A Comparative Study of Aqueous and Non-Aqueous Solvents to Be Used in Low-Temperature Serial Molecular–Electronic Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
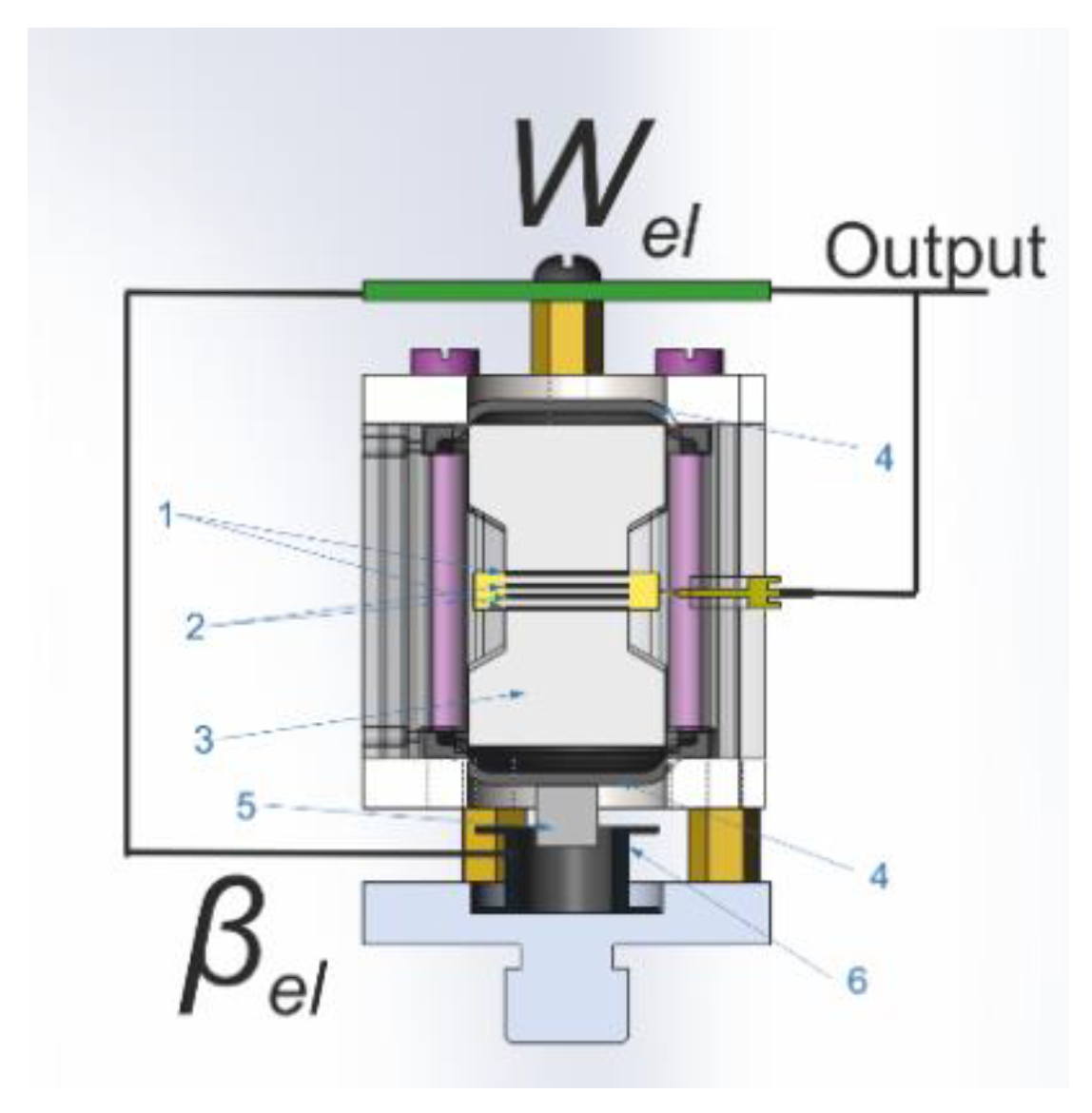
2.2. MET Principles of Operation
- #1: [BMIM][I]/PC/LiI—5/90/5 (non-aqueous solution) are the ratios in %mol. Considering molar masses [BMIM][I] = 266.12 g/mol, PC = 102.09 g/mol, and LiI = 133.85 g/mol for convenience, the mass ratio in the form [BMIM][I]/PC/LiI can be obtained—11.8/82.2/6 and + 0.0128 g of iodine
- #2: [BMIM][I]/Water/LiI 40/55/5, weight ratio—[BMIM][I]/Water/LiI = 85.5/8.05/5.44. Based on about 10 mL, the following amounts are obtained in grams: [BMIM][I]/Water/LiI = 10.812/1.006/0.68 and + 0.0128 g of iodine
- #3: PC/LiI—4 mol/L per 20 mL − 535.6 g LiI/50 = 10.712 g LiI + PC and + 0.0128 g.
