Anti-Atherogenic Properties of Allium ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits
Abstract
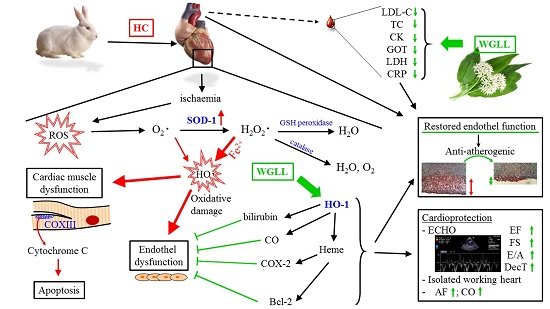
:1. Introduction
2. Results
2.1. Bioanalytical Analysis of Wild Garlic Leaf Lyophilisate
2.2. Echocardiographic Analyses
2.3. Cardiac Function in Isolated Working Hearts
2.4. Western Blot Analysis for Biomarkers of Cardiac Tissue Function
2.5. Rabbit Aortic Histology
2.6. Serological Correlates of Experimental Treatments
3. Discussion
4. Experimental Section (Methodology)
4.1. Sample Lyophilization and Bioanalytics
4.2. Animals
4.3. Echocardiography
4.4. Measurement of Serum Parameters
4.5. Isolated Working Heart Preparation (Langendorff Method)
4.6. Histological Analysis of the Aortic Root
4.7. Extraction of Myocardial Protein
4.8. Western Blot Assays for Protein Expression in Cardiac Tissue
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kilian, R.; Hanelt, P.; Research, I.P.G.C.P.; Kilian, W. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops: Except Ornamentals; Springer: Berlin & Heidelberg, Germany, 2001. [Google Scholar]
- Assessment, G.F.I.F.R. Risk of mix-up with bear’s garlic. Available online: http://www.bfr.bund.de/en/press_information/2005/10/risk_of_mix_up_with_bears_garlic-6228.html (accessed on 20 April 2016).
- Yoo, K.; Pike, L. Determination of flavor precursor compound s-alk(en)yl-l-cysteine sulfoxides by an HPLC method and their distribution in Allium species. Sci. Hortic. 1998, 75, 1–10. [Google Scholar]
- Djurdjevic, L.; Dinic, A.; Pavlovic, P.; Mitrovic, M.; Karadzic, B.; Tesevic, V. Allelopathic potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 32, 533–544. [Google Scholar] [CrossRef]
- Gitin, L.; Dinica, R.; Parnavel, R. The influence of extraction method on the apparent content of bioactive compounds in romanian Allium spp. Leaves. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 93–97. [Google Scholar]
- Gođevac, D.; Vujisić, L.; Mojović, M.; Ignjatović, A.; Spasojević, I.; Vajs, V. Evaluation of antioxidant capacity of Allium ursinum L. Volatile oil and its effect on membrane fluidity. Food Chem. 2008, 107, 1692–1700. [Google Scholar] [CrossRef]
- Sendl, A.; Elbl, G.; Steinke, B.; Redl, K.; Breu, W.; Wagner, H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 1992, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Nagy, N.; Varga, E.; Varadi, J.; Gesztelyi, R.; Szabo, G.; Szendrei, L.; Bacskay, I.; et al. Cardioprotective mechanisms of prunus cerasus (sour cherry) seed extract against ischemia-reperfusion-induced damage in isolated rat hearts. Am. J. Physiol.. Heart Circ. Physiol. 2006, 291, H1329–H1336. [Google Scholar] [CrossRef] [PubMed]
- Lekli, I.; Szabo, G.; Juhasz, B.; Das, S.; Das, M.; Varga, E.; Szendrei, L.; Gesztelyi, R.; Varadi, J.; Bak, I.; et al. Protective mechanisms of resveratrol against ischemia-reperfusion-induced damage in hearts obtained from zucker obese rats: The role of glut-4 and endothelin. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H859–H866. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P. Dyslipidaemia. Lancet 2003, 362, 717–731. [Google Scholar] [CrossRef]
