Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
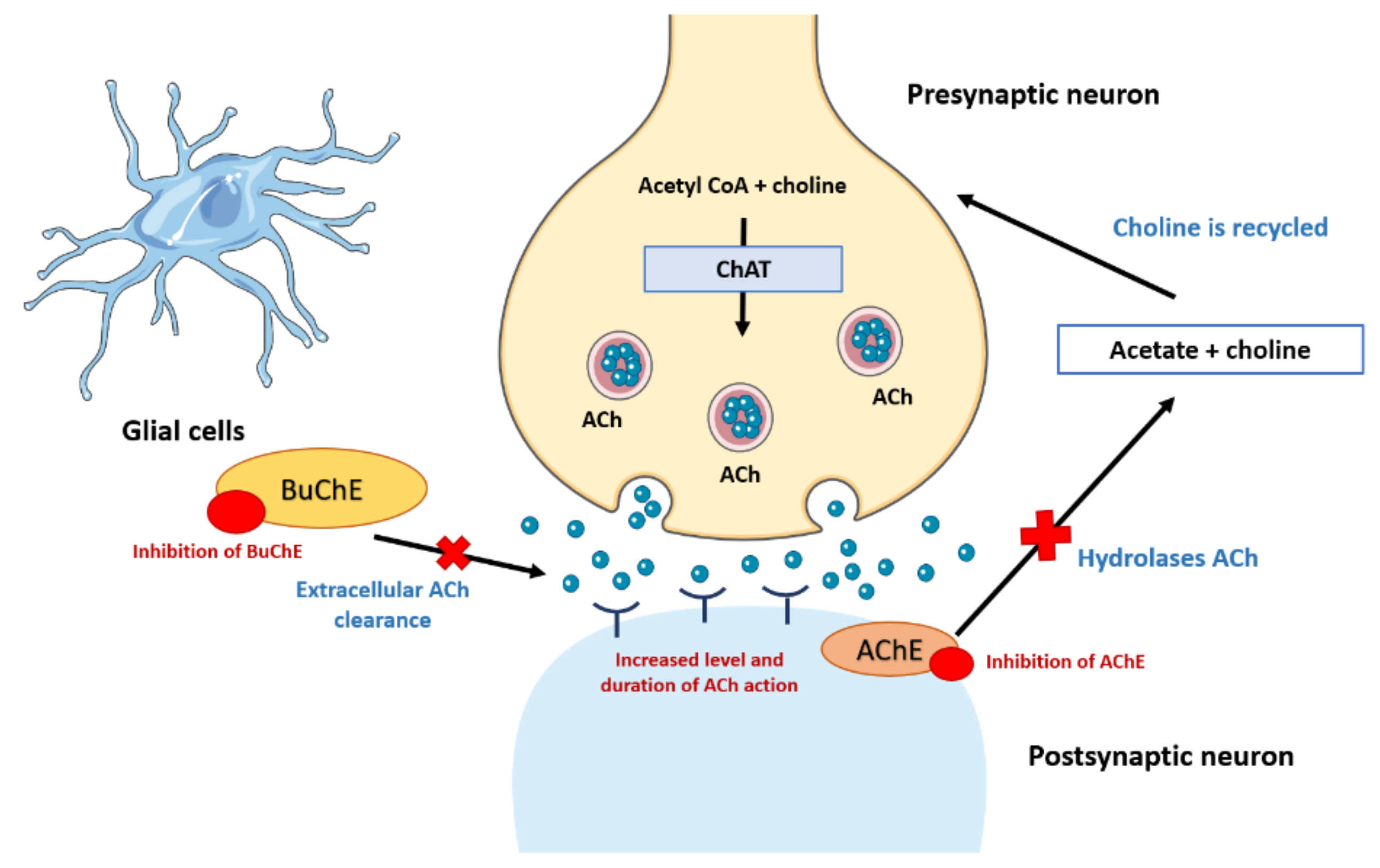
2. Cholinergic Hypothesis in Pathogenesis and Treatment of AD
3. Diterpenoids
3.1. Abietane Diterpenoids
3.2. Tanshinones
| Species | Plant Parts | Extraction Method | Types of Compound | Chemical Constituent | Cholinesterase Inhibition (µM) (A: AChE, B: BuChE) | References |
|---|---|---|---|---|---|---|
| Caryopteris mongolica | Aerial part | Extract with acetone water (4:1), quenched with diethyl ether and water, diethyl ether fraction was passed through HP20 column | Abietane diterpenoid | 12-O-demethylcryptojaponol (7) | 50.8 (A) 70.1 (B) | [14] |
| 6α-hydroxydemethylcryptojaponol (8) | 12.3 (A) 7.7 (B) | |||||
| Lycopodiastrum casuarinoides | Aerial part | Extract with 75% ethanol, partitioned with EtOAc and 3% tartaric acid, column chromatography | Abietane diterpenoid | Lycocasuarinone A (9) | 26.8 (A) | [15] |
| Perovskia atriplicifolia Salvia glutinosa | Root | Sonicate with hexane, column chromatography | Nor-abietanoid | Arucadiol (10) | 4.0 (B) | [22] |
| Miltirone (11) | 0.90 μg/mL (B) | |||||
| Tanshinone IIa (12) | 2.79 μg/mL (B) | |||||
| 1-oksomiltirone (13) | 5.06 μg/mL (B) | |||||
| Cryptotanshinone (14) | 1.15 μg/mL (B) | |||||
| 1,2-didehydromiltirone (15) | 1.12 μg/mL (B) | |||||
| 1,2-didehydrotanshinone IIa (16) | 5.98 μg/mL (B) | |||||
| 1β-OH-cryptotanshinone (17) | 1.21 μg/mL (B) | |||||
| 15,16-dihydrotanshinone (18) | 1.71 μg/mL (B) | |||||
| Tanshinone I (19) | 11.24 μg/mL (B) | |||||
| Isotanshinone II (20) | 9.16 μg/mL (B) | |||||
| 1(S)-OH-tanshinone IIa (21) | 5.71 μg/mL (B) |
4. Triterpenoids
4.1. Serratene-Type Triterpenoids
4.2. Cucurbitane-Type Diterpenoids
4.3. Limonoids
4.4. Lanostane Triterpenoids
4.5. Friedelanes
4.6. Other Triterpenoids
| Species | Plant Parts | Extraction Method | Types of Compound | Chemical Constituent | Cholinesterase Inhibition (µM) (A: AChE, B: BuChE) | References |
|---|---|---|---|---|---|---|
| Lycopodiella cernua | Whole plant | Reflux with methanol, partitioned with hexane and EtOH, EtOH fraction subjected to column chromatography | Serratene | 21β-hydroxyserrat-14-en-3,16-dione (22) | 10.67 (A) | [25] |
| 3β,14α,15α,21β-tetrahydroxyserratan-24-oic acid-3β-yl-(4′-methoxy-5′-hydroxybenzoate) (23) | 9.98 (A) | |||||
| 3β,21α-diacetoxyserratan-14β-ol (24) | 0.91 (A) | |||||
| 3β,21β,29-trihydroxyserrat-14-en-3β-yl p-dihydrocoumarate (25) | 1.69 (A) 0.42 (B) | |||||
| serrat-14-en-3α,21β-diol (26) | 1.37 (B) | |||||
| Citrullus colocynthis | Fruits | Extract with methanol, fractionated, column chromatography | Cucurbitane | Colocynthenin A (27) | 2.6 (A) | [29] |
| Colocynthenin C (28) | 3.1 (A) | |||||
| Trichilia welwitschii | Seeds | Extract with dichloromethane methanol, flash chromatography | Limonoid | Trichilia lactone D5 (31) | 28.55 (A) | [33] |
| Rohituka (30) | 57.5 (A) | |||||
| Dregeanin DM4 (29) | 78.37 (A) | |||||