- #4: Water/KI/PC; 1 volume of an aqueous solution of KI with a concentration of 4 mol/liter with a concentration of I2 = 0.1 mol/liter is mixed with 9 volumes of the inorganic solvent propylene carbonate, PC.
- #5: Water/LiI/PC; 1 volume of an ordinary aqueous solution of LiI with the concentration 4 mol/liter is mixed with a solution with the concentration of I2 = 0.1 mol/L and with 9 volumes of another solvent, propylene carbonate, PC. The concentration of I2 in the resulting solution is 0.01 mol/L.
- #6: Water/KI/PC; 1 volume of an aqueous solution of LiI with the concentration 4 mol/liter is mixed with a solution with the concentration of I2 = 0.1 mol/L and with 1 volume of another solvent, propylene carbonate, PC. The concentration of J2 in the resulting solution is 0.05 mol/L.
- #7: Water/LiI/PC; 1 volume of an aqueous solution of KI with the concentration 4 mol/liter is mixed with a solution with the concentration of I2 = 0.1 mol/L, and with 1 volume of another solvent, propylene carbonate, PC. The concentration of I2 in the resulting solution is 0.05 mol/L.
- #8: An aqueous solution of KI 0.1 is mixed with alcohol (C2H5OH) in a ratio of 1 to 1 by volume. The concentration of I2 in the resulting solution is 0.05 mol/L.
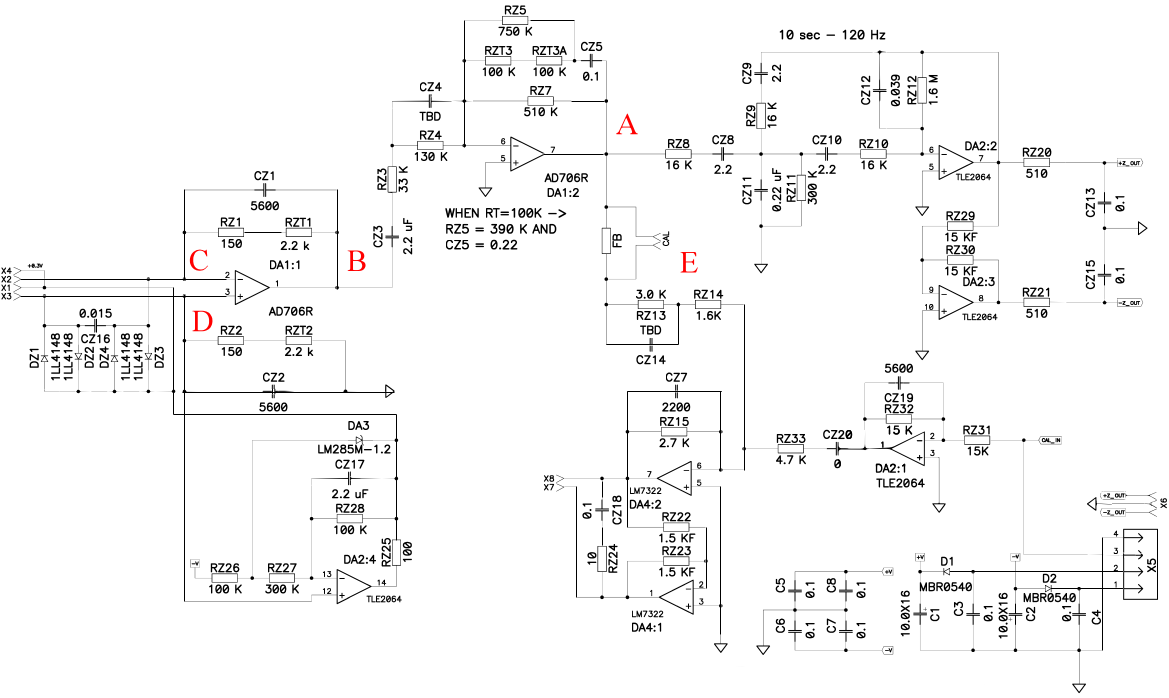
2.3. Experimental Setup
3. Results
4. Conclusions
- -
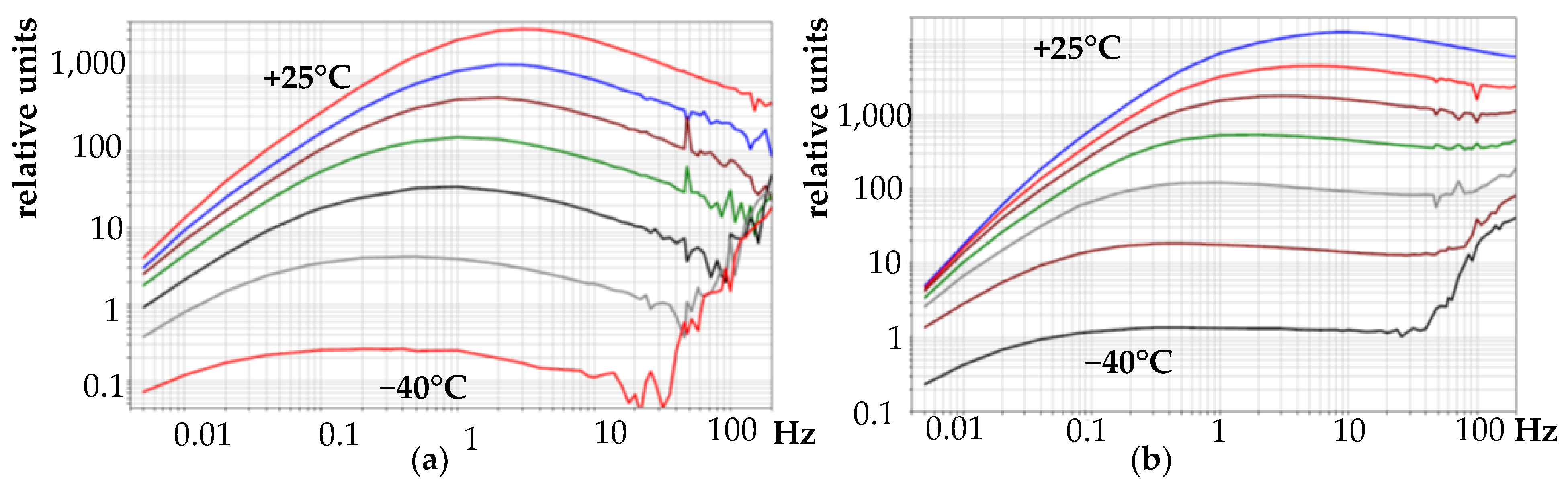
- An aqueous solution of the electrolyte sample # 2 turned out to be unsuitable for use in traditional designs of electrochemical seismic receivers due to low sensitivity and too high temperature sensitivity of the frequency response. Therefore, it was not applicable in potential devices.
