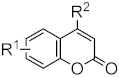
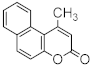
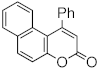
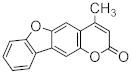
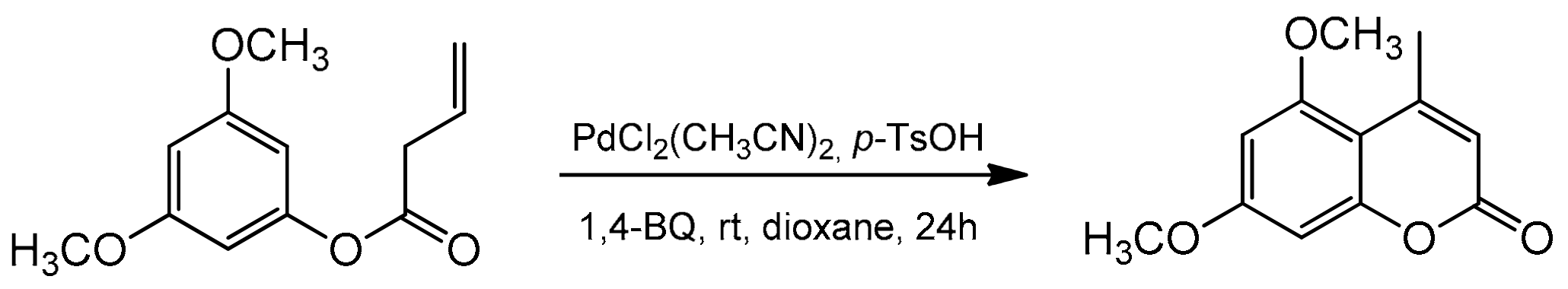Abstract
For several decades, coumarins have attracted considerable attention due to the fact of their application in diverse fields such as medical science and biomedical research as well as several industrial branches. Recently, many compounds containing the coumarin moiety have been intensively studied, mainly due to the fact of their biological activities such as antitumor, antioxidative, anti-HIV, vasorelaxant, antimicrobial, and anticancer. They are also widely used as fluorescent dyes and probes because of their great structural flexibility and large fluorescent quantum yields. For this reason, numerous attempts have been made to develop new and more practical methods for the synthesis of these compounds. This review aims at providing a comprehensive overview of coumarin synthesis methods by direct C–H bond activation in order to demonstrate the current state-of-the-art methods as well as the current limitations.
1. Introduction to Coumarins
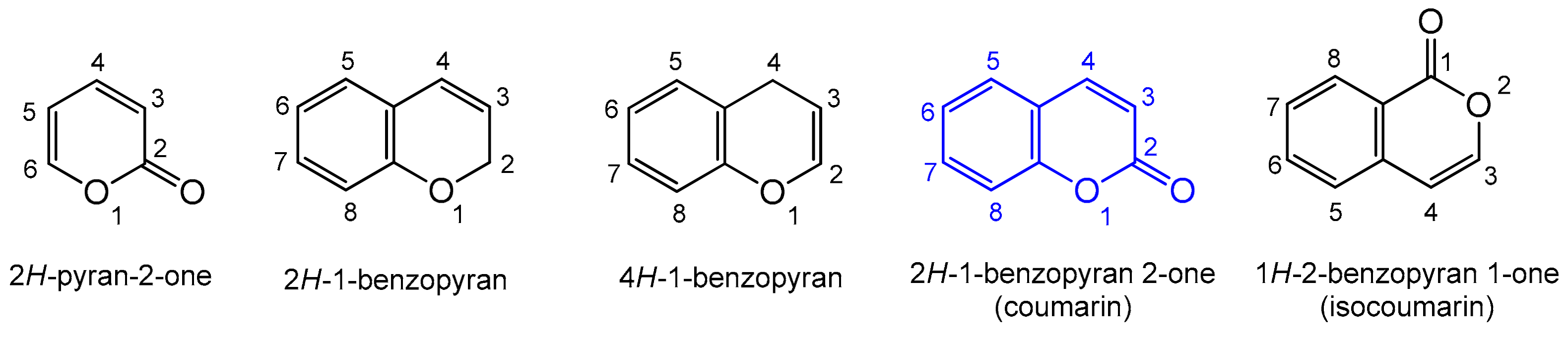
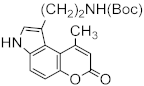
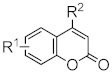
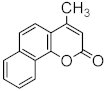
Heterocyclic compounds are an integral part of many biologically active molecules. Currently, many commercial drugs have a heterocyclic scaffold in their structure. Among them, bicyclic oxygen heterocycles resulting from fusion with the benzene ring constitute a large group of biologically active compounds (Figure 1).
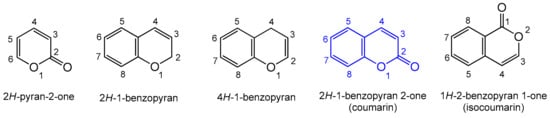
Figure 1.
Biologically important oxygen heterocycles.
The 2H-pyran-2-one ring is commonly found in nature, and as a privileged biological scaffold, it exhibits a broad spectrum of actions including antifungal, antibiotic, and cytotoxic activities [1,2]. A condensed form of 2H-pyran-2-one with the benzyl ring is known as coumarin. Coumarin is an organic heterocyclic compound belonging to the lactone subgroup with a benzo-α-pyrone (2H-1-benzopyran-2-one) skeleton; the systematic nomenclature was established by the. IUPAC (International Union of Pure and Applied Chemistry) [3]. Natural coumarin is obtained from tonka bean trees, which grow in Venezuela, Colombia, Guyana, and Brazil. The larger yields of coumarin can be obtained from the trees aged 7–10 years. Yellow–green fallen, mango-like fruits containing seeds are harvested from February to April. Pure coumarin is isolated by the alcoholic extraction of dry seeds or whole fruit.
Since the 19th century, coumarins have been a large class of natural compounds. It is estimated that more than 1300 natural coumarins isolated from the plants, fungi, and bacteria are known at present [4]. In nature, coumarins are secondary metabolites and often play a protective role in inhibiting various biological processes. From a chemical point of view, they are molecules with several attractive features, such as a small molecular weight, simple structure, high bioavailability, good solubility in most organic solvents, and low toxicity. These features, together with the broad spectrum of biological activities, contribute to their significant role as leading compounds in the potential drugs study [5,6,7].
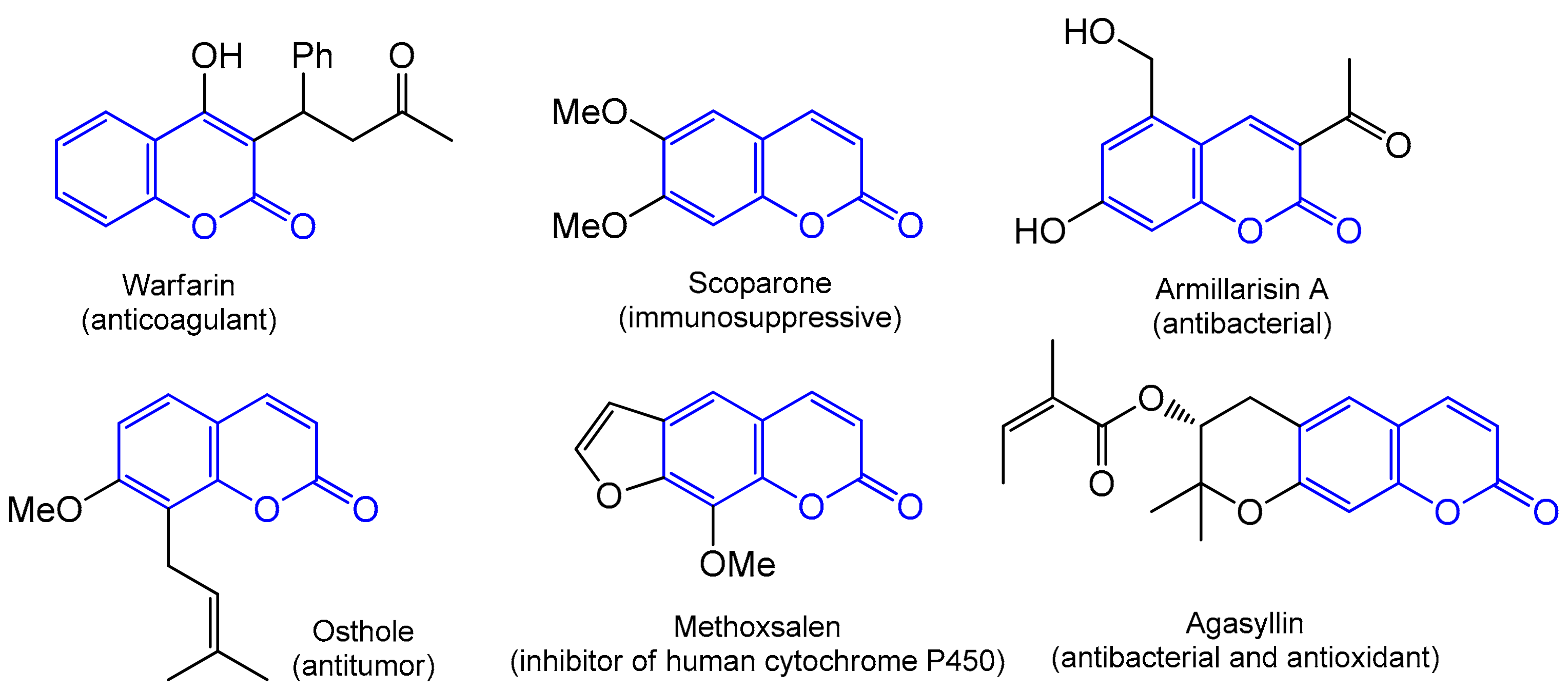
The wide and diverse area of application for coumarins in medical sciences, biomedical research, and in many industries, including perfumery, is impressive. The pharmacological profile of these phytochemicals includes antibacterial (agasyllin, felamidin, and armillarisin A) [8,9], anti-inflammatory (coumarin and esculetin) [10], anticancer [11,12,13,14], antiviral [15,16], antioxidant (Esculetin) [17,18,19,20], antifungal [21], anticoagulant (warfarin) [22,23], anti-HIV (inophyllums and calanolides) [24,25,26], and other activities [27]. Coumarins also act as selective enzyme inhibitors; they are able to interact with targets in the treatment of diseases such as Parkinson’s and Alzheimer’s [28,29] and, more recently, they have been widely adopted in the design of small-molecule fluorescent chemosensors [30]. Some examples of biologically active coumarin derivatives are presented in Figure 2.
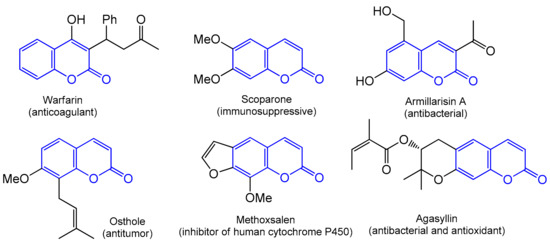
Figure 2.
Examples of coumarin skeleton-based drugs.
Coumarin and its derivatives can be obtained from a variety of natural resources; however, this is a time-consuming process. For this reason, its preparation has attracted the significant attention of organic chemists. So far, several methods have been developed for the synthesis of coumarins including metal-free reaction as well the metal catalytic route.
2. Methods for the Synthesis of the Coumarin Core
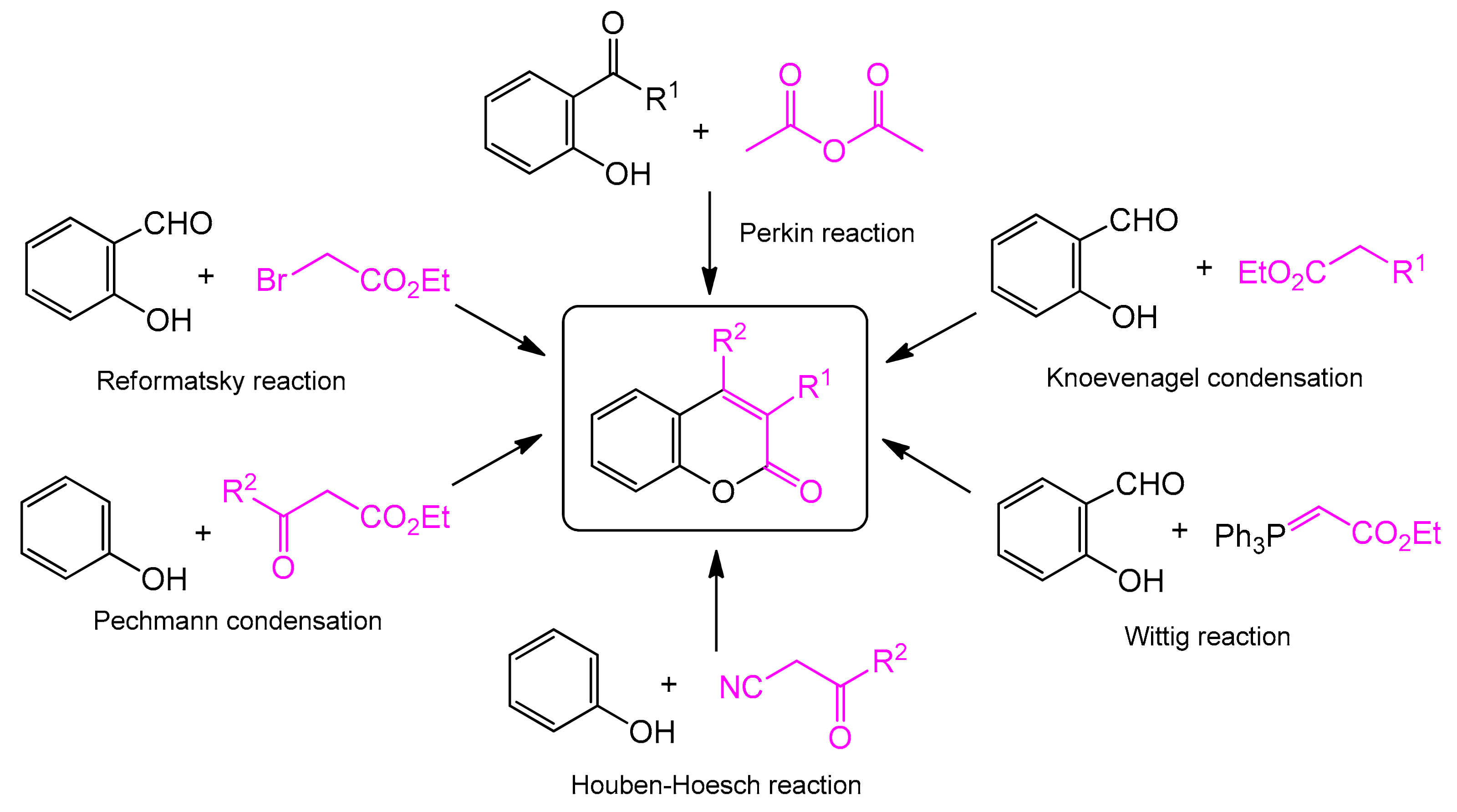
The classic and most frequently used one-step methods for the synthesis of coumarin derivatives include the Perkin reaction, von Pechmann condensation, Knoevenagel condensation, Baylis–Hillman reaction, Michael addition, Kostanecki reaction, and Heck lactonization reaction [31] (Scheme 1). The coumarin synthesis was first described by Perkin in 1868 [32,33]. The reaction consisted of heating sodium salt of salicylaldehyde with acetic anhydride. Further research into this process led to the preparation of cinnamic acid and its analogs using general synthesis that became known as the Perkin reaction. One of the most studied approaches to the synthesis of coumarins is the Pechmann reaction. It is based on the condensation of various phenols with β-ketoesters. It requires the presence of homogeneous catalysts, such as trifluoroacetic acid (TFA) [34] or Lewis acids (LAs) (e.g., AlCl3 [35], ZnCl2 [36], ZrCl4 [37], and TiCl4 [38]) or heterogeneous catalysts such as cation exchange resins [39], silica composites [40], and zeolites [41]. In the case of the Knoevenagel reaction, the transformation usually occurs via condensation of various o-hydroxyaldehydes with active methylene compounds in the presence of a base catalyst [42]. In turn, Houben–Hoesch condensation is based on the reaction of β-ketonitriles and phenol derivatives, and in the Wittig reaction, coumarin derivatives are formed as a result of the reaction of aromatic aldehydes or ketones with a phosphonate or phosphorous ylide. More details on the abovementioned and other metal-free synthetic strategies resulting in coumarins preparation can be found in a number of reviews [43,44,45,46,47,48,49,50,51].
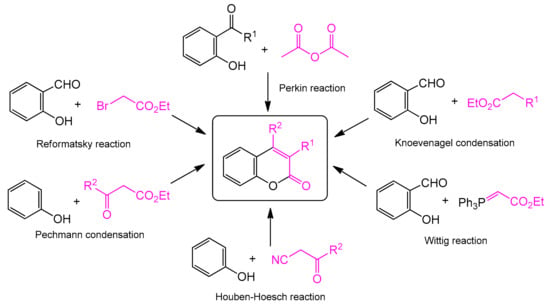
Scheme 1.
Traditional methods of coumarin synthesis.
The chemistry of transition metal complexes is widely applied in catalysis. The transition metals, united in the d-block, have an excellent ability to form coordination complexes consisting of a central metal atom and ligands. These complexes can induce numerous organic reactions, often under mild conditions and even in a stereoselective manner. For this reason, reactions in the presence of transition metals that are compatible with many functional groups have been successfully applied to the synthesis of coumarins.
This review paper discusses various new methodologies using metals as catalysts in the production of coumarins through C–H activation. This includes reactions that allow for the construction of a de novo coumarin backbone and those that introduce new substituents to the already formed heterocyclic backbone. Particular attention is focused on C–H functionalization reactions supported by the transition metal complexes of palladium, ruthenium, cobalt, platinum, gold, iron, etc.
3. C–H Bond Activation in Coumarin Synthesis
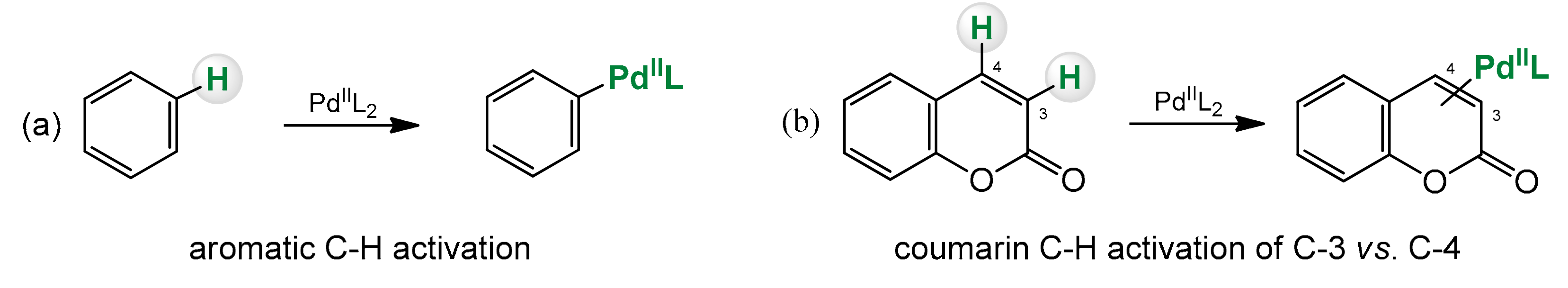
Organometallic reactions based on the activation of the C–H bond are a type of reactions where the strong C–H bond is broken and replaced with a C-X bond, where X can be anything else then H (Scheme 2) [52,53].
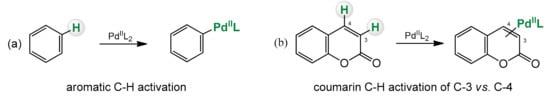
Scheme 2.
C–H bond activation of benzene (a) and coumarin (b).
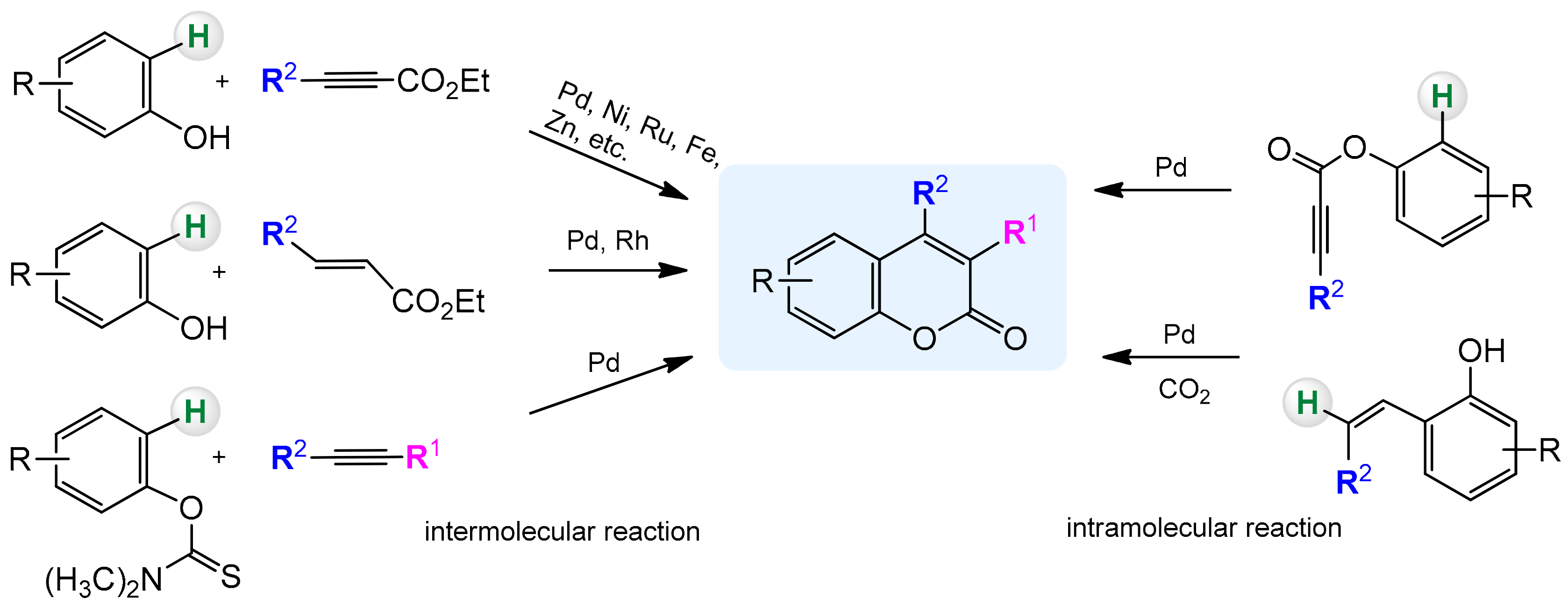
In recent years, C–H activation has become a versatile and effective tool in the preparation of heterocyclic compounds including coumarins and their derivatives. The traditional methods rely mainly on the condensation reactions of phenols with the carbonyl compounds and are catalyzed by a strong acid or base. Regioselectivity of these processes is not ideal and often leads to mixtures of compounds. Formation of C–C and C–O bonds in the presence of transition metal complexes via C–H activation is a good alternative to classical methods and partially solves this problem. In addition, it provides an efficient and cost-effective method for the direct synthesis of substituted coumarins from readily available arenes (Scheme 3).
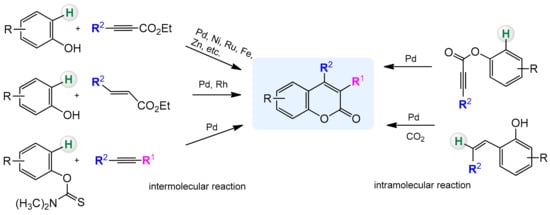
Scheme 3.
Direct synthesis of coumarins by C–H bond activation (selected examples).
It has been reported that the therapeutic applications of coumarins depend on the nature of the substituent present in the benzene or pyrone ring. Almost all natural coumarins possess an oxidized substituent at the C-7 position. The coumarins substituted at C-3 and C-5 exhibit more pronounced anticancer and antioxidant activities. In turn, those substituted at C-4, C-6, and C-7 show acetylcholinesterase inhibitory activities as well anti-inflammatory properties [14,54]. For this reason, it seems to be of significant importance to develop methods for efficient and regioselective syntheses resulting in specified products.
The coumarin backbone has six C–H sites and a C=C unit that are susceptible to functionalization. The aryl subunit of the coumarin ring system is not as reactive as in the case of the normal benzene derivative, and modifications in this region are quite difficult chemically, although possible by such reaction as C–H activation. The conjugated C=C bond between the C-3 and C-4 carbon is fixed in the cis conformation and contributes to strong fluorescence emission and good photostability of coumarins. This bond is reactive and chemically available; hence, it is possible to obtain coumarins modified at C-3 and C-4 and even larger fused coumarin heterocyclic derivatives. The Kumada, Stille, Heck, Sonogashira, Negishi, Suzuki, or Buchwald–Hartwig coupling reactions are most often used for this purpose [55,56,57,58,59,60,61,62,63]. The substrates in the abovementioned reactions are mostly coumarin derivatives such as vinyl coumarin, bromocoumarin, coumarin-3-carboxylic acid, or triflate-substituted coumarin. This is where the direct functionalization of the C–H bond emerges as an atom-economical and environmentally friendly synthetic tool that eliminates the need to prefunctionalize the coupling partners.
