Root Foraging Behavior of Two Agronomical Herbs Subjected to Heterogeneous P Pattern and High Ca Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Commencement
2.2. Experiment Design and Arrangement
2.3. Plant Culture and Sampling
2.4. Calculation and Statistical Analysis
3. Results
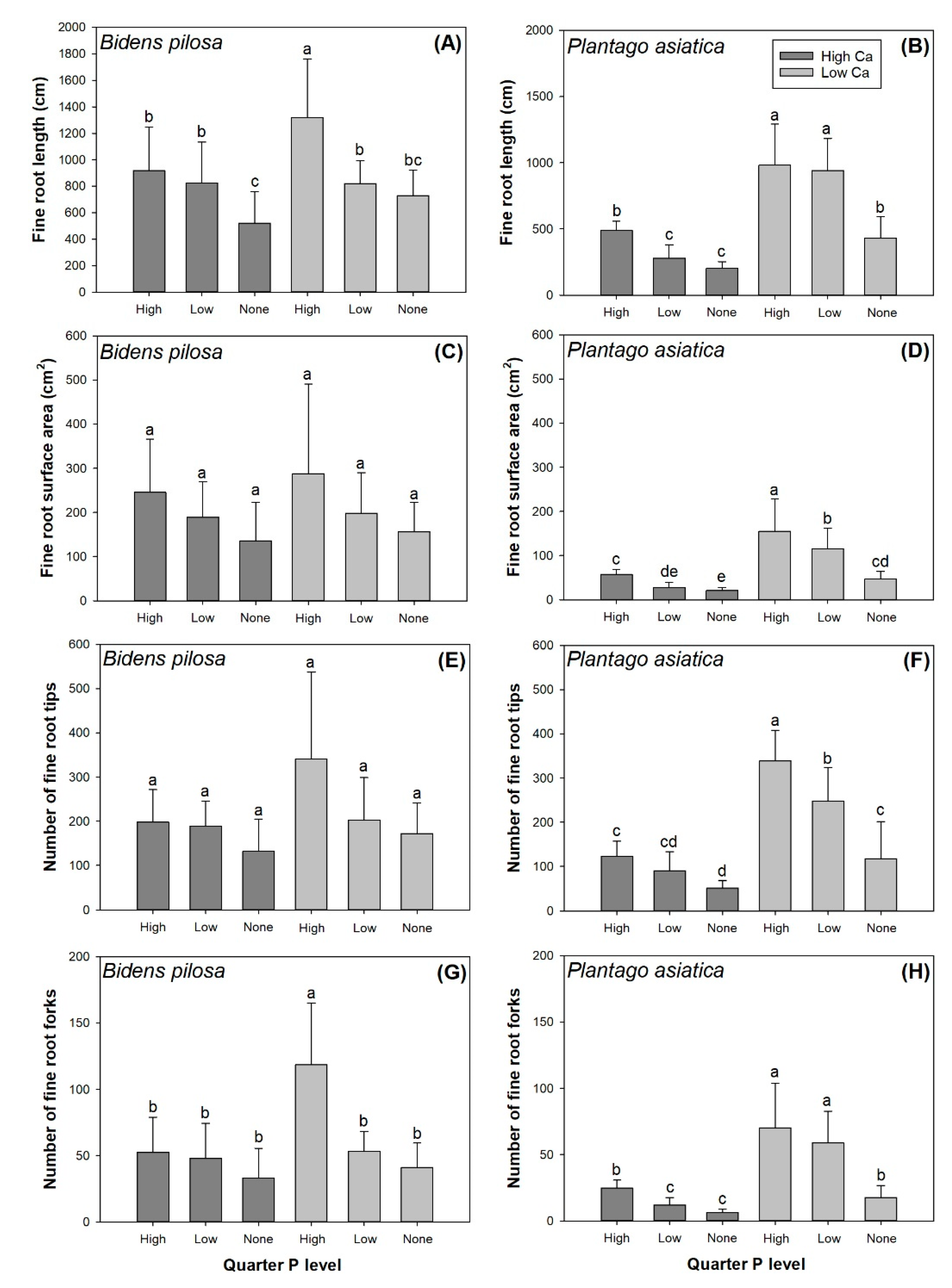
3.1. Fine Root Morphology Per Pot Section
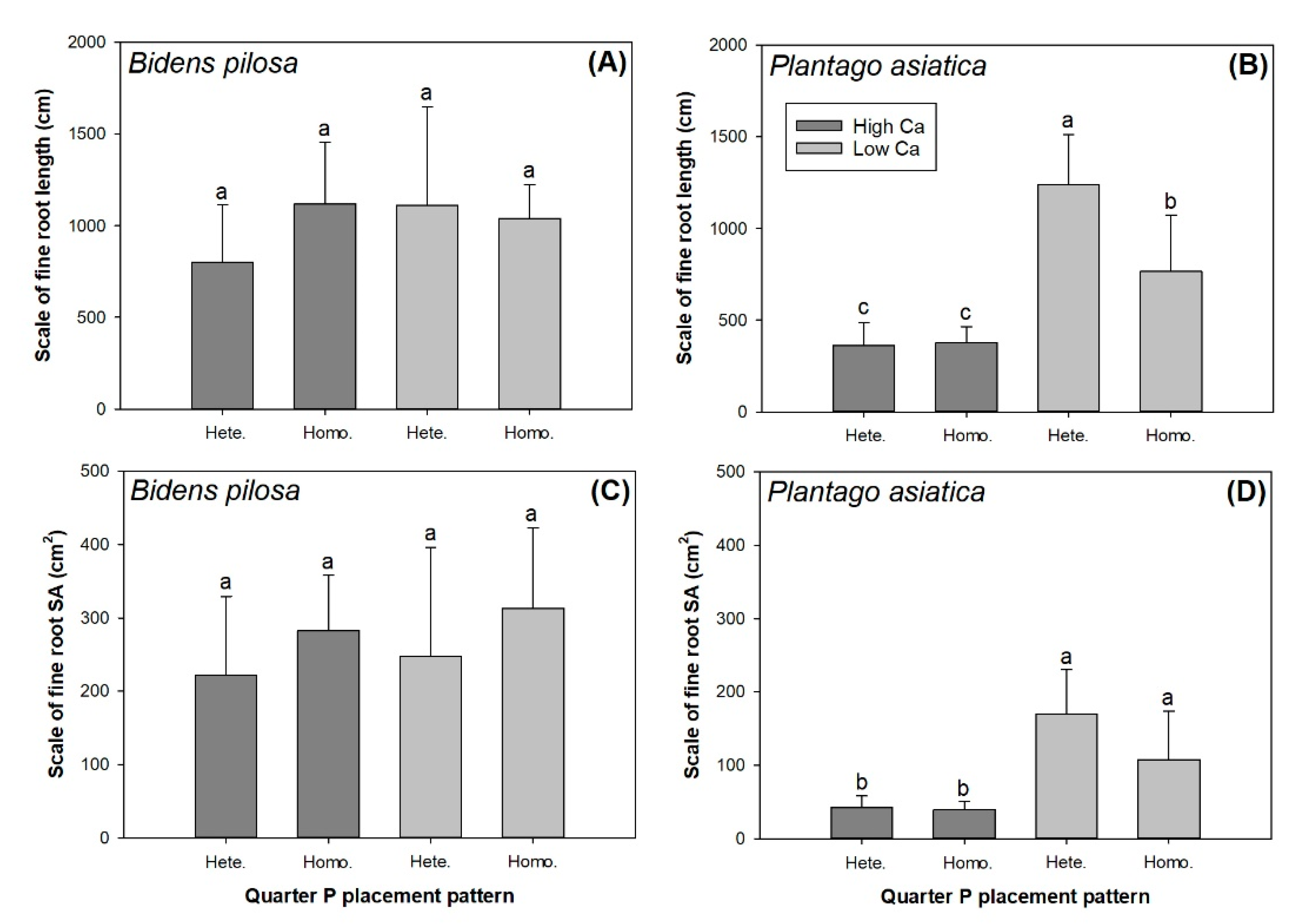
3.2. Root Foraging Scale
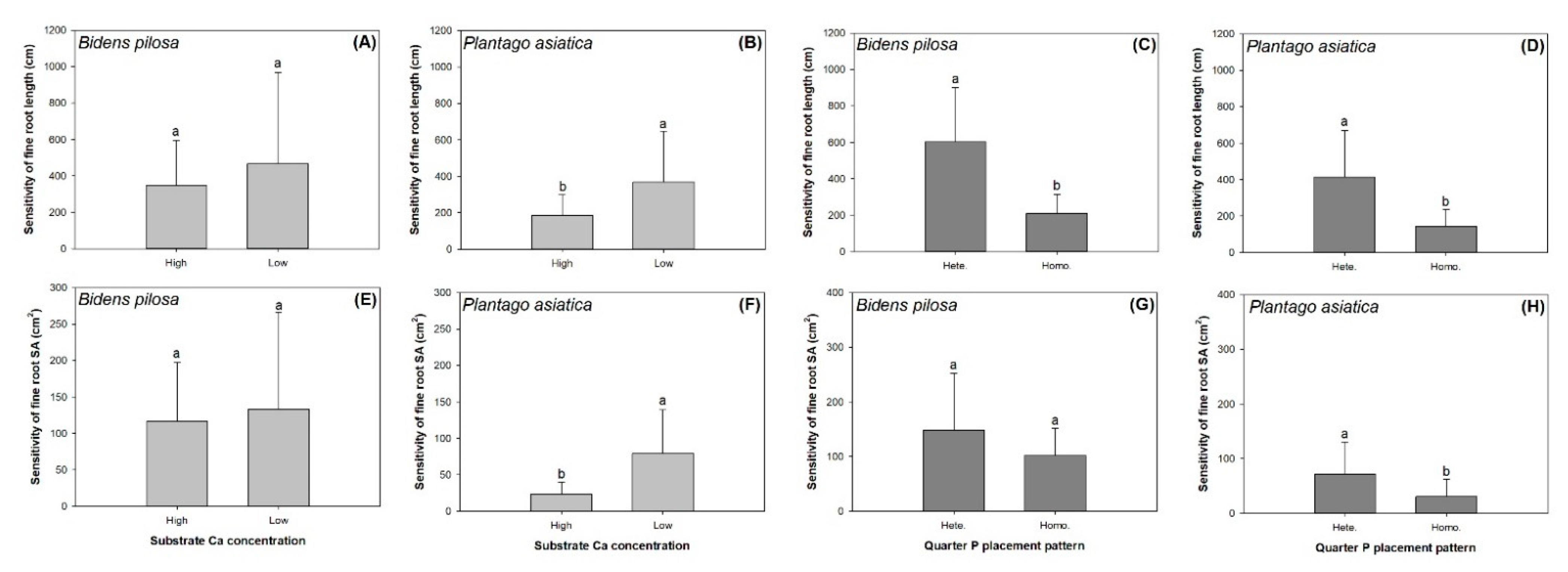
3.3. Foot Foraging Sensitivity
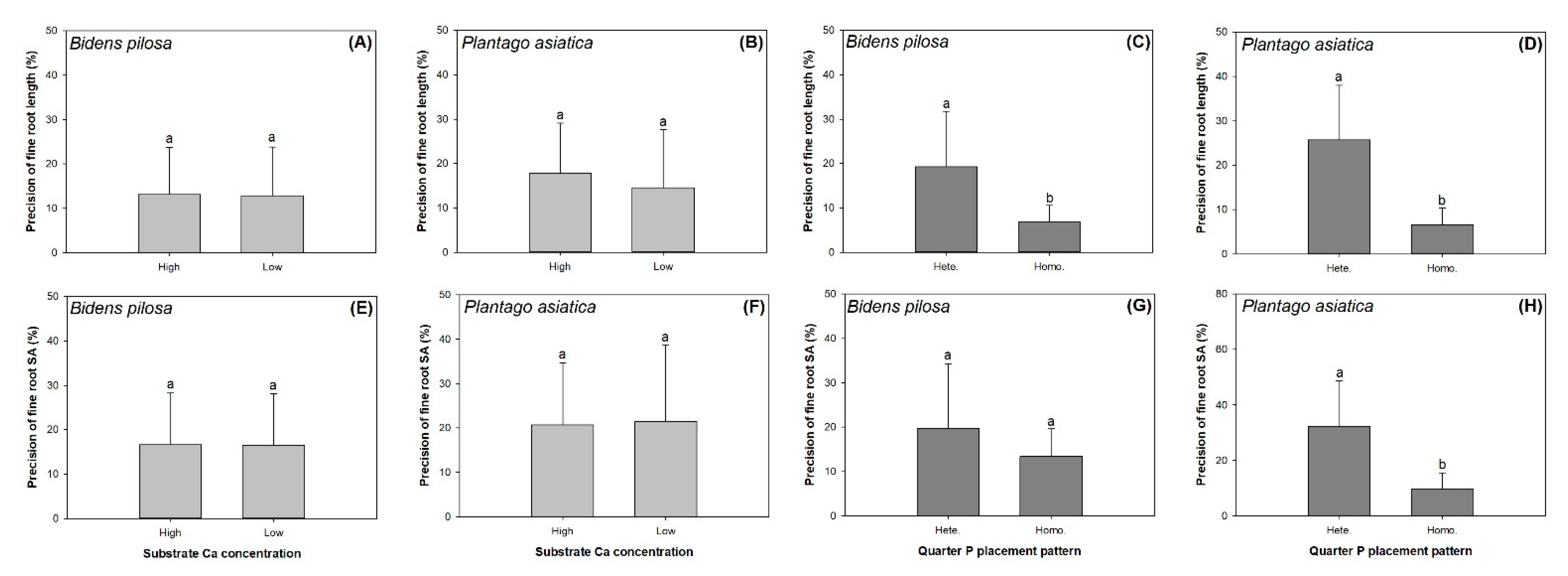
3.4. Foot Foraging Precision
3.5. Regressions of Root Foraging Parameters against Fine Root Morphologies
3.6. Relationship between Root Foraging Scale and Precision
4. Discussion
4.1. Fine Root Placement in Heterogenous P Patches in High Ca Soils
4.2. Root Foraging Behavior and Driving Forces from Fine Root Morphology
4.3. Relationship between Root Foraging Scale and Precision
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, X.; Deng, X.; Xiang, W.; Lei, P.; Ouyang, S.; Wen, H.; Chen, L. Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan, China. Biogeosciences 2018, 15, 2991–3002. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.L.; Price, R.M.; Schedlbauer, J.L.; Saha, A.; Gaiser, E.E. The Influence of Hydrologic Restoration on Groundwater-Surface Water Interactions in a Karst Wetland, the Everglades (FL, USA). Wetlands 2014, 34, 23–35. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Luo, G.; Yang, H.; Wang, S. The Evolution of a Karst Rocky Desertification Land Ecosystem and Its Driving Forces in the Houzhaihe Area, China. Open J. Ecol. 2015, 5, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Tang, X.; Qin, Y.; He, Q.; Yi, Y.; Ji, Z. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2019, 649, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Song, A.; Yang, H.; Müller, W.E.G. Impact of Rocky Desertification Control on Soil Bacterial Community in Karst Graben Basin, Southwestern China. Front. Microbiol. 