- -
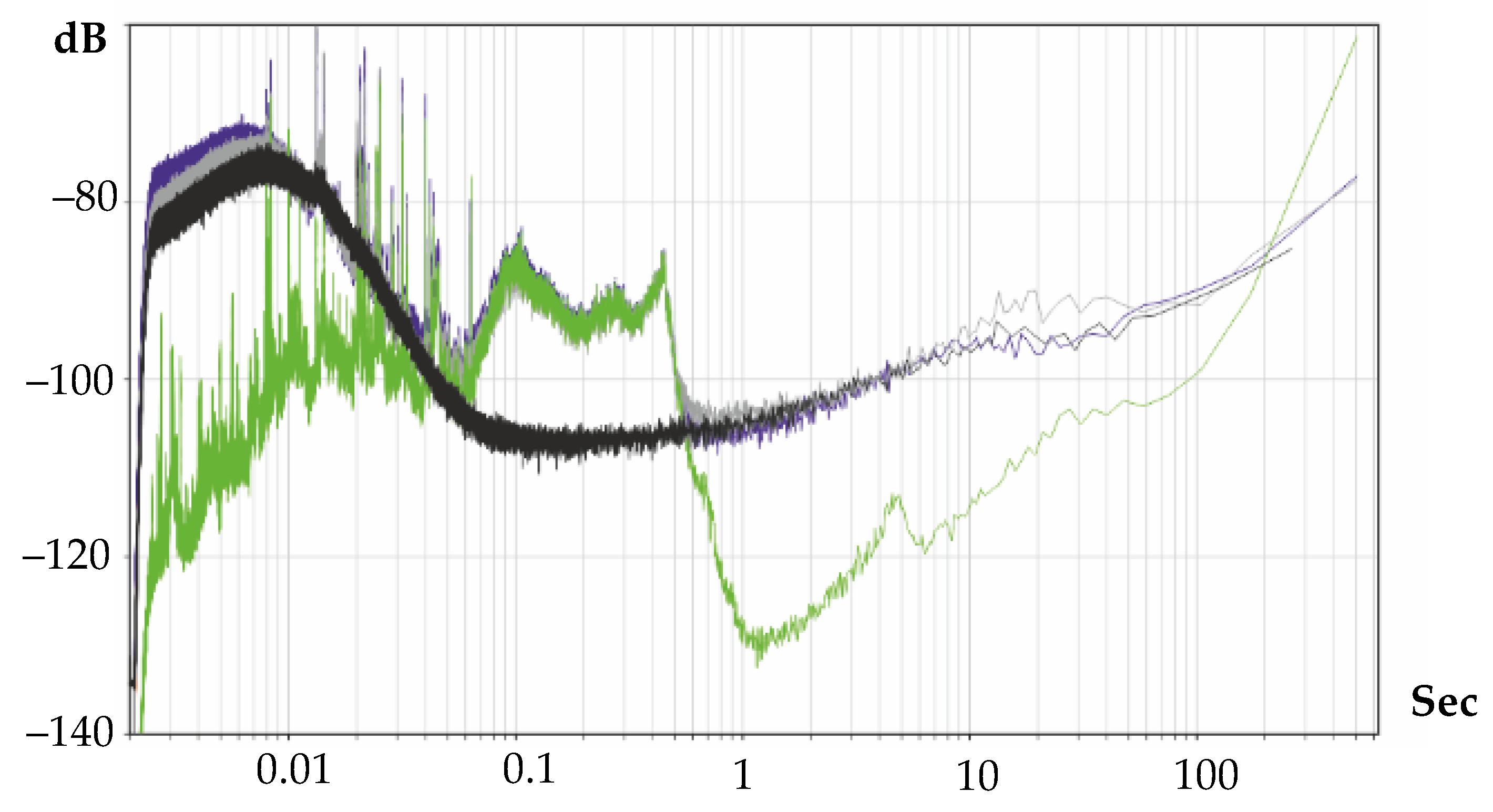
- Non-aqueous solutions #1 and #3 show relatively high conversion factors and sensitivities for their concentration of major carriers. There is a comparatively higher temperature sensitivity for sample #3 AFC compared with conventional solutions based on LiI. At the same time, the noise characteristics for these electrolytes are at least in the middle frequency band (1–10 Hz correspond to traditional meters). At the same time, as the experience of operating devices with electrolytes #1 and #3 has shown, they are subject to precipitation of undissolved iodide salts, due to which their characteristics have significantly degraded over time. Thus, working with these electrolytes requires additional research, and, therefore, they cannot yet be used in measuring instruments.
- -
- The use of the addition of a non-aqueous propylene carbonate solvent generally makes it possible to reduce the traditional freezing point of the electrolytes based on potassium iodide, but, at the same time, with a decrease in the PC concentration, the activation energy of the diffusion coefficient increases significantly, and the temperature dependence of the frequency response becomes more significant.
- -
- Unfortunately, the use of non-aqueous propylene carbonate solvent in combination with an aqueous solution of lithium iodide leads to a significant increase in the temperature sensitivity of the frequency response, as a result, it can hardly be acceptable for use in real measuring systems.