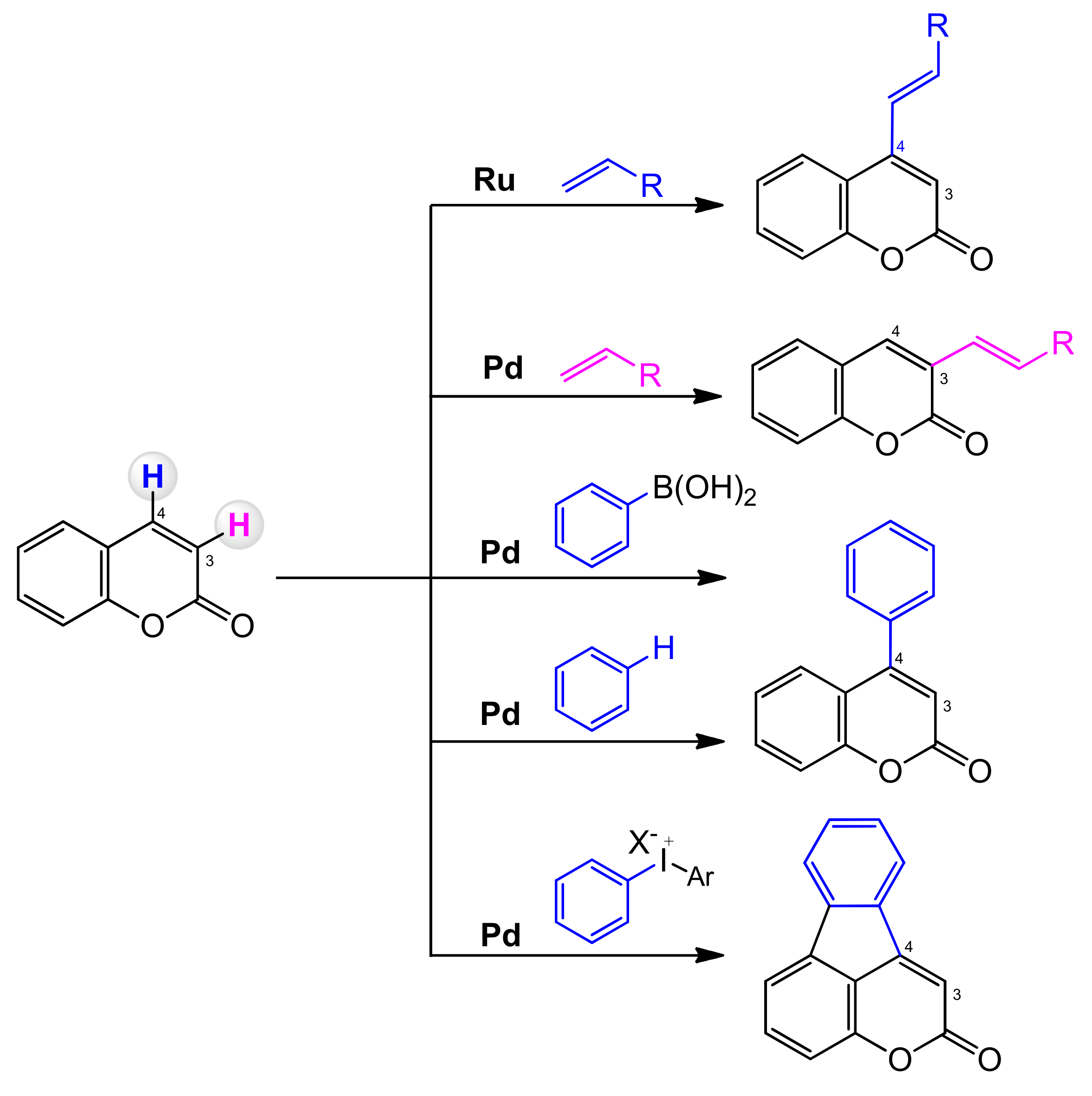
In coumarin, the two C–H bonds at C-3 and C-4 are chemically different. Direct activation of the C–H bond can sometimes be difficult and demanding. The C-3 position can undergo electrophilic palladation to form a C-3 palladium intermediate that allows regionally controlled arylation or alkenylation. It is also possible to predict the regioselectivity of the arylation and alkenylation reactions of the palladium catalyzed coumarins (Scheme 4). The carbopalladation favors the delivery of the aryl group to the C-4 position, and the alkenylation process is usually C-3 selective [64].
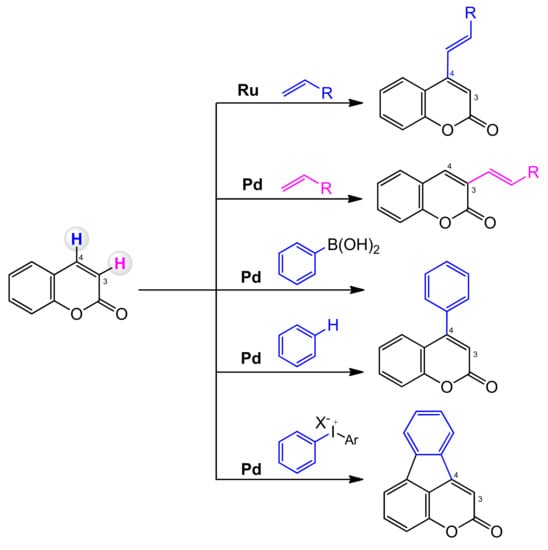
Scheme 4.
Regioselectivity of coumarin C–H functionalization.
The selective functionalization of the C-4 position is more difficult, and its main limitation is the large dependence on palladium chemistry and the use of arenes as coupling partners. A solution to the problems of the selective functionalization of the C=C bond of coumarins is often the introduction of directing groups covalently tethered to the substrate at C-3 or C-4, which can coordinate to the transition metal center and direct the C–H activation process to the target site.
3.1. Direct Synthesis of Coumarins through Intermolecular Hydroarylation of Alkynes
Phenol and naphthol derivatives are proper substrates for the production of 4-arylcoumarins. The reaction of their C–H functionalization takes place in the ortho position, and the metals used as catalysts in this process are, first of all, palladium, iron, and platinum.
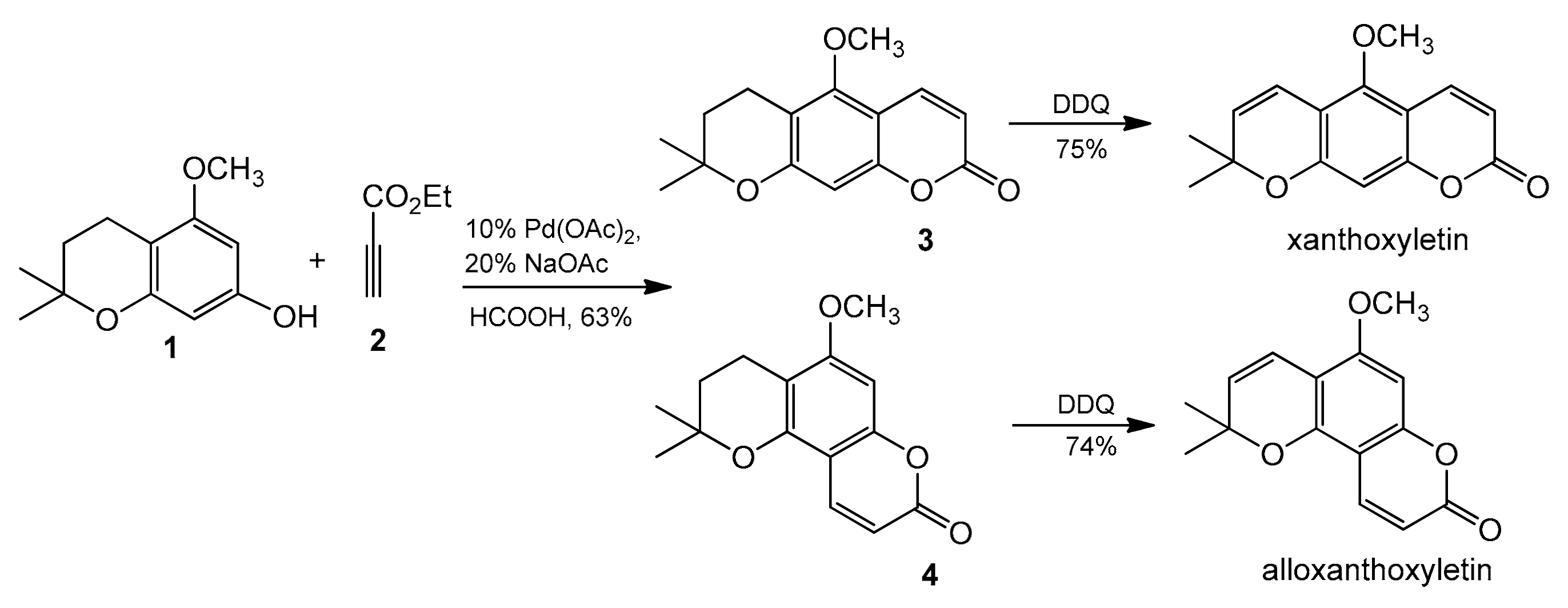
The palladium-catalyzed addition of phenols to alkynoates was initially demonstrated by Trost and co-workers [65,66]. The reactions proceeded with good yields at 35 °C with 10 mol% Pd(OAc)2 as a metal source and the addition of sodium acetate as a base and formic acid as a solvent was of key importance. Formic acid was proposed to influence the cyclization reaction by reducing PdII to Pd0. It was soon found that better results were achieved when using Pd2(dba)3 instead of Pd(OAc)2. The reaction proceeded effectively at room temperature with unsubstituted as well as alkyl- and aryl-substituted alkynoates (Table 1) [66,67,68,69,70,71]. The applicability of this methodology was demonstrated by the efficient synthesis of natural coumarins such as ayapin (Table 1, Entry 29) and fraxinol methyl ether (Table 1, Entry 23). The Trost group also reported that the reaction of chroman 1 with ethyl propynoate (2) catalyzed by palladium acetate produced a mixture of regioisomers 3 and 4 at the ratio 1.0:3.3 (Scheme 5). The DDQ oxidation of the chromans 3 and 4 to the chromenes completes the synthesis of xanthoxyletin and alloxanthoxyletin—natural coumarins that display anticancer and anti-HIV activities.

Table 1.
Synthesis of coumarins via metal-catalyzed hydroarylation.
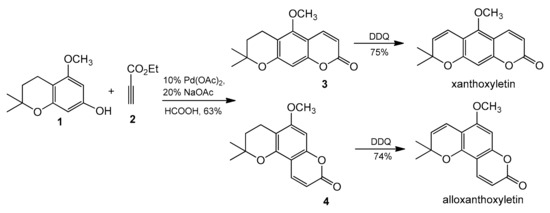
Scheme 5.
Synthesis of xanthoxyletin and alloxanthoxyletin.
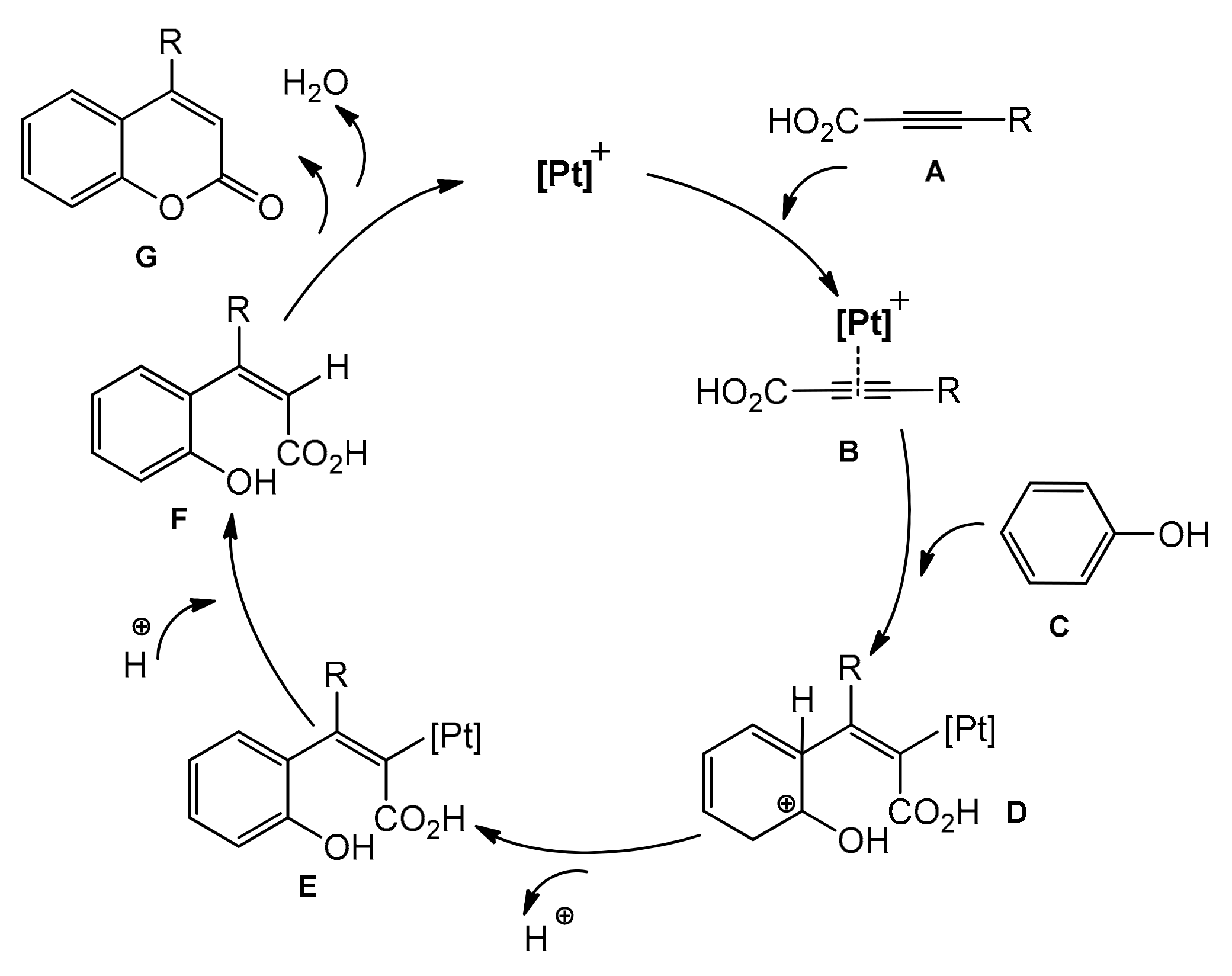
The Kitamura group presented the direct hydroarylation of alkynes using a Pd(OAc)2 catalyst in TFA/DCM [67,68,69]. This involved a reaction of substituted phenols with phenylpropiolic acid. The synthesis was totally regioselective, required no other additives, and water was the only side product. Although the reaction yields were not impressive, this was the first example of the palladium-catalyzed protocol for direct synthesis of the coumarin skeleton. A few years later, the same authors reported a catalytic system based on platinum, such as PtCl2/AgOTf, K2PtCl4/AgOTf, and K2PtCl4/AgOAc, for the synthesis of 4-arylcoumarins from phenols, phenylpropiolic, 2-octynoic as well propiolic acids [70]. The synthesis of substituted coumarins proceeded with moderate to good yields (Table 1). The authors presented a possible reaction mechanism where the triple bond is activated in the first step, followed by intramolecular hydroarylation and formation of hydroxy substituted cinnamic acids. In the last step, intramolecular esterification took place to form the final product (Scheme 6).
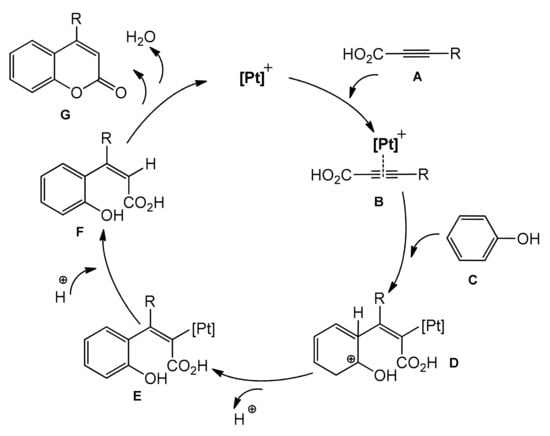
Scheme 6.
Proposed mechanism of Pt-catalyzed hydroarylation.
Iron-catalyzed hydroarylation offers an interesting and improved method for the synthesis of 4-substituted coumarins [71]. The reaction proceeded efficiently in the TFA/1,2-DCE solvent system. The yields reported with the iron catalyst, in some cases, were much higher than those obtained with palladium or platinum complexes and are summarized in Table 1.
In the hydroarylation reaction the catalytic system based on palladium, iron, and platinum exhibited variable activity. The reactions proceeded even with unactivated and deactivated phenol, albeit with low yield. Among the propiolic acid derivatives, the highest yields were obtained for both phenylpropionic acid and propiolic acids.
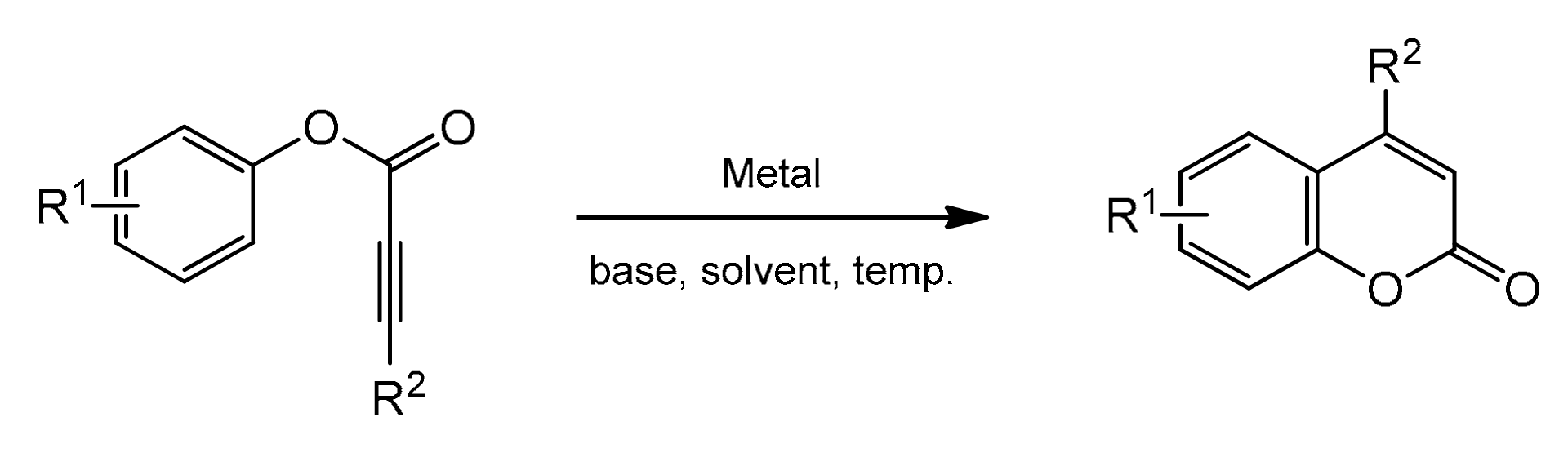
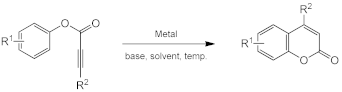
3.2. Direct Synthesis of Coumarins through the Intramolecular Hydroarylation of Alkynes
Metal-catalyzed intramolecular hydroarylation of arylpropiolates via the activation of the C–H bond is an alternative one-step method resulting in theto coumarin preparation (Scheme 7).
Scheme 7.
Intramolecular hydroarylation of alkynes.
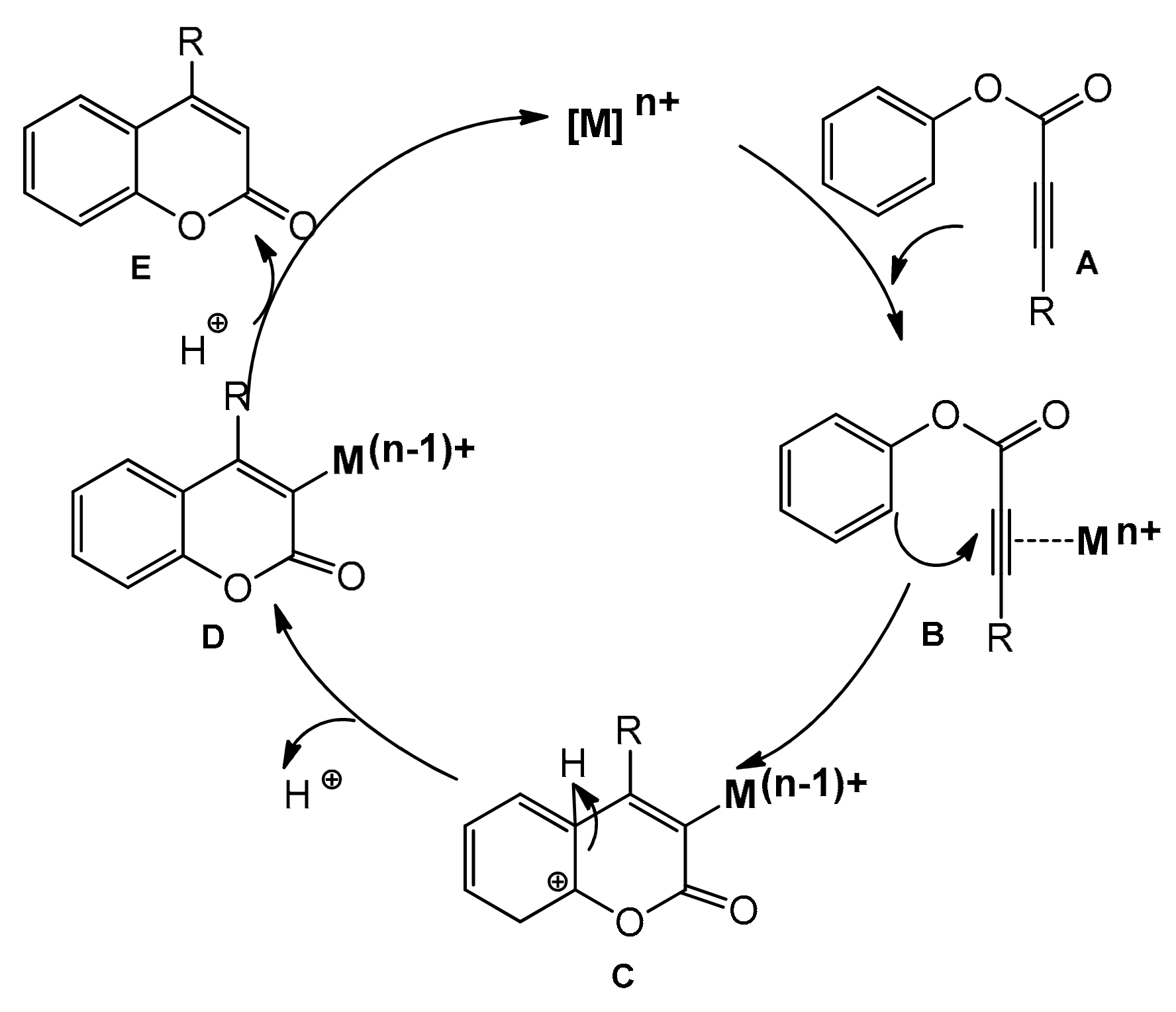
Recently, several improved versions of the reaction for the synthesis of 4-arylcoumarins with metal complexes as catalysts have been reported. In the course of the intramolecular hydroarylation process, the triple bond of A is activated by the alkynophilic metal catalyst (Pd, Pt, Au), and the formed intermediate B undergoes intramolecular cyclization leading to C. The proton elimination produces the complex D, which releases the coumarin derivative E after the proto-demetallation and regenerates the catalyst (Scheme 8).
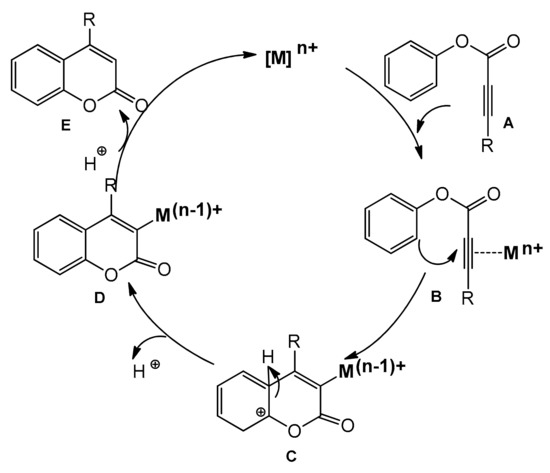
Scheme 8.
Intramolecular hydroarylation of C≡C.
In 2000, Fujiwara and co-workers reported the palladium-catalyzed general method for the preparation of coumarins by intramolecular hydroarylation [72,73]. Various aryl alkynoates and alkynanilides reacted at room temperature in the presence of catalytic amounts of Pd(OAc)2 in TFA/DCM to give the desired oxygen-containing heterocycles in high yields and regioselectivity and with more than 1000 TON. This method appeared to tolerate functional groups such as Br and CHO; however, it was limited to electron-rich aromatic compounds. The most successful reactions were those with the donor groups on the aryl moiety (Table 2). The reaction mechanism was experimentally proved by NMR monitoring and isotopic labeling experiments.

Table 2.
Coumarins obtained via intramolecular hydroarylation.
Ferric chloride as a catalyst was reported by Lu and co-workers for coupling of aryl-substituted alkynes with the electron-rich arene [74]. The reaction ran in nitromethane for 3 days at 80 °C resulting in a satisfactory yield of the corresponding coumarin (Table 2). In turn, the reactivity and scope of PtCl4 andPtCl2 in the intramolecular hydroarylation of alkynes was presented by the Sames group [75,76]. Alkynoate esters were converted to the corresponding coumarins with a yield up to 73% with 5 mol % of PtCl4 at 70 °C (Table 2).