- Posa, A.; Szabo, R.; Kupai, K.; Csonka, A.; Szalai, Z.; Veszelka, M.; Torok, S.; Daruka, L.; Varga, C. Exercise training and calorie restriction influence the metabolic parameters in ovariectomized female rats. Oxid. Med. Cell. Longev. 2015, 2015, 787063. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Soran, H.; Durrington, P.N. Hypercholesterolaemia and its management. BMJ 2008, 337, a993. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Epure, L.I.; Roman, G.V.; Mărăcineanu, R. Studies on medicinal and aromatic plants used in the therapeutic recipes in the bucharest university hospital. Sci. Pap. Univ. Agron. Sci. Vet. Med. Buchar. Ser. A Agron. 2011, LIV, 304–313. [Google Scholar]
- Hiyasat, B.; Sabha, D.; Grotzinger, K.; Kempfert, J.; Rauwald, J.W.; Mohr, F.W.; Dhein, S. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology 2009, 83, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sabha, D.; Hiyasat, B.; Grotzinger, K.; Hennig, L.; Schlegel, F.; Mohr, F.W.; Rauwald, H.W.; Dhein, S. Allium ursinum L.: Bioassay-guided isolation and identification of a galactolipid and a phytosterol exerting antiaggregatory effects. Pharmacology 2012, 89, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Clouatre, D.; Mohamadi, A.; Jarrell, S.T. Wild garlic has a greater effect than regular garlic on blood pressure and blood chemistries of rats. Int. Urol. Nephrol. 2001, 32, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Rietz, B.; Isensee, H.; Strobach, H.; Makdessi, S.; Jacob, R. Cardioprotective actions of wild garlic (Allium ursinum) in ischemia and reperfusion. Mol. Cell. Biochem. 1993, 119, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Juhasz, B.; Tosaki, A. Management of multicellular senescence and oxidative stress. J. Cell. Mol. Med. 2013, 17, 936–957. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Porcel, M.; Lerman, L.O.; Holmes, D.R., Jr.; Richardson, D.; Napoli, C.; Lerman, A. Chronic antioxidant supplementation attenuates nuclear factor-κβ activation and preserves endothelial function in hypercholesterolemic pigs. Cardiovasc. Res. 2002, 53, 1010–1018. [Google Scholar] [CrossRef]
- Gordts, S.C.; van Craeyveld, E.; Muthuramu, I.; Singh, N.; Jacobs, F.; de Geest, B. Lipid lowering and HDL raising gene transfer increase endothelial progenitor cells, enhance myocardial vascularity, and improve diastolic function. PLoS ONE 2012, 7, e46849. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Jacobs, F.; Gordts, S.C.; de Geest, B. Low-density lipoprotein receptor gene transfer in hypercholesterolemic mice improves cardiac function after myocardial infarction. Gene Ther. 2012, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Iloki-Assanga, S.B.; Lewis-Lujan, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of bucida buceras l. And phoradendron californicum. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G.M. Anti-inflammatory effects of a stabilized lipid extract of Perna canaliculus (Lyprinol®). Allerg. Immunol. 2000, 32, 272–278. [Google Scholar]