- -
- The use of an alcohol-containing solvent, although it also reduces the absolute freezing point of traditional solutions based on potassium iodide salts (up to −35 °C) but, probably, is also not a promising direction of research due to the presence of a phase transition at a point up to the boundary of the industrial range (−40 °C) and also due to the noticeably worsening temperature sensitivity of the frequency response compared with the classical electrolytes based on potassium and lithium iodides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bugaev, A.S.; Antonov, A.N.; Agafonov, B.M.; Belotelov, K.S.; Vergeles, S.S.; Dudkin, P.V.; Egorov, E.V.; Egorov, I.V.; Zhevnenko, D.A.; Zhabin, S.N.; et al. Measuring Devices Based on Molecular-Electronic Transducers. J. Commun. Technol. Electron. 2018, 63, 1339–1351. [Google Scholar] [CrossRef]
- Krishtop, T.V.; Zhevnenko, D.A.; Kokhanovsky, S.V.; Dudkin, P.V.; Zlobin, A.S.; Belyaev, A.Y.; Krishtop, V.G. High-sensitive ultra-low frequency hydrophone. In Proceedings of the SPIE 11022, International Conference on Micro- and Nano-Electronics, Zvenigorod, Russian, 1–5 October 2018; p. 1102210. [Google Scholar]
- Egorov, E.; Shabalina, A.; Zaitsev, D.; Kurkov, S.; Gueorguiev, N. Frequency Response Stabilization and Comparative Studies of MET Hydrophone at Marine Seismic Exploration Systems. Sensors 2020, 20, 1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhong, A.; Lu, Y.; Chen, J.; Chen, D.; Wang, J. A MEMS Electrochemical Angular Accelerometer Leveraging Silicon-Based Three-Electrode Structure. Micromachines 2022, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Krylov, A.; Egorov, I.; Kovachev, S.; Ilinskiy, D.; Ganzha, O.; Timashkevich, G.; Roginskiy, K.; Kulikov, M.; Novikov, M.; Ivanov, V.; et al. Ocean-Bottom Seismographs Based on Broadband MET Sensors: Architecture and Deployment Case Study in the Arctic. Sensors 2021, 21, 3979. [Google Scholar] [CrossRef] [PubMed]
- Egorov, I.; Bugaev, A.; Chikishev, D. Strong motion molecular-electronic accelerometer International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, 2019. In Proceedings of the 19th International Multidisciplinary Scientific Geoconference, SGEM 2019, Albena, Bulgaria, 28 June–6 July 2019; Volume 19, pp. 959–966. [Google Scholar]
- Yudahin, F.; Antonovskaya, G.; Kapustian, N.; Egorov, E.; Klimov, A. An Investigation of an External Impact Conversion into the Strained Rotation Inside Ancient Boulder Structures (Solovky Islands, White Sea). Geotech. Geol. Earthq. Eng. 2013, 24, 3–14. [Google Scholar] [CrossRef]
- Bernauer, F.; Wassermann, J.; Igel, H. Rotational sensors—a comparison of different sensor types. J. Seism. 2012, 16, 595–602. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Sun, J.; Chen, H.; Ju, C.; Lin, T.; Tian, B.; Zheng, F. Design, Modeling and Simulation of a Liquid Jet Gyroscope Based on Electrochemical Transducers. Micromachines 2021, 12, 1008. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wang, J.; Chen, D.; Liu, B.; She, X.; Xu, C.; Qi, W.; Agafonov, V.; Egorov, E.; Chen, J. A MEMS-Based Electrochemical Angular Accelerometer With a Force-Balanced Negative Feedback. IEEE Sens. J. 2021, 21, 15972–15978. [Google Scholar] [CrossRef]
- Huang, H.; Carande, B.; Tang, R.; Oiler, J.; Dmitriy, Z.; Vadim, A.; Yu, H. Development of a micro seismometer based on molecular electronic transducer technology for planetary exploration. In Proceedings of the IEEE 26th International Conference on Micro Electro Mechanical Systems (MEMS), Taipei, Taiwan, 20–24 January 2013; pp. 629–632. [Google Scholar] [CrossRef]