2021, 12, 636405. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Huang, Y.; Wu, F.; Liu, W.; Ning, Y.; Huang, Z.; Tang, S.; Liang, Y. Plant adaptability in karst regions. J. Plant Res. 2021, 134, 889–906. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.R. The Role of Rock Weathering in the Phosphorus Budget of Terrestrial Watersheds. Biogeochemistry 1990, 11, 97–110. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Niinemets, Ü.; Kull, K. Co-limitation of plant primary productivity by nitrogen and phosphorus in a species-rich wooded meadow on calcareous soils. Acta Oecologica 2005, 28, 345–356. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Meng, W.P.; Dai, Q.H.; Ren, Q.Q.; Tu, N.; Leng, T.J. Ecological stoichiometric characteristics of soil-moss C, N, and P in restoration stages of karst rocky desertification. PLoS ONE 2021, 16, e0252838. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, D.Q.; Bai, X.Y.; Zeng, C.; Xiao, J.Y.; Qian, Q.H.; Luo, G.J. Responses of Soil Physical and Chemical Properties to Karst Rocky Desertification Evolution in Typical Karst Valley Area. In Proceedings of the 3rd International Conference on Environmental Science and Material Application (ESMA), Chongqing, China, 25–26 November 2017; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Nord, E.A.; Shea, K.; Lynch, J.P. Optimizing reproductive phenology in a two-resource world: A dynamic allocation model of plant growth predicts later reproduction in phosphorus-limited plants. Ann. Bot. 2011, 108, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Schenk, M.K.; Barber, S.A. Phosphate Uptake by Corn as Affected by Soil Characteristics and Root Morphology. Soil Sci. Soc. Am. J. 1979, 43, 880–883. [Google Scholar] [CrossRef]