| Ganoderma lucidum | Fruiting body | Extract with ethanol, fractionated and column chromatography | Lanostane | Ganolucidic acid E (32) | 13.8 (A) | [34] |
| 11β-hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oic acid (33) | 10.8 (A) | |||||
| Ganoderic Am1 (34) | 183 (A) | |||||
| Methyl ganoderate C (35) | 148 (A) | |||||
| Ganodernoid C1 (36) | 142 (A) | |||||
| 12β-hydroxyganodernic F (37) | 102 (A) | |||||
| Methyl ganoderate E (38) | 45.8 (A) | |||||
| Ganoderic acid C6 (39) | 147.5 (A) | |||||
| Methyl Ganoderic acid C6 (40) | 145.2 (A) | |||||
| Gaodernoid B2 (41) | 102.4 (A) | |||||
| Ganoderlactone G (42) | 130.5 (A) | |||||
| Ganodernoid A (43) | 149.0 (A) | |||||
| Malpighia emarginata | Branches and roots | Extract with acetone, fractionated, column chromatography | Norfriedelane | Norfriedelin A (44) | 10.3 (A) | [37] |
| Norfriedelin B (45) | 28.7 (A) | |||||
| Patrinia scabiosaefolia | Whole plant | Extract with ethanol, partitioned with ethyl acetate, column chromatography | Triterpenoid | 3β-hydroxy-24-nor-urs-4(23)-12-dien-28-oic acid (46) | 10.1 (A) | [38] |
| Callicarpa maingayi | Leaves | Extract with methanol, fractionated, column chromatography | Triterpenoid | Euscaphic acid (47) | 35.9 (A) | [39] |
| Arjunic acid (48) | 37.5 (A) | |||||
| Ursolic acid (49) | 21.5 (A) | |||||
| Garcinia hombroniana | Barks | Sequential extraction, column chromatography | Triterpenoid | 2-hydroxy-3-O-caffeoyltaraxar-14-en-28-oic acid (50) | 13.5 (A) 10.6 (B) | [40] |
| Taraxerol (51) | 17.8 (B) | |||||
| Betulin (52) | 28.5 (A) | |||||
| Betulinic acid (53) | 24.2 (A) 19.1 (B) |
5. Sesquiterpenoids
5.1. Sesquiterpene Lactones
5.2. Sesquiterpene with Agarofuran Skeletons
5.3. Caryophyllene-Type Terpenoids
5.4. Other Sesquiterpenes
| Species | Plant Parts | Extraction Methods | Types of Compounds | Chemical Constituents | Cholinesterase Inhibition (µM) (A: AChE, B: BuChE) | References |
|---|---|---|---|---|---|---|
| Cynara cornigera | Aerial parts | Extract with methanol, column chromatography | Sesquiterpene lactone | Cornigeraline A (54) | 20.5 (A) | [41] |
| Sibthorpine (55) | 35.8 (A) | |||||
| 3-hydroxy-grosheimin (56) | 30.5 (A) | |||||
| Grosheimin (57) | 61.8 (A) | |||||
| Solstitalin A (58) | 25.7 (A) | |||||
| 13-chlorosolstitialine (59) | 62.1 (A) | |||||
| Cyanaropicrin (60) | 31.3 (A) | |||||
| Maytenus disticha | Seeds | Extract with methanol, fractionated, chloroform fraction, further column chromatography | Agarofuran | 1α,6β,8α-triacetoxy-9β-furoyloxy-β-agarofuran (61) | 248 (A) | [44] |
| 1α-hydroxy-6β,8α-diacetoxy-9β-furoyloxy-β-agarofuran (62) | 738 (A) | |||||
| 1α,6β-diacetoxy-8α-hydroxy-9β-furoyloxy-β-agarofuran (63) | 161 (A) | |||||
| 1α-acetoxy-6β,8α-dihydroxy-9β-furoyloxy-β-agarofuran (64) | 312 (A) | |||||
| 1α,2α,6β,8α,15-pentaacetoxy-9β-benzoyloxy-β-agarofuran (65) | 122 (A) | |||||
| 1α-acetoxy-6β,9β-difuroyloxy-4β-hydroxy-β-agarofuran (66) | 738 (A) | |||||