- Huang, H.; Liang, M.; Tang, R.; Oiler, J.; Yu, H. Molecular Electronic Transducer-Based Low-Frequency Accelerometer Fabricated With Post-CMOS Compatible Process Using Droplet as Sensing Body. IEEE Electron Device Lett. 2013, 34, 1304–1306. [Google Scholar] [CrossRef]
- Huang, H.; Liang, M.; Tang, R.; Oiler, J.; Ma, T.; Yu, H. An electrolyte droplet-based low frequency accelerometer based on molecular electronic transducer. In Proceedings of the Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 924–927. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, D.; Chen, J.; Deng, T.; Li, G.; Wang, J. A MEMS based electrochemical seismometer with a novel integrated sensing unit. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 247–250. [Google Scholar]
- Li, G.; Sun, Z.; Wang, J.; Chen, D.; Chen, J.; Chen, L.; Xu, C.; Qi, W.; Zheng, Y. A Flexible Sensing Unit Manufacturing Method of Electrochemical Seismic Sensor. Sensors 2018, 18, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishtop, V.G.; Agafonov, V.; Bugaev, A.S. Technological principles of motion parameter transducers based on mass and charge transport in electrochemical microsystems. Russ. J. Electrochem. 2012, 48, 746–755. [Google Scholar] [CrossRef]
- Agafonov, V.; Shabalina, A.; Ma, D.; Krishtop, V. Modeling and experimental study of convective noise in electrochemical planar sensitive element of MET motion sensor. Sens. Actuators A Phys. 2019, 293, 259–268. [Google Scholar] [CrossRef]
- He, W.T.; Chen, D.Y.; Wang, J.B.; Zhang, Z.Y. MEMS based broadband electrochemical seismometer. Opt. Precis. Eng. 2015, 23, 444–451. [Google Scholar]
- Deng, T.; Chen, D.; Chen, J.; Sun, Z.; Li, G.; Wang, J. Microelectromechanical Systems-Based Electrochemical Seismic Sensors with Insulating Spacers Integrated Electrodes for Planetary Exploration. IEEE Sens. J. 2016, 16, 650–653. [Google Scholar] [CrossRef]
- Krishtop, V.G. Experimental modeling of the temperature dependence of the transfer function of rotational motion sensors based on electrochemical transducers. Russ. J. Electrochem. 2014, 50, 350–354. [Google Scholar] [CrossRef]
- Chikishev, D.A.; Zaitsev, D.L.; Belotelov, K.S.; Egorov, I.V. The Temperature Dependence of Amplitude- Frequency Response of the MET Sensor of Linear Motion in a Broad Frequency Range. IEEE Sens. J. 2019, 19, 9653–9661. [Google Scholar] [CrossRef]
- Fokina, A.; Zaitsev, D.; Egorov, E. A simple and cheap method of met geophones and seismic accelerometers temperature sensitivity stabilization in a wide temperature band. In Proceedings of the 20th International Multidisciplinary Scientific Geoconference: Science and Technologies in Geology, Exploration and Mining, SGEM 2020, Albena, Bulgaria, 2–11 July 2020; pp. 411–418. [Google Scholar]
- Zaitsev, D.; Shabalina, A. The fiatures of low-temperature operation for electrochemical sensors of motion parameters for the economic development of the Arctic Region of the Russian Federation in the fields of geophysics, seismology and seismic exploration. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM 2021, Albena, Bulgaria, 2–11 July 2021. [Google Scholar]
- Evseev, I.; Zaitsev, D.; Agafonov, V. Study of Transfer Characteristics of a Molecular Electronic Sensor for Borehole Surveys at High Temperatures and Pressures. Sensors 2019, 19, 2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, M.; Yu, H.; Ngan, M.; Nickerson, S.; Nofen, E.; Dai, L. MEMS accelerometer based on Molecular Electronic Transducers using Ionic Liquid. In Proceedings of the IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015; pp. 1167–1170. [Google Scholar]