Cyclization of aryl alkynoates was also tested in the action of gold catalysts. The operationally simple Au(I)-catalyzed transformation was developed by Banwell [77]. The commercially available gold complex Au(I) was JohnPhosAu(MeCN)SbF6 with a weakly coordinating acetonitrile ligand. The process involved low catalyst loadings, mild reaction temperatures, short reaction times, and allowed the formation of a number of important heterocyclic motifs including coumarin The gold catalyst, Au(PPh3)Cl, was also applied in the cyclization reaction [78]. The catalyst required activation with AgOTf and anhydrous conditions for optimal activity. Water was found to play a critical role in the selectivity of the reaction. Anhydrous conditions allowed to obtain the coumarin derivatives, while the addition of 1 equiv. of water resulted in the spirocyclic product. The same research group also developed the aminocoumarins synthesis method [79]. For the cyclization reactions, 5 mol% of Au(PPh3)Cl/AgSbF6 or 10 mol% of PtCl4 was used. For both catalytic systems, a mixture of 1,4-dioxane/DCE was used as a solvent mixture. The gold catalyst was effective at room temperature while the platinum catalyst required heating at 80 °C. The intramolecular hydroarylation reaction was developed in the presence of Au(III) ions by the Kim group [80]. The coumarin with diethylamino group at C-7 obtained with 70% yield exhibited strong fluorescence. An efficient hydroarylation reaction of electron-deficient alkynes under the “solventless” conditions was also described by Shi and He [81]. The method applied the AuCl3/AgOTf catalytic system and was used to construct coumarins from various aryl alkynoates in good to excellent yields. The reaction worked for aryl alkynoates with different substituted groups including the electron-withdrawing ones. The isotopic labeling experiments appeared to confirm the involvement of direct metalation of electron-rich aryl groups by the gold(III) species to produce an arylgold(III) complex.
3.3. Direct Synthesis of Coumarins via the Intermolecular Hydroarylation of Alkenes
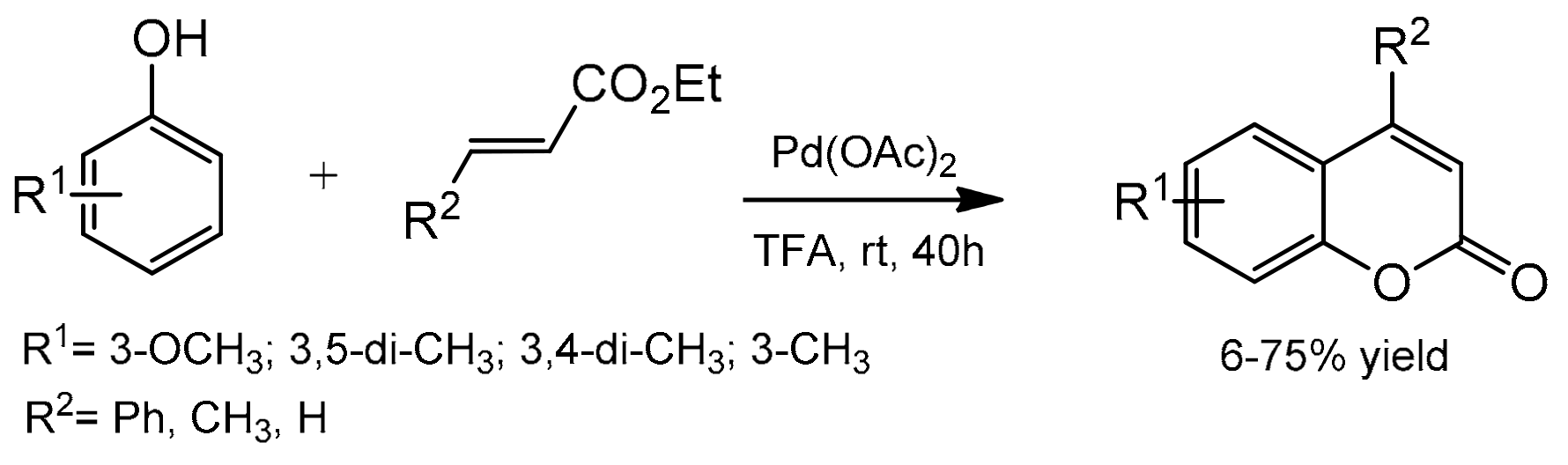
Direct C–H bond alkenylation provides another attractive strategy for coumarin synthesis, and some successful examples have been developed including Pd-catalyzed arylation of acrylates. The first palladium-catalyzed reaction was reported in 2005 by Kitamura and co-workers [82]. The coumarin formation was accomplished at room temperature in TFA as the solvent and K2S2O8 as the oxidant (Scheme 9). The reaction of several phenols with ethyl cinnamate, ethyl crotonate, and ethyl acrylate gave the corresponding products in moderate to good yields. The oxidative coupling of electron-rich phenols was a competitive reaction under the reaction conditions thereby reducing product yields.
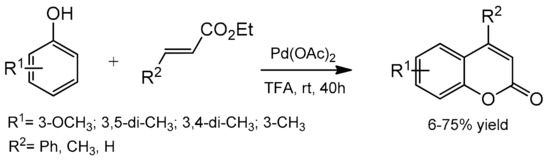
Scheme 9.
Synthesis of coumarins from the phenol derivative and acrylates.
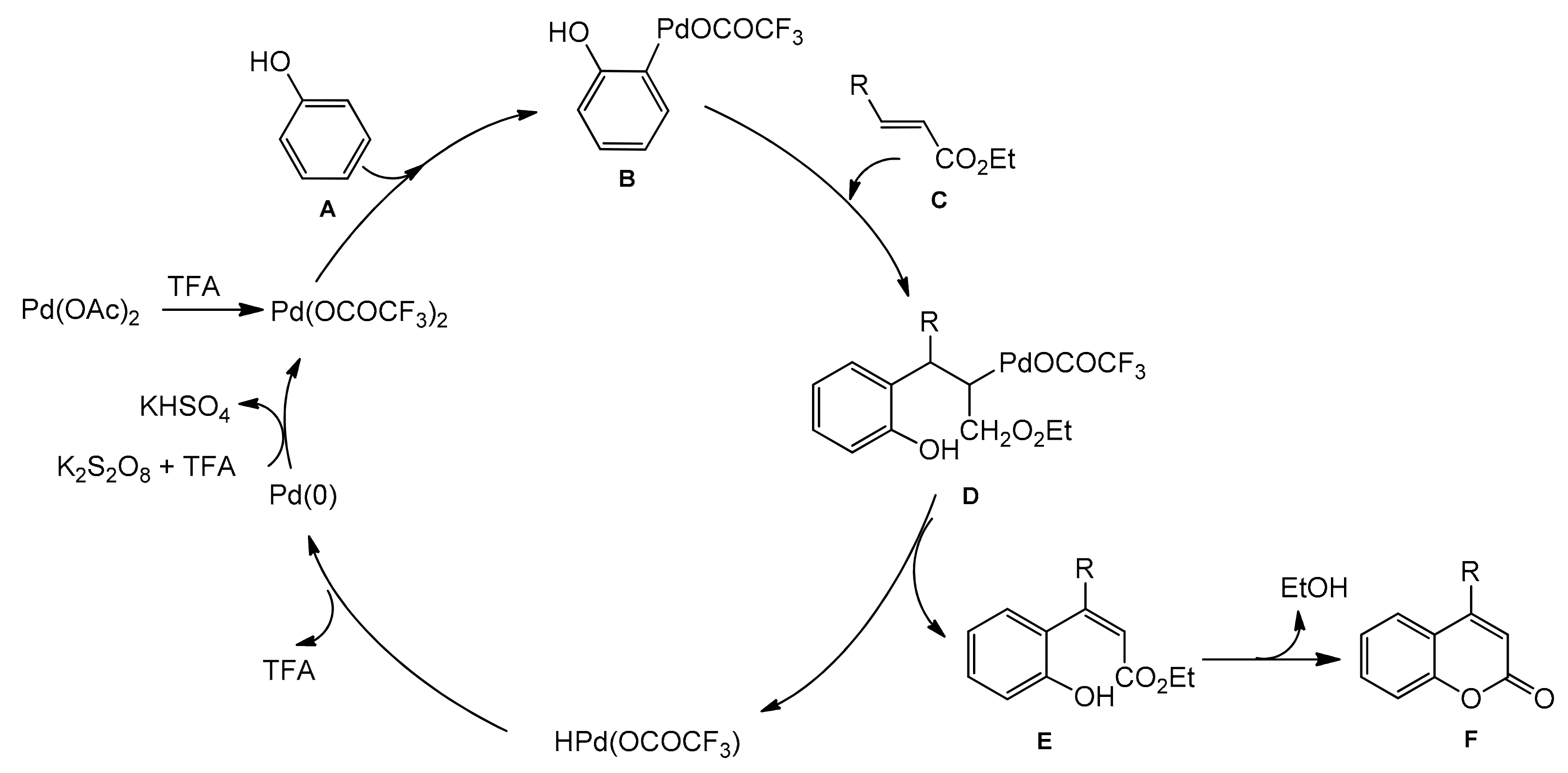
A possible reaction mechanism for the formation of coumarins is shown in Scheme 10. It assumes that an arylpalladium species undergoes addition to the double bond of acrylate followed by the palladium hydride elimination instead of protonation by TFA. Therefore, an oxidant is needed to regenerate the palladium(II) moiety.
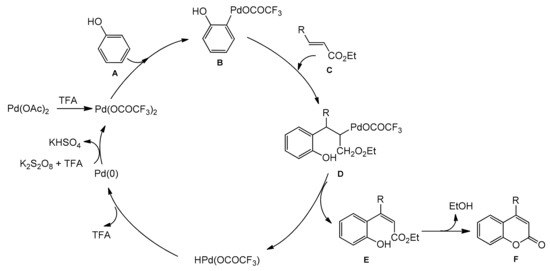
Scheme 10.
Mechanism of Pd-catalyzed arylation of acrylates.
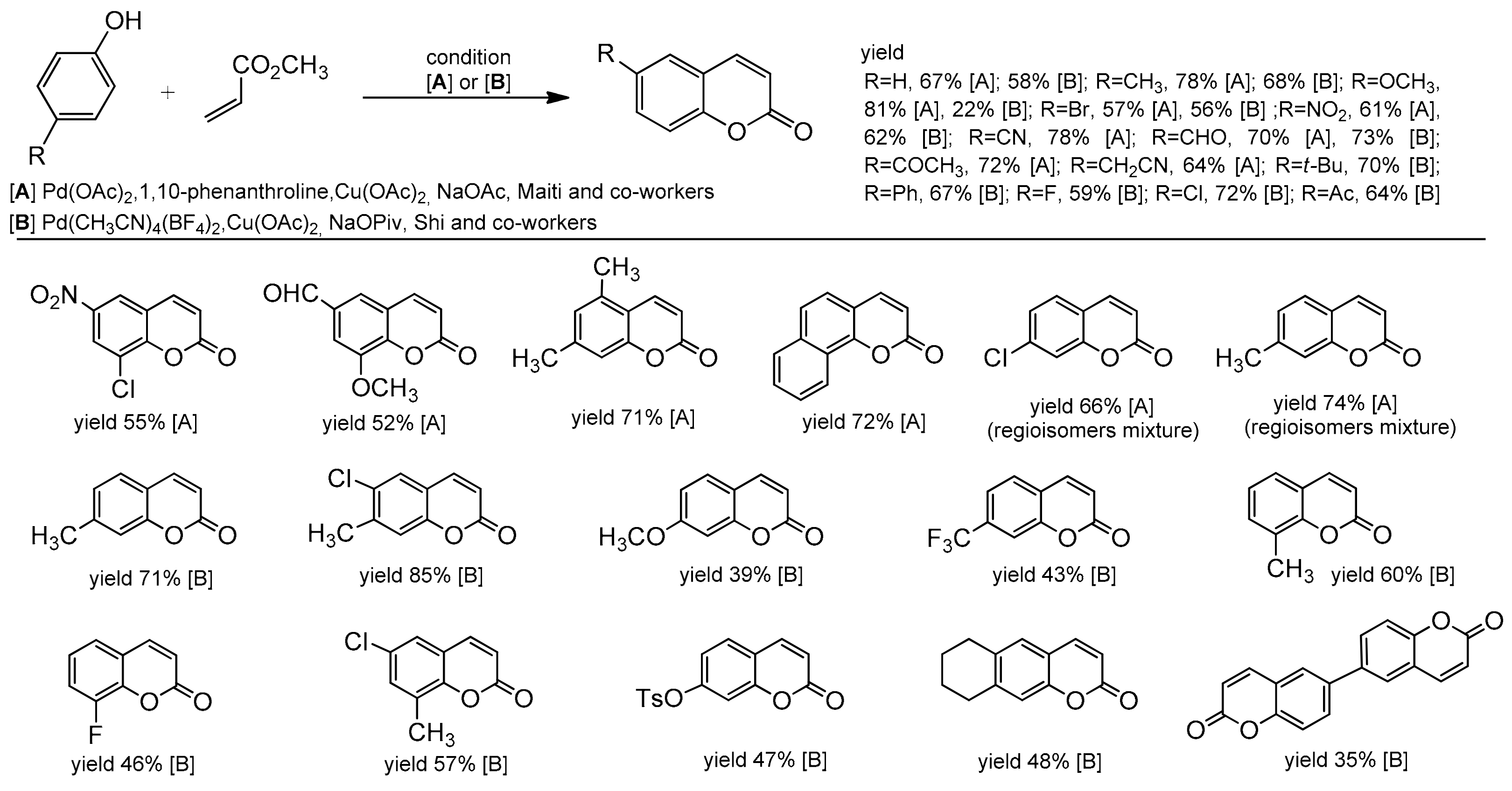
Another example of Pd-catalyzed alkenylation of phenols can be found in the paper by Maiti and co-workers [83]. They established a versatile protocol for the synthesis of coumarins and 2-substituted benzofurans by reacting simple phenols with methyl acrylate. The reaction worked well with Pd(OAc)2/1,10-phenanthroline as a catalyst and in the presence of Cu(OAc)2 and sodium acetate at 110 °C. Phenols with the electron-donating as well with electron-withdrawing groups were successfully applied for the synthesis of target coumarins (Scheme 11).
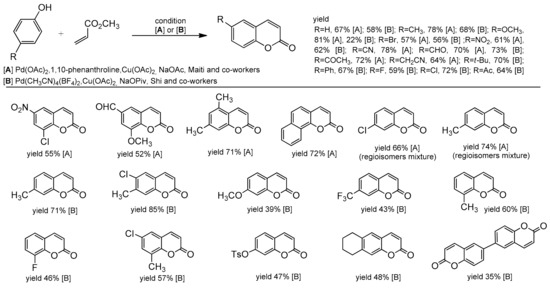
Scheme 11.
Palladium-catalyzed coumarin synthesis from the phenol derivative and methyl acrylate.
Later, Shi developed a C–H alkenylation reaction using Pd(CH3CN)4(BF4)2 [84] which was a great complement to the previous methods of coumarin synthesis described by Maiti (Scheme 11). The substrate scope for phenol was very wide and both electron-rich and electron-deficient phenols were evaluated with electron-deficient ones affording better yields. Apart from the alkyl and aryl groups attached to phenol, functional groups, such as NO2, CHO, Ac, OTs, or halide, were also well tolerated and provided the possibility for further modification of the coumarin skeleton. Moreover, the authors investigated the substrate scope of the alkenes. They reported that more sterically hindered acrylates gave the corresponding coumarin with low yield, similar to 1,2-disubstituted alkenes (Scheme 12).
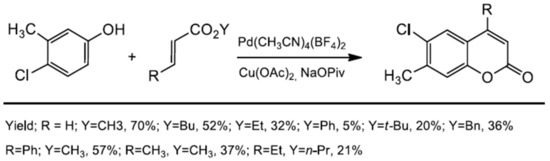
Scheme 12.
Palladium-catalyzed coumarin synthesis from the phenol derivative and 1,2-di-substituted alkenes.
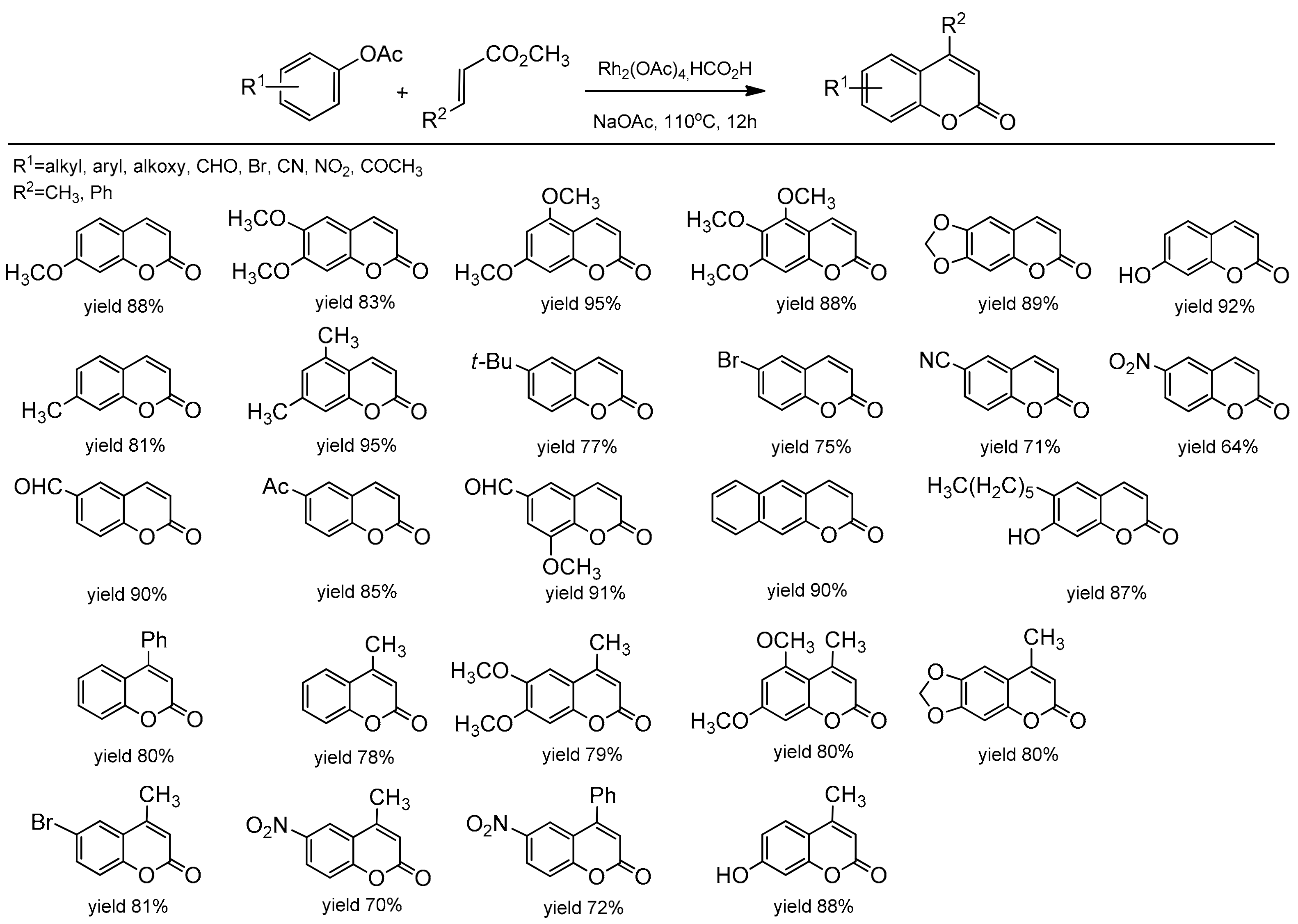
The rhodium-based catalytic system for the synthesis of C-4-substituted coumarin was reported by Gadakh et al. [85]. Rh2(OAc)4 in the presence of NaOAc as a base and HCO2H as a reducing agent catalyzed the C–H bond activation of phenolic acetate and acrylates (Scheme 13). The reaction was general for aryl acetates with strong electron-withdrawing groups. The use of 1,2-disubstituted alkenes as a coupling partner also provided C-4 substituted coumarins with high yields. Ortho-metalation of aryl acetate proceeded through rhodacycle as an intermediate, and it was well supported by the authors due to the deuterium labeling studies.
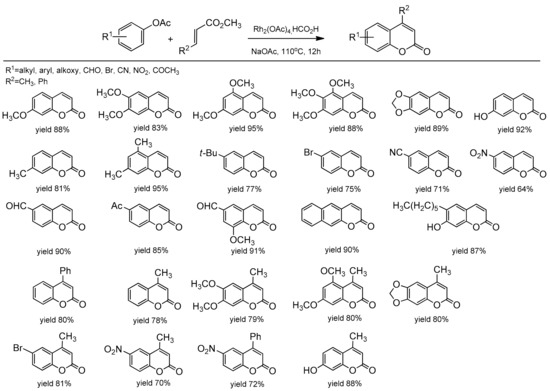
Scheme 13.
Rh-catalyzed coumarin synthesis.
3.4. Direct Synthesis of Coumarins via Intramolecular Hydroarylation of Alkenes
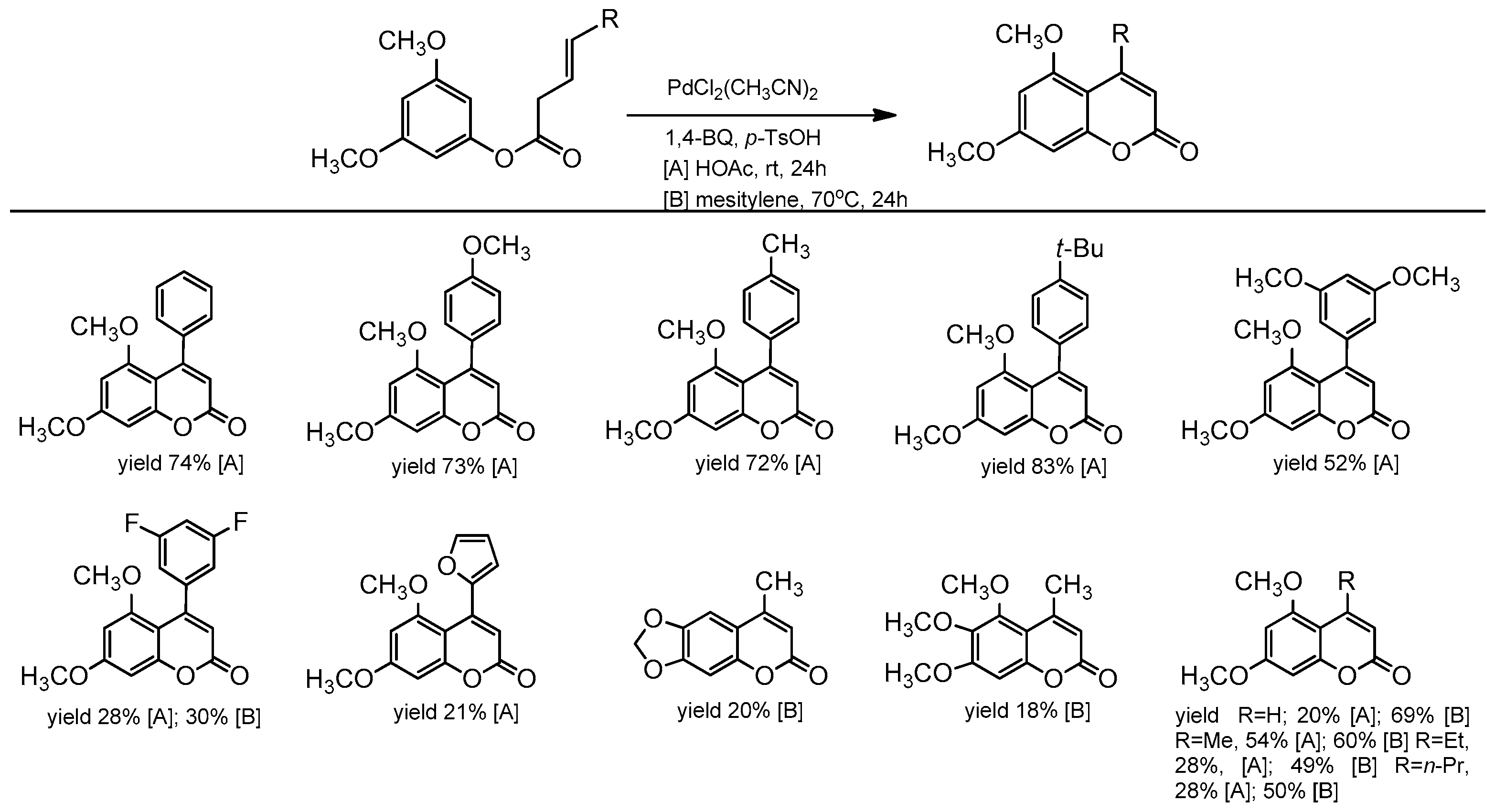
Recently, the Lete’ group presented the intramolecular Pd(II)-catalyzed alkenylation of aryl homoallyl ethers allowing access to substituted flavenes and isoflavenes [86]. The reaction was conducted in the presence of PdCl2(CH3CN)2 and 1,4-benzoquinone (1,4-BQ). The addition of p-TsOH enhanced the system’s reactivity, and the synthesis could be performed in dioxane at room temperature for 24 h. The catalytic process was versatile, and the reaction condition also allowed for the synthesis of 5,7-dimethoxy-4-methylcoumarin (Scheme 14).
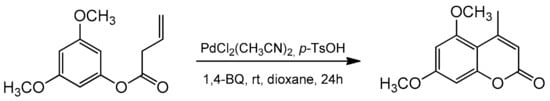
Scheme 14.
Synthesis of 5,7-dimethoxy-4-methylcoumarin.