- Do Nascimento, C.A.C.; Pagliari, P.H.; Faria, L.d.A.; Vitti, G.C. Phosphorus Mobility and Behavior in Soils Treated with Calcium, Ammonium, and Magnesium Phosphates. Soil Sci. Soc. Am. J. 2018, 82, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.J.; de Kroon, H. Foraging in Plants: The Role of Morphological Plasticity in Resource Acquisition. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MA, USA, 1994; Volume 25, pp. 159–238. [Google Scholar]
- Werner, F.; Mueller, C.W.; Thieme, J.; Gianoncelli, A.; Rivard, C.; Höschen, C.; Prietzel, J. Micro-scale heterogeneity of soil phosphorus depends on soil substrate and depth. Sci. Rep. 2017, 7, 3203. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- He, C.; Gao, J.; Zhao, Y.; Liu, J. Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra. Plants 2021, 10, 1482. [Google Scholar] [CrossRef]
- Wei, H.X.; Guo, P.; Zheng, H.F.; He, X.Y.; Wang, P.J.; Ren, Z.B.; Zhai, C. Micro-scale heterogeneity in urban forest soils affects fine root foraging by ornamental seedlings of Buddhist pine and Northeast yew. Urban For. Urban Green 2017, 28, 63–72. [Google Scholar] [CrossRef]
- Tan, L.; Fan, R.; Sun, H.; Guo, S. Root foraging of birch and larch in heterogeneous soil nutrient patches under water deficit. PLoS ONE 2021, 16, e0255848. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Liang, Y.; Wang, K.; Zhang, W. Responses of Fine Root Functional Traits to Soil Nutrient Limitations in a Karst Ecosystem of Southwest China. Forests 2018, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wei, X.; Zhou, Z.; Shao, C.; Su, S. Influence of Heterogeneous Karst Microhabitats on the Root Foraging Ability of Chinese Windmill Palm (Trachycarpus fortunei) Seedlings. Int. J. Environ. Res. Public Health 2020, 17, 434. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.D.; Grime, J.P.; Mackey, J.M.L. A trade-off between scale and precision in resource foraging. Oecologia 1991, 87, 532–538. [Google Scholar] [CrossRef]