| Pulicaria vulgaris | Aerial part | Extract with acetone–water (1:1), fractionated, column chromatography | Caryophyllene Sesquiterpene | Pulicaryenne A (67) | 214.85 (A) | [45] |
| (1S,6R,9S,11R)-13,14-dihdroxycaryophyll-2(15)-en-7-one (68) | 39.97 (A) | |||||
| (5Z)-14-hydroxycaryophyllen-7-one (69) | 108.26 (A) | |||||
| (1S,5Z,9R)-12-acetoxy-14-hydroxycaryophylla-2(15),5-dien-7-one (70) | 101.22 (A) | |||||
| (1S,5Z,9R)-12,14-dihydroxycaryophylla-2(15),5-dien-7-one (71) | 25.78 (A) | |||||
| Lycopodiastrum casuarinoides | Sesquiterpene acid | Megatigma-7, 9-diene-1,4-epoxy-2-hydroxy-10-carboxylic acid (72) | 9.49 (A) 9.34 (B) | [15] | ||
| Artemisia annua | Leaves | Extract with ethanol, column chromatography, Sephadex LH-20 | Sesquiterpene lactone | Artemisinin (2) | 104 (A) | [46] |
| Aquilaria sinensis | Woods | Diethyl ether extract, column chromatography | 5,11-epoxyguaiane | Qinanol A (73) | 100.7 (A) | [47] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadra, S.; Dalai, M.K.; Chanda, J.; Mukherjee, P.K. Chapter 13—Evaluation of bioactive compounds as acetylcholinesterase inhibitors from medicinal plants. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 273–306. [Google Scholar]
- LaLonde, R.T. Terpenes and terpenoids. In Van Nostrand’s Encyclopedia of Chemistry; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Perveen, S. Introductory Chapter: Terpenes and terpenoids. In Terpenes and Terpenoids; BoD–Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Brahmkshatriya, P.P.; Brahmkshatriya, P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. Nat. Prod. 2013, 12, 2665–2691. [Google Scholar]
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their growing importance in medicine. Trends Pharmacol. Sci. 2008, 29, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products; Humana Press: Clifton, NJ, USA, 2005. [Google Scholar]
- Lahiri, D.K.; Farlow, M.R.; Greig, N.H.; Sambamurti, K. Current drug targets for Alzheimer’s disease treatment. Drug Dev. Res. 2002, 56, 267–281. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterase Inhibitors Stabilize Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2000, 920, 321–327. [Google Scholar] [CrossRef]
- Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules 2021, 26, 728. [Google Scholar] [CrossRef]
- Tayeb, H.O.; Yang, H.D.; Price, B.H.; Tarazi, F.I. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol. Ther. 2012, 134, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R. Chapter 28—Phytochemicals as anti-inflammatory nutraceuticals and phytopharmaceuticals. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 363–388. [Google Scholar]
- Smanski, M.J.; Peterson, R.M.; Shen, B. Chapter eight—Platensimycin and platencin biosynthesis in streptomyces platensis. In Showcasing Discovery and Characterization of Novel Bacterial Diterpene Synthases; Hopwood, D.A., Ed.; Methods in Enzymology; Academic Press: Cambrige, MA, USA, 2012; pp. 163–186. [Google Scholar]