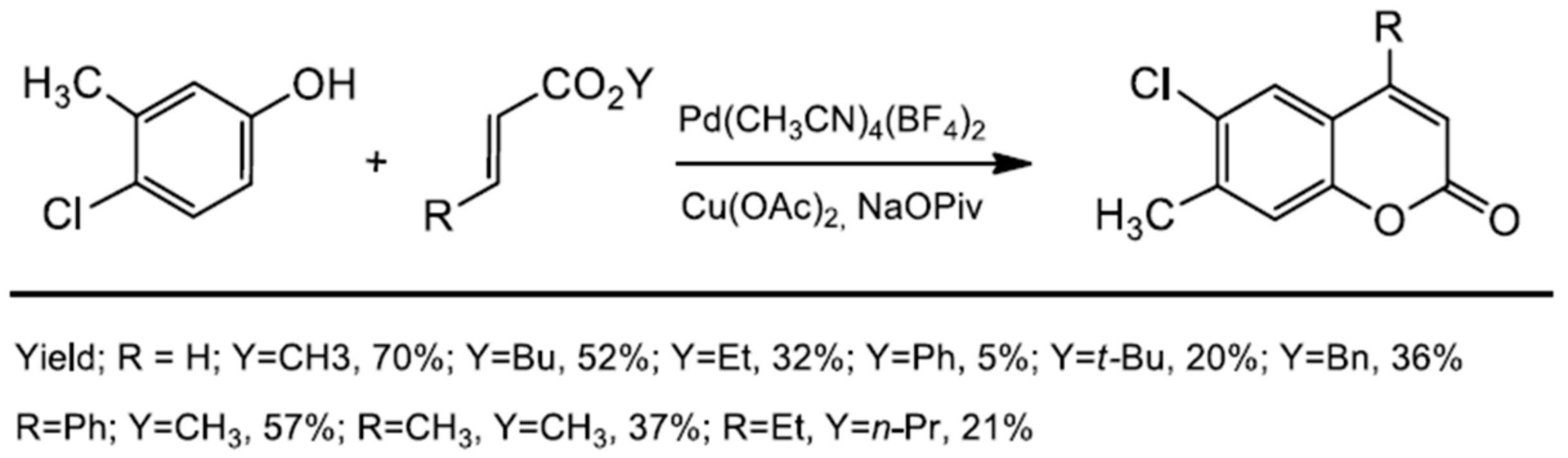
Following this trend, two years later, the same group further investigated the intramolecular C–H alkenylation variant towards the synthesis of coumarin derivatives [87]. To obtain 4-aryl as well 4-alkylcoumarins, PdCl2(CH3CN)2 was used with N-fluoro-2,4,6- trimethylpyridinium triflate (F+) and Cu(OAc)2 as oxidants. The catalytic process proceeded in acetic acid at room temperature or 70 °C, and the presence of p-TsOH was essential. The product yield depended on the substrate structure (Scheme 15). High yields were observed for coumarins in which the aryl group in the C-4 position of coumarin was para substituted (yield 72–83%). Unsubstituted or 4-alkylcoumarins were obtained with low yields, and an increase in the temperature did not improve the reaction conversion. Changing the solvent to mesitylene slightly improved the reaction yield (Scheme 15).
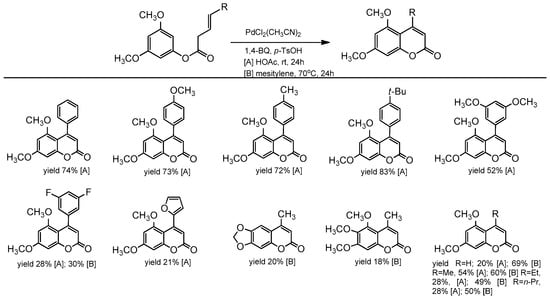
Scheme 15.
Pd-catalyzed synthesis of C-4 substituted coumarins.
3.5. Intramolecular Cyclocarbonylation and Cyclocarboxylation
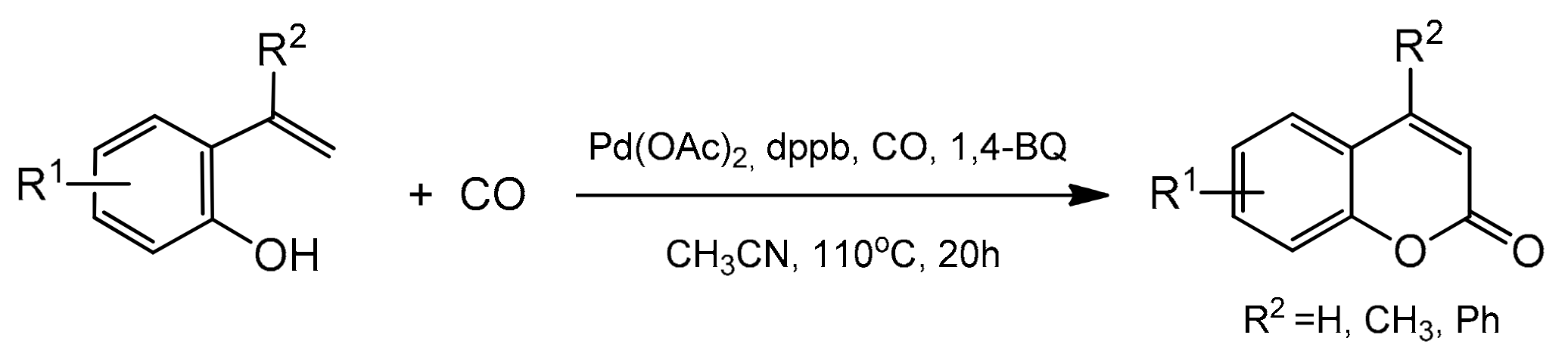
The cyclocarbonylation and cyclocarboxylation reactions have been demonstrated as a powerful route for the direct synthesis of the heterocyclic compounds [88] including the coumarin skeleton. In 2012, a novel method for the synthesis of 4-methylcoumarin and 4-phenylcoumarin was developed by the Alper group [89]. The reaction was based on the palladium-catalyzed oxidative cyclocarbonylation (Scheme 16).
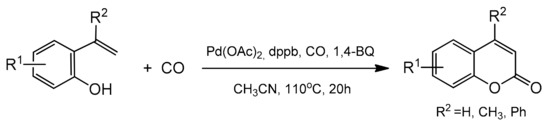
Scheme 16.
Pd-catalyzed oxidative cyclocarbonylation of 2-vinylphenol.
Terminal alkenes were coupled with 2-vinylphenol at CO (100 psi) and 1,4-benzoquinone (1,4-BQ) or air as the oxidant (Table 3). The process took place in acetonitrile at 110 °C in the presence of a ligand, and DPPB (1,4-butylenebis(diphenylphosphine)) acted effectively. This was the first report of an intramolecular oxidative alkoxycarbonylation involving sp2 C–H bond activation where coumarins were selectively formed from the adjacent vinyl and phenol groups on an aromatic ring. The possible mechanism of oxidative cyclocarbonylation was also presented by the authors.

Table 3.
Results of the carbonylation of 2-vinylphenol derivatives.
Mascareñas and Gulías reported the rhodium-catalyzed cycloaddition reaction involving the C–H bond activation process that provided a practical and efficient route to the coumarins as well benzoxepines [90]. The reaction resulted in heteroaryl formation in CH3CN at 85 °C with the yields up to 85%. In addition to carbon monoxide (balloon pressure), the catalytic amounts of [Cp*RhCl2]2 (Cp* = pentamethylcyclopentadienyl) and Cu(OAc)2 as the oxidant were used (Table 3).
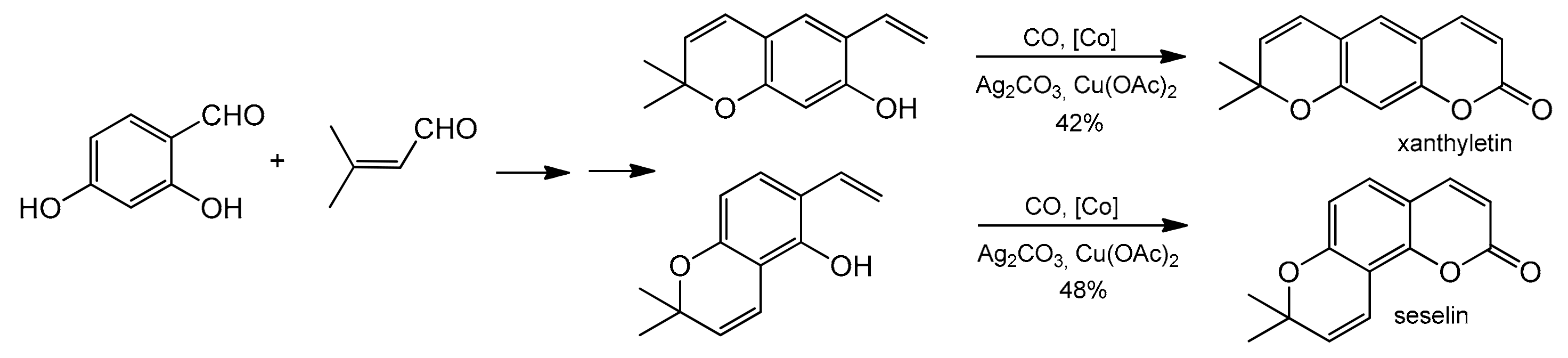
Cp*Co(CO)I2-catalyzed annulations of 2-alkenylphenols with CO for the synthesis of coumarin derivatives were developed by Wang and co-workers [91]. The reaction featured mild reaction conditions, broad substrate scope, and good functional group tolerance (Table 3). In addition to the cobalt catalyst, the authors used a system of two oxidants in the reaction, Ag2CO3/Cu(OAc)2xH2O. This allowed the reaction to perform with good yields after 20 h under mild temperature conditions (30 °C) in o-xylene. The practical utility of this new strategy was demonstrated in the total synthesis of natural coumarin xanthyletin and seselin (Scheme 17).
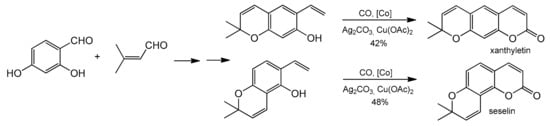
Scheme 17.
Total synthesis of the natural coumarin xanthyletin and seselin.
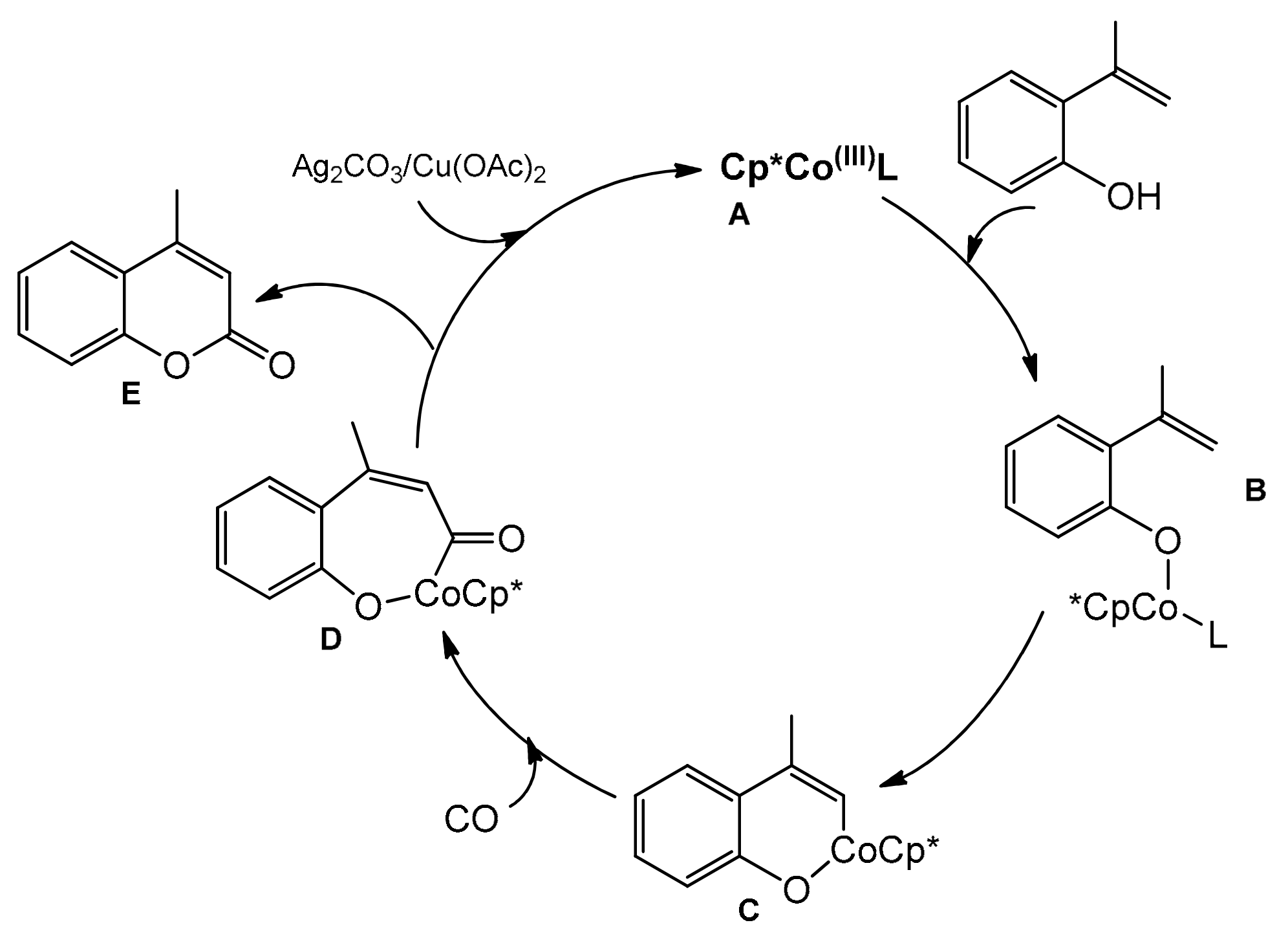
The reaction mechanism proposed by the authors is outlined in Scheme 18. The active catalyst A was generated by the reaction of Cp*Co(CO)I2 with Ag2CO3 or Cu(OAc)2. Replacement of the ligand (L) with 2-vinylphenol gives the intermediate B from which the cyclometalated intermediate C could be obtained. Then, after the CO coordination, a migratory insertion took place to give D. The final product E was formed by reductive elimination and the copper or silver salt regenerated the active catalyst.
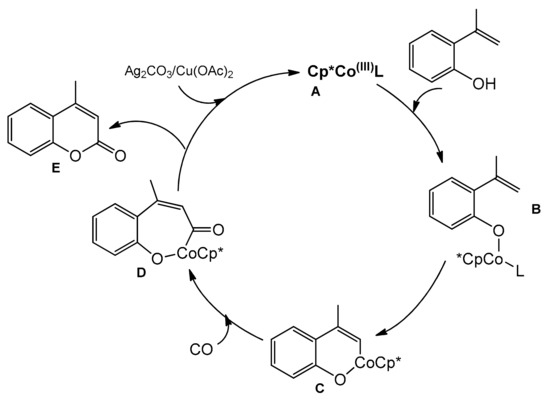
Scheme 18.
Mechanism of the Co-catalyzed carbonylation reaction.
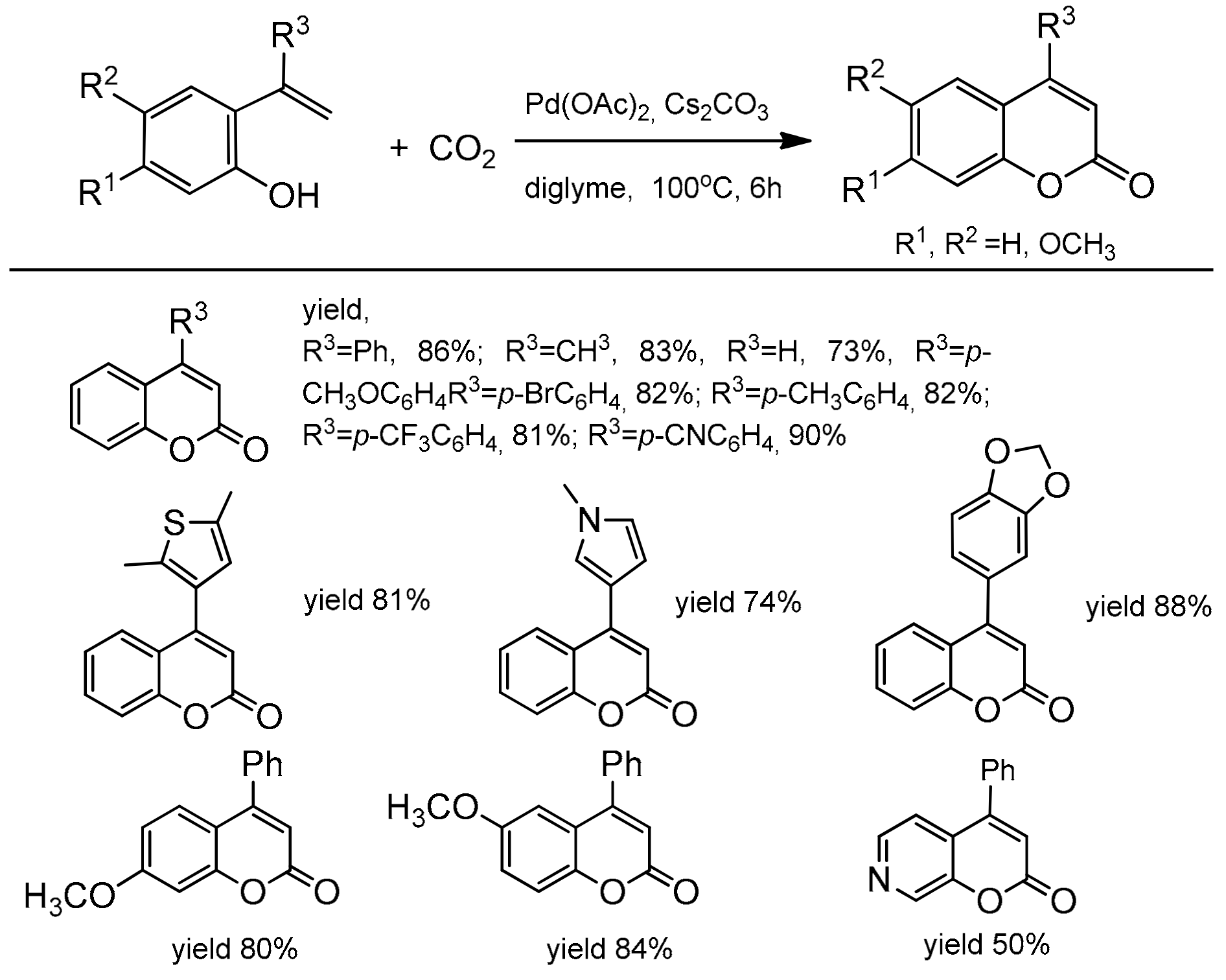
The catalytic, direct carboxylation of C–H bonds under the atmospheric carbon dioxide pressure presented by Iwasawa and co-workers [92] is also an attractive method for the coumarins synthesis. For the first time, the Pd(II)-catalyzed alkenyl C–H bond functionalization with nucleophilic carboxylation was developed. The reaction employed various functionalized 2-hydroxystyrenes with Pd(OAc)2, Cs2CO3 in diglyme at 100 °C under 1 atm CO2 (Scheme 19).
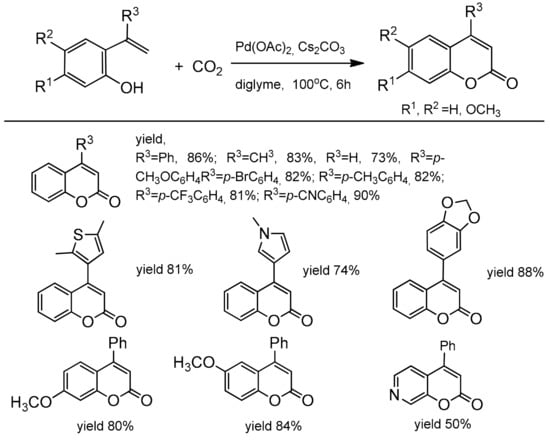
Scheme 19.
Pd-catalyzed reaction of 2-hydroxystyrenes with carbon dioxide.
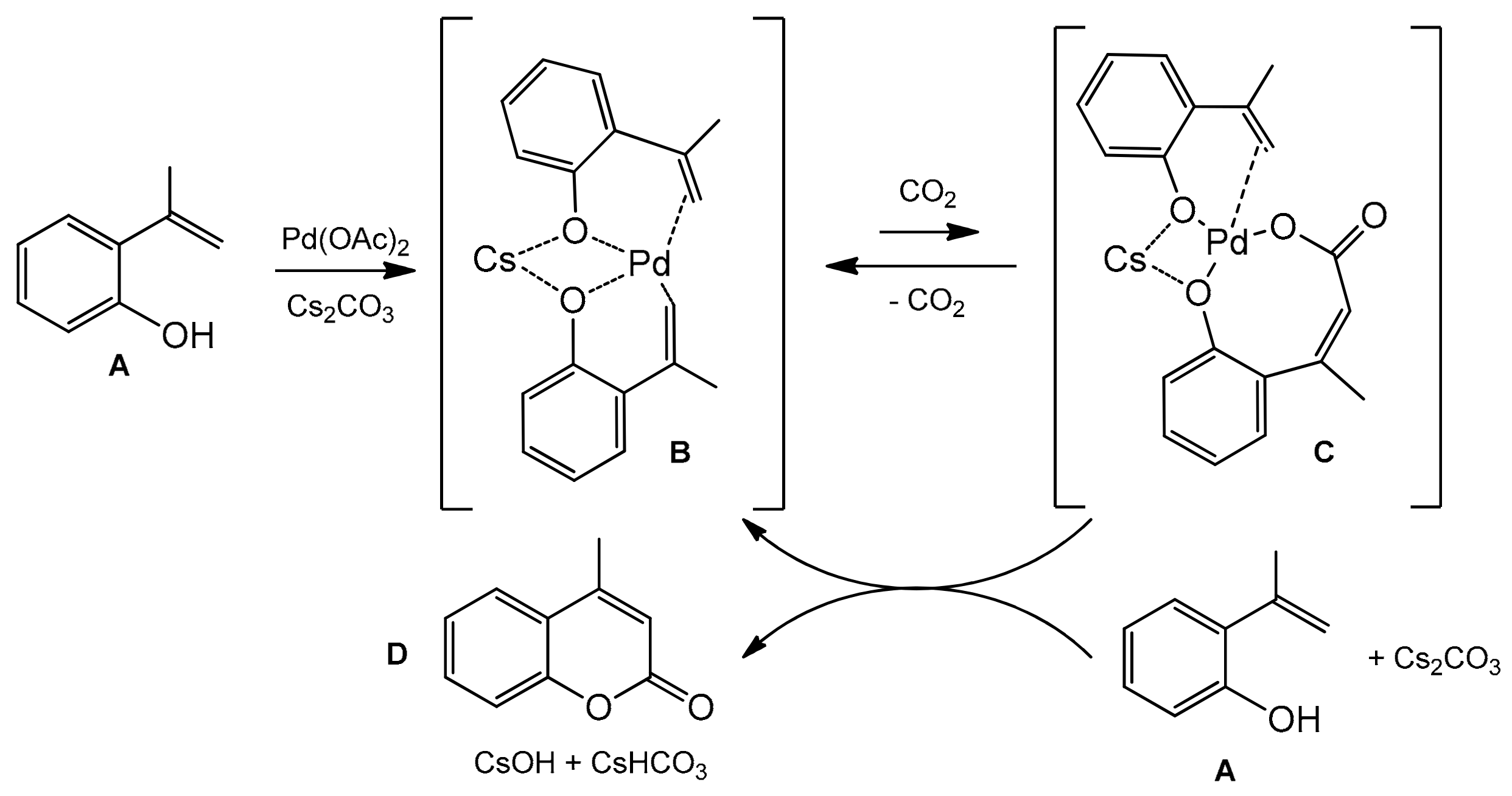
Substrates with an electron-donating or an electron-withdrawing group in the phenyl ring at α-position of 2-hydroxystyrene gave the corresponding C-4-substituted coumarins in good yields (78–90%). The substituents, such as 4-cyanophenyl, 3,4-methylenedioxyphenyl, pyrrole, and thiophene as well as bromophenyl moiety, were not affected under the reaction condition and also provided the desired carboxylation products. Only β-substituted 2-hydroxystyrenes did not give the desired reaction products. Based on the observation of the reaction intermediates, the authors proposed the mechanism of carboxylation process (Scheme 20). In the first step, the alkenylpalladium complex B was formed which then underwent reversible nucleophilic carboxylation to give the intermediate C. Reaction of C with the second molecule of 2-hydroxystyrene A and a base produces the coumarin D and regenerates the alkenylpalladium complex.
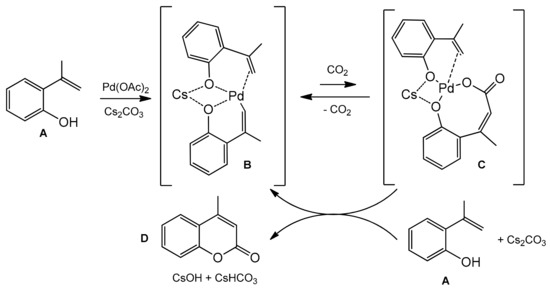
Scheme 20.
Mechanism of Pd-catalyzed reaction of 2-hydroxystyrenes with carbon dioxide.
