Pure Alloy Additive or Preliminary Alloy: A Comparative Study on Obtaining High-Strength Copper Magnesium Alloys Designed for Electrical Power Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metallurgical Synthesis and Chemical Composition
2.2. Mechanical Properties
2.3. Physical Properties
2.4. SEM Analysis and XRD Observations
3. Results and Discussion
3.1. Metallurgical Synthesis and Chemical Composition
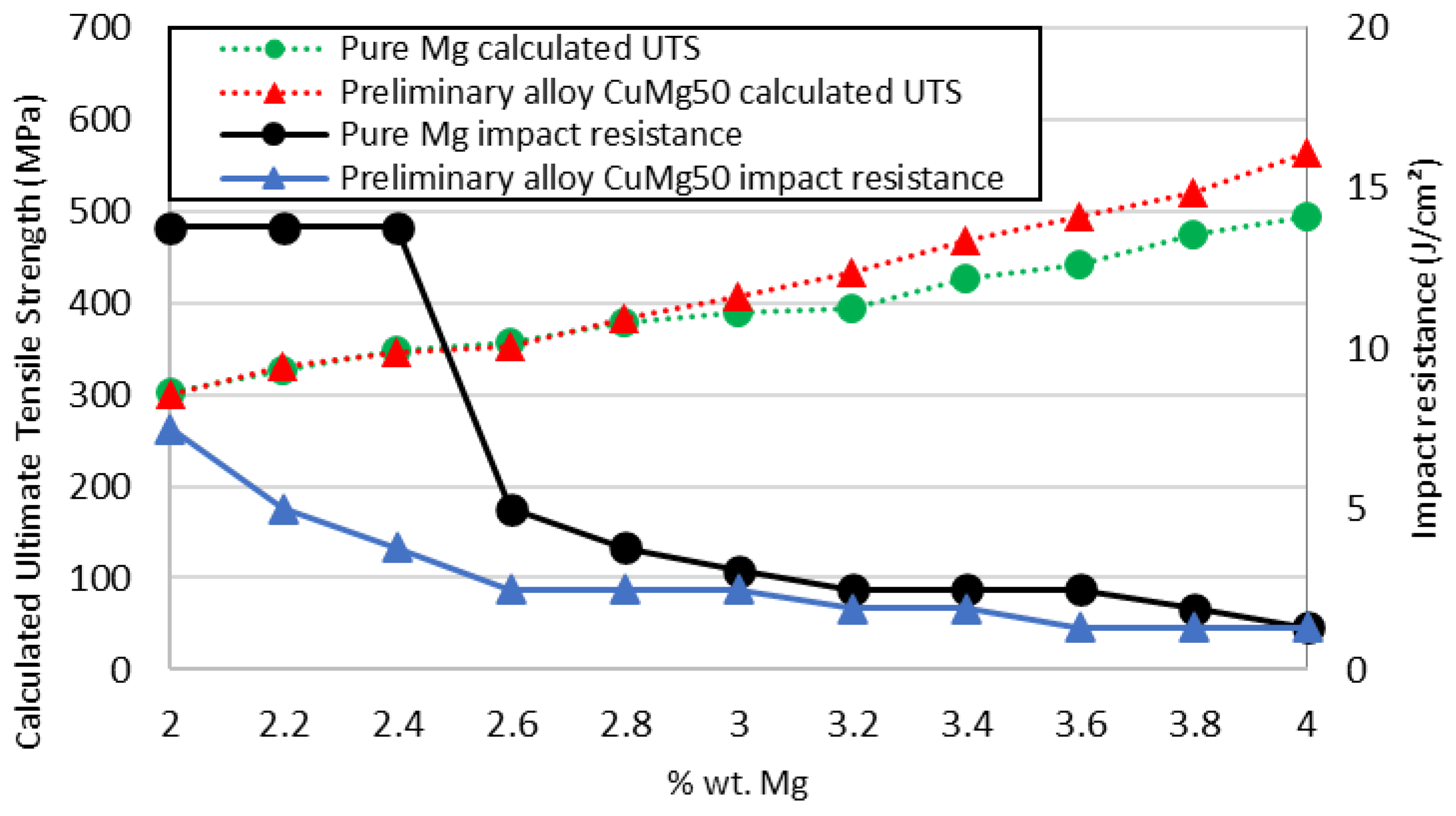
3.2. Mechanical Properties
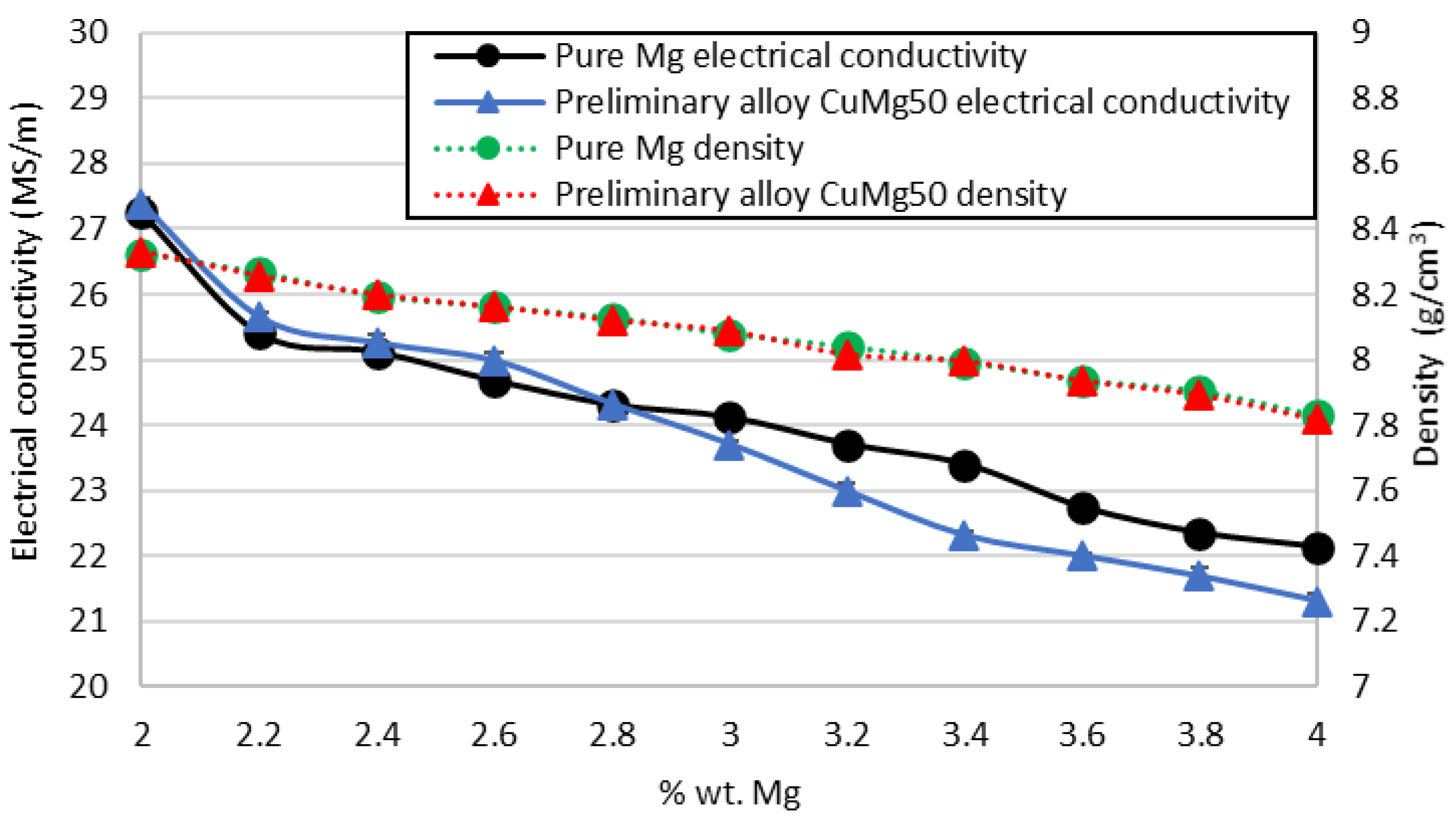
3.3. Physical Properties
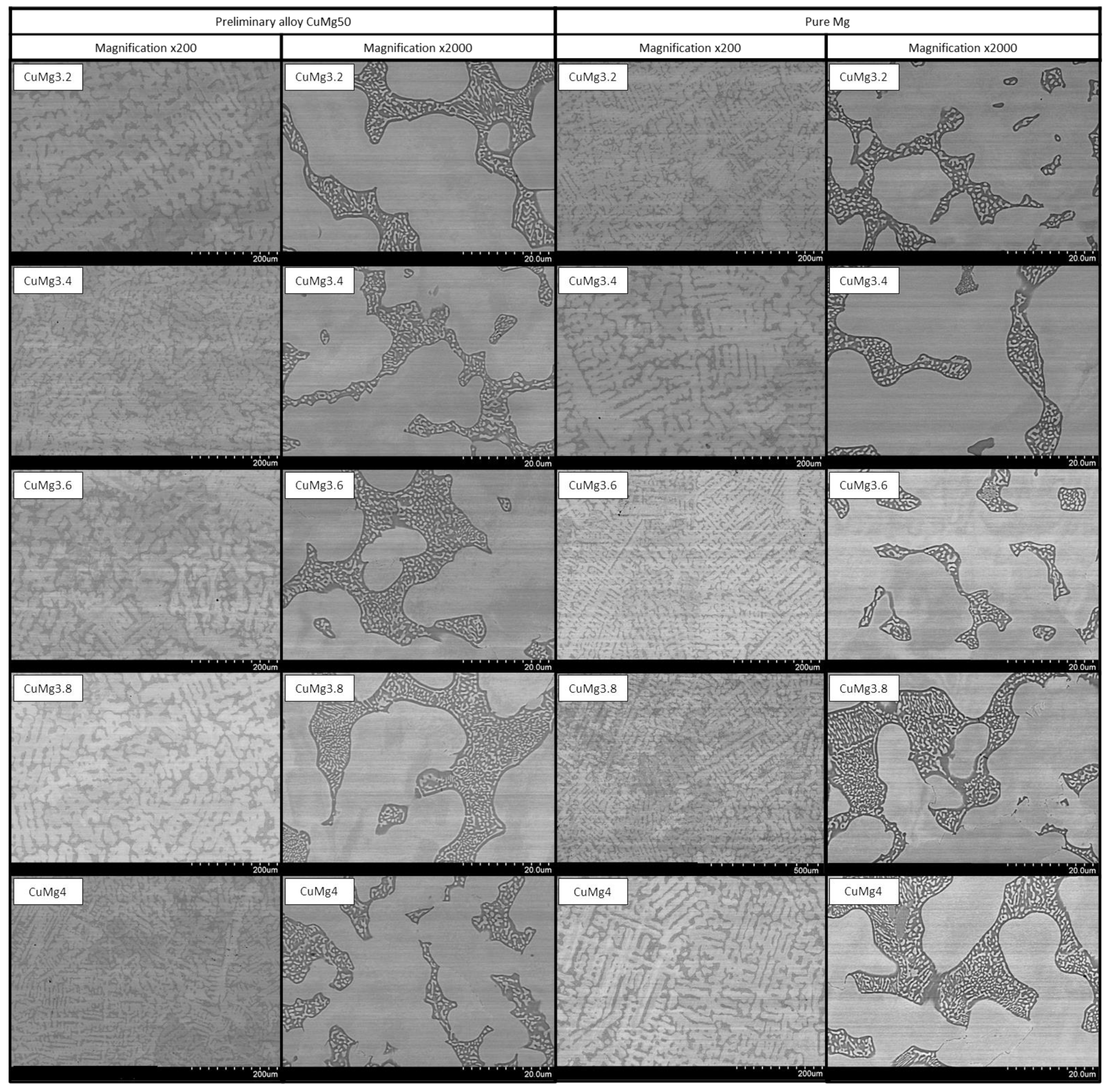
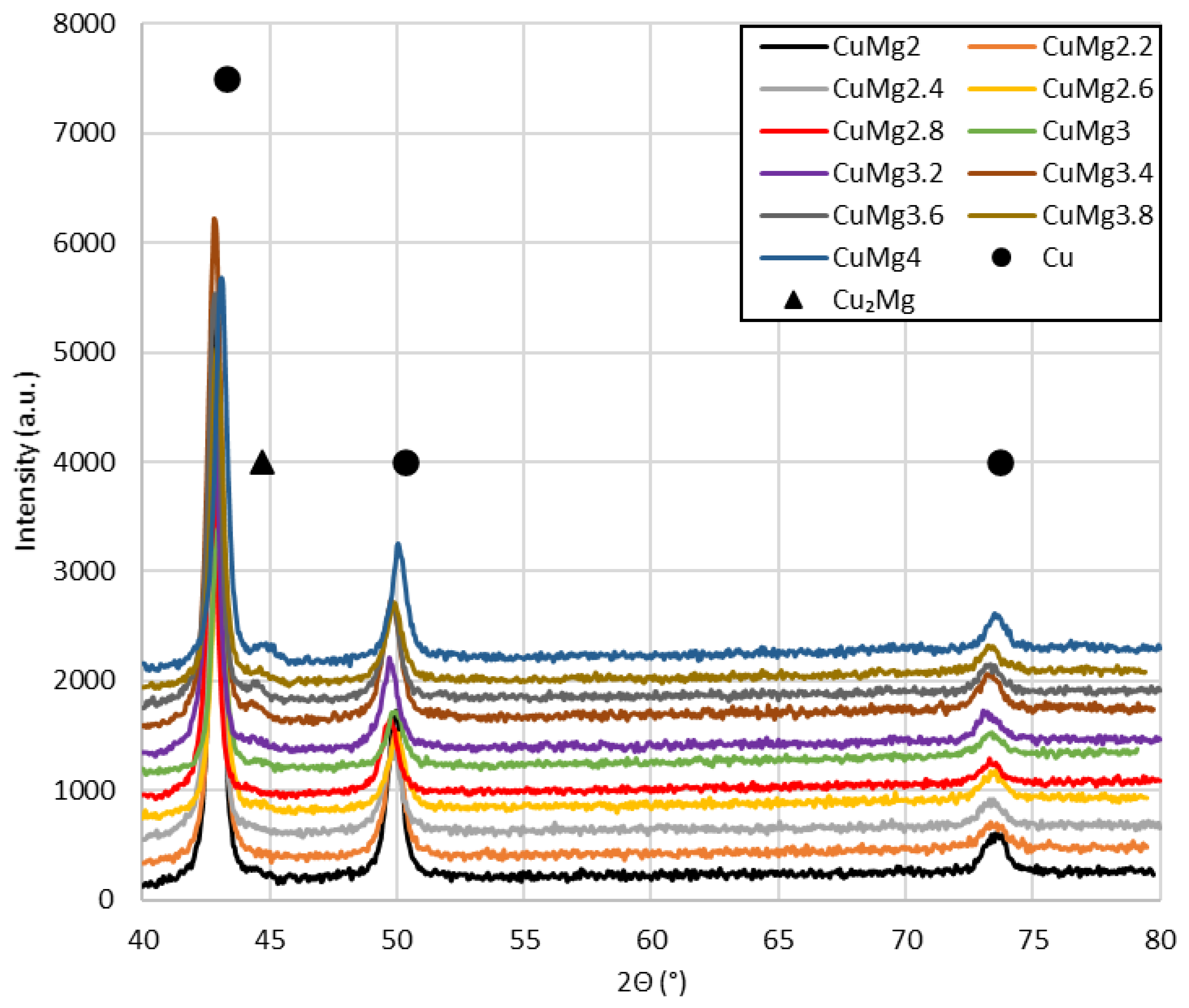
3.4. SEM Analysis and XRD Observations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric car battery: An overview on global demand, recycling and future approaches towards sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef] [PubMed]
- Zasadzińska, M.; Knych, T.; Strzępek, P.; Jurkiewicz, B.; Franczak, K. Analysis of the strengthening and recrystallization of electrolytic copper (Cu-ETP) and oxygen free copper (Cu-OF). Arch. Civ. Mech. Eng. 2019, 19, 186–193. [Google Scholar] [CrossRef]
- Zasadzińska, M.; Knych, T.; Smyrak, B.; Strzępek, P. Investigation of the dendritic structure influence on the electrical and mechanical properties diversification of the continuously casted copper strand. Materials 2020, 13, 5513. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Zhang, Y.; Guo, Y.; Zhao, Y. Enhanced electrical conductivity and mechanical properties in thermally stable fine-grained copper wire. Commun. Mater. 2021, 2, 46. [Google Scholar] [CrossRef]