- Austen Angell, C.; Ansari, Y.; Zhao, Z. Ionic Liquids: Past, present and future. Faraday Discuss. 2012, 154, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, W.J.; Gliege, M.; Gunckel, R.; Zhao, Z.; Yu, H.; Dai, L.L. A Dual Ionic Liquid-Based Low-Temperature Electrolyte System. J. Phys. Chem. B 2018, 122, 12077–12086. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, S.; Nofen, E.M.; Chen, H.; Ngan, M.; Shindel, B.; Yu, H.; Dai, L.L. A Combined Experimental and Molecular Dynamics Study of Iodide-Based Ionic Liquid and Water Mixtures. J. Phys. Chem. B 2015, 119, 8764–8772. [Google Scholar] [CrossRef]
- Lin, W.J.; Xu, Y.; MacDonald, S.; Gunckel, R.; Zhao, Z.; Dai, L.L. Tailoring intermolecular interactions to develop a low-temperature electrolyte system consisting of 1-butyl-3-methylimidazolium iodide and organic solvents. RSC Adv. 2019, 9, 36796–36807. [Google Scholar] [CrossRef] [Green Version]
- Egorov, I.V.; Nguyen, N.C.; Nguyen, T.S.; Chuvakhov, P.V. Simulation of the Laminar–Turbulent Transition by Applying Dissipative Numerical Schemes. Comput. Math. Math. Phys. 2021, 61, 254–266. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Chen, D.; Liang, T.; Xu, C.; Qi, W.; She, X.; Agafonov, V.M.; Shabalina, A.S.; Chen, J. An Electrochemical Angular Micro-Accelerometer Based on Miniaturized Planar Electrodes Positioned in Parallel. IEEE Sens. J. 2021, 21, 21305–21313. [Google Scholar] [CrossRef]
- Egorov, I.V.; Zaytsev, D.L.; Agafonov, V.M. Study of the Prospects for the Use of Ionic Liquids and Non-Aqueous Salt Solutions for Low-Temperature Operation of Serial Electrochemical Geophysical Sensors. In Proceedings of the 7th International Conference on Sensors Engineering and Electronics Instrumentation Advances (SEIA’ 2021), Mallorca, Spain, 22–24 September 2021. [Google Scholar]
- Carl Roth GmbH + Co, KG. Available online: https://www.carlroth.de (accessed on 20 January 2022).
- Sigmaaldrich. Available online: https://www.sigmaaldrich.com/RU/en (accessed on 20 January 2022).
- Lanhit. Available online: https://www.lanhit.ru/ (accessed on 20 January 2022).
- R-sensors. Available online: https://www.R-sensors.ru/ (accessed on 20 January 2022).
- Egorov, I.V.; Shabalina, A.S.; Agafonov, V.M. Design and Self-Noise of MET Closed-Loop Seismic Accelerometers. IEEE Sens. J. 2017, 17, 2008–2014. [Google Scholar] [CrossRef]
- Shabalina, A.S.; Krishtop, V.G. The precision seismometer based on planar electrochemical transducer. In Proceedings of the SPIE 10224, International Conference on Micro- and Nano-Electronics 2016, Zvenigorod, Russian, 30 December 2016; Volume 10224. [Google Scholar]
- Miroborudovaniya. Available online: https://miroborudovaniya.ru/product/klimaticheskaya-ispytatelnaya-kamera-teplo-vlaga-holod-climcontrol-m-60-100-80-ktvh/ (accessed on 20 January 2022).
- Guralp Systems. Available online: https://www.guralp.com/ (accessed on 20 January 2022).






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, D.; Egorov, I.; Agafonov, V. A Comparative Study of Aqueous and Non-Aqueous Solvents to Be Used in Low-Temperature Serial Molecular–Electronic Sensors. Chemosensors 2022, 10, 111. https://doi.org/10.3390/chemosensors10030111
Zaitsev D, Egorov I, Agafonov V. A Comparative Study of Aqueous and Non-Aqueous Solvents to Be Used in Low-Temperature Serial Molecular–Electronic Sensors. Chemosensors. 2022; 10(3):111. https://doi.org/10.3390/chemosensors10030111
Chicago/Turabian StyleZaitsev, Dmitry, Ivan Egorov, and Vadim Agafonov. 2022. "A Comparative Study of Aqueous and Non-Aqueous Solvents to Be Used in Low-Temperature Serial Molecular–Electronic Sensors" Chemosensors 10, no. 3: 111. https://doi.org/10.3390/chemosensors10030111
APA StyleZaitsev, D., Egorov, I., & Agafonov, V. (2022). A Comparative Study of Aqueous and Non-Aqueous Solvents to Be Used in Low-Temperature Serial Molecular–Electronic Sensors. Chemosensors, 10(3), 111. https://doi.org/10.3390/chemosensors10030111