3.6. Other Methods for Direct Synthesis of Coumarins by C–H Bond Activation
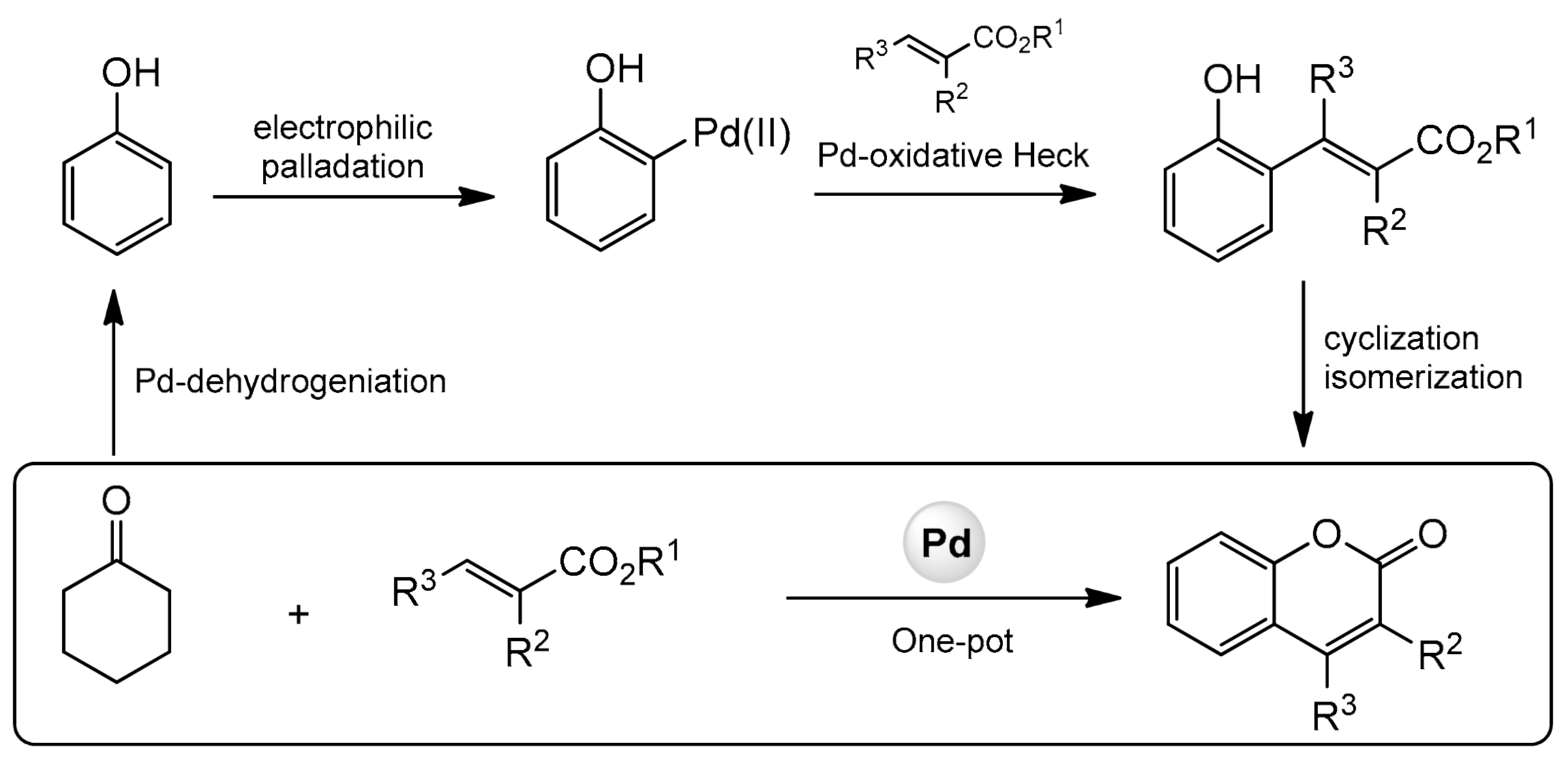
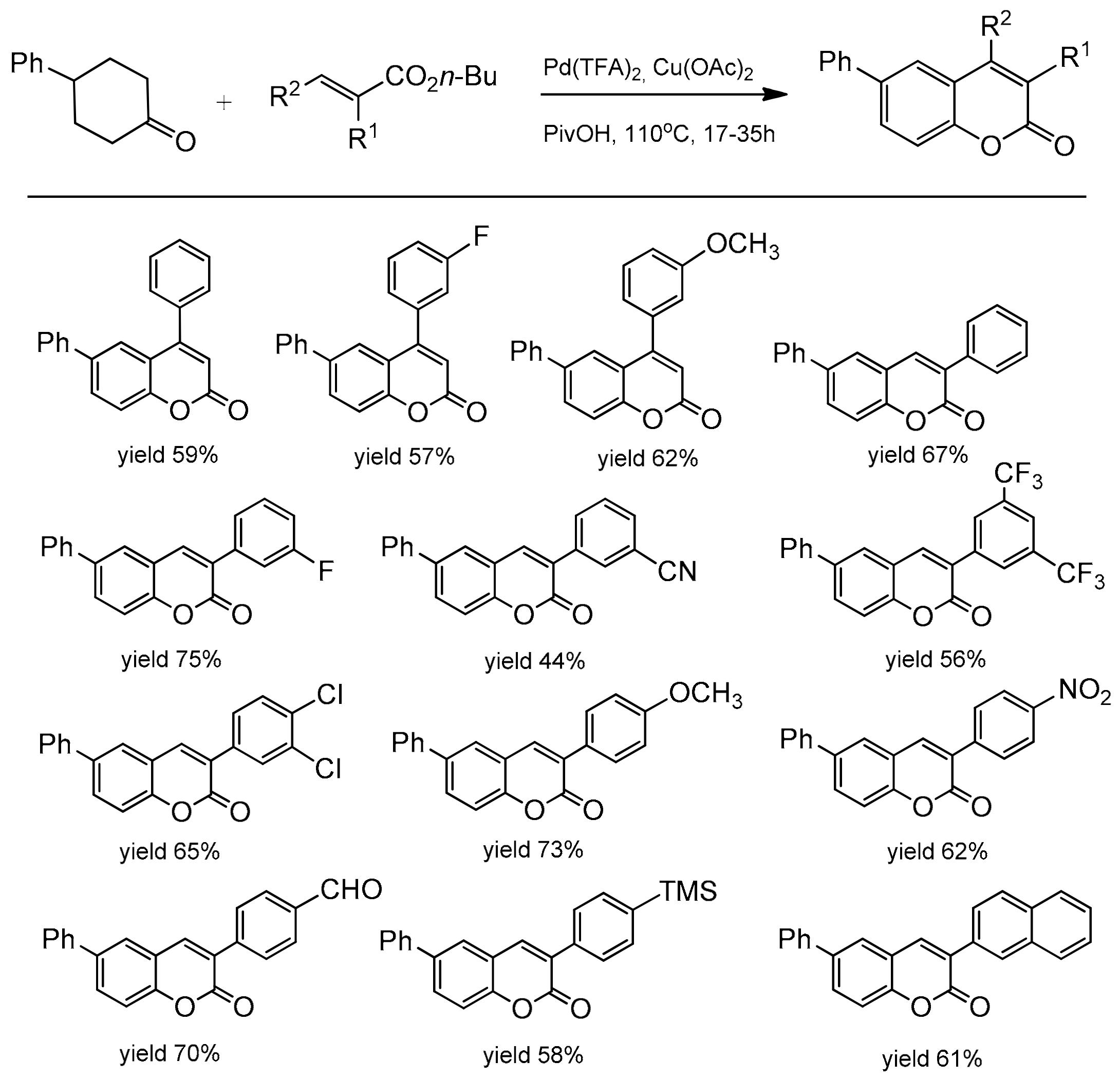
In 2013, the Hong group presented a very distinguished paper on the synthesis of C-3, C-4, and C-7-substituted coumarins [93]. The reaction was catalyzed by palladium(II) using cyclohexanones and alkenes as substrates and consisted of a dehydrogenation-oxidative Heck-cyclization sequence (Scheme 21).
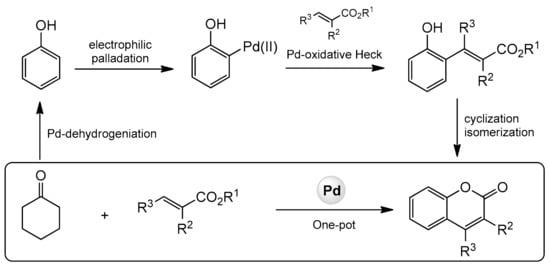
Scheme 21.
Sequence of reactions for construction of substituted coumarins.
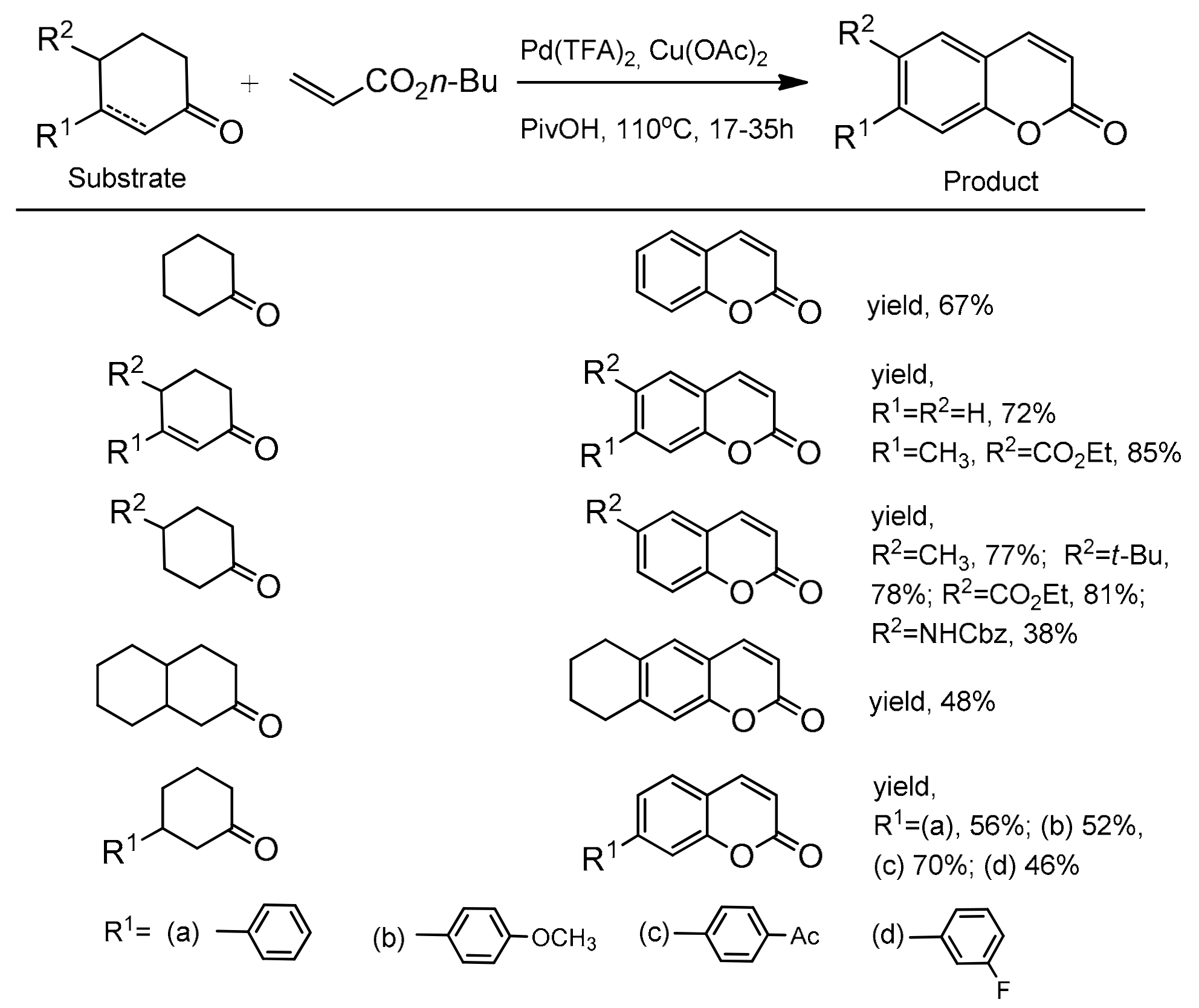
The optimal conditions for the reaction were: Pd(TFA)2, Cu(OAc)2 to facilitate the re-oxidation of Pd(0) to Pd(II), 1 atm O2, pivalic acid as a solvent, and 110 °C. The scope and generality of the methodology was explored using cyclic ketones and butyl acrylate. It was found that a variety of C-3 and C-4 substituted cyclohexanones as well as cyclohexenones worked very well in this process yielding the corresponding coumarins in the 38–85% yield (Scheme 22). Furthermore, the catalytic method was also successfully applied to the synthesis of various C-3 and C-4 substituted coumarins starting with different activated alkenes and 4-phenylcyclohexanone. The formation of these synthetically and biologically useful C-3 and C-4-phenyl and -naphthyl substituted coumarins was observed with moderate to good yields (Scheme 23).
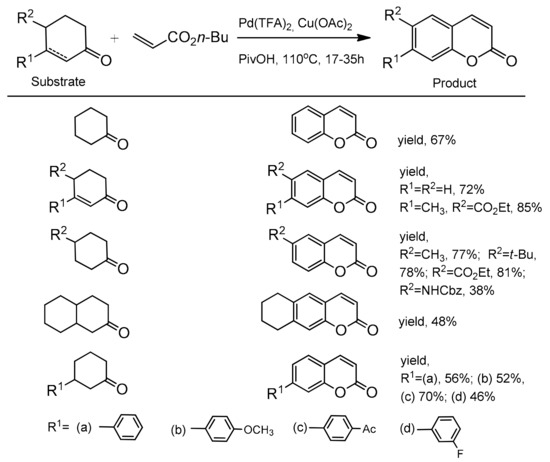
Scheme 22.
Palladium-catalyzed reaction of cyclic ketones with butyl acrylate.
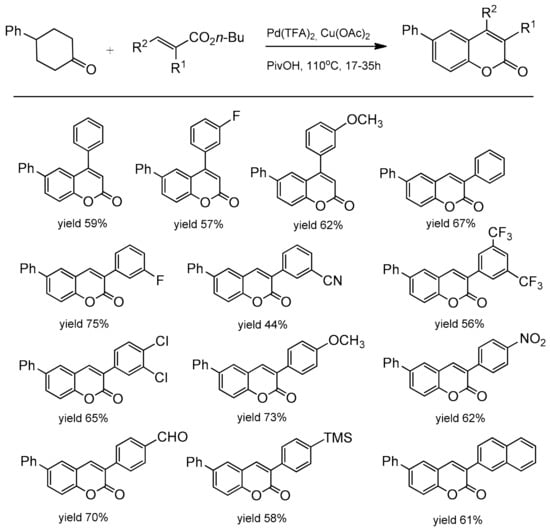
Scheme 23.
Palladium-catalyzed reaction of 4-phenylcyclohexanone with the alkene substrate.
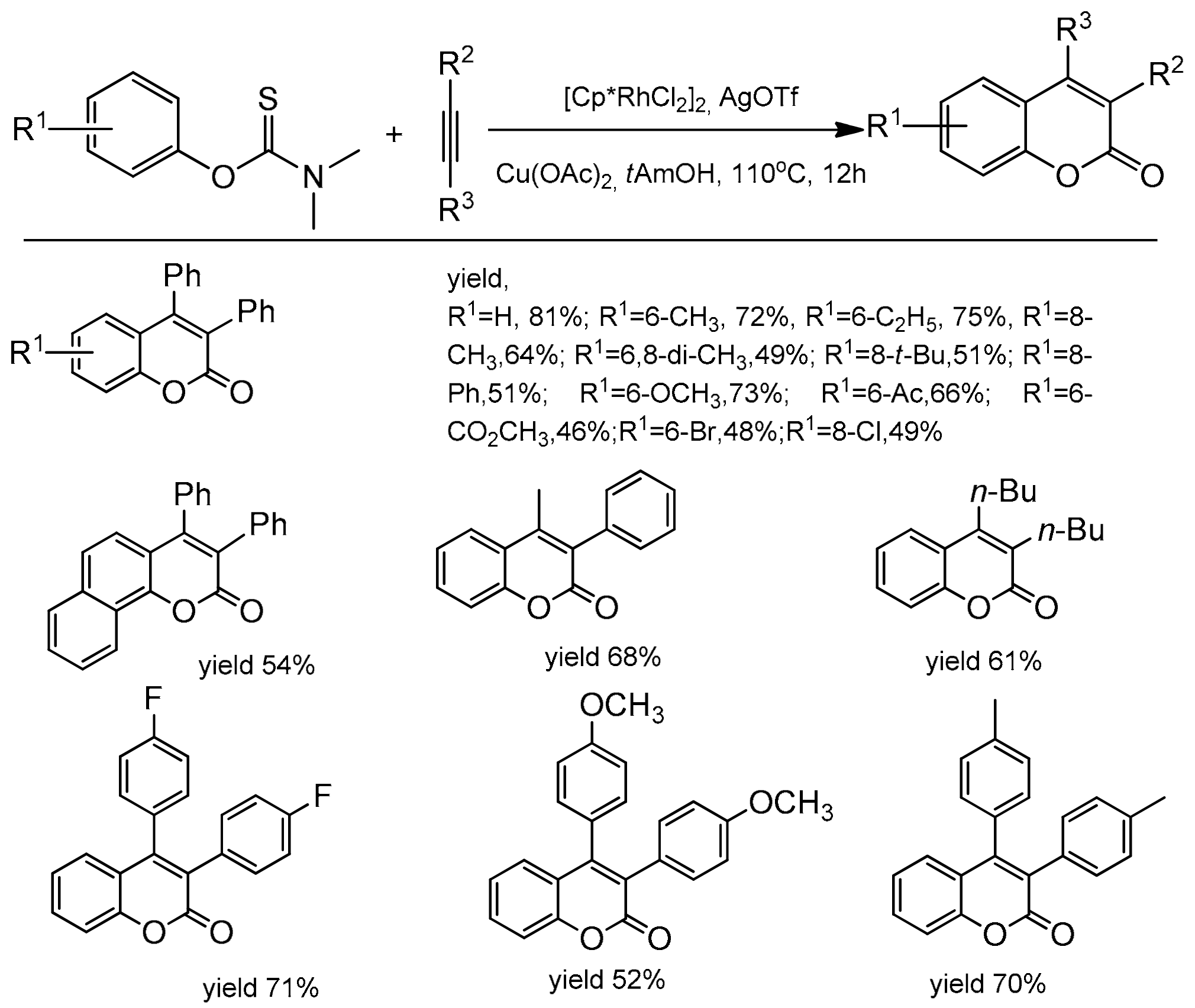
Xia and co-workers extended the access to C-3 and C-4 substituted coumarins making use of aryl thiocarbamates as the starting material [94]. The reaction was catalyzed by [Cp*RhCl2]2 with a catalytic amount of AgOTf. Copper acetate was applied as an oxidant and t-AmOH as a solvent. The reaction was heated for 12 h at 110 °C for complete conversion. In the reaction of diphenylacetylene with aryl thiocarbamates substituted at the para position with the electron-donating groups the corresponding products were obtained with good yields (up to 82%). The same substituents at the ortho position gave lower yields of products. The electron-deficient thiocarbamates also exhibited diminished reactivity. The process was compatible with ester, ketone, and halide functional groups (Scheme 24). To obtain insight into the reaction mechanism, some control experiments and mechanism studies were performed and revealed that Cu(OAc)2 acts both as the oxidant for Rh(I) and as the oxygen source for the carbonyl group.
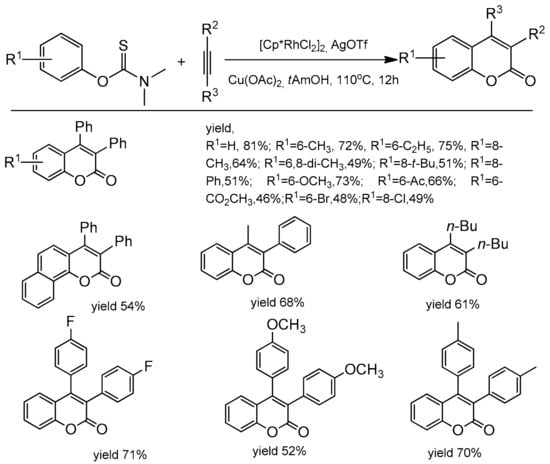
Scheme 24.
Rhodium-catalyzed reaction of aryl thiocarbamates with diarylacetylenes.
3.7. C–H bond Activation Strategy for the Synthesis of C-3 and C-4 Substituted Coumarins
In the scientific literature, transition metal-catalyzed functionalization of (hetero)arenes is reported as a simple and environmentally friendly tool. In recent years, significant progress in the installation of aryl groups and olefins into the coumarin skeleton has been made. Any of the six carbons can be modified in the coumarin scaffold; however, the C-3 and C-4 functionalization has been performed most commonly. One of the processes applied for the preparation of C-3 coumarin derivatives is the cross-dehydrogenative coupling reaction [95].
3.7.1. C-3 Selective Reactions
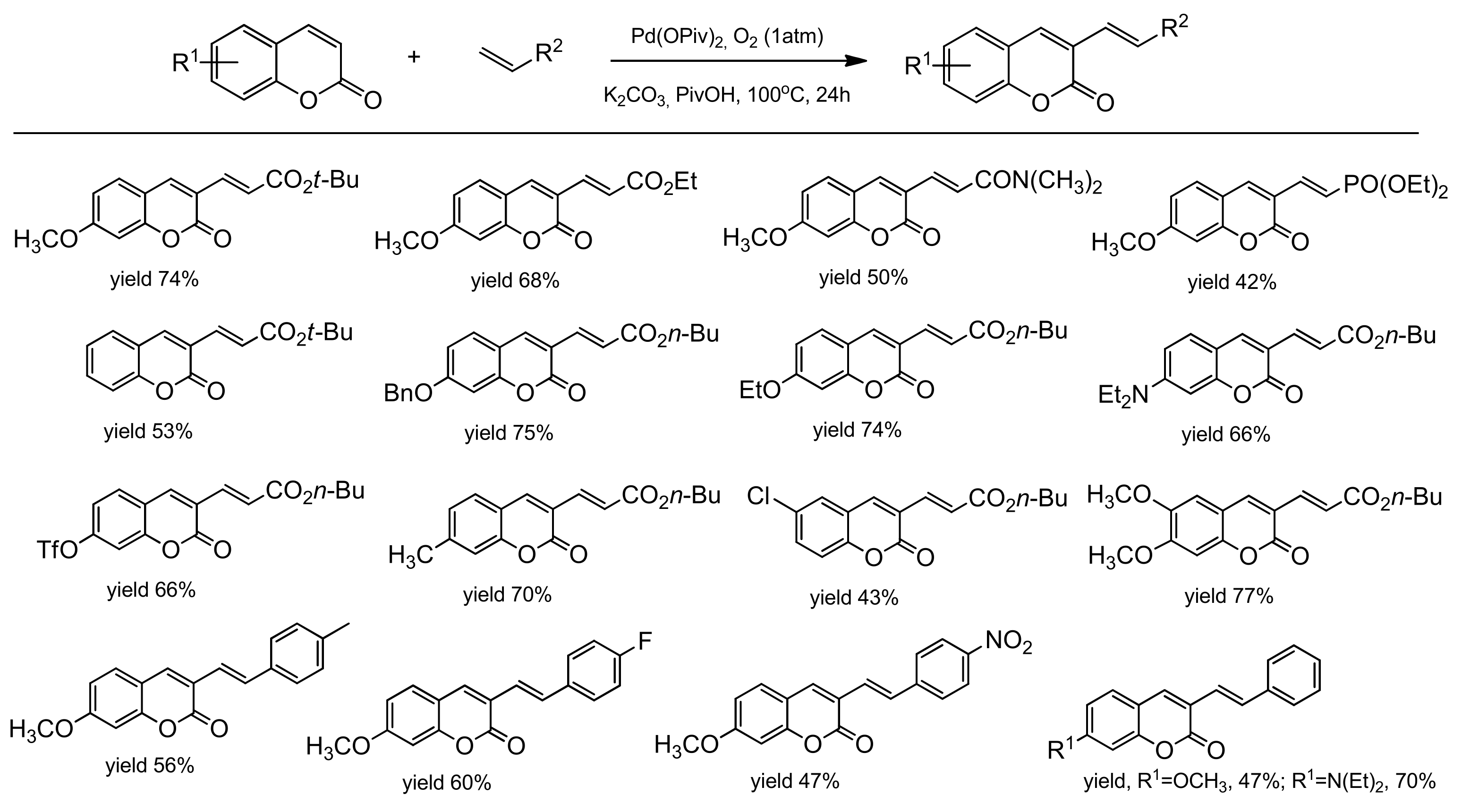
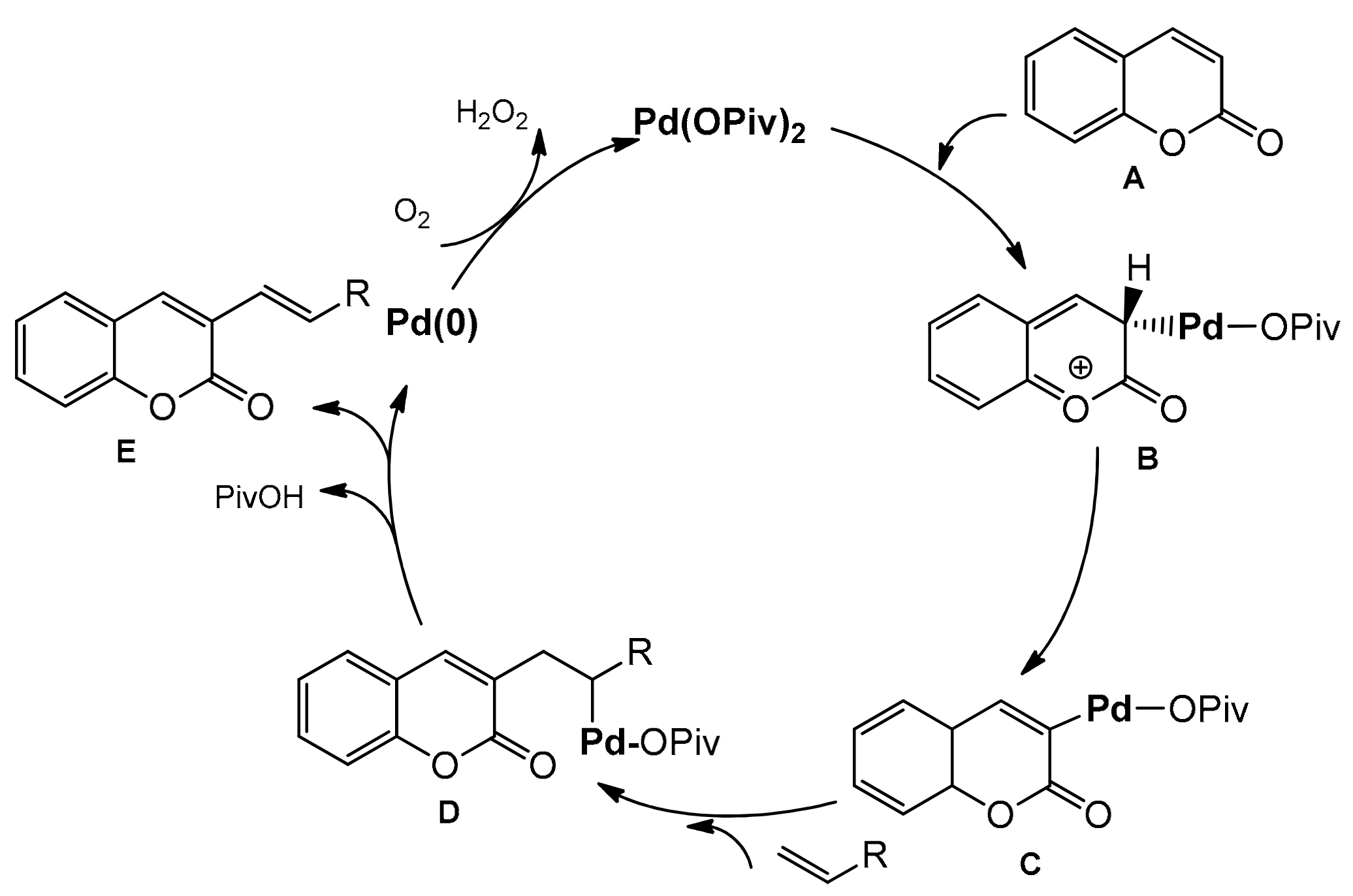
The palladium-catalyzed coupling reaction of coumarin with alkenes was explored by the Hong group [96]. The desired coupling products were obtained using a catalytic system composed of Pd(OPiv)2 and K2CO2 as a base. The C-3 alkenylation worked well in the open air and/or under 1 atm O2 as the sole oxidant in PivOH at 100 °C. Various arylcoumarins with such functional groups as alkyl, chloro, benzoxy, methoxy, triflate, ethoxy as well as the coumarins substituted at C-3 with alkenes moiety, ester, phosphonate, and amide, were prepared in good yields. Their results are summarized in Scheme 25. To elucidate the alkenylation process, a mechanistic analysis was made leading to the proposed mechanism (Scheme 26). The electrophilic palladation of coumarin at C-3 was favorable due to the fact of its more nucleophilic nature and an intermediate B was formed. Then, an intermediate was inserted into the olefin followed by the reductive elimination of a Pd/alkyl in D to provide the product E. Molecular O2 regenerated the palladium(II) catalyst.
