Multidisciplinary Care of a Vertebral Fracture in a Patient with Hematopoietic Stem Cell Transplant: Safety Appropriateness in Interventional Pain Management and Rehabilitation Considerations
Abstract
:1. Introduction
2. Materials and Methods
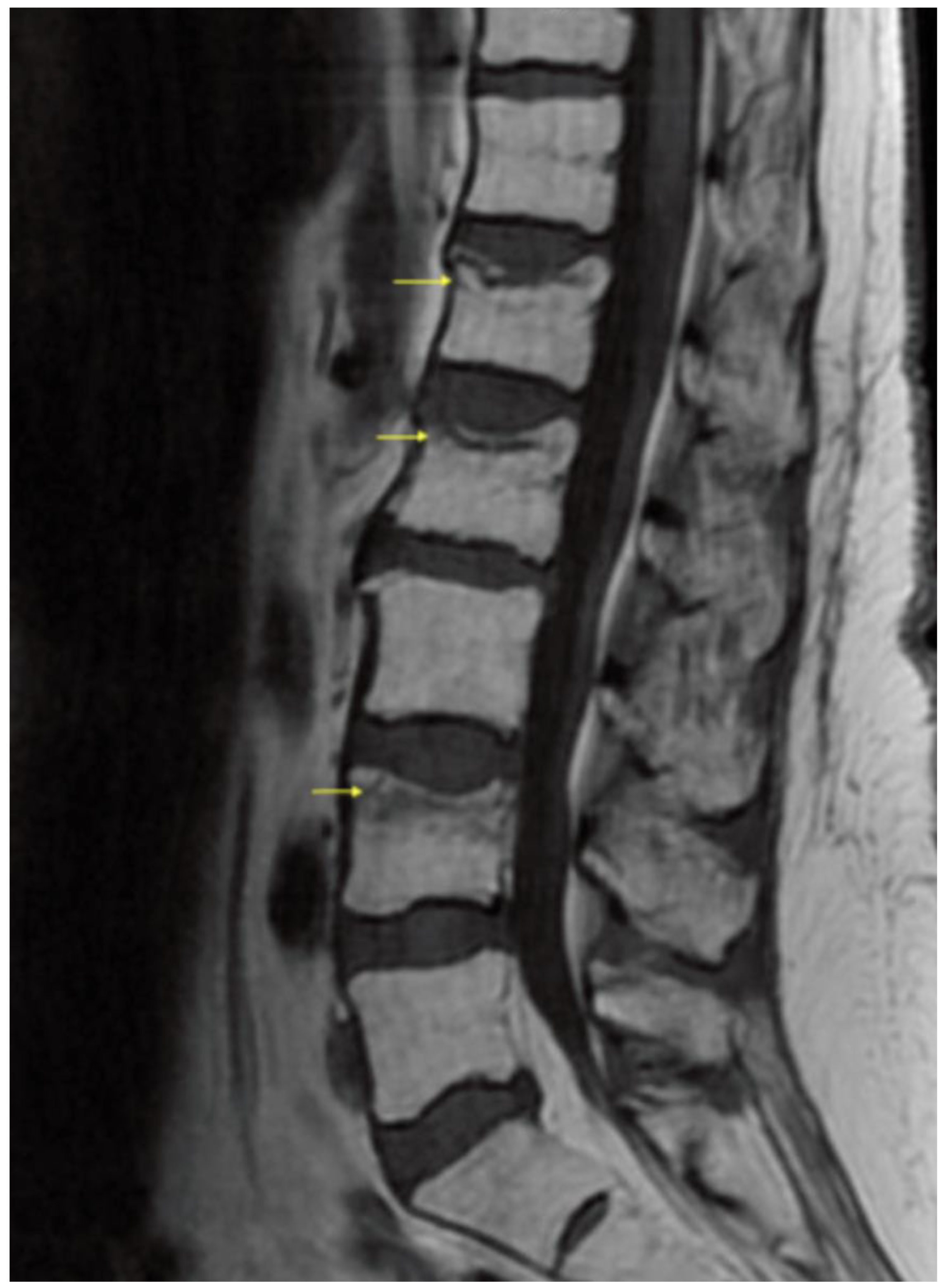
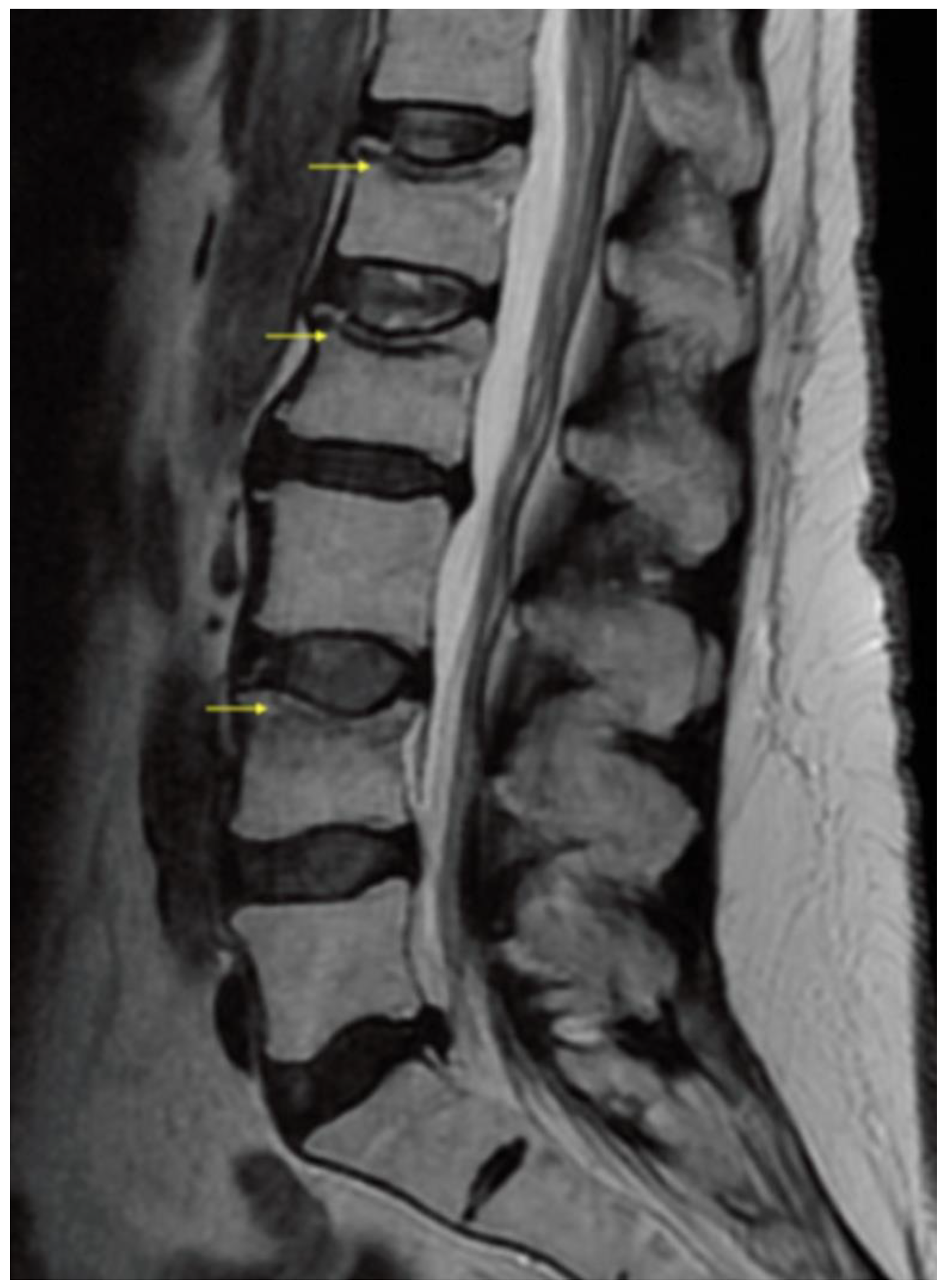
3. Results/Case Report
4. Discussion
4.1. Multidisciplinary Rehabilitation
4.2. Interventional Pain Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pundole, X.N.; Barbo, A.G.; Lin, H.; Champlin, R.E.; Lu, H. Increased incidence of fractures in recipients of hematopoietic stem cell transplantation. J. Clin. Oncol. 2015, 33, 1364–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; McCarthy, P.L.; Dunford, L.M.; Roy, D.M.; Brown, K.; Paplham, P.; Syta, M.; LaMonica, D.; Smiley, S.L.; Battiwalla, M.; et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant. 2008, 41, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Body, J.J.; Brandi, M.L.; Broady, R.; Cannata-Andia, J.; Cannata-Ortiz, M.J.; el Maghraoui, A.; Guglielmi, G.; Hadji, P.; Pierroz, D.D.; et al. Osteoporosis management in hematologic stem cell transplant recipients: Executive summary. J. Bone Oncol. 2021, 28, 100361. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.; Bouchud, P.Y. Risk stratification and immunogenic risk for infections following stem cell transplantation. Virulence 2016, 7, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Ebeling, P.R. Approach to the patient with transplantation related bone-loss. J. Clin. Endocrinol. Metab. 2009, 91, 1283–1490. [Google Scholar] [CrossRef] [Green Version]
- Edidin, A.A.; Ong, K.; Lau, E.; Kurtz, S.M. Morbidity and Mortality after Vertebral Fractures. Spine 2015, 40, 1228–1241. [Google Scholar] [CrossRef]
- Steinberg, A.; Sher, A.; Bailey, C.; Fu, J.B. The role of physical rehabilitation in stem cell transplantation patients. Support. Care Cancer 2015, 23, 2447–2460. [Google Scholar] [CrossRef]