- Hung, T.M.; Luan, T.C.; Vinh, B.T.; Cuong, T.D.; Min, B.S. Labdane-type diterpenoids from Leonurus heterophyllus and their cholinesterase inhibitory activity. Phytother. Res. 2011, 25, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Selenge, E.; Oikawa, S.; Ageishi, K.; Batkhuu, J.; Sasaki, K.; Yoshizaki, F. Cholinesterase-inhibitory diterpenoids and chemical constituents from aerial parts of Caryopteris mongolica. J. Nat. Med. 2015, 69, 471–478. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, D.; Li, X.-M.; Li, D.; Zhou, G.; Xu, K.-P.; Kang, F.-H.; Zou, Z.-X.; Xu, P.-S.; et al. Anti-cholinesterase activities of constituents isolated from Lycopodiastrum casuarinoides. Fitoterapia 2019, 139, 104366. [Google Scholar] [CrossRef]
- De Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Zhang, Y.; Cao, H.; Wang, Y.; Teng, T.; Ma, G.; Li, Y.; Li, K.; Zhang, Y. Ferulic acid inhibits the transition of amyloid-β42 monomers to oligomers but accelerates the transition from oligomers to fibrils. J. Alzheimers Dis. 2013, 37, 19–28. [Google Scholar] [CrossRef]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, M.A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgarbossa, A.; Giacomazza, D.; di Carlo, M. Ferulic Acid: A Hope for Alzheimer’s Disease Therapy from Plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, F.-Y.; Feng, C.-Q.; Yang, X.-F. Studies on the active constituents in radix salviae miltiorrhizae and their protective effects on cerebral ischemia reperfusion injury and its mechanism. Pharmacogn. Mag. 2015, 11, 69–73. [Google Scholar] [PubMed] [Green Version]
- Pinho, B.R.; Ferreres, F.; Valentão, P.; Andrade, P.B. Nature as a source of metabolites with cholinesterase-inhibitory activity: An approach to Alzheimer’s disease treatment. J. Pharm. Pharmacol. 2013, 65, 1681–1700. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Slusarczyk, S.; Matkowski, A.; Perez-Garrido, A.; Giron-Rodriguez, F.; Ceron-Carrasco, J.P.; den-Haan, H.; Pena-Garcia, J.; Perez-Sanchez, H.; Domaradzki, K.; et al. Selective in vitro and in silico butyrylcholinesterase inhibitory activity of diterpenes and rosmarinic acid isolated from Perovskia atriplicifolia Benth. and Salvia glutinosa L. Phytochemistry 2017, 133, 33–44. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeon, S.J.; Jung, J.W.; Lee, S.; Yoon, B.H.; Shin, B.Y.; Son, K.H.; Cheong, J.H.; Kim, Y.S.; Kang, S.S.; et al. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur. J. Pharmacol. 2007, 574, 140–147. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Alho, D.P.S.; Gonçalves, B.M.F.; Valdeira, A.S.; Mendes, V.I.S.; Jing, Y. Chapter 2—Highlights of pentacyclic triterpenoids in the cancer settings. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 33–73. [Google Scholar]