- Rybak, M.E.; Calvey, E.M.; Harnly, J.M. Quantitative determination of allicin in garlic: Supercritical fluid extraction and standard addition of alliin. J. Agric. Food Chem. 2004, 52, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Seki, T. Antithrombotic and anticancer effects of garlic-derived sulfur compounds: A review. BioFactors 2006, 26, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Bergman, M.; Bessler, H.; Punsky, I.; Djaldetti, M. Effect of a garlic derivative (alliin) on peripheral blood cell immune responses. Int. J. Immunopharmacol. 1999, 21, 589–597. [Google Scholar] [CrossRef]
- Juhasz, B.; Kertesz, A.; Balla, J.; Balla, G.; Szabo, Z.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Varga, B.; Haines, D.D.; et al. Cardioprotective effects of sour cherry seed extract (SCSE) on the hypercholesterolemic rabbit heart. Curr. Pharm. Des. 2013, 19, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Grigioni, F.; Avierinos, J.F.; Barbieri, A.; Rusinaru, D.; Szymanski, C.; Ferlito, M.; Tafanelli, L.; Bursi, F.; Trojette, F.; et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J. Am. Coll. Cardiol. 2009, 54, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Zuchi, C.; Bardelli, G.; Murrone, A.; Lauciello, R.; D’Addario, S.; Mengoni, A.; Alunni, G.; Ambrosio, G. Fibrosis assessment by integrated backscatter and its relationship with longitudinal deformation and diastolic function in heart failure with preserved ejection fraction. Int. J. Cardiovasc. Imaging 2016, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Czompa, A.; Gyongyosi, A.; Czegledi, A.; Csepanyi, E.; Bak, I.; Haines, D.D.; Tosaki, A.; Lekli, I. Cardioprotection afforded by sour cherry seed kernel: The role of heme oxygenase-1. J. Cardiovasc. Pharmacol. 2014, 64, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Bak, I.; Ferdinandy, P.; Mahmoud, F.F.; Al-Harbi, S.A.; Blasig, I.E.; Tosaki, A. Cardioprotective effects of the calcineurin inhibitor FK506 and the PAF receptor antagonist and free radical scavenger, EGb 761, in isolated ischemic/reperfused rat hearts. J. Cardiovasc. Pharmacol. 2000, 35, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Isoyama, S.; Maruyama, Y.; Ashikawa, K.; Sato, S.; Suzuki, H.; Watanabe, J.; Shimizu, Y.; Ino-Oka, E.; Takishima, T. Effects of afterload reduction on global left ventricular and regional myocardial functions in the isolated canine heart with stenosis of a coronary arterial branch. Circulation 1983, 67, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Lekli, I.; Teissier, P.; Bak, I.; Tosaki, A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: Focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012, 204, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Kupai, K.; Szabo, R.; Veszelka, M.; Awar, A.A.; Torok, S.; Csonka, A.; Barath, Z.; Posa, A.; Varga, C. Consequences of exercising on ischemia-reperfusion injury in type 2 diabetic Goto-Kakizaki rat hearts: Role of the HO/NOS system. Diabetol. Metab. Syndr. 2015, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Posa, A.; Kupai, K.; Menesi, R.; Szalai, Z.; Szabo, R.; Pinter, Z.; Palfi, G.; Gyongyosi, M.; Berko, A.; Pavo, I.; et al. Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid. Med. Cell. Longev. 2013, 2013, 521563. [Google Scholar] [PubMed]