- Stubblefield, M.D.; O’Dell, M.W. Cancer Rehabilitation Principles and Practice, 1st ed.; Demos Medical: New York, NY, USA, 2009. [Google Scholar]
- Hacker, E.D.; Larson, J.L.; Peace, D. Exercise in patients receiving hematopoietic stem cell transplantation: Lessons learned and results from a feasibility study. Oncol. Nurs. Forum 2011, 38, 215–223. [Google Scholar] [CrossRef]
- De Lisio, M.; Baker, J.M.; Parise, F. Exercise promotes bone marrow cell cervical and recipient reconstitution post-bone marrow transplantation, which is associated with increased survival. Exp. Hematol. 2013, 41, 143–154. [Google Scholar] [CrossRef]
- Kerezoudis, P.; Rinaldo, L.; Alvi, M.A.; Hunt, C.L.; Qu, W.; Maus, T.P.; Bydon, M. The effect of epidural steroid injections on bone mineral density and vertebral fracture risk: A systematic review and critical appraisal of current literature. Pain Med. 2018, 19, 569–579. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Bellone, T.; Scardapane, M.; De Marini, P.; Autrusseau, P.-A.; Auloge, P.; Garnon, J.; Jennings, J.W.; Gangi, A. Vertebral augmentation reduces the 12-month mortality and morbidity in patients with osteoporotic vertebral compression fractures. Eur. Radiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.; Maingard, J.; Hirsch, J.A.; Phan, K.; Asadi, H.; Chandra, R.V. Mortality Outcomes of Vertebral Augmentation (Vertebroplasty and/or Balloon Kyphoplasty) for Osteoporotic Vertebral Compression Fractures: A Systematic Review and Meta-Analysis. Radiology 2020, 295, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Beall, D.P.; Lorio, M.P.; Yun, B.M.; Runa, M.J.; Ong, K.L.; Warner, C.B. Review of vertebral augmentation: An updated meta-analysis of the effectiveness. Int. J. Spine Surg. 2018, 12, 295–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beall, D.P.; Chambers, M.F.; Thomas, S.M.; Amburgy, J.; Webb, J.R.; Goodman, B.S.; Datta, D.K.; Easton, R.W.; Linville, D.; Talati, S.; et al. Prospective and multicenter evaluation of outcomes for quality of life and activities of daily living for balloon