- Huang, C.-L.; Xu, M.; Cui, S.; Li, Z.; Fang, H.; Wang, P. Copper-induced ripple effects by the expanding electric vehicle fleet: A crisis or an opportunity. Resour. Conserv. Recycl. 2020, 161, 104861. [Google Scholar] [CrossRef]
- Henckens, M.; Driessen, P.P.J.; Worrel, E. Metal scarcity and sustainability, analyzing the necessity to reduce the extraction of scarce metals. Resour. Conserv. Recycl. 2014, 93, 1–8. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Copper demand, supply, and associated energy use to 2050. Glob 2016, 39, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until 2100 with regression and stock Dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Maki, K.; Ito, Y.; Matsunaga, H.; Mori, H. Solid-solution copper alloys with high strength and high electrical conductivity. Scr. Mater. 2013, 68, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Ružić, J.; Stašić, J.; Rajković, V.; Božić, D. Strengthening effects in precipitation and dispersion hardened powder metallurgy copper alloys. Mater. Des. 2013, 49, 746–754. [Google Scholar] [CrossRef]
- Gorsse, S.; Ouvrad, B.; Goune, M.; Poulon-Quintin, A. Microstructural design of new high conductivity—High strength Cu-based alloy. J. Alloys Compd. 2015, 633, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Im, Y.-D.; Matsumoto, R.; Utsunomiya, H. Strength and electrical conductivity of Cu-Al alloy sheets by cryogenic high-speed rolling. Mater. Sci. Eng. A 2020, 799, 139815. [Google Scholar] [CrossRef]
- Prashanth, M.; Satish, N.; Ajay kumar, B.S. Effect of Brass and Silver on Mechanical Properties of Copper. Mater. Today Proc. 2018, 5, 25404–25411. [Google Scholar] [CrossRef]