- Wijesinghe, D.K.; John, E.A.; Beurskens, S.; Hutchings, M.J. Root system size and precision in nutrient foraging: Responses to spatial pattern of nutrient supply in six herbaceous species. J. Ecol. 2001, 89, 972–983. [Google Scholar] [CrossRef]
- Grime, J.P. The Scale–Precision Trade-off in Spacial Resource Foraging by Plants: Restoring Perspective. Ann. Bot. 2007, 99, 1017–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macgillivray, C.W.; Grime, J.P.; Band, S.R.; Booth, R.E.; Campbell, B.; Hendry, G.A.F.; Hillier, S.H.; Hodgson, J.G.; Hunt, R.; Jalili, A.; et al. Testing Predictions of the Resistance and Resilience of Vegetation Subjected to Extreme Events. Funct. Ecol. 1995, 9, 640–649. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Fritschi, F.B.; Juenger, T.E. The genetic basis of the root economics spectrum in a perennial grass. Proc. Natl. Acad. Sci. USA 2021, 118, e2107541118. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yan, W.; Quan, G.; Zhang, J.; Liang, K. Soil microbial carbon utilization, enzyme activities and nutrient availability responses to Bidens pilosa and a non-invasive congener under different irradiances. Sci. Rep. 2017, 7, 11309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISSG. Global Invasive Species Database: Bidens pilosa in Terrestrial System. Available online: http://www.iucngisd.org/gisd/speciesname/Bidens+pilosa (accessed on 10 January 2022).
- Rojas-Sandoval, J. Bidens pilosa (Blackjack); Invasive Species Compendium; CABI: Wallingford, UK, 2020. [Google Scholar]