kyphoplasty in the treatment of vertebral compression fractures: The EVOLVE Trial. Neurosurgery 2019, 84, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Bar, M.; Ott, S.M.; Lewiecki, E.M.; Sarafoglou, K.; Wu, J.Y.; Thompson, M.J.; Vaux, J.J.; Dean, D.R.; Saag, K.G.; Hashmi, S.K.; et al. Bone health management after hematopoietic cell transplantation: An expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2020, 26, 1784–1802. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Fink, H.A.; Mirigian, L.; Guañabens, N.; Eastell, R.; Akesson, K.; Bauer, D.C.; Ebeling, P.R. Pain, quality of life and safety outcomes of kyphoplasty for vertebral compression fractures: Report of a task force of the American Society of Bone and Mineral Research. J. Bone Miner. Res. 2017, 32, 1935–1944. [Google Scholar] [CrossRef] [Green Version]
- Chandra, R.V.; Yoo, A.J.; Hirsch, J.A. Vertebral augmentation: Update on safety, efficacy, cost effectiveness and increased survival? Pain Physician 2013, 16, 309–320. [Google Scholar] [CrossRef]
- Helm, S.; Harmon, P.C.; Noe, C.; Calodney, A.K.; Abd-Elsayed, A.; Knezevic, N.N.; Racz, G.B. Transforaminal epidural steroid injections: A systematic review and meta-analysis of efficacy and safety. Pain Physician 2021, 24, S209–S232. [Google Scholar]
- Tauchmanova, L.; Cola, A.; Lombardi, G.; Rotoli, B.; Selleri, C. Bone Loss and Its Management in Long-Term Survivors from Allogeneic Stem Cell Transplantation. J. Clin. Endocrinol. Metab. 2007, 92, 4536–4545. [Google Scholar] [CrossRef] [Green Version]
- Kendler, D.L.; Body, J.J.; Brandi, M.L.; Broady, R.; Cannata-Andia, J.; Cannata-Ortiz, M.J.; El Maghraoui, A.; Guglielmi, G.; Hadji, P.; Pierroz, D.D.; et al. Bone management in hematologic stem cell transplant recipients. Osteoporos. Int. 2018, 29, 2597–2610. [Google Scholar] [CrossRef]