- Tardieua, S.; Mesguich, D.; Lonjon, A.; Lecouturier, F.; Ferreira, N.; Chevalliera, G.; Proietti, A.; Estournèsa, C.; Laurent, C. Nanostructured 1% silver-copper composite wires with a high tensile strength and a high electrical conductivity. Mater. Sci. Eng. A 2019, 761, 138048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.B.; Tao, N.R.; Lu, K. A high strength and high electrical conductivity bulk Cu-Ag alloy strengthened with nanotwin. Scr. Mater. 2017, 129, 39–43. [Google Scholar] [CrossRef]
- Strzępek, P.; Mamala, A.; Zasadzińska, M.; Franczak, K.; Jurkiewicz, B. Research on the drawing process of Cu and CuZn wires obtained in the cryogenic conditions. Cryogenics 2019, 100, 11–17. [Google Scholar] [CrossRef]
- Li, R.; Guo, E.; Chen, Z.; Kang, H.; Wang, W.; Zou, C.; Li, T.; Wang, T. Optimization of the balance between high strength and high electrical conductivity in CuCrZr alloys through two-step cryorolling and aging. J. Alloys Compd. 2019, 771, 1044–1051. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Sun, L.X.; Tao, N.R. Nanostructures and nanoprecipitates induce high strength and high electrical conductivity in a CuCrZr alloy. J. Mater. Sci. Technol. 2020, 48, 18–22. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Z.; Li, Z.; Zhou, Z.; Yang, Z.; Lei, Q. Microstructure and properties of a novel Cu-Mg-Ca alloy with high strength and high electrical conductivity. J. Alloys Compd. 2017, 723, 1162–1170. [Google Scholar] [CrossRef]
- Cao, Y.; Han, S.Z.; Choi, E.-A.; Ahn, J.H.; Mi, X.; Lee, S.; Shin, H.; Kim, S.; Lee, J. Effect of inclusion on strength and conductivity of CuNiSi alloys with discontinuous precipitation. J. Alloys Compd. 2020, 843, 156006. [Google Scholar] [CrossRef]