- Li, F.; He, X.; Sun, Y.; Zhang, X.; Tang, X.; Li, Y.; Yi, Y. Distinct endophytes are used by diverse plants for adaptation to karst regions. Sci. Rep. 2019, 9, 5246. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, J.; Li, S.H.; Song, H.Y.; Wang, J.M.; Tao, J.P.; Liu, J.C. Synergistic aboveground-belowground growth of Bidens pilosa L. in heterogeneous karst habitats. Plant Sci. J. 2020, 38, 762–772, (In Chinese with English abstract). [Google Scholar]
- Chen, B.M.; Su, J.Q.; Liao, H.X.; Peng, S.L. A greater foraging scale, not a higher foraging precision, may facilitate invasion by exotic plants in nutrient-heterogeneous conditions. Ann. Bot. 2018, 121, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.-Y.; Wang, P.; Liu, L.; Dong, B.-C.; Yu, F.-H. Root responses to nitrogen pulse frequency under different nitrogen amounts. Acta Oecologica 2017, 80, 32–38. [Google Scholar] [CrossRef]
- Takematsu, T.; Ichizen, N. Weeds of the World, Syspetalae; Zenkoku Noson Kyoiku Kyokai: Tokyo, Japan, 1987; Volume 1. [Google Scholar]
- Yang, Z.Q. Ecology analysis of invasive plants after 12 years of natural restoration in karst desertification area. In Land Reclamation in Ecological Fragile Areas, 1st ed.; Hu, Z., Ed.; CRC Press: London, UK, 2017. [Google Scholar]
- Yamawo, A.; Ohsaki, H.; Cahill, J.F. Damage to leaf veins suppresses root foraging precision. Am. J. Bot. 2019, 106, 1126–1130. [Google Scholar] [CrossRef]
- Zhou, C.W.; Shang, C.F.; Chen, F.Y.; Bao, J.Z.; Yu, L.F.; Guo, P. Light-emitting diode spectra modify nutritional status, physiological response, and secondary metabolites in Ficus hirta and Alpinia oxyphylla. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12314. [Google Scholar] [CrossRef]
- LY/T 1840-2009; People’s Republic of China Forestry Industry Standard: Technology Regulations of Vegetation Resotration in Karst Deserification Zone (Forestry Industry Standard). State Forestry Bureau, No. 8. Chinese Academy of Forestry Research Institute of Tropical Forestry: Beijing, China, 2009; p. B65.