- Nguyen, V.T.; To, D.C.; Tran, M.H.; Oh, S.H.; Kim, J.A.; Ali, M.Y.; Woo, M.-H.; Choi, J.S.; Min, B.S. Isolation of cholinesterase and β-secretase 1 inhibiting compounds from Lycopodiella cernua. Bioorg. Med. Chem. 2015, 23, 3126–3134. [Google Scholar] [CrossRef]
- Cao, H.; Chai, T.-T.; Wang, X.; Morais-Braga, M.F.B.; Yang, J.-H.; Wong, F.-C.; Wang, R.; Yao, H.; Cao, J.; Cornara, L.; et al. Phytochemicals from fern species: Potential for medicine applications. Phytochem. Rev. 2017, 16, 379–440. [Google Scholar] [CrossRef]
- Boonya-udtayan, S.; Thasana, N.; Jarussophon, N.; Ruchirawat, S. Serratene triterpenoids and their biological activities from Lycopodiaceae plants. Fitoterapia 2019, 136, 104181. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Chen, X.; Chen, S.-X.; Gan, L.-S.; Yuan, T. Colocynthenins A–D, Ring-A seco-Cucurbitane Triterpenoids from the Fruits of Citrullus colocynthis. J. Nat. Prod. 2018, 81, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.S.; Ali, L.; Al-Rawahi, S.S.; Al-Harrasi, A. New p-Terphenyl from the Fruit of Citrullus colocynthis (Cucurbitaceae). Nat. Prod. Commun. 2017, 12, 1934578X1701200724. [Google Scholar] [CrossRef] [Green Version]
- Drijfhout, F.P.; David Morgan, E. 4.11—Terrestrial natural products as antifeedants. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 457–501. [Google Scholar]
- Sato, R. Chapter eighteen—Nomilin as an anti-obesity and anti-hyperglycemic agent. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 425–439. [Google Scholar]
- Dzoyem, J.P.; Tsamo, A.T.; Melong, R.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biol. Res. 2015, 48, 57. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Chen, Y.; Zhang, L.; Xing, M. Chapter ten—Ganoderma lucidum polysaccharide used for treating physical frailty in China. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambrige, MA, USA, 2019; pp. 179–219. [Google Scholar]
- Wei, J.C.; Wang, A.H.; Wei, Y.L.; Huo, X.K.; Tian, X.G.; Feng, L.; Ma, X.C.; Wang, C.; Huang, S.S.; Jia, J.M. Chemical characteristics of the fungus Ganoderma lucidum and their inhibitory effects on acetylcholinesterase. J. Asian Nat. Prod. Res. 2018, 20, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, N.; Ferro, E.A. Bioactive triterpenes and related compounds from celastraceae. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Boston, MA, USA, 2006; pp. 239–307. [Google Scholar]
- Liu, J.Q.; Peng, X.R.; Li, X.Y.; Li, T.Z.; Zhang, W.M.; Shi, L.; Han, J.; Qiu, M.H. Norfriedelins A-C with acetylcholinesterase inhibitory activity from acerola tree (Malpighia emarginata). Org. Lett. 2013, 15, 1580–1583. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Ma, R.-J.; Yang, L.; Li, J.-Y.; Hou, B.; Hu, J.-M.; Zhou, J. Triterpenoids and iridoids from Patrinia scabiosaefolia. Fitoterapia 2017, 119, 130–135. [Google Scholar] [CrossRef]
- Ado, M.A.; Maulidiani, M.; Ismail, I.S.; Ghazali, H.M.; Shaari, K.; Abas, F. Acetylcholinesterase and α-glucosidase inhibitory compounds from Callicarpa maingayi. Nat. Prod. Res. 2021, 35, 2992–2996. [Google Scholar] [CrossRef]