- Geng, G.; Wang, D.; Zhang, W.; Liu, L.; Laptev, A.M. Fabrication of Cu-Ni-Si alloy by melt spinning and its mechanical and electrical properties. Mater. Sci. Eng. A 2020, 776, 138979. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Z.; Sheng, X.; Gao, Y.; Lei, Q. Grain refinement and mechanical properties improvements in a high strength Cu-Ni-Si alloy during multidirectional forging. Fusion Eng. Des. 2020, 159, 111766. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Z.; Dai, J.; Li, Z.; Jiang, H.; Wang, W.; Zhang, X. Microstructure and properties of a novel CuNiCoSiMg alloys with super high strength and conductivity. Mater. Sci. Eng. A 2019, 744, 754–763. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Z.; Li, Z.; Qiu, W.; Jiang, H.; Lei, Q.; Liu, Z.; Jiang, Y.; Zhang, S. Microstructure and properties of a CuNiSiCoCr alloy with high strength and high conductivity. Mater. Sci. Eng. A 2019, 759, 396–403. [Google Scholar] [CrossRef]
- Yu, X.; Hou, Y.; Ren, X.; Sun, C.; Wang, M. Research progress on the removal, recovery and direct high-value materialization of valuable metal elements in electroplating/electroless plating waste solution. J. Water Process. Eng. 2022, 46, 102577. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lu, Z. In situ investigation of atmospheric corrosion behavior of copper under thin electrolyte layer and static magnetic field. Microelectron. Reliab. 2020, 108, 113630. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Lu, Z. Atmospheric corrosion behavior of copper under static magnetic field environment. Mater. Lett. 2020, 266, 127472. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Q.; Qiang, L.; Zhang, B.; Zhang, J. Comparison study of gold coatings prepared by traditional and modified galvanic replacement deposition for corrosion prevention of copper. Microelectron. Reliab. 2020, 110, 113695. [Google Scholar] [CrossRef]