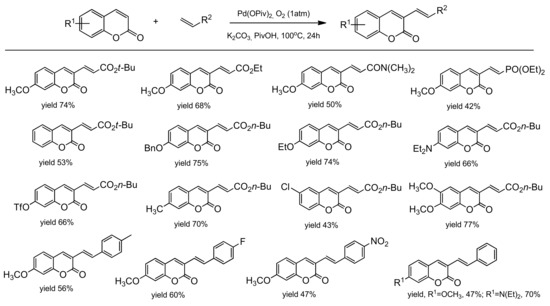
Scheme 25.
Pd-catalyzed C-3 functionalization.
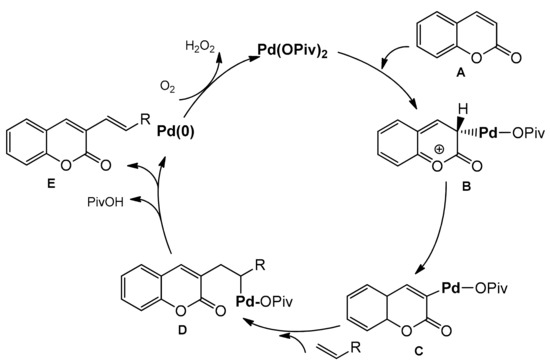
Scheme 26.
Proposed mechanism of Pd-catalyzed C-3 functionalization.
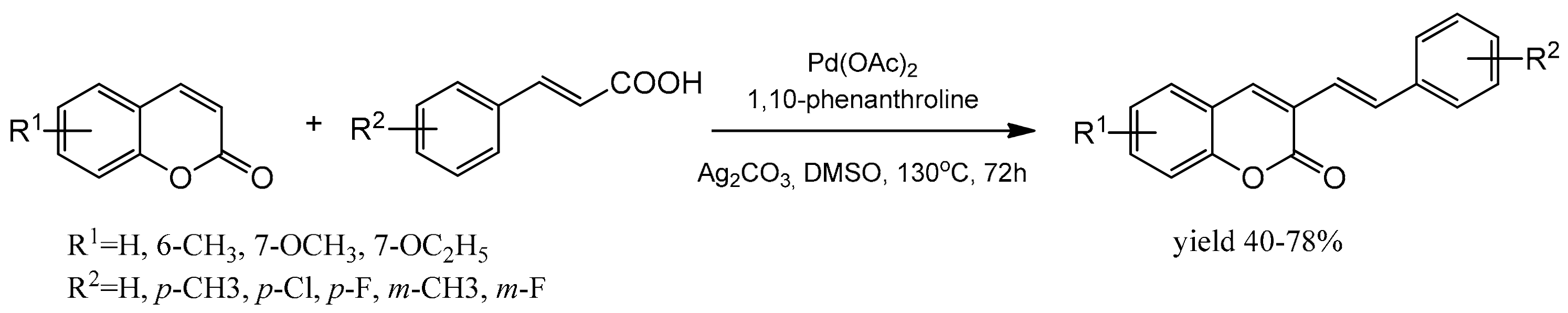
The efficient protocol for the regioselective synthesis of 3-styrylcoumarins was presented by Wang et al. [97]. The reaction of coumarin with cinnamic acid was catalyzed by the palladium complex and proceeded via the decarboxylative cross-coupling route. Ag2CO3 was added as the oxidant and 1,10-phenanthroline as a ligand. The solvent appeared to influence the reaction yield, and DMSO was the most appropriate. With the optimized reaction conditions, coumarins reacted efficiently with cinnamic acids after 72 h at 130 °C (Scheme 27). 3-Styrylcoumarins were obtained in moderate to good yields with complete selectivity and proved to have good fluorescence quantum yields.
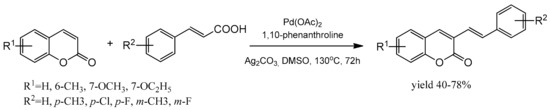
Scheme 27.
Reaction of coumarin with cinnamic acid.
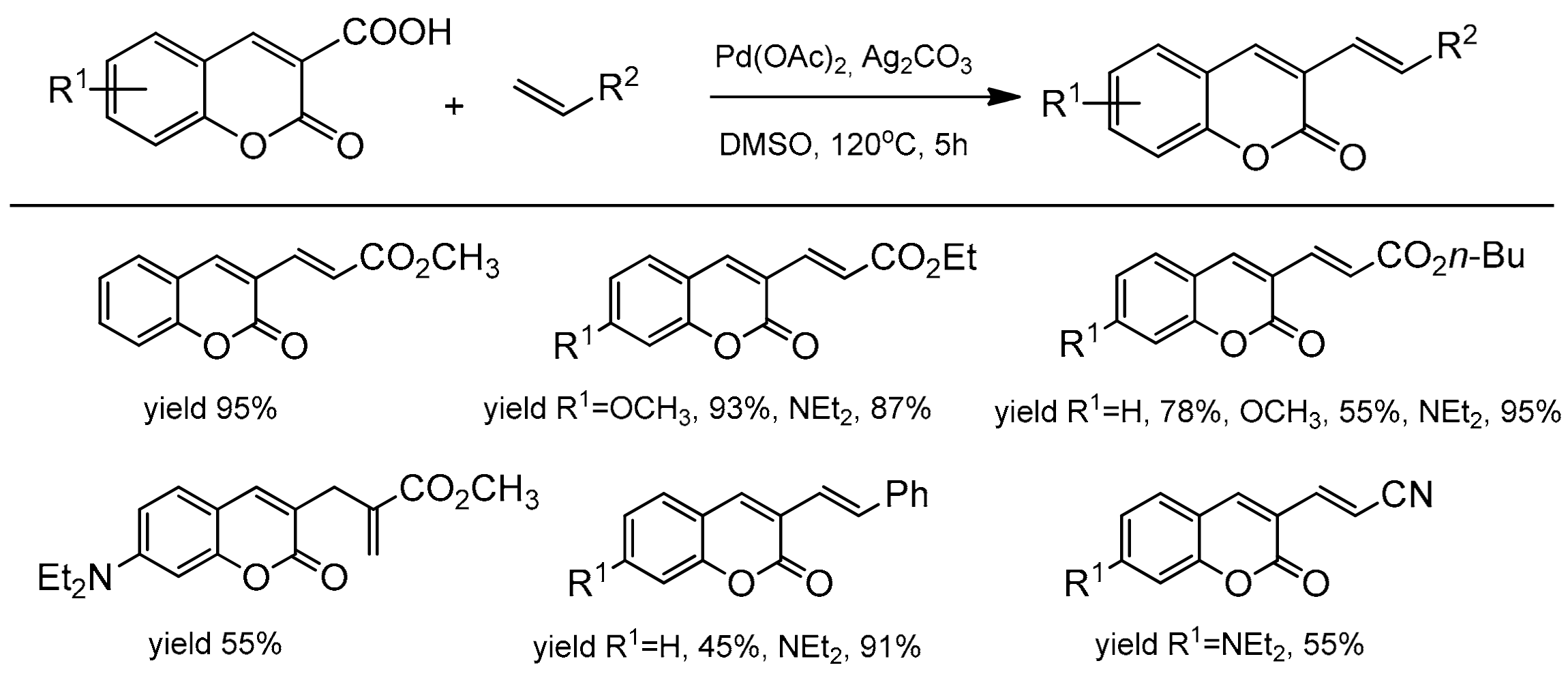
Pd-catalyzed decarboxylative alkenylation of coumarin-3-carboxylic acids was presented by Jafarpour et al. [98] for the synthesis of various C-3 substituted coumarins. The optimal reaction conditions were PdCl2, Ag2CO3, and DMSO at 120 °C for 5 h. The desired 3-vinylcoumarins were obtained with the yields up to 95% (Scheme 28). The reaction of coumarin with alkenes having the ester group led to alkenylated coumarin with good to excellent yields. In turn, a reaction with acrylonitrile and styrene finished with moderate yields of products. In the paper, the authors presented the decarboxylation reaction of coumarins with aryl iodides, obtaining 4-arylcoumarins with efficiency up to 95%. It was observed that the decarboxylation process in both arylation and alkenylation reactions proceeded with high selectivity.
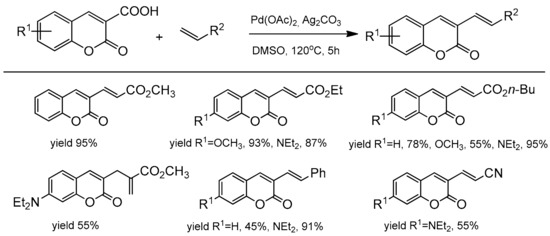
Scheme 28.
Reaction decarboxylative alkenylation of coumarins.
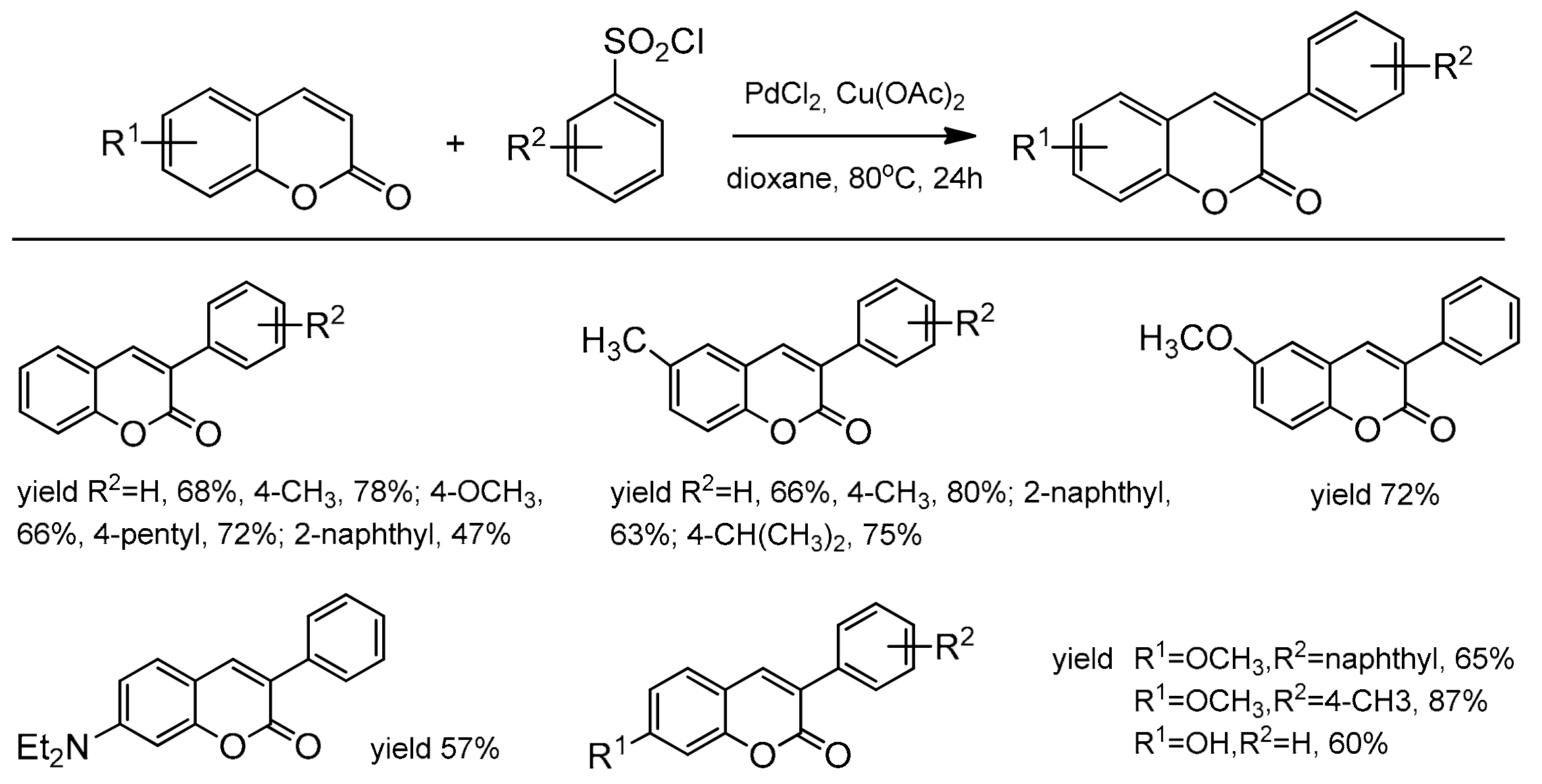
Jafarpour et al. also presented an alternative route for the direct C–H functionalization of the coumarin skeleton [99]. The reaction of coumarins with arenesulfonyl chloride or sodium arenesulfinate proceeded via the Pd-catalyzed C–H bond activation. The coumarins substituted with the electron-donating as well as electron-withdrawing groups were explored, and 3-aryl substituted products were constructed with good isolated yields and high regioselectivity. The results of this desulfonative arylation are presented in Scheme 29.
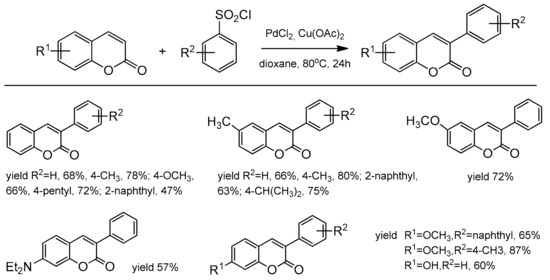
Scheme 29.
C-3 arylation of coumarins.
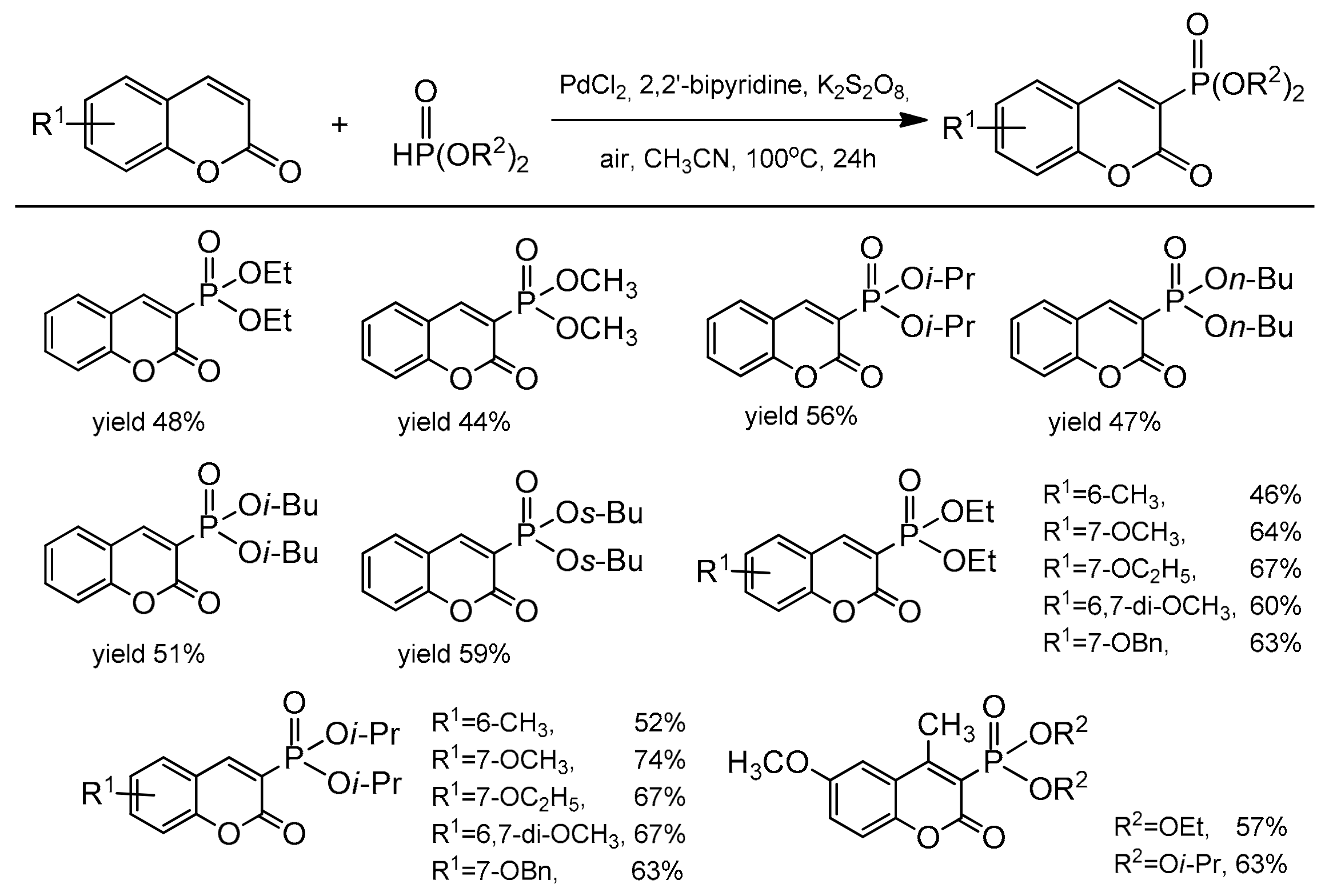
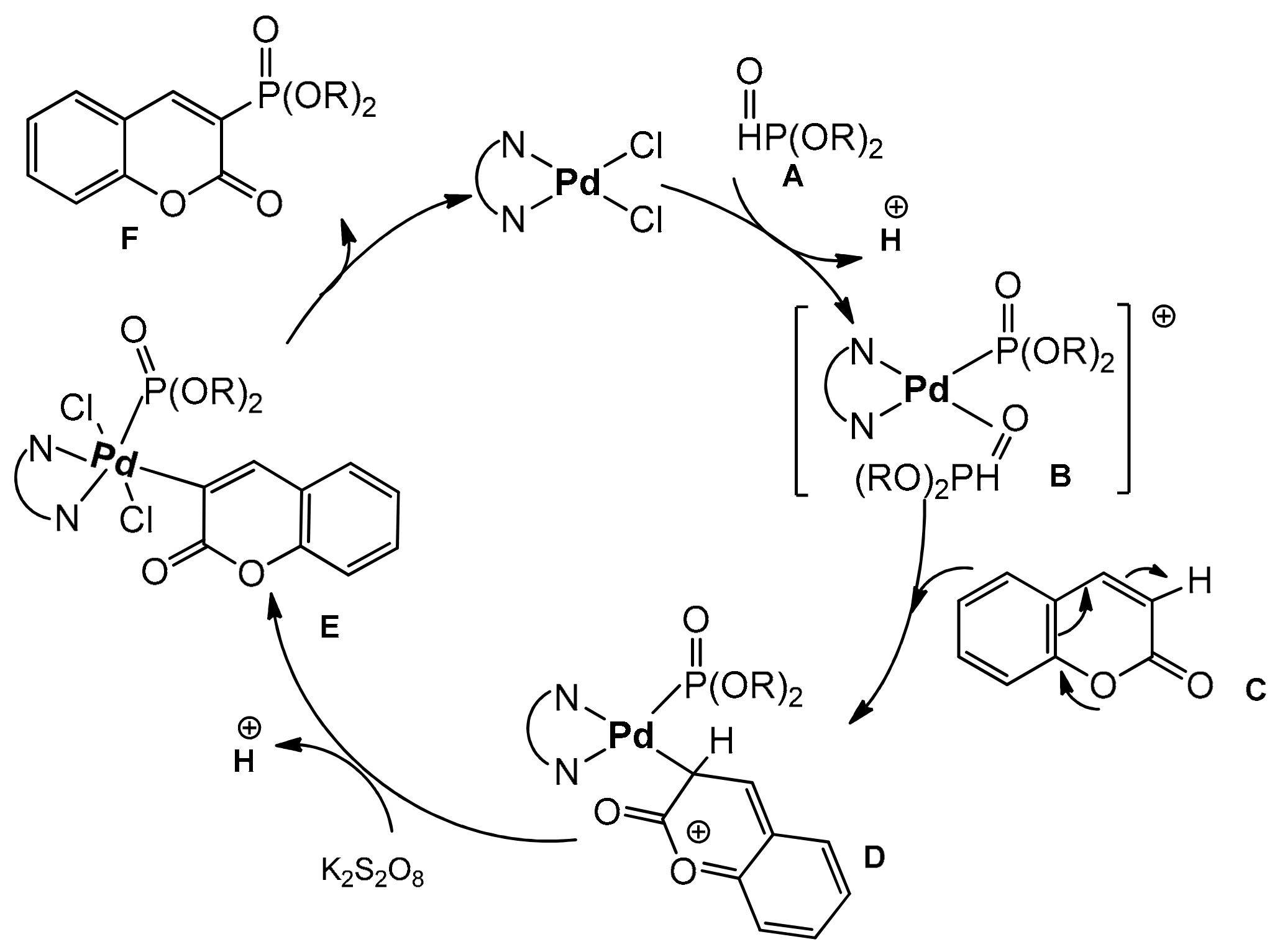
The catalytic reaction conditions were PdCl2, Cu(OAc)2, 1,4-dioxane as a solvent at 80 °C for 20 h. It was postulated that the copper salt plays the role of an oxidant and contributes to the desulfonation process. Although the exact mechanism of this reaction was unclear, the authors have proposed a possible path for this process. In 2013, regioselective phosphonation at the C-3 position was reported by Wu and co-workers [100]. This direct C–H functionalization was accomplished in the presence of Pd(OAc)2 and K2S2O8 in acetonitrile at 100 °C with the access of air. The bidentate nitrogen ligand, 2,2’-bipyridine was necessary to complete the reaction after 24 h. For the test reactions, the substituted coumarins at C-6 and C-7 and different dialkyl H-phosphonates were applied. Generally, coumarins with the electron-donor groups gave higher yields than with the electron-deficient ones. It was also found that HP(O)(Oi-Pr)2 and HP(O)(OBu)2 were the best partners for phosphonation of coumarins. The results of the phosphonation reaction are summarized in Scheme 30.
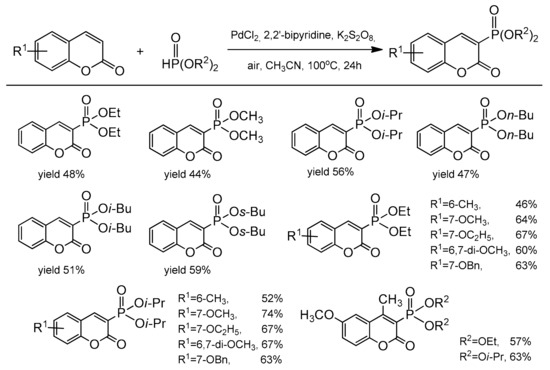
Scheme 30.
Coumarin phosphonation process.
The authors investigated the mechanism of phosphonation reaction. Using TEMPO as a radical scavenger, they excluded the radical mechanism. The ESI-MS technique was applied to monitor the reaction intermediates. Based on this observation, the plausible mechanism was estimated as shown in Scheme 31.
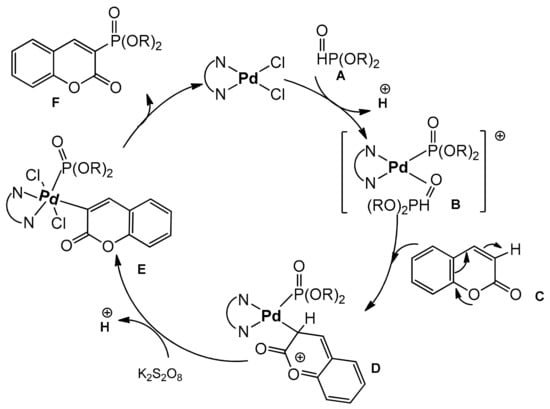
Scheme 31.
Proposed mechanism of coumarin phosphonation.