- Allen, C.S.; Yeung, J.H.; Vandermeer, B.; Homik, J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst. Rev. 2016, 10, CD001347. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Cenzer, I.; Nguyen, B.; Lee, S.J. Time to Benefit of Bisphosphonate Therapy for the Prevention of Fractures Among Postmenopausal Women with Osteoporosis: A Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kobza, A.O.; Herman, D.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Understanding and Managing Corticosteroid-Induced Osteoporosis. Open Access Rheumatol. 2021, 13, 177–190. [Google Scholar] [CrossRef]
- Pinto, D.; Alshahrani, M.; Chapurlat, R.; Chevalley, T.; Dennison, E.; Camargos, B.M.; Papaioannou, A.; Silverman, S.; Kaux, J.-F.; Lane, N.E.; et al. The global approach to rehabilitation following osteoporotic fragility fracture: A review of the rehabilitation working group of the International Osteoporosis Foundation (IOF) committee of scientific advisors. Osteoporos. Int. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. Practice Guidelines for Spinal Diagnostic and Treatment Procedures; International Spine Intervention Society: San Francisco, CA, USA, 2013. [Google Scholar]
- Cobos, E.; Cruz, J.C.; Day, M. Etiology and management of coagulation abnormalities in the pain management patient. Curr. Rev. Pain 2000, 4, 413–419. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.M.; Schaefer, M.P.; Malkamaki, D.M. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011, 12, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Friedly, J.L.; Comstock, B.A.; Standaert, C.J.; Nedeljkovic, S.; Kennedy, D.J.; Sibell, D.M.; Suri, P.; Akuthota, V.; Annaswamy, T.M.; Bauer, Z.; et al. Patient and procedural risk factors for cortisol suppression following epidural steroid injections for spinal stenosis. PM R 2016, 8, S159–S160. [Google Scholar] [CrossRef]
- Spine Intervention Society (SIS) Patient Safety Committee. Spinal injections in immunocompromised patients and the risk associated with procedural care: To inject or not to inject? Fact finders for patient safety. Spine Interv. Soc. 2019. Available online: https://www.spineintervention.org/page/FactFinders#POTENTIAL (accessed on 10 February 2022).
