efflux
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 9-cis-βc | 9-cis-β-carotene |
| RA | Retinoic Acid |
| ABCA1 | ATP Binding Cassette transporter A1 |
| ABCG1 | ATP Binding Cassette transporter G1 |
| APOE | Apolipoprotein E |
| RXR | Retinoid X Receptor |
References
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rader, D.J. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 2007, 22, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Larrede, S.; Quinn, C.M.; Jessup, W.; Frisdal, E.; Olivier, M.; Hsieh, V.; Kim, M.-J.; Van Eck, M.; Couvert, P.; Carrie, A.; et al. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-D.; Franklin, V.; Marcel, Y.L. In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, Á.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, I.; Pedrelli, M.; Potì, F.; Stomeo, G.; Gomaraschi, M.; Calabresi, L.; Bernini, F. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Evans, R.M. The RXR heterodimers and orphan receptors. Cell 1995, 83, 841–850. [Google Scholar] [CrossRef]
- De Lera, A.R.; Krezel, W.; Ruhl, R. An endogenous mammalian retinoid X receptor ligand, at last! Chem. Med. Chem. 2016, 11, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Czimmerer, Z.; Nagy, L. The role of lipid-activated nuclear receptors in shaping macrophage and dendritic cell function: From physiology to pathology. J. Allergy Clin. Immunol. 2013, 132, 264–286. [Google Scholar] [CrossRef] [PubMed]
- Costet, P.; Lalanne, F.; Gerbod-Giannone, M.C.; Molina, J.R.; Fu, X.; Lund, E.G.; Gudas, L.J.; Tall, A.R. Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol. Cell. Biol. 2003, 23, 7756–7766. [Google Scholar] [CrossRef] [PubMed]
- Ayaori, M.; Yakushiji, E.; Ogura, M.; Nakaya, K.; Hisada, T.; Uto-Kondo, H.; Takiguchi, S.; Terao, Y.; Sasaki, M.; Komatsu, T.; et al. Retinoic acid receptor agonists regulate expression of ATP-binding cassette transporter G1 in macrophages. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki-Mogami, T.; Tamehiro, N.; Sato, Y.; Okuhira, K.-i.; Sai, K.; Kagechika, H.; Shudo, K.; Abe-Dohmae, S.; Yokoyama, S.; Ohno, Y.; et al. The RXR agonists PA024 and HX630 have different abilities to activate LXR/RXR and to induce ABCA1 expression in macrophage cell lines. Biochem. Pharmacol. 2008, 76, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Langmann, T.; Liebisch, G.; Moehle, C.; Schifferer, R.; Dayoub, R.; Heiduczek, S.; Grandl, M.; Dada, A.; Schmitz, G. Gene expression profiling identifies retinoids as potent inducers of macrophage lipid efflux. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1740, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Sennoune, S.R.; Martinez-Zaguilan, R.; Slominski, A.T.; Pruitt, K. Regulation of retinoid mediated cholesterol efflux involves liver X receptor activation in mouse macrophages. Biochem. Biophys. Res. Commun. 2015, 464, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lin, J.; Chen, H.; Wang, J.; Liu, Y.; Xia, M. Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in APOE-deficient mice. Br. J. Nutr. 2015, 114, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. 2012, 58, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zolberg Relevy, N.; Bechor, S.; Harari, A.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. The inhibition of macrophage foam cell formation by 9-cis β-carotene is driven by BCMO1 activity. PLoS ONE 2015, 10, e0115272. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Kurl, S.; Makikallio, T.H.; Ronkainen, K.; Laukkanen, J.A. Serum β-carotene concentrations and the risk of congestive heart failure in men: A population-based study. Int. J. Cardiol. 2013, 168, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.; Virtamo, J.; Korhonen, P.; Albanes, D.; Pietinen, P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 2000, 31, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Krinsky, N.I.; Benotti, P.N.; Russell, R.M. Biosynthesis of 9-cis-retinoic acid from 9-cis-β-carotene in human intestinal mucosa in vitro. Arch. Biochem. Biophys. 1994, 313, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Shaish, A.; Harari, A.; Hananshvili, L.; Cohen, H.; Bitzur, R.; Luvish, T.; Ulman, E.; Golan, M.; Ben-Amotz, A.; Gavish, D.; et al. 9-cis β-carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis 2006, 189, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Kamari, Y.; Gonen, A.; Gerber, Y.; Ben-Amotz, A.; Shaish, A. A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J. Nutr. 2008, 138, 1923–1930. [Google Scholar] [PubMed]