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; Osman, H.; Murugaiyah, V. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2015, 30, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Awouafack, M.D.; Tane, P.; Kuete, V.; Eloff, J.N. 2—Sesquiterpenes from the medicinal plants of Africa. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 33–103. [Google Scholar]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 11—Terpenoids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 233–266. [Google Scholar]
- Hegazy, M.E.; Ibrahim, A.Y.; Mohamed, T.A.; Shahat, A.A.; El Halawany, A.M.; Abdel-Azim, N.S.; Alsaid, M.S.; Pare, P.W. Sesquiterpene Lactones from Cynara cornigera: Acetyl Cholinesterase Inhibition and In Silico Ligand Docking. Planta Med. 2016, 82, 138–146. [Google Scholar]
- Alarcon, J.; Cespedes, C.L.; Munoz, E.; Balbontin, C.; Valdes, F.; Gutierrez, M.; Astudillo, L.; Seigler, D.S. Dihydroagarofuranoid Sesquiterpenes as Acetylcholinesterase Inhibitors from Celastraceae Plants: Maytenus disticha and Euonymus japonicus. J. Agric. Food Chem. 2015, 63, 10250–10256. [Google Scholar] [CrossRef] [PubMed]
- Zardi-Bergaoui, A.; Znati, M.; Harzallah-Skhiri, F.; Jannet, H.B. Caryophyllene Sesquiterpenes from Pulicaria vulgaris Gaertn.: Isolation, Structure Determination, Bioactivity and Structure−Activity Relationship. Chem. Biodivers. 2019, 16, e1800483. [Google Scholar] [CrossRef]
- Chougouo, R.D.K.; Nguekeu, Y.M.M.; Dzoyem, J.P.; Awouafack, M.D.; Kouamouo, J.; Tane, P.; McGaw, L.J.; Eloff, J.N. Anti-inflammatory and acetylcholinesterase activity of extract, fractions and five compounds isolated from the leaves and twigs of Artemisia annua growing in Cameroon. Springerplus 2016, 5, 1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.L.; Li, W.; Dong, W.H.; Wang, J.; Mei, W.L.; Dai, H.F. Five new 5,11-epoxyguaiane sesquiterpenes in agarwood “Qi-Nan” from Aquilaria sinensis. Fitoterapia 2016, 112, 191–196. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review). Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai Shi Min, S.; Liew, S.Y.; Chear, N.J.Y.; Goh, B.H.; Tan, W.-N.; Khaw, K.Y. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology 2022, 11, 307. https://doi.org/10.3390/biology11020307
Lai Shi Min S, Liew SY, Chear NJY, Goh BH, Tan W-N, Khaw KY. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology. 2022; 11(2):307. https://doi.org/10.3390/biology11020307
Chicago/Turabian StyleLai Shi Min, Shereen, Sook Yee Liew, Nelson Jeng Yeou Chear, Bey Hing Goh, Wen-Nee Tan, and Kooi Yeong Khaw. 2022. "Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy" Biology 11, no. 2: 307. https://doi.org/10.3390/biology11020307
APA StyleLai Shi Min, S., Liew, S. Y., Chear, N. J. Y., Goh, B. H., Tan, W.-N., & Khaw, K. Y. (2022). Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology, 11(2), 307. https://doi.org/10.3390/biology11020307