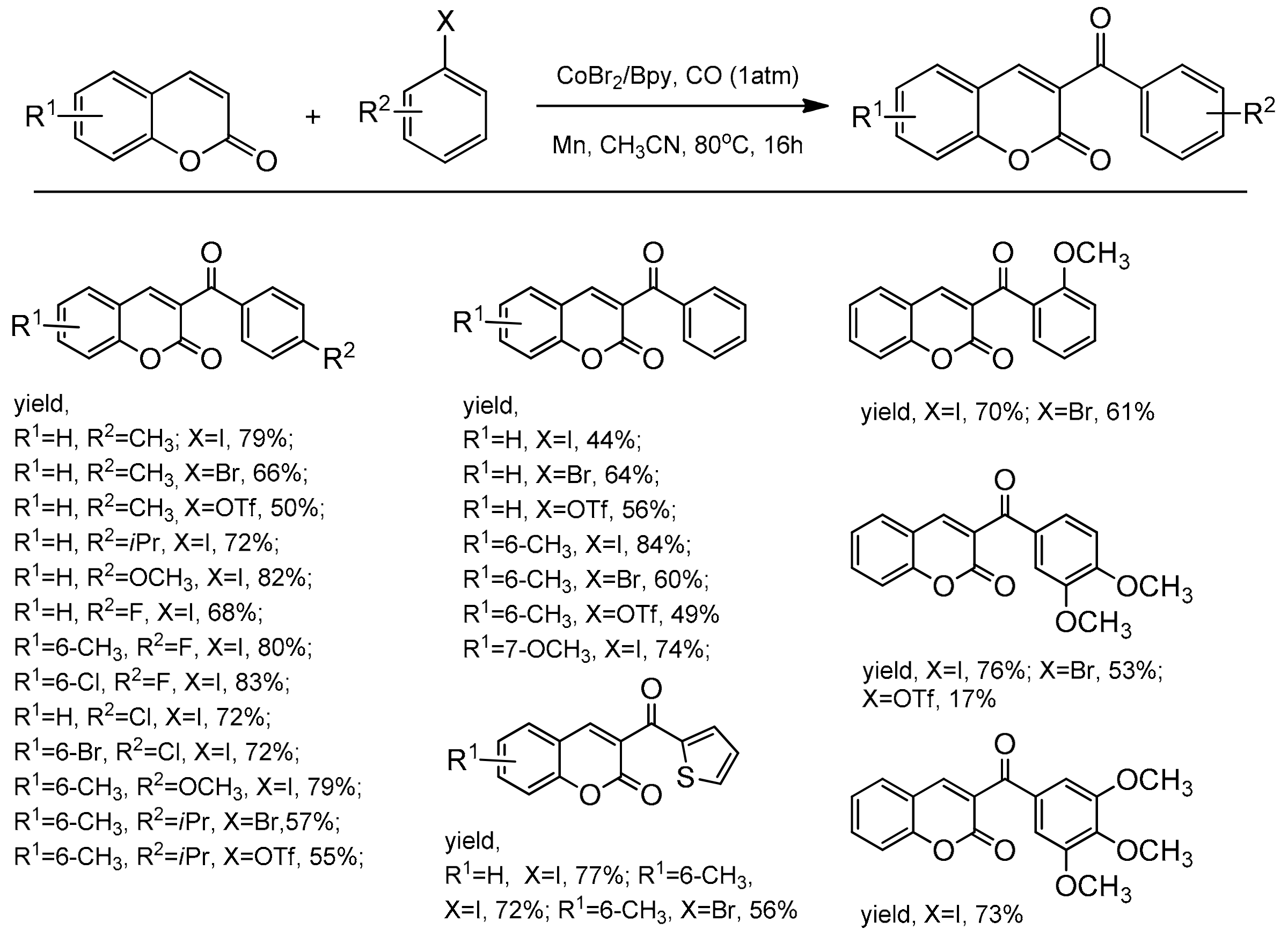
The cobalt-catalyzed direct C-3 functionalization of coumarins with aryl triflates, aryl iodides, and aryl bromides was revealed by Shabanian and co-workers [101]. The process represented the aroylation cross-coupling reactions between coumarin skeleton and the carbon monoxide via a regioselective C–H functionalization. The reactions were carried out under CO atmosphere (1 atm) in the presence of Co-Br2/Bpy10 (Bpy = 2,2’-bipyridine) and manganese powder as the reducing agent. After 16 h at 80 °C in acetonitrile, the substituted C-3 coumarins were isolated in yields up to 84% (Scheme 32).
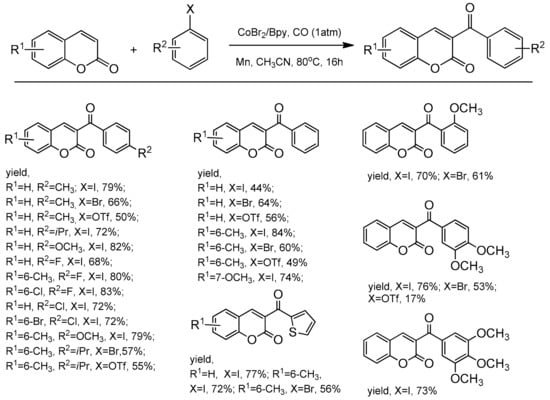
Scheme 32.
C-3 selective the Co-catalyzed carbonylation reaction.
3.7.2. C-4 Selective Reactions
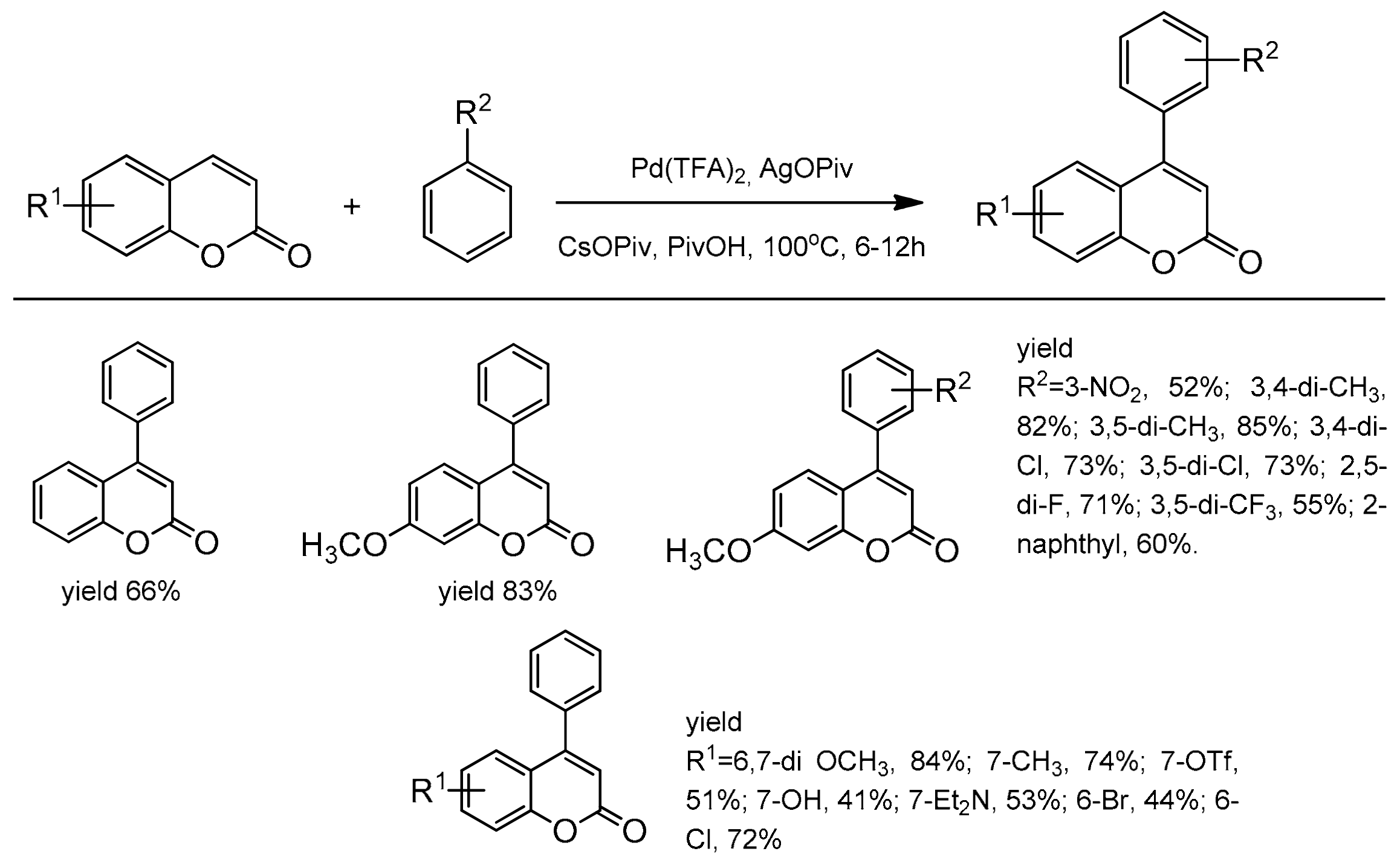
One of the first successful example of C-4 regiocontrolled C–H functionalization of coumarins was presented by Min and Hong [102]. The simple non-activated arenes and coumarins participated in the Pd-catalyzed oxidative Heck coupling reaction. Among the palladium species, Pd(OPiv)2 was the most effective in promoting coupling in the presence of AgOPiv and CsOPiv in pivalic acid at 100 °C. A series of 4-arylcoumarins (neoflavones) were obtained with very good yield and selectivity (Scheme 33).
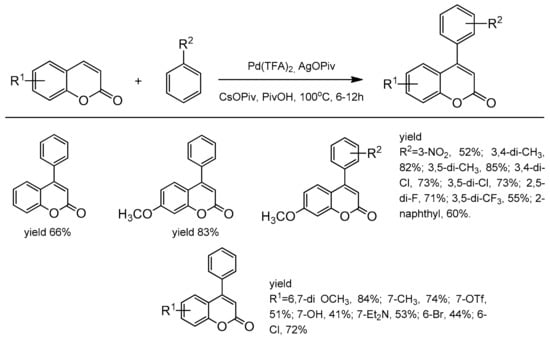
Scheme 33.
C-4 selective arylation.
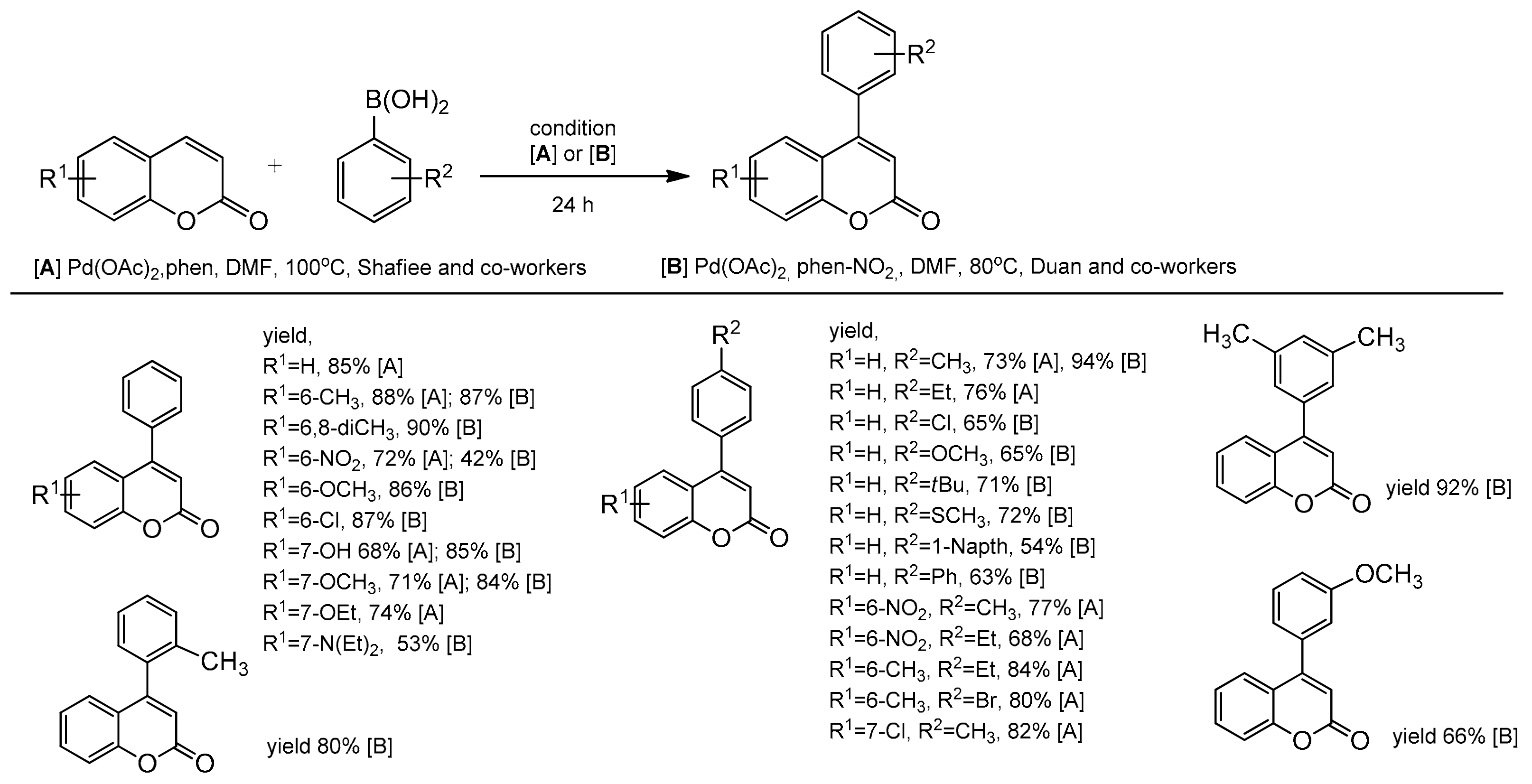
An efficient protocol for the direct construction of 4-arylcoumarins via the Pd-catalyzed oxidative Heck reaction was developed independently by two groups of Duan and co-workers [103] and Shafiee and co-workers [104]. The coumarins were treated with phenylboronic acid and alkyl substituted arylboronic acids, under oxygen (balloon pressure) in the presence of the palladium catalyst in DMF at 80 or 100 °C for 24 h. For the coupling reaction the Shafiee group used 1,10-phenanthroline (phen) as the ligand, while Duan and co-workers applied phen-NO2 (5-nitro-1,10-phenanthroline). The cross-coupling reaction of arylboronic acids and coumarins with the electron-donating or the electron-withdrawing groups proceeded smoothly, resulting in variously functionalized 4-arylcoumarins in moderate to excellent yields (Scheme 34).
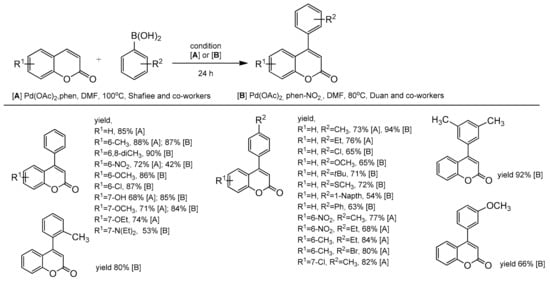
Scheme 34.
Pd-catalyzed oxidative Heck reaction.
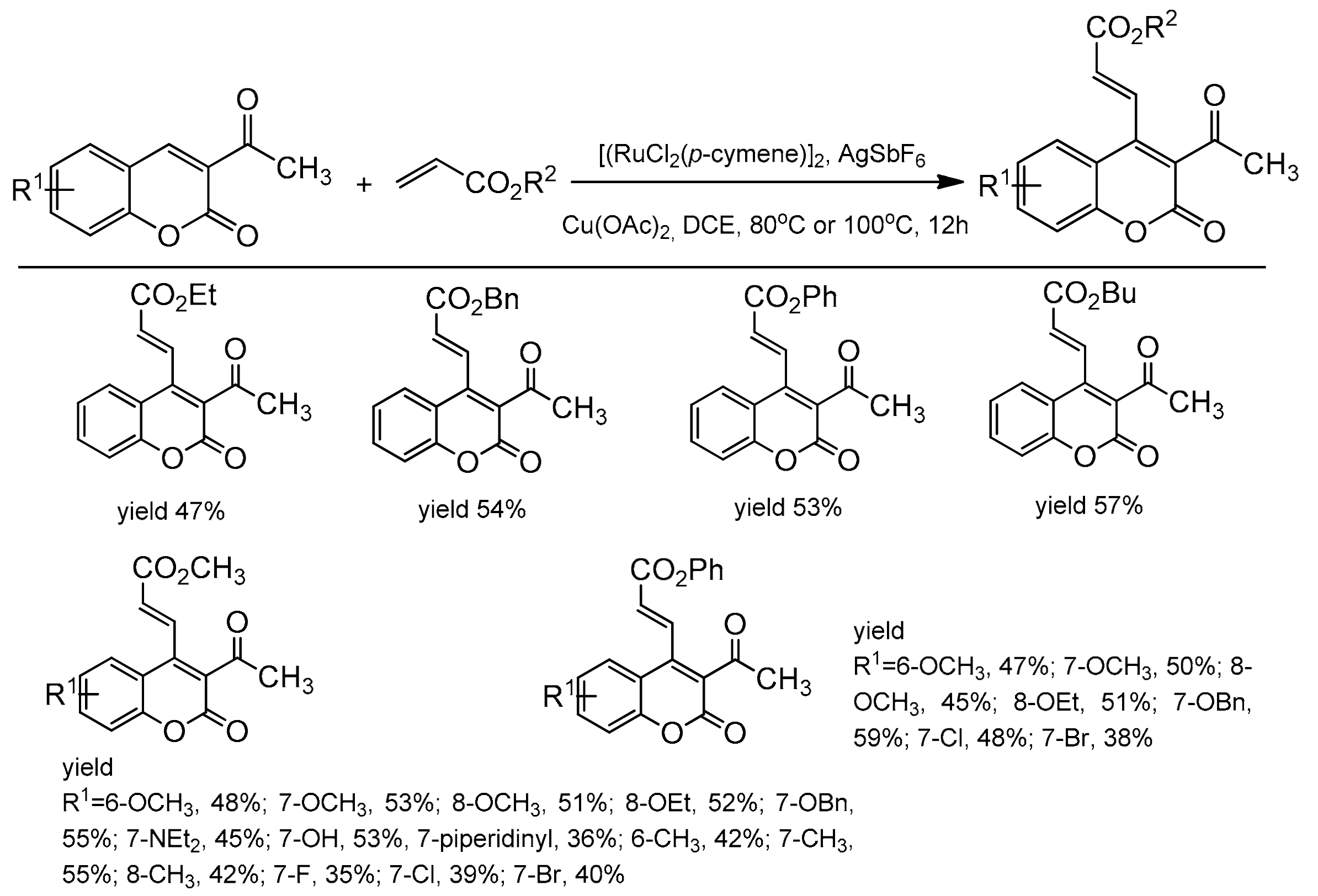
3-Carbonyl coumarins were used as substrates for the synthesis of C-3 and C-4 substituted products [105]. The presence of the directing carbonyl group was used to install the alkenyl moiety at the C-4 position. This was achieved in the presence of the ruthenium catalyst, [RuCl2(p-cymene)]2, AgSbF6, and Cu(OAc)2. The reaction was conducted in dichloroethane at 80 or 100 °C under air. The alkenylation was performed with various alkyl and phenyl acrylates with 3-acetylcoumarins substituted at the C-6, C-7, and C-8 positions as substrates. The results of alkenylation reaction are presented in Scheme 35.
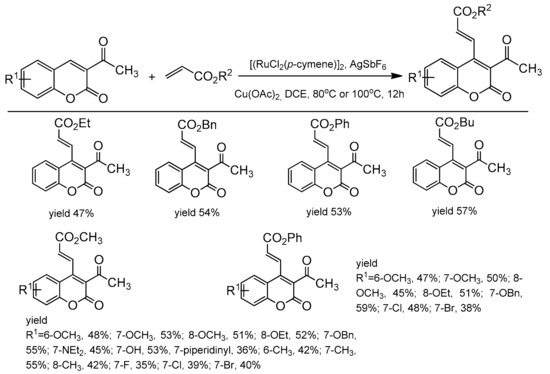
Scheme 35.
Ru-catalyzed alkenylation of 3-acetylcoumarins.
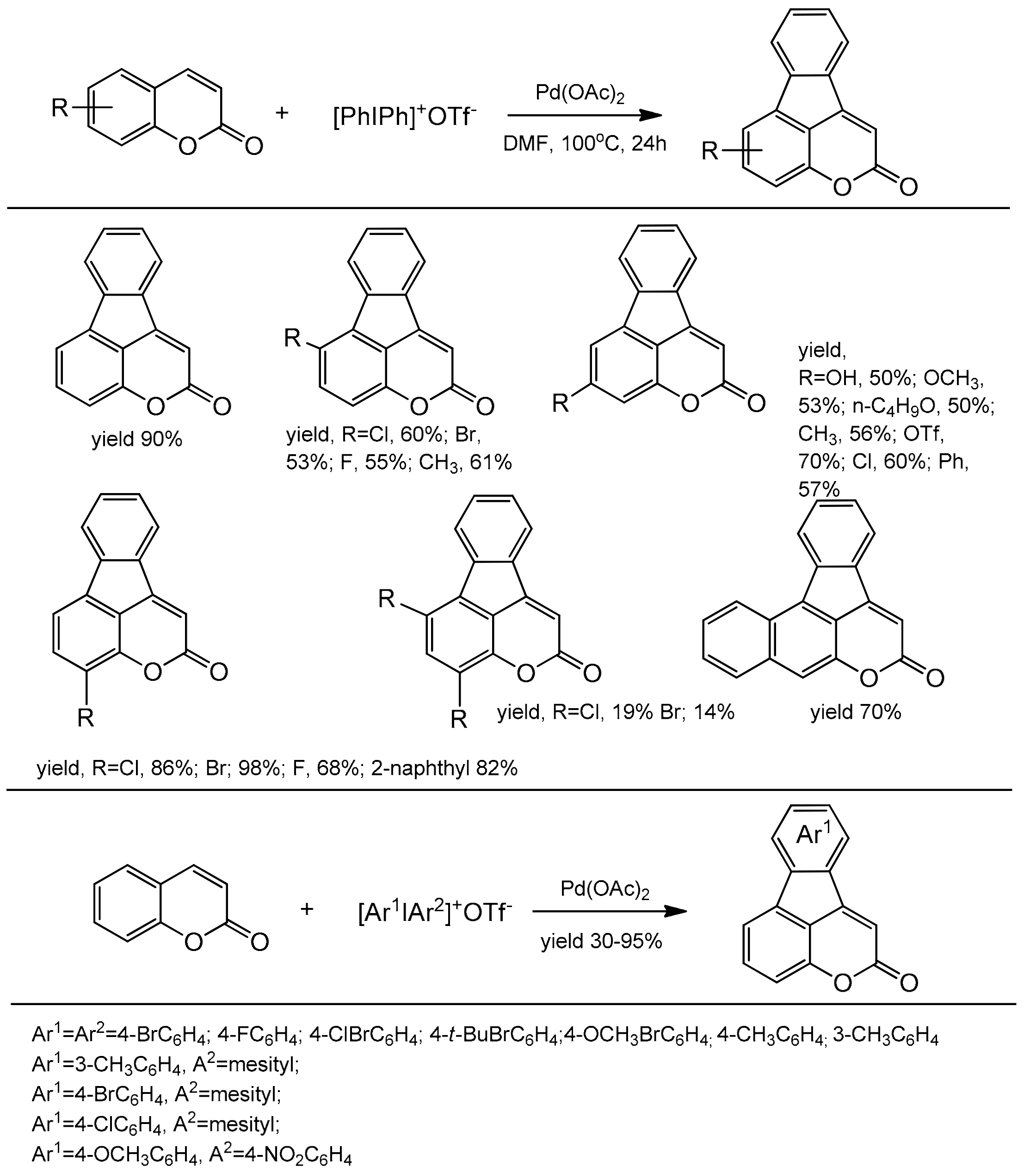
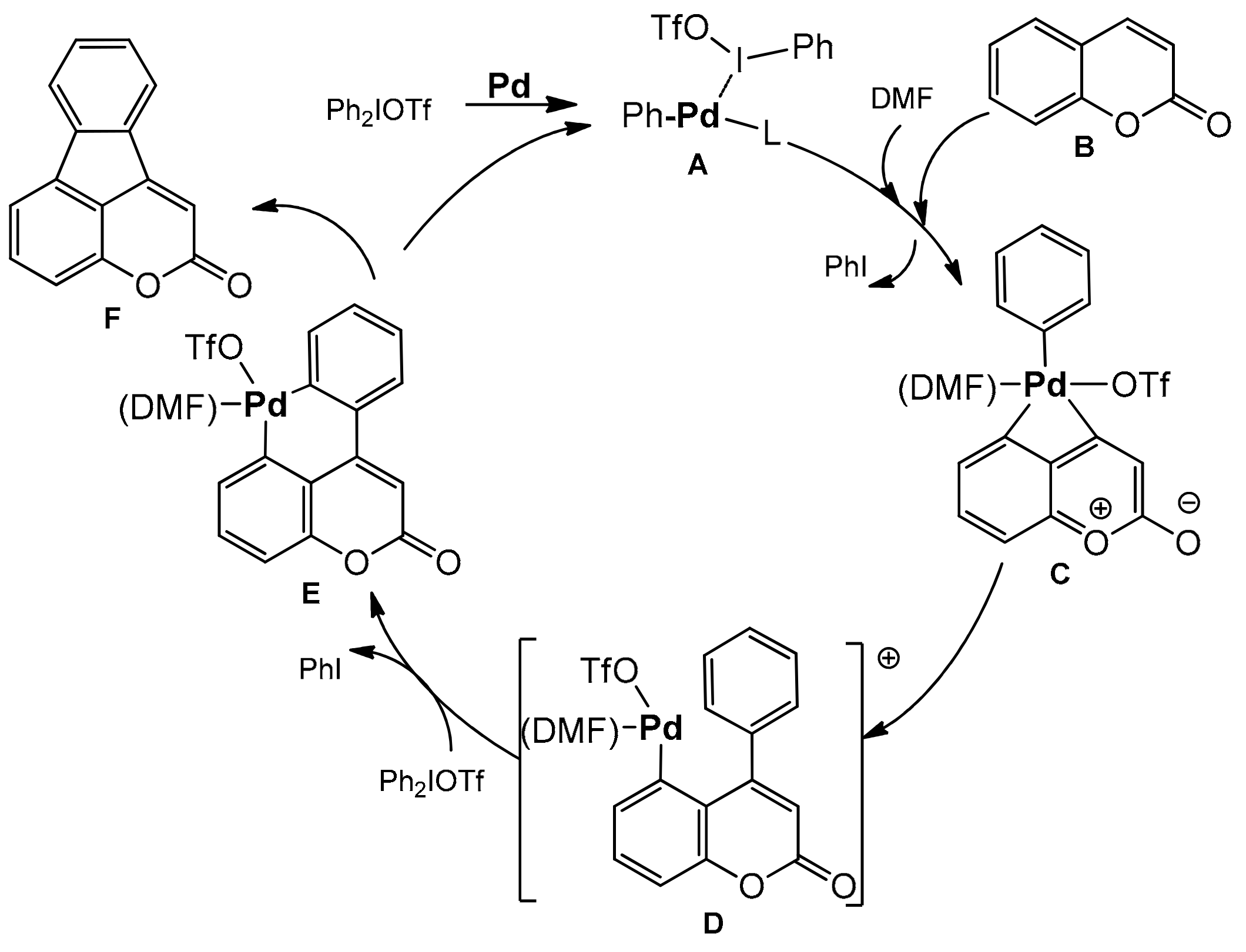
Large access to a series of 4,5-benzocoumarins with the potential fluorescent properties synthesized with good yields by Wang et al. [106]. This method involved simultaneous activation of the C-I bond and the vicinal C–H bond in diarylyliodonium salts. The reaction did not require the use of ligands or an oxidant. The diarylation product was formed in the presence of palladium acetate in DMF at 100 °C after 24 h. The coumarins substituted at C-6, C-7, and/or C-8 with halogens, phenyl, alkoxy, and methyl groups were well tolerated. In the case of diaryliodium salts, both symmetrical and unsymmetrical compounds with various functional groups on the aromatic ring worked well in this reaction (Scheme 36). In the reaction mechanism the process is initiated by the cleavage of iodine-aryl bond to generate a palladium intermediate A which can react as a Lewis acid with activation of the carbonyl group of coumarin B. In the next step an oxidative attack at C-4 takes place with the activation of the C–H bond of coumarin to form palladacycle C. The reductive elimination breaks one aryl bond leading to D. The second oxidative addition in D produces the palladium(IV) complex E and the second reductive elimination regenerates the catalyst A with the formation of a product F possessing two newly generated carbon–carbon bonds (Scheme 37).