- Nie, Y.-P.; Chen, H.-S.; Wang, K.-L.; Ding, Y.-L. Rooting characteristics of two widely distributed woody plant species growing in different karst habitats of southwest China. Plant Ecol. 2014, 215, 1099–1109. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.S.; He, C.X.; Wang, Q.R. Effects of light spectra and N-15 pulses on growth, leaf morphology, physiology, and internal nitrogen cycling in Quercus variabilis Blume seedlings. PLoS ONE 2021, 16, e0243954. [Google Scholar] [CrossRef]
- Chu, X.L.; Luo, X.Y.; Zhou, Z.C. Exponential fertilization on red-seed tree (Ormosia hosiei) seedlings subjected to contrasting light conditions: Do we really need intensive nutrient loading? Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12244. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Xu, S.J.; Li, Y.J.; He, C.X. Nutrient assimilation and utilization in korean pine (Pinus koraiensis) seedlings exposed to exponential fertilization under contrasting spectra. Commun. Soil Sci. Plant Anal. 2020, 51, 2414–2428. [Google Scholar] [CrossRef]
- Zhou, C.W.; Cui, W.J.; Yuan, T.; Cheng, H.Y.; Su, Q.; Guo, P. Water content, carbohydrate accumulation, and secondary metabolites in Allium victorialis sprouts exposed to shoot cutting in varied irradiations. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12524. [Google Scholar] [CrossRef]
- Wei, H.X.; Guo, P. Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: Post-transplant response to nursery-applied inorganic fertilizer and organic amendment. Iforest-Biogeosci. For. 2017, 10, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Xu, C.; Ren, J.; Ma, L.; Duan, J.; Jiang, L. Newly transplanted Larix olgensis Henry stock with greater root biomass has higher early nitrogen flux rate. Soil Sci. Plant Nutr. 2013, 59, 740–749. [Google Scholar] [CrossRef]
- Warren, C.R.; Livingston, N.J.; Turpin, D.H. Response of Douglas-fir seedlings to a brief pulse of 15N-labeled nutrients. Tree Physiol. 2003, 23, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Christian, N.; Herre, E.A.; Clay, K. Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol. 2019, 222, 1573–1583. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Zhang, D.H.; Wei, H.X.; Guo, J. Using morphological attributes for the fast assessment of nutritional responses of Buddhist pine (Podocarpus macrophyllus Thunb. D. Don) seedlings to exponential fertilization. PLoS ONE 2019, 14, e0225708. [Google Scholar] [CrossRef]
- Rajaniemi, T.K.; Reynolds, H.L. Root foraging for patchy resources in eight herbaceous plant species. Oecologia 2004, 141, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; Cahill, J.F. Plant phenotypic plasticity belowground: A phylogenetic perspective on root foraging trade-offs. Am. Nat. 2005, 166, 216–230. [Google Scholar] [CrossRef]
- Kramer, C.Y. Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications. Biometrics 1956, 12, 307–310. [Google Scholar] [CrossRef]
- Zhang, D.S.; Lyu, Y.; Li, H.B.; Tang, X.Y.; Hu, R.; Rengel, Z.; Zhang, F.S.; Whalley, W.R.; Davies, W.; Cahill, J.F.; et al. Neighbouring plants modify maize root foraging for phosphorus: Coupling nutrients and neighbours for improved nutrient-use efficiency. New Phytol. 2020, 226, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Tigabu, M.; Zhang, Y.; Ma, X.Q.; Cai, L.P.; Wu, P.F.; Liu, A.Q.; Wang, C.; Qiu, H.Y. Root plasticity, whole plant biomass, and nutrient accumulation of Neyraudia reynaudiana in response to heterogeneous phosphorus supply. J. Soils Sediments 2017, 17, 172–180. [Google Scholar] [CrossRef]