- Liao, J.C.; Lai, P.L.; Chen, L.H.; Niu, C.-C. Surgical outcomes of infectious spondylitis after vertebroplasty, and comparisons between pyogenic and tuberculosis. BMC Infect. Dis. 2018, 18, 555. [Google Scholar] [CrossRef]
- Park, J.W.; Park, S.M.; Lee, H.J.; Lee, C.K.; Chang, B.S.; Kim, H. Infection following percutaneous vertebral augmentation with polymethylmethacrylate. Arch. Osteoporos. 2018, 13, 47. [Google Scholar] [CrossRef]
- Santiago, K.; Cheng, J.; Jivanelli, B.; Lutz, G. Infections Following Interventional Spine Procedures: A Systematic Review. Pain Physician 2021, 24, 101–116. [Google Scholar] [PubMed]
- Zhu, R.S.; Kan, S.L.; Ning, G.Z.; Chen, G.Z.; Cao, L.X.; Jiang, Z.H.; Zhang, X.L.; Hu, W. Which is the best treatment of osteoporotic vertebral compression fractures: Balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos. Int. 2019, 30, 287–298. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tieppo Francio, V.; Barndt, B.; Latif, U.; Eickmeyer, S.M. Multidisciplinary Care of a Vertebral Fracture in a Patient with Hematopoietic Stem Cell Transplant: Safety Appropriateness in Interventional Pain Management and Rehabilitation Considerations. Healthcare 2022, 10, 497. https://doi.org/10.3390/healthcare10030497
Tieppo Francio V, Barndt B, Latif U, Eickmeyer SM. Multidisciplinary Care of a Vertebral Fracture in a Patient with Hematopoietic Stem Cell Transplant: Safety Appropriateness in Interventional Pain Management and Rehabilitation Considerations. Healthcare. 2022; 10(3):497. https://doi.org/10.3390/healthcare10030497
Chicago/Turabian StyleTieppo Francio, Vinicius, Brandon Barndt, Usman Latif, and Sarah M. Eickmeyer. 2022. "Multidisciplinary Care of a Vertebral Fracture in a Patient with Hematopoietic Stem Cell Transplant: Safety Appropriateness in Interventional Pain Management and Rehabilitation Considerations" Healthcare 10, no. 3: 497. https://doi.org/10.3390/healthcare10030497
APA StyleTieppo Francio, V., Barndt, B., Latif, U., & Eickmeyer, S. M. (2022). Multidisciplinary Care of a Vertebral Fracture in a Patient with Hematopoietic Stem Cell Transplant: Safety Appropriateness in Interventional Pain Management and Rehabilitation Considerations. Healthcare, 10(3), 497. https://doi.org/10.3390/healthcare10030497