- Harari, A.; Abecassis, R.; Relevi, N.; Levi, Z.; Ben-Amotz, A.; Kamari, Y.; Harats, D.; Shaish, A. Prevention of atherosclerosis progression by 9-cis-β-carotene rich alga Dunaliella in APOE-deficient mice. BioMed Res. Int. 2013, 2013, 169517. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Sacha, T.; Zawada, M.; Hartwich, J.; Lach, Z.; Polus, A.; Szostek, M.; Zdziowska, E.; Libura, M.; Bodzioch, M.; Dembińska-Kieć, A.; et al. The effect of β-carotene and its derivatives on cytotoxicity, differentiation, proliferative potential and apoptosis on the three human acute leukemia cell lines: U-937, HL-60 and TF-1. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1740, 206–214. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.M.; Woods, J.A.; O’Brien, N.M. Use of Tween 40 and Tween 80 to deliver a mixture of phytochemicals to human colonic adenocarcinoma cell (CaCo-2) monolayers. Br. J. Nutr. 2004, 91, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-W.; Chang, T.-J.; Yang, D.-J.; Chen, Y.-C.; Wang, M.; Chang, Y.-Y. Regulation of virus-induced inflammatory response by β-carotene in RAW264.7 cells. Food Chem. 2012, 134, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Nakayama, C.; Suzuki, K. Dietary β-carotene regulates interleukin-1β-induced expression of apolipoprotein E in astrocytes isolated from stroke-prone spontaneously hypertensive rats. Neurochem. Int. 2013, 62, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, F.B.; Chen, Y.D.; Lopez, R.D.; Reaven, G.M. Effects of noninsulin-dependent diabetes mellitus on the uptake of very low density lipoproteins by thioglycolate-elicited mouse peritoneal macrophages. J. Clin. Endocrinol. Metab. 1985, 61, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yeum, K.J.; Booth, S.L.; Sadowski, J.A.; Liu, C.; Tang, G.; Krinsky, N.I.; Russell, R.M. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am. J. Clin. Nutr. 1996, 64, 594–602. [Google Scholar] [PubMed]
- Argmann, C.A.; Sawyez, C.G.; McNeil, C.J.; Hegele, R.A.; Huff, M.W. Activation of peroxisome proliferator–activated receptor gamma and retinoid X receptor results in net depletion of cellular cholesteryl esters in macrophages exposed to oxidized lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Petkovich, P.M. Retinoic acid metabolism. J. Am. Acad. Dermatol. 2001, 45, S136–S142. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ross, A.C. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J. Lipid Res. 2010, 51, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Loudig, O.; Maclean, G.A.; Dore, N.L.; Luu, L.; Petkovich, M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem. J. 2005, 392, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.J.; Bain, G.; Yao, M.; Gottlieb, D.I. CYP26, a novel mammalian cytochrome P450, is induced by retinoic acid and defines a new family. J. Biol. Chem. 1997, 272, 18702–18708. [Google Scholar] [CrossRef] [PubMed]
- Adorni, M.P.; Zimetti, F.; Billheimer, J.T.; Wang, N.; Rader, D.J.; Phillips, M.C.; Rothblat, G.H. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 2007, 48, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Turley, S.D.; Lobaccaro, J.-M.A.; Medina, J.; Li, L.; Lustig, K.; Shan, B.; Heyman, R.A.; Dietschy, J.M.; Mangelsdorf, D.J. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 2000, 289, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, B.A.; Repa, J.J.; Joseph, S.B.; Wilpitz, D.C.; Kast, H.R.; Mangelsdorf, D.J.; Tontonoz, P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, B.A.; Joseph, S.B.; Walczak, R.; Pei, L.; Wilpitz, D.C.; Collins, J.L.; Tontonoz, P. Autoregulation of the human liver X receptor α promoter. Mol. Cell. Biol. 2001, 21, 7558–7568. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, A.; Repa, J.J.; Lobaccaro, J.-M.A.; Bronson, A.; Mangelsdorf, D.J.; Edwards, P.A. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages A transcriptional role for specific oxysterols. J. Biol. Chem. 2000, 275, 14700–14707. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-L.; Chen, W.-J.; Yin, K.; Zhao, G.-J.; Mo, Z.-C.; Lv, Y.-C.; Ouyang, X.-P.; Yu, X.-H.; Kuang, H.-J.; Jiang, Z.-S. PAPP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRα through the IGF-I-mediated signaling pathway. Atherosclerosis 2012, 222, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Song, Z.; Zhang, Q. Cholesterol efflux is LXR α isoform-dependent in human macrophages. BMC Cardiovasc. Disord. 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ranalletta, M.; Matsuura, F.; Peng, F.; Tall, A.R. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Russell, R.M. Distribution of orally administered beta-carotene among lipoproteins in healthy men. Am. J. Clin. Nutr. 1992, 56, 128–135. [Google Scholar] [PubMed]