- Xia, Z.C.; He, Y.; Yu, L.; Lv, R.B.; Korpelainen, H.; Li, C.Y. Sex-specific strategies of phosphorus (P) acquisition in Populus cathayana as affected by soil P availability and distribution. New Phytol. 2020, 225, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.Y.; Zhou, B.Z.; Ge, X.G.; Cao, Y.H.; Brunner, I.; Shi, J.X.; Li, M.H. Species-Specific Responses of Root Morphology of Three Co-existing Tree Species to Nutrient Patches Reflect Their Root Foraging Strategies. Front. Plant Sci. 2021, 11, 618222. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.A.D.; Guimaraes, F.A.R.; Goncalves, V.A.; Benevenute, S.D.; Ferreira, G.L.; Ferreira, L.R.; Ferreira, F.A. Accumulation of macronutrients by weed and corn in coexistence in soil with different fertility managements. Semin.-Cienc. Agrar. 2015, 36, 669–681. [Google Scholar] [CrossRef]
- Becker, B.; Terrones, F.; Horchler, P. Weed communities in Andean cropping systems of northern Peru. J. Appl. Bot.-Angew. Bot. 1998, 72, 113–130. [Google Scholar]
- Cho, J.Y.; Park, Y.J.; Yul, Y.S.; Kim, H. Effects of the Different Concentration of the Nutrient Solution on the Growth and the Inorganic Matter Contents of Three Kinds of Fall Planting Namul Resources in Water Culture. Prot. Hortic. Plant Fact. 2007, 16, 7–12, (In Korean with Englilsh Abstract). [Google Scholar]
- Li, H.B.; Zhang, D.S.; Wang, X.X.; Li, H.G.; Rengel, Z.; Shen, J.B. Competition between Zea mays genotypes with different root morphological and physiological traits is dependent on phosphorus forms and supply patterns. Plant Soil 2019, 434, 125–137. [Google Scholar] [CrossRef]
- Holopainen, T.; HeinonenTanski, H.; Halonen, A. Injuries to Scots pine mycorrhizas and chemical gradients in forest soil in the environment of a pulp mill in Central Finland. Water Air Soil Pollut. 1996, 87, 111–130. [Google Scholar] [CrossRef]
- Tchiaze, A.I.; Taffouo, V.D.; Fankem, H.; Kenne, M.; Baziramakenga, R.; Ekodeck, G.E.; Antoun, H. Influence of Nitrogen Sources and Plant Growth-Promoting Rhizobacteria Inoculation on Growth, Crude Fiber and Nutrient Uptake in Squash (Cucurbita moschata Duchesne ex Poir.) Plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Bibikova, T.N.; Zhigilei, A.; Gilroy, S. Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity. Planta 1997, 203, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; de Kroon, H.; Cahill, J.F.; Mommer, L. Improving the scale and precision of hypotheses to explain root foraging ability. Ann. Bot. 2008, 101, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Mou, P.; Mitchell, R.J.; Jones, R.H. Root Distribution of Two Tree Species Under a Heterogeneous Nutrient Environment. J. Appl. Ecol. 1997, 34, 645–656. [Google Scholar] [CrossRef]

| Fine Root Parameter | Source of Variance | ||
|---|---|---|---|
| Ca | P | Ca × P | |
| Bidens pilosa | |||
| Length | 5.34 * | 16.12 *** | 3.38 * |
| Surface area | 0.99 | 9.07 | 0.17 |
| Number of tips | 4.43 * | 7.66 *** | 2.37 |
| Number of forks | 3.82 | 8.92 *** | 3.94 * |
| Plantago asiatica | |||
| Length | 228.54 *** | 51.14 *** | 20.25 *** |
| Surface area | 129.5 *** | 35.43 *** | 14.54 *** |
| Number of tips | 132.05 *** | 39.98 *** | 11.10 *** |
| Number of forks | 132.81 *** | 41.04 *** | 17.21 *** |
| Root Foraging Parameter | Source of Variance | ||
|---|---|---|---|
| Ca | Pattern | Ca × Pattern | |
