Premature Vertebral Mineralization in hmx1-Mutant Zebrafish
Abstract
:1. Introduction
2. Material and Methods
2.1. Zebrafish and Mouse Maintenance and Breeding
2.2. RNA Extraction, cDNA Synthesis, and RT-PCR
2.3. Statistical Analysis
2.4. Alcian Blue and Alizarin Red Staining
2.5. Whole-Mount in Situ Hybridization
2.6. DMH1 Treatment
3. Results
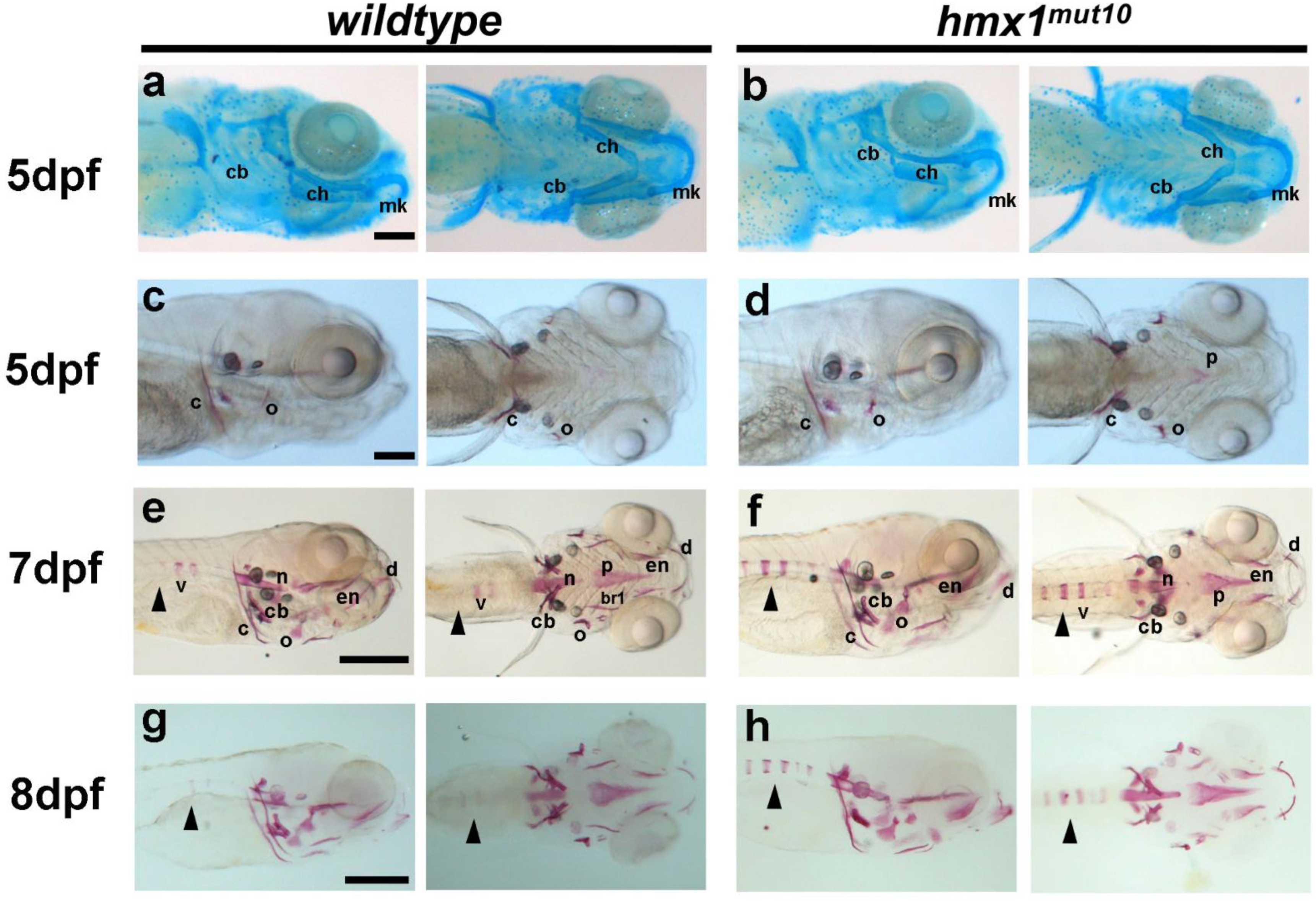
3.1. Hmx1mut10 Zebrafish Present Premature Vertebrae Mineralization
3.2. Hmx1mut150 Zebrafish Recapitulate Premature Vertebral Mineralization Similarly to Hmx1mut10 Mutants