- Mashtalyar, D.; Gnedenkov, S.; Sinebryukhov, S.; Imshinetskiy, I.; Gnedenkov, A.; Bouznik, V. Composite coatings formed using plasma electrolytic oxidation and fluoroparaffin materials. J. Alloy. Compd. 2018, 767, 353–360. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.; Shtansky, D.V.; Levashov, E.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Gnedenkov, S.; Sinebryukhov, S.; Egorkin, V.; Mashtalyar, D.; Vyaliy, I.; Nadaraia, K.; Imshinetskiy, I.; Nikitin, A.; Subbotin, E.; Gnedenkov, A. Magnesium fabricated using additive technology: Specificity of corrosion and protection. J. Alloy. Compd. 2019, 808, 151629. [Google Scholar] [CrossRef]
- Karavai, O.; Bastos, A.; Zheludkevich, M.; Taryba, M.; Lamaka, S.V.; Ferreira, M.G.S. Localized electrochemical study of corrosion inhibition in microdefects on coated AZ31 magnesium alloy. Electrochimica Acta 2010, 55, 5401–5406. [Google Scholar] [CrossRef]
- Liu, G.; Jia, S.; Hong, S.; Lim, J.; Zhu, Y.; Mimura, K.; Isshiki, M. Corrosion resistance of dilute CuMg alloys at elevated temperature. Corros. Sci. 2009, 51, 463–468. [Google Scholar] [CrossRef]
- Rodriguez-Calvillo, P.; Ferrer, N.; Cabrera-Marrero, J.-M. Analysis of microstructure and strengthening in CuMg alloys deformed by equal channel angular pressing. J. Alloy. Compd. 2015, 626, 340–348. [Google Scholar] [CrossRef]
- Tong, Y.-X.; Li, S.-Y.; Zhang, D.-T.; Li, L.; Zheng, Y.-F. High strength and high electrical conductivity CuMg alloy prepared by cryorolling. Trans. Nonferrous Met. Soc. China 2019, 29, 595–600. [Google Scholar] [CrossRef]