- Blankenhorn, D.H. The infiltration of carotenoids into human atheromas and xanthomas. Ann. Intern. Med. 1960, 53, 944–954. [Google Scholar]
- Gopal, K.; Nagarajan, P.; Jedy, J.; Raj, A.T.; Gnanaselvi, S.K.; Jahan, P.; Sharma, Y.; Shankar, E.M.; Kumar, J.M. β-Carotene attenuates angiotensin II-induced aortic aneurysm by alleviating macrophage recruitment in APOE−/− mice. PLoS ONE 2013, 8, e67098. [Google Scholar] [CrossRef] [PubMed]
- Mokady, S.; Avron, M.; Ben-Amotz, A. Accumulation in chick livers of 9-cis versus all-trans beta-carotene. J. Nutr. 1990, 120, 889–892. [Google Scholar] [PubMed]
- Ben-Amotz, A.; Mokady, S.; Edelstein, S.; Avron, M. Bioavailability of a natural isomer mixture as compared with synthetic all-trans-beta-carotene in rats and chicks. J. Nutr. 1989, 119, 1013–1019. [Google Scholar] [PubMed]
- Erdman, J.W.; Thatcher, A.J.; Hofmann, N.E.; Lederman, J.D.; Block, S.S.; Lee, C.M.; Mokady, S. All-trans β-carotene is absorbed preferentially to 9-cis β-carotene, but the latter accumulates in the tissues of domestic ferrets (Mustela putorius puro). J. Nutr. 1998, 128, 2009–2013. [Google Scholar] [PubMed]
- Matsumoto, A.; Mizukami, H.; Mizuno, S.; Umegaki, K.; Nishikawa, J.-I.; Shudo, K.; Kagechika, H.; Inoue, M. Β-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem. Pharmacol. 2007, 74, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Catalano, A.; Parrone, N.; Monego, G.; Ranelletti, F.O. Lycopene regulation of cholesterol synthesis and efflux in human macrophages. J. Nutr. Biochem. 2011, 22, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.T.; Edwards, P.A. ABCG1 and ABCG4 are coexpressed in neurons and astrocytes of the CNS and regulate cholesterol homeostasis through SREBP-2. J. Lipid Res. 2008, 49, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Jian, B.; Wang, N.; Sun, Y.; Moya, M.D.L.L.; Phillips, M.C.; Rothblat, G.H.; Swaney, J.B.; Tall, A.R. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 1997, 272, 20982–20985. [Google Scholar] [CrossRef] [PubMed]
- Krivospitskaya, O.; Elmabsout, A.A.; Sundman, E.; Söderström, L.Å.; Ovchinnikova, O.; Gidlöf, A.C.; Scherbak, N.; Norata, G.D.; Samnegård, A.; Törmä, H. A CYP26B1 polymorphism enhances retinoic acid catabolism and may aggravate atherosclerosis. Mol. Med. 2012, 18, 712. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.M.; Wang, S.W.; Chen, C.L.; Wu, P.H.; Wu, W.B. Effects of all-trans retinoic acid, retinol, and β-carotene on murine macrophage activity. Food Funct. 2014, 5, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Sung, M.K. Effects of major dietary antioxidants on inflammatory markers of RAW 264.7 macrophages. Biofactors 2004, 21, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, N.; Schulman, I.G. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1120–1126. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechor, S.; Zolberg Relevy, N.; Harari, A.; Almog, T.; Kamari, Y.; Ben-Amotz, A.; Harats, D.; Shaish, A. 9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients 2016, 8, 435. https://doi.org/10.3390/nu8070435
Bechor S, Zolberg Relevy N, Harari A, Almog T, Kamari Y, Ben-Amotz A, Harats D, Shaish A. 9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients. 2016; 8(7):435. https://doi.org/10.3390/nu8070435
Chicago/Turabian StyleBechor, Sapir, Noa Zolberg Relevy, Ayelet Harari, Tal Almog, Yehuda Kamari, Ami Ben-Amotz, Dror Harats, and Aviv Shaish. 2016. "9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages" Nutrients 8, no. 7: 435. https://doi.org/10.3390/nu8070435
APA StyleBechor, S., Zolberg Relevy, N., Harari, A., Almog, T., Kamari, Y., Ben-Amotz, A., Harats, D., & Shaish, A. (2016). 9-cis β-Carotene Increased Cholesterol Efflux to HDL in Macrophages. Nutrients, 8(7), 435. https://doi.org/10.3390/nu8070435