| Bidens pilosa | |||
| Scale of root length | 2.36 | 1.00 | 2.42 |
| Scale of root surface area | 0.58 | 2.82 | 0.01 |
| Sensitivity of root length | 0.83 | 8.85 ** | 1.46 |
| Sensitivity of root surface area | 0.16 | 1.27 | 0.01 |
| Precision of root length | 0.01 | 12.93 *** | 0.18 |
| Precision of root surface area | 0.01 | 2.26 | 0.25 |
| Plantago asiatica | |||
| Scale of root length | 69.70 *** | 11.90 ** | 10.65 ** |
| Scale of root surface area | 39.74 *** | 4.13 * | 4.79 * |
| Sensitivity of root length | 8.02 ** | 17.56 *** | 1.03 |
| Sensitivity of root surface area | 13.60 *** | 7.47 * | 0.97 |
| Precision of root length | 0.90 | 31.97 *** | 0.01 |
| Precision of root surface area | 0.03 | 23.83 *** | 0.03 |
| Root Foraging | Root Morphology | Length | Surface Area | ||||
|---|---|---|---|---|---|---|---|
| Estimate | F Value | p Value | Estimate | F Value | p Value | ||
| Bidens pilosa | |||||||
| Scale | Intercept | −24.14 | 0.07 | 0.7925 | −3.53 | 0.02 | 0.8802 |
| Length | 1.33 | 141.22 | <0.0001 | ||||
| Surface area | 1.11 | 26.56 | <0.0001 | ||||
| Tip number | |||||||
| Fork number | |||||||
| Sensitivity | Intercept | −28.34 | 0.03 | 0.86 | −22.17 | 0.28 | 0.6006 |
| Length | |||||||
| Surface area | |||||||
| Tip number | |||||||
| Fork number | 2.3 | 8.53 | 0.0058 | 0.78 | 14.74 | 0.0005 | |
| Precision | Intercept | ||||||
| Length | |||||||
| Surface area | |||||||
| Tip number | |||||||
| Fork number | |||||||
| Plantago asiatica | |||||||
| Scale | Intercept | 42.59 | 2.64 | 0.113 | 99.69 | 9.56 | 0.0039 |
| Length | 0.63 | 13.77 | 0.0007 | −0.18 | 17.08 | 0.0002 | |
| Surface area | 5.15 | 17.96 | 0.0001 | 3.15 | 87.62 | <0.0001 | |
| Tip number | −291.42 | 9.2 | 0.0045 | ||||
| Fork number | |||||||
| Sensitivity | Intercept | −698.46 | 4.82 | 0.0343 | −280.88 | 23.79 | <0.0001 |
| Length | |||||||
| Surface area | |||||||
| Tip number | 2747.15 | 9.54 | 0.0037 | 935.26 | 33.75 | <0.0001 | |
| Fork number | |||||||
| Precision | Intercept | 25.25 | 35.87 | <0.0001 | |||
| Length | |||||||
| Surface area | |||||||
| Tip number | |||||||
| Fork number | −0.06 | 6.27 | 0.0167 | ||||
| Foraging Parameter | Correlation Coefficients | Scale | Sensitivity | Precision | Scale | Sensitivity | Precision |
|---|---|---|---|---|---|---|---|
| Root Length | Root Surface Area | ||||||
| Scale | R | 1 | 0.5317 | 0.2225 | 1 | 0.5832 | 0.2626 |
| p | - | 0.0004 | 0.1676 | - | <0.0001 | 0.1017 | |
| Sensitivity | R | 0.8532 | 1 | 0.8797 | 0.2665 | 1 | 0.8656 |
| p | <0.0001 | - | <0.0001 | 0.0965 | - | <0.0001 | |
| Precision | R | 0.2626 | 0.8656 | 1 | −0.2258 | 0.7872 | 1 |
| p | 0.1017 | <0.0001 | - | 0.1612 | <0.0001 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Cui, W.; Yuan, T.; Cheng, H.; Su, Q.; Wei, H.; Guo, P. Root Foraging Behavior of Two Agronomical Herbs Subjected to Heterogeneous P Pattern and High Ca Stress. Agronomy 2022, 12, 624. https://doi.org/10.3390/agronomy12030624
Zhou C, Cui W, Yuan T, Cheng H, Su Q, Wei H, Guo P. Root Foraging Behavior of Two Agronomical Herbs Subjected to Heterogeneous P Pattern and High Ca Stress. Agronomy. 2022; 12(3):624. https://doi.org/10.3390/agronomy12030624
Chicago/Turabian StyleZhou, Changwei, Wenjing Cui, Ting Yuan, Huayan Cheng, Qian Su, Hongxu Wei, and Peng Guo. 2022. "Root Foraging Behavior of Two Agronomical Herbs Subjected to Heterogeneous P Pattern and High Ca Stress" Agronomy 12, no. 3: 624. https://doi.org/10.3390/agronomy12030624