- Ito, Y.; Matsunaga, H.; Mori, H.; Maki, K. Effect of Plastic Deformation on the Proof Strength and Electrical Conductivity of Copper-Magnesium Supersaturated Solid-Solution Alloys. Mater. Trans. 2014, 55, 1738–1741. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, K.I.; Semboshi, S.; Konno, T.J. Transmission Electron Microscopy Observations on Cu-Mg Alloy Systems. SSP 2007, 127, 103–108. [Google Scholar] [CrossRef]
- Strzępek, P.; Mamala, A.; Zasadzińska, M.; Noga, P.; Sadzikowski, M. The influence of the continuous casting conditions on the properties of high-strength two-phase CuMg alloys. Materials 2020, 13, 4805. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Tian, B.; Yakubov, V.; An, J.; Volinsky, A.A.; Liu, Y.; Song, K.; Li, L.; Fu, M. Effects of Ce and Y addition on microstructure evolution and precipitation of Cu-Mg alloy hot deformation. J. Alloy. Compd. 2019, 781, 118–130. [Google Scholar] [CrossRef]
- Tabor, D. The hardness and strength of metals. J. Inst. Met. 1951, 79, 1–18. [Google Scholar]
- Tabor, D. The hardness of solids. Rev. Phys. Technol. 1970, 1, 145–179. [Google Scholar] [CrossRef]
- Cahoon, J.R. An improved equation relating hardness to ultimate strength. Metall. Mater. Trans. B 1972, 3, 3040. [Google Scholar] [CrossRef] [Green Version]
- Nordheim, L. Zur Elektronentheorie der Metalle. I. Ann. Phys. 1931, 401, 607–640. [Google Scholar] [CrossRef]



| Alloy Additive | Preliminary Alloy CuMg50 | Pure Mg | ||||
|---|---|---|---|---|---|---|
| Nominal Chemical Composition | Cu | Mg | Other | Cu | Mg | Other |
| wt.% | ||||||