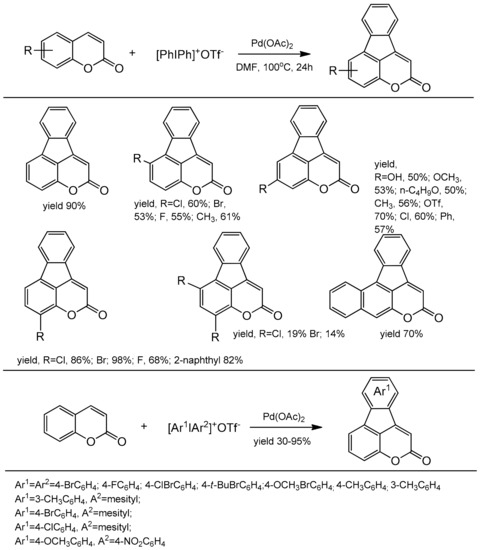
Scheme 36.
Direct synthesis of 4,5 benzocoumarins.
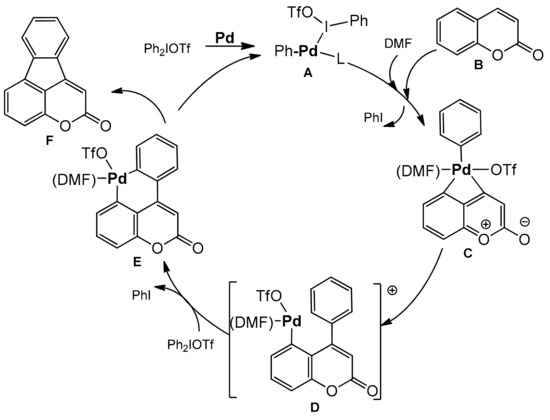
Scheme 37.
Proposed mechanism of the 4,5 benzocoumarins synthesis.
4. Summary
Coumarin compounds are attractive organic compounds with many practical applications. Among them there are compounds with biological activity, pharmaceuticals, agrochemicals, dyes, and optoelectronic materials. For this reason, enormous and continuous attempts were made to develop new synthetic pathways and protocols to facilitate the key cyclization reaction of heterocyclic ring and its regioselective functionalization. Of the numerous proposed reactions for the preparation of coumarins, those based on transition metal catalysts have been frequently used recently. Such processes as intramolecular and intermolecular hydroarylation of alkenes or alkynes can be mentioned among the most effective reactions proceeding via the activation of the C–H bond. Knowledge about the mechanistic foundations of catalytic processes seems to be of significant importance in order to improve them and simplify the conditions. Direct functionalization of the coumarin skeleton seems to be one of the more difficult tasks in recent times. Therefore, further progress in this area is extremely important for improving the process as regards the best efficiency and selectivity of the reaction with a broad range of substrates.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fairlamb, I.J.S.; Marrison, L.R.; Dickinson, J.M.; Lu, F.-J.; Schmidt, J.P. 2-Pyrones possessing antimicrobial and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 4285–4299. [Google Scholar] [CrossRef] [PubMed]
- McGlacken, G.P.; Fairlamb, I.J.S. 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Nat. Prod. Rep. 2005, 22, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013; Royal Society of Chemistry Publishing: London, UK, 2014. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds-literature. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [Green Version]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar] [CrossRef]
- Balewski, Ł.; Szulta, S.; Jalińska, A.; Kornicka, A. A Mini-review: Recent advances in coumarin-metal complexes with biological properties. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Peng, X.-M.; Damu, G.L.V.; Zhou, C.-H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Ahmed, F.S.; Abd El-Salam, A.M.; Rady, M.A.; Latif, M.S.A. Synthesis and biological activity of some new 3-and 6-substituted coumarin amino acid derivatives. Part I. J. Heterocycl. Chem. 1981, 18, 1203–1207. [Google Scholar] [CrossRef]
- Grover, J.; Jachak, S.M. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015, 5, 38892–38905. [Google Scholar] [CrossRef]
- Emami, S.; Dadashpour, S. Current developments of coumarin based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.; Jia, H.; Zhou, Y.-D.; Nagle, D.G. Benzochromenones from the marine crinoid comantheria rotula inhibit hypoxia-inducible factor-1 (HIF-1) in cell-based reporter assays and differentially suppress the growth of certain tumor cell lines. J. Nat. Prod. 2007, 70, 1462–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Hsieh, H.-P.; Hsu, T.-A.; Yeh, J.-Y.; Horng, J.-T.; Shih, S.-R.; Chang, S.-T.; Chao, Y.-S. Coumarin Compounds and Their Use for Treating Viral Infection. U.S. Patent Application No. 12/481,789, 17 December 2009. [Google Scholar]
- Arora, R.K.; Kaur, N.; Bansal, Y.; Bansal, G. Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B 2014, 4, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Pu, W.; Lin, Y.; Zhang, J.; Wang, F.; Wang, C.; Zhang, G. 3-Arylcoumarins: Synthesis and potent anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2014, 24, 5432–5434. [Google Scholar] [CrossRef]
- Chen, L.Z.; Sun, W.; Bol, W.; Wang, J.Q.; Xiu, C.; Tang, W.J.; Shi, J.B.; Zhou, H.P.; Liu, X.H. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 138, 170–181. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef]
- Satish, G. Chapter 8—Anticoagulant agents. In Advances in Structure and Activity Relationship of Coumarin Derivatives; Penta, S., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 151–159. [Google Scholar]
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A.; Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378. [Google Scholar] [CrossRef]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.-H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Coumarins as inhibitors of HIV reverse transcriptase. Curr. HIV Res. 2006, 4, 347–363. [Google Scholar] [CrossRef]
- Wadhwa, P.; Priti, J.; Santosh, R.; Hemant, R.A.J. Quinoline, coumarin and other heterocyclic analogs based HIV-1 integrase inhibitors. Curr. Drug Discov. Technol. 2018, 15, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Dorababu, A. Pharmacological report of recently designed multifunctional coumarin and coumarin-heterocycle derivatives. Arch. Pharm. 2021, 355, e2100345. [Google Scholar] [CrossRef]
- Edmondson, D.E.; Mattevi, A.; Binda, C.; Li, M.; Hubalek, F. Structure and mechanism of monoamine oxidases. Curr. Med. Chem. 2004, 11, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Wimbiscus, M.; Kostenko, O.; Malone, D. MAO inhibitors: Risks, benefits, and lore. Clevel. Clin. J. Med. 2010, 77, 859–882. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Z.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Ansary, I.; Taher, A. One-pot synthesis of coumarin derivatives. In Phytochemicals in Human Health; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Perkin, W.H. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868, 21, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Perkin, W.H. On the hydride of aceto-salicyl. J. Chem. Soc. 1868, 21, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.L.; Sapp, J. A new one-step synthesis of substituted coumarins. J. Org. Chem. 1962, 27, 3703–3705. [Google Scholar] [CrossRef]
- Sethna, S.M.; Shah, N.M.; Shah, R.C. Aluminium chloride, a new reagent for the condensation of β-ketonic esters with phenols. Part I. The condensations of methyl β-resorcylate, β-resorcylic acid, and resacetophenone with ethyl acetoacetate. J. Chem. Soc. 1938, 228–232. [Google Scholar] [CrossRef]
- Corrie, J.E.T. A convenient synthesis of N-(7-dimethylamino-4-methylcoumarin-3-yl)-maleimide incorporating a novel variant of the Pechmann reaction. J. Chem. Soc. Perkin Trans. 1990, 1, 2151–2152. [Google Scholar] [CrossRef]
- Smitha, G.; Reddy, S.C. ZrCl4- catalyzed Pechmann reaction: Synthesis of coumarins under solvent-free conditions. Synth. Commun. 2004, 34, 3997–4003. [Google Scholar] [CrossRef]
- Valizadeh, H.; Shockravi, A. An efficient procedure for the synthesis of coumarin derivatives using TiCl4 as catalyst under solvent-free conditions. Tetrahedron Lett. 2005, 46, 3501–3503. [Google Scholar] [CrossRef]
- John, E.; Israelstam, S. Notes. Use of cation exchange resins in organic reactions. I. The Von Pechmann reaction. J. Org. Chem. 1961, 26, 240–242. [Google Scholar] [CrossRef]
- Laufer, M.C.; Hausmann, H.; Hölderich, W.F. Synthesis of 7-hydroxycoumarins by Pechmann reaction using nafion resin/silica nanocomposites as catalysts. J. Catal. 2003, 218, 315–320. [Google Scholar] [CrossRef]
- Hoefnagel, A.J.; Gunnewegh, E.A.; Downing, R.S.; van Bekkum, H. Synthesis of 7-hydroxycoumarins catalysed by solid acid catalysts. J. Chem. Soc. Chem. Commun. 1995, 225–226. [Google Scholar] [CrossRef]
- Salem, M.A.; Helal, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M. An overview on synthetic strategies to coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent advances in the synthesis of coumarin derivatives via Knoevenagel condensation: A review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Heravi, M.M.; Khaghaninejad, S.; Mostofi, M. Pechmann reaction in the synthesis of coumarin derivatives. Adv. Heterocycl. Chem. 2014, 112, 1–50. [Google Scholar] [CrossRef]
- Lanman, B.A. Pechmann coumarin synthesis. In Name Reactions in Heterocyclic Chemistry II; Li, J.J., Ed.; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Vekariya, R.H.; Patel, H.D. Synthesis of bromocarbonyl compounds: Recent advances. Tetrahedron 2014, 70, 3949–3961. [Google Scholar] [CrossRef]
- Ibrahem, I.; Sundén, H.; Rios, R.; Zhao, G.-L.; Córdova, A. One-pot pyrrolidine-catalyzed synthesis of benzopyrans, benzothiopyranes, and dihydroquinolidines. Chimia 2007, 61, 219–223. [Google Scholar] [CrossRef]
- Bouhaoui, A.; Eddahmi, M.; Dib, M.; Khouili, M.; Aires, A.; Catto, M.; Bouissane, L. Synthesis and biological properties of coumarin derivatives. A review. Chem. Select 2021, 6, 5848–5870. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.; Lee, K. Metal-free, Brønsted acid-mediated synthesis of coumarin derivatives from phenols and propiolic acids. Tetrahedron Lett. 2016, 57, 3600–3603. [Google Scholar] [CrossRef]
- Gouda, M.A.; Hussein, B.H.M.; El-Demerdash, A.; Ibrahim, M.E.; Salem, M.A.; Helal, M.H.; Hamama, W.S. A Review: Synthesis and medicinal importance of coumarins and their analogues. Curr. Bioact. Compd. 2020, 16, 993–1008. [Google Scholar] [CrossRef]
- Shilov, E.; Shul’pin, G.B. Activation of C−H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef]
- Kuhl, N.; Hopkinson, M.N.; Wencel-Delord, J.; Glorius, F. Beyond directing groups: Transition-metal-catalyzed C-H activation of simple arenes. Angew. Chem. Int. Ed. 2012, 51, 10236–10254. [Google Scholar] [CrossRef]
- Pratap, R.; Ram, V.J. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef]
- Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Ahn, K.H.; Gryko, D.T. π-Expanded coumarins: Synthesis, optical properties and applications. J. Mater. Chem. C 2015, 3, 1421–1446. [Google Scholar] [CrossRef]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; Teissier García, A.G.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Kim, N.-J.; Yun, H.; Han, Y.T. Recent advances in synthesis of 4-arylcoumarins. Molecules 2018, 23, 2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdou, M.M.; Abu-Rayyanb, A.; Bedira, A.G.; Abdel-Fattaha, S.; Omara, A.M.A.; Ahmedc, A.A.; El-Desoky, E.-S.I.; Ghaithd, E.A. 3-(Bromoacetyl)coumarins: Unraveling their synthesis, chemistry, and applications. RSC Adv. 2021, 11, 38391–38433. [Google Scholar] [CrossRef]
- Koleva, A.I.; Petkova-Yankova, N.I.; Nikolova, R.D. Synthesis and chemical properties of 3-phosphono-coumarins and 1,2-benzoxaphosphorins as precursors for bioactive compounds. Molecules 2019, 24, 2030. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lan, J.; You, J. Oxidative C−H/C−H coupling reactions between two (hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef]
- Bhatia, R.; Pathania, S.; Singh, V.; Rawal, R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Hetero. Comp. 2018, 54, 280–291. [Google Scholar] [CrossRef]
- Kanchana, U.S.; Diana, E.J.; Mathew, T.V.; Anilkumar, G. Palladium-catalyzed cross-coupling reactions of coumarin derivatives: An overview. Appl. Organomet. Chem. 2020, 34, e5983. [Google Scholar] [CrossRef]
- Sharma, R.K.; Katiyar, D. Recent advances in transition-metal-catalyzed synthesis of coumarins. Synthesis 2016, 48, 2303–2322. [Google Scholar] [CrossRef]
- Choi, H.; Min, M.; Peng, Q.; Kang, D.; Paton, R.S.; Hong, S. Unraveling innate substrate control in site-selective palladium-catalyzed C–H heterocycle functionalization. Chem. Sci. 2016, 7, 3900–3909. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.M.; Toste, F.D. A new palladium-catalyzed addition: A mild method for the synthesis of coumarins. J. Am. Chem. Soc. 1996, 118, 6305–6306. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D.; Greenman, K. Atom economy. Palladium-catalyzed formation of coumarins by addition of phenols and alkynoates via a net C-H insertion. J. Am. Chem. Soc. 2003, 125, 4518–4526. [Google Scholar] [CrossRef]
- Jia, C.; Lu, W.; Oyamada, J.; Kitamura, T.; Matsuda, K.; Irie, M.; Fujiwara, Y. Novel Pd(II)- and Pt(II)-catalyzed regio- and stereoselective trans-hydroarylation of alkynes by simple arenes. J. Am. Chem. Soc. 2000, 122, 7252–7263. [Google Scholar] [CrossRef]
- Juzo, O.; Chengguo, J.; Yuzo, F.; Tsugio, K. Direct synthesis of coumarins by Pd(II)-catalyzed reaction of alkoxyphenols and alkynoates. Chem. Lett. 2002, 380–381. [Google Scholar] [CrossRef]
- Kotani, M.; Yamamoto, K.; Oyamada, J.; Fujiwara, Y.; Kitamura, T. A Convenient synthesis of coumarins by palladium(II)-catalyzed reaction of phenols with propiolic acids. Synthesis 2004, 1466–1470. [Google Scholar] [CrossRef]
- Oyamada, J.; Kitamura, T. Synthesis of coumarins by Pt-catalyzed hydroarylation of propiolic acids with phenols. Tetrahedron 2006, 62, 6918–6925. [Google Scholar] [CrossRef]
- Kutubi, S.; Hashimoto, T.; Kitamura, T. Improved synthesis of coumarins by iron(III)-catalyzed cascade reaction of propiolic acids and phenols. Synthesis 2011, 8, 1283–1289. [Google Scholar] [CrossRef]
- Jia, C.; Piao, D.; Kitamura, T.; Fujiwara, Y. New method for preparation of coumarins and quinolinones via Pd-catalyzed intramolecular hydroarylation of C-C triple bonds. J. Org. Chem. 2000, 65, 7516–7522. [Google Scholar] [CrossRef]
- Jia, C.; Piao, D.; Oyamada, J.; Lu, W.; Kitamura, T.; Fujiwara, Y. Efficient activation of aromatic C–H bonds for addition to C–C multiple bonds. Science 2000, 287, 1992–1995. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.R.; Lu, W. FeCl3-Catalyzed alkenylation of simple arenes with aryl-substituted alkynes. Org. Lett. 2007, 9, 2219–2222. [Google Scholar] [CrossRef]
- Pastine, S.J.; Youn, S.W.; Sames, D. Pt-IV-Catalyzed cyclization of arene-alkyne substrates via intramolecular electrophilic hydroarylation. Org. Lett. 2003, 7, 1055–1058. [Google Scholar] [CrossRef]
- Pastine, S.J.; Youn, S.W.; Sames, D. Pt(IV)-catalyzed cyclization of arene–alkyne substrates via C–H bond functionalization. Tetrahedron 2003, 59, 8859–8868. [Google Scholar] [CrossRef]
- Menon, R.S.; Findlay, A.D.; Bissember, A.C.; Banwell, M.G. The Au(I)-catalyzed intramolecular hydroarylation of terminal alkynes under mild conditions: Application to the synthesis of 2H-chromenes, coumarins, benzofurans, and dihydroquinolines. J. Org. Chem. 2009, 74, 8901–8903. [Google Scholar] [CrossRef] [PubMed]
- Aparece, M.D.; Vadola, P.A. Gold-catalyzed dearomative spirocyclization of aryl alkynoate esters. Org. Lett. 2014, 16, 6008–6011. [Google Scholar] [CrossRef] [PubMed]
- Vadola, P.A.; Sames, D. Catalytic coupling of arene C–H bonds and alkynes for the synthesis of coumarins: Substrate scope and application to the development of neuroimaging agents. J. Org. Chem. 2012, 77, 7804–7814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, J.H.; Kim, H.N.; Yoon, J.; Kim, J.S.; Kim, H.-J. A rationally designed fluorescence turn-on probe for the gold(III) ion. Org. Lett. 2010, 12, 932–934. [Google Scholar] [CrossRef]
- Shi, Z.; He, C. Efficient functionalization of aromatic C-H bonds catalyzed by gold(III) under mild and solvent-free conditions. J. Org. Chem. 2004, 69, 3669–3671. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Oyamada, J.; Kitamura, T. Formation of coumarins by palladium(II)-catalyzed reaction of phenols with ethyl acrylates. Bull. Chem. Soc. Jpn. 2005, 78, 468–472. [Google Scholar] [CrossRef]
- Sharma, U.; Naveen, T.; Maji, A.; Manna, S.; Maiti, D. Angew. Palladium-catalyzed synthesis of benzofurans and coumarins from phenols and olefins. Chem. Int. Ed. 2013, 52, 12669–12673. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Li, Z.-W.; Shi, Z.-J. Palladium-catalyzed base-accelerated direct C–H bond alkenylation of phenols to synthesize coumarin derivatives. Org. Chem. Front. 2014, 1, 44–49. [Google Scholar] [CrossRef]
- Gadakh, S.K.; Dey, S.; Sudalai, A. Rh-Catalyzed synthesis of coumarin derivatives from phenolic acetates and acrylates via C–H bond activation. J. Org. Chem. 2015, 80, 11544–11550. [Google Scholar] [CrossRef]
- Carral-Menoyo, A.; Misol, A.; Gomez-Redondo, M.; Sotomayor, N.; Lete, E. Palladium(II)-catalyzed intramolecular C-H alkenylation for the synthesis of chromanes. J. Org. Chem. 2019, 84, 2048–2060. [Google Scholar] [CrossRef]
- Ortiz-de-Elguea, V.; Carral-Menoyo, A.; Simón-Vidal, L.; Martinez-Nunes, M.; Barbolla, I.; Lete, M.G.; Sotomayor, N.; Lete, E. Pd(II)-catalyzed Fujiwara−Moritani reactions for the synthesis and functionalization of substituted coumarins. ACS Omega 2021, 6, 29483–29494. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-F.; Neumann, H.; Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2012, 113, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Zeng, F.; Alper, H. Synthesis of coumarins via Pd-catalyzed oxidative cyclocarbonylation of 2-vinylphenols. Org. Lett. 2012, 14, 5602–5605. [Google Scholar] [CrossRef] [PubMed]
- Seoane, A.; Casanova, N.; Quiñones, N.; Mascareñas, J.L.; Gulías, M. Straightforward assembly of benzoxepines by means of a rhodium(III)-catalyzed C–H functionalization of o-vinylphenols. J. Am. Chem. Soc. 2014, 136, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Zhang, S.-S.; Jiang, C.-Y.; Wu, J.-Q.; Li, Q.; Wang, H. Cp*Co(III)-Catalyzed annulations of 2-alkenylphenols with CO: Mild access to coumarin derivatives. Org. Lett. 2015, 17, 5404–5407. [Google Scholar] [CrossRef]
- Sasano, K.; Takaya, J.; Iwasawa, N. Palladium(II)-catalyzed direct carboxylation of alkenyl C−H Bonds with CO2. J. Am. Chem. Soc. 2013, 135, 10954–10957. [Google Scholar] [CrossRef]
- Kim, D.; Min, M.; Hong, S. One-pot catalysis of dehydrogenation of cyclohexanones to phenols and oxidative Heck coupling: Expedient synthesis of coumarins. Chem. Commun. 2013, 49, 4021–4022. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, F.; Yang, L.; Xia, C. Access to coumarins by rhodium-catalyzed oxidative annulation of aryl thiocarbamates with internal alkynes. Org. Lett. 2015, 17, 1477–1480. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Mao, F.; Kwong, F.Y. Palladium-catalyzed cross-dehydrogenative functionalization of C (sp2)-H bonds. Chem. Asian J. 2014, 9, 26–47. [Google Scholar] [CrossRef]
- Min, M.; Kim, Y.; Hong, S. Regioselective palladium-catalyzed olefination of coumarins via aerobic oxidative Heck reactions. Chem. Commun. 2013, 49, 196–198. [Google Scholar] [CrossRef]
- Wang, X.; Pan, S.; Li, Y.; Wang, H.; Chen, Z.; Huang, K. Regioselective palladium-catalyzed decarboxylative cross-coupling reaction of alkenyl acids with coumarins: Synthesis of 3-styrylcoumarin compounds. J. Org. Chem. 2015, 80, 2407–2411. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Zarei, S.; Olia, M.B.A.; Jalalimanesh, N.; Rahiminejadan, S. Palladium-catalyzed decarboxylative cross-coupling reactions: A route for regioselective functionalization of coumarins. J. Org. Chem. 2013, 78, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, F.; Olia, M.B.A.; Hazrati, H. Highly regioselective α-arylation of coumarins via palladium- catalyzed C-H activation/desulfitative coupling. Adv. Synth. Catal. 2013, 355, 3407–3412. [Google Scholar] [CrossRef]
- Mi, X.; Huang, M.; Zhang, J.; Wang, C.; Wu, Y. Regioselective palladium-catalyzed phosphonation of coumarins with dialkyl H-phosphonates via C-H functionalization. Org. Lett. 2013, 15, 6266–6269. [Google Scholar] [CrossRef]
- Pashazadeh, R.; Rajai-Daryasarei, S.; Mirzaei, S.; Soheilizad, M.; Ansari, S.; Shabanian, M. A regioselective approach to C3-aroylcoumarins via cobalt catalyzed C(sp2)–H activation carbonylation of coumarin. Synthesis 2019, 51, 3014–3020. [Google Scholar] [CrossRef]
- Min, M.; Hong, S. Regioselective palladium-catalyzed direct cross-coupling of coumarins with simple arenes. Chem. Commun. 2012, 48, 9613–9615. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Z.; Wang, H.; Fu, X.; Duan, C. Palladium-catalyzed oxidative Heck coupling reaction for direct synthesis of 4-arylcoumarins using coumarins and arylboronic acids. J. Org. Chem. 2012, 77, 2053–2057. [Google Scholar] [CrossRef]
- Khoobi, M.; Alipour, M.; Zarei, S.; Jafarpour, F.; Abbas Shafiee, A. A facile route to flavone and neoflavone backbones via a regioselective palladium catalysed oxidative Heck reaction. Chem. Comm. 2012, 48, 2985–2987. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, T.-T.; Yao, L.; Wang, Q.-L.; Zhao, L.-M. Access to 4-alkenylated coumarins via ruthenium-catalyzed olefinic C−H alkenylation of coumarins with modifiable and removable directing groups. J. Org. Chem. 2020, 85, 9514–9524. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Y.; Han, J.; Wang, L. Palladium catalyzed C–I and vicinal C–H dual activation of diaryliodonium salts for diarylation: Synthesis of 4,5-benzocoumarins. Org. Lett. 2015, 17, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).