- Posa, A.; Pavo, I.; Varga, C. Heme oxygenase contributes to estradiol and raloxifene-induced vasorelaxation in estrogen deficiency. Int. J. Cardiol. 2015, 189, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Maslov, L.N.; Naryzhnaia, N.V.; Podoksenov Iu, K.; Prokudina, E.S.; Gorbunov, A.S.; Zhang, I.; Pei Zh, M. Reactive oxygen species are triggers and mediators of an increase in cardiac tolerance to impact of ischemia-reperfusion. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova 2015, 101, 3–24. (In Russian) [Google Scholar]
- Mao, G.D.; Thomas, P.D.; Lopaschuk, G.D.; Poznansky, M.J. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the fenton reaction in sod toxicity. J. Biol. Chem. 1993, 268, 416–420. [Google Scholar] [PubMed]
- Bagatini, M.D.; Martins, C.C.; Battisti, V.; Gasparetto, D.; da Rosa, C.S.; Spanevello, R.M.; Ahmed, M.; Schmatz, R.; Schetinger, M.R.; Morsch, V.M. Oxidative stress versus antioxidant defenses in patients with acute myocardial infarction. Heart Vessels 2011, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Stange, E.; Agostini, B.; Paenberg, J. Changes in rabbit lipoprotein properties by dietary cholesterol, and saturated and polyunsaturated fats. Atherosclerosis 1975, 22, 125–148. [Google Scholar] [CrossRef]
- Cohen, E.; Aviram, M.; Khatib, S.; Volkova, N.; Vaya, J. Human carotid atherosclerotic plaque protein(s) change HDL protein(s) composition and impair HDL anti-oxidant activity. BioFactors 2016, 42, 115–128. [Google Scholar] [PubMed]
- Kim, E.J.; Kim, B.H.; Seo, H.S.; Lee, Y.J.; Kim, H.H.; Son, H.H.; Choi, M.H. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS ONE 2014, 9, e97841. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Like, W.; Yan, F.; Tian, X.; Qiuyan, W.; Lifeng, H. S-Allyl-l-cysteine sulfoxide inhibits tumor necrosis factor-α induced monocyte adhesion and intercellular cell adhesion molecule-1 expression in human umbilical vein endothelial cells. Anat. Rec. 2010, 293, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; Bombicz, M.; Priksz, D.; Balla, J.; Balla, G.; Gesztelyi, R.; Varga, B.; Haines, D.D.; Tosaki, A.; Juhasz, B. Adverse impact of diet-induced hypercholesterolemia on cardiovascular tissue homeostasis in a rabbit model: Time-dependent changes in cardiac parameters. Int. J. Mol. Sci. 2013, 14, 19086–19108. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Fulop, G.A.; Kovacs, A.; Csipo, T.; Bodi, B.; Priksz, D.; Juhasz, B.; Beke, L.; Hendrik, Z.; Mehes, G.; et al. Renin overexpression leads to increased titin-based stiffness contributing to diastolic dysfunction in hypertensive mRen2 rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1671–H1682. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Varga, B.; Czompa, A.; Bak, I.; Lekli, I.; Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Antal, M.; Szendrei, L.; et al. Postischemic cardiac recovery in heme oxygenase-1 transgenic ischemic/reperfused mouse myocardium. J. Cell. Mol. Med. 2011, 15, 1973–1982. [Google Scholar] [CrossRef] [PubMed]





| Mean ± SEM | HR (bpm) | Ao (cm) | LV ESD (cm) | LV EDD (cm) | FS PLAX (%) |
|---|---|---|---|---|---|
| Control | 180.8 ± 4.145 | 0.946 ± 0.024 | 1.016 ± 0.091 | 1.655 ± 0.050 | 39.370 ± 5.021 |
| HC | 150.2 ± 4.303 * | 0.919 ± 0.025 | 1.242 ± 0.045 * | 1.756 ± 0.063 | 29.220 ± 0.803 * |
| HCT | 185.0 ± 7.053 ** | 0.898 ± 0.012 | 1.184 ± 0.020 | 1.793 ± 0.031 | 33.820 ± 1.312 ** |