| CuMg2 | 97.92 | 2.01 | 0.07 | 97.95 | 2 | 0.05 |
| CuMg2.2 | 97.70 | 2.21 | 0.09 | 97.71 | 2.18 | 0.11 |
| CuMg2.4 | 97.51 | 2.41 | 0.08 | 97.5 | 2.39 | 0.11 |
| CuMg2.6 | 97.31 | 2.60 | 0.09 | 97.31 | 2.6 | 0.09 |
| CuMg2.8 | 97.10 | 2.81 | 0.09 | 97.12 | 2.8 | 0.08 |
| CuMg3 | 96.90 | 3.00 | 0.10 | 96.91 | 3.01 | 0.08 |
| CuMg3.2 | 96.72 | 3.21 | 0.07 | 96.72 | 3.2 | 0.08 |
| CuMg3.4 | 96.50 | 3.42 | 0.08 | 96.5 | 3.46 | 0.04 |
| CuMg3.6 | 96.31 | 3.60 | 0.09 | 96.31 | 3.6 | 0.09 |
| CuMg3.8 | 96.10 | 3.81 | 0.09 | 96.14 | 3.79 | 0.07 |
| CuMg4 | 95.90 | 4.01 | 0.09 | 95.9 | 4.01 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzępek, P.; Zasadzińska, M. Pure Alloy Additive or Preliminary Alloy: A Comparative Study on Obtaining High-Strength Copper Magnesium Alloys Designed for Electrical Power Systems. Energies 2022, 15, 2093. https://doi.org/10.3390/en15062093
Strzępek P, Zasadzińska M. Pure Alloy Additive or Preliminary Alloy: A Comparative Study on Obtaining High-Strength Copper Magnesium Alloys Designed for Electrical Power Systems. Energies. 2022; 15(6):2093. https://doi.org/10.3390/en15062093
Chicago/Turabian StyleStrzępek, Paweł, and Małgorzata Zasadzińska. 2022. "Pure Alloy Additive or Preliminary Alloy: A Comparative Study on Obtaining High-Strength Copper Magnesium Alloys Designed for Electrical Power Systems" Energies 15, no. 6: 2093. https://doi.org/10.3390/en15062093
APA StyleStrzępek, P., & Zasadzińska, M. (2022). Pure Alloy Additive or Preliminary Alloy: A Comparative Study on Obtaining High-Strength Copper Magnesium Alloys Designed for Electrical Power Systems. Energies, 15(6), 2093. https://doi.org/10.3390/en15062093