| EF PLAX (%) | LV mass PLAX (g) | FS SAX (%) | EF SAX (%) | LV mass SAX (g) | |
| Control | 56.910 ± 1.294 | 6.632 ± 0.478 | 32.310 ± 0.717 | 54.130 ± 0.961 | 6.573 ± 0.351 |
| HC | 49.810 ± 1.140 * | 8.218 ± 0.628 | 29.010 ± 1.056 * | 49.430 ± 1.517 * | 8.315 ± 0.792 * |
| HCT | 55.990 ± 1.756 ** | 8.769 ± 0.169 * | 32.970 ± 1.131 ** | 54.930 ± 1.522 ** | 8.195 ± 0.226 * |
| E/A | DecT (ms) | E/E’ | LVOTVmax (cm/s) | LVOTVTI (cm) | |
| Control | 1.376 ± 0.045 | 71.250 ± 4.101 | 1.417 ± 0.058 | 84.280 ± 2.131 | 0.071 ± 0.002 |
| HC | 1.207 ± 0.037 * | 87.440 ± 3.534 * | 1.775 ± 0.101 | 87.940 ± 5.719 | 0.080 ± 0.005 |
| HCT | 1.344 ± 0.076 | 69.540 ± 4.787 ** | 1.718 ± 0.155 | 77.150 ± 2.157 | 0.0685 ± 0.002 |
| E’/A’ (lateral) | MAPSE (cm) | RV S′ (cm/s) | RV E′/A′ | TAPSE (cm) | |
| Control | 1.303 ± 0.058 | 0.527 ± 0.018 | 8.935 ± 0.273 | 1.336 ± 0.051 | 0.576 ± 0.012 |
| HC | 1.109 ± 0.071 | 0.571 ± 0.025 | 8.103 ± 0.215 * | 1.233 ± 0.092 | 0.576 ± 0.015 |
| HCT | 1.065 ± 0.117 | 0.592 ± 0.030 * | 9.156 ± 0.210 ** | 1.055 ± 0.077 * | 0.644 ± 0.020 ** |
| Groups | TC | LDL-C | HDL-C | ApoA | ApoB |
|---|---|---|---|---|---|
| Control | 0.793 ± 0.067 | 0.230 ± 0.023 | 0.523 ± 0.045 | 0.042 ± 0.005 | 0.015 ± 0.003 |
| HC | 26.370 ± 3.660 *,# | 23.550 ± 3.032 *,# | 4.427 ± 0.656 * | 0.022 ± 0.003 *,# | 0.280 ± 0.063 *,# |
| HCT | 20.030 ± 1.947 *,** | 17 ± 1.942 *,** | 3.314 ± 0.369 * | 0.056 ± 0.008 ** | 0.172 ± 0.019 *,** |
| TG | CRP | GOT | LDH | CK | |
| Control | 0.788 ± 0.035 | 0.100 ± 0.014 | 29.670 ± 2.895 | 726.400 ± 170.700 | 4213 ± 623.200 |
| HC | 1.367 ± 0.335 | 0.728 ± 0.362 * | 48.800 ± 16.220 * | 791.900 ± 325.400 | 4955 ± 1353 |
| HCT | 1.052 ± 0.339 | 0.596 ± 0.231 | 24.910 ± 2.708 ** | 316.600 ± 37.170 ** | 2851 ± 600.800 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombicz, M.; Priksz, D.; Varga, B.; Gesztelyi, R.; Kertesz, A.; Lengyel, P.; Balogh, P.; Csupor, D.; Hohmann, J.; Bhattoa, H.P.; et al. Anti-Atherogenic Properties of Allium ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits. Int. J. Mol. Sci. 2016, 17, 1284. https://doi.org/10.3390/ijms17081284
Bombicz M, Priksz D, Varga B, Gesztelyi R, Kertesz A, Lengyel P, Balogh P, Csupor D, Hohmann J, Bhattoa HP, et al. Anti-Atherogenic Properties of Allium ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits. International Journal of Molecular Sciences. 2016; 17(8):1284. https://doi.org/10.3390/ijms17081284
Chicago/Turabian StyleBombicz, Mariann, Daniel Priksz, Balazs Varga, Rudolf Gesztelyi, Attila Kertesz, Peter Lengyel, Peter Balogh, Dezso Csupor, Judit Hohmann, Harjit Pal Bhattoa, and et al. 2016. "Anti-Atherogenic Properties of Allium ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits" International Journal of Molecular Sciences 17, no. 8: 1284. https://doi.org/10.3390/ijms17081284
APA StyleBombicz, M., Priksz, D., Varga, B., Gesztelyi, R., Kertesz, A., Lengyel, P., Balogh, P., Csupor, D., Hohmann, J., Bhattoa, H. P., Haines, D. D., & Juhasz, B. (2016). Anti-Atherogenic Properties of Allium ursinum Liophylisate: Impact on Lipoprotein Homeostasis and Cardiac Biomarkers in Hypercholesterolemic Rabbits. International Journal of Molecular Sciences, 17(8), 1284. https://doi.org/10.3390/ijms17081284