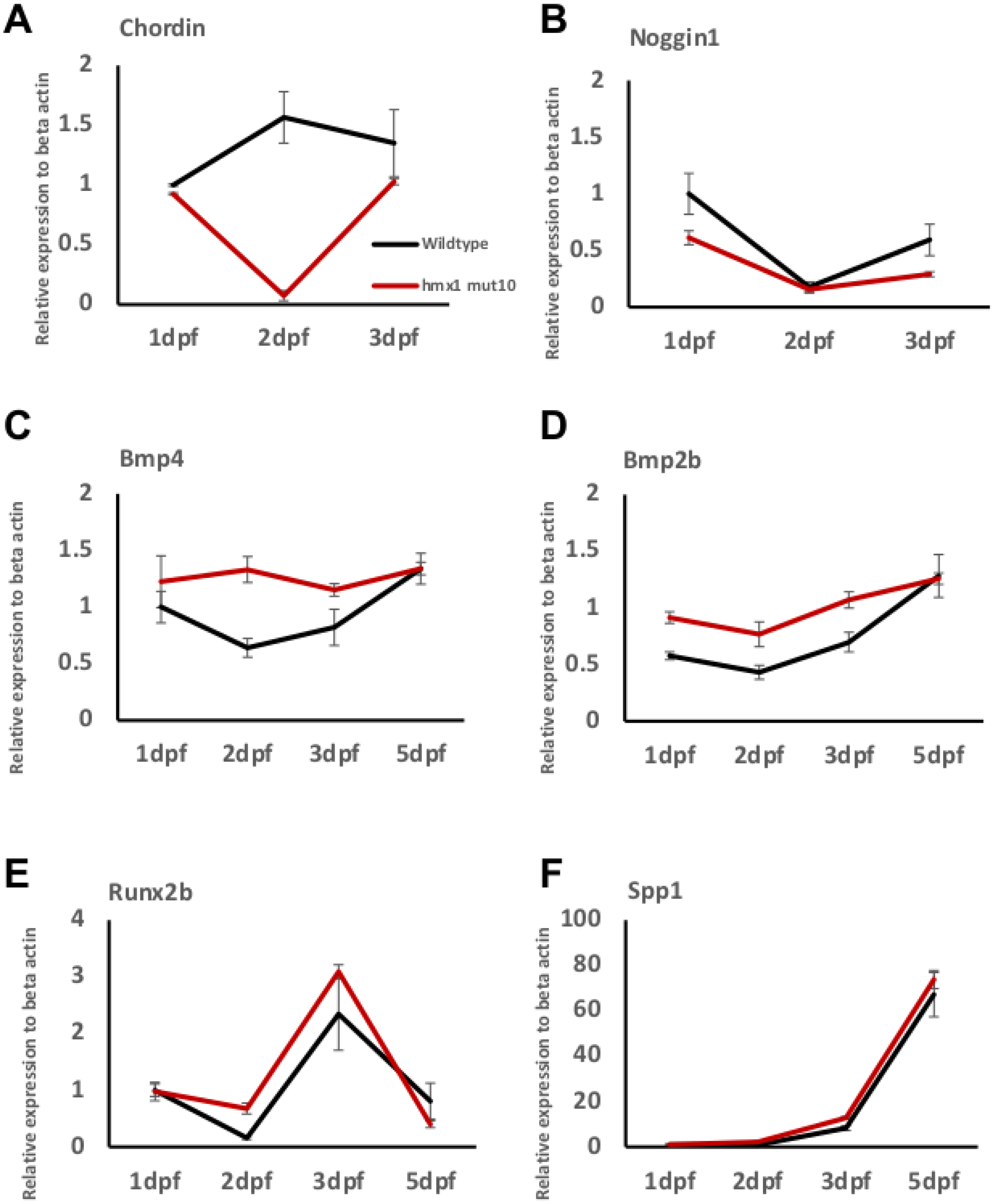
3.3. Osteogenic Signaling during Early Bone Development in Wildtype and Hmx1mut10 Zebrafish
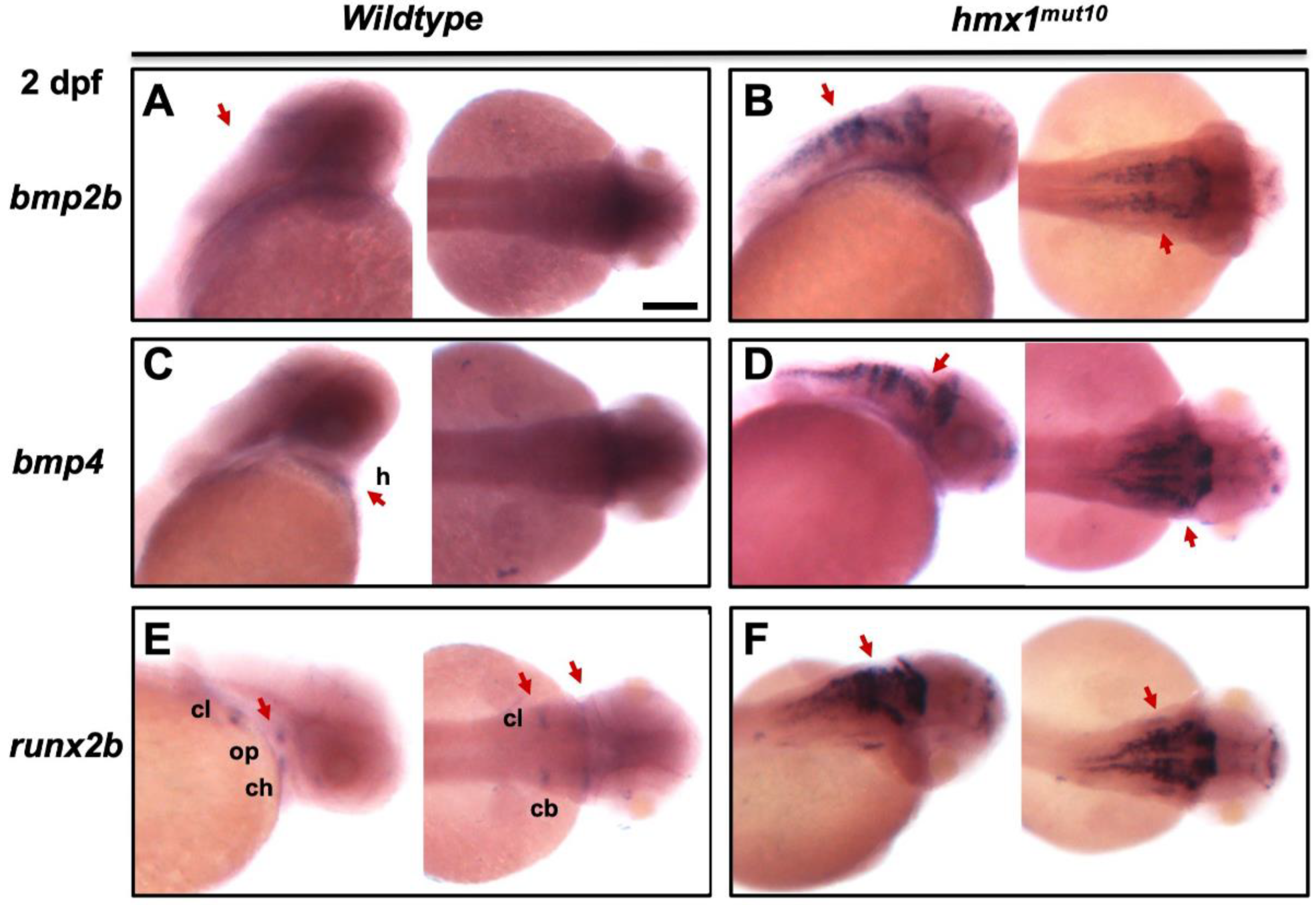
3.4. Bmp2b and bmp4 Are Expressed in the Dorsal Region in Hmx1mut10 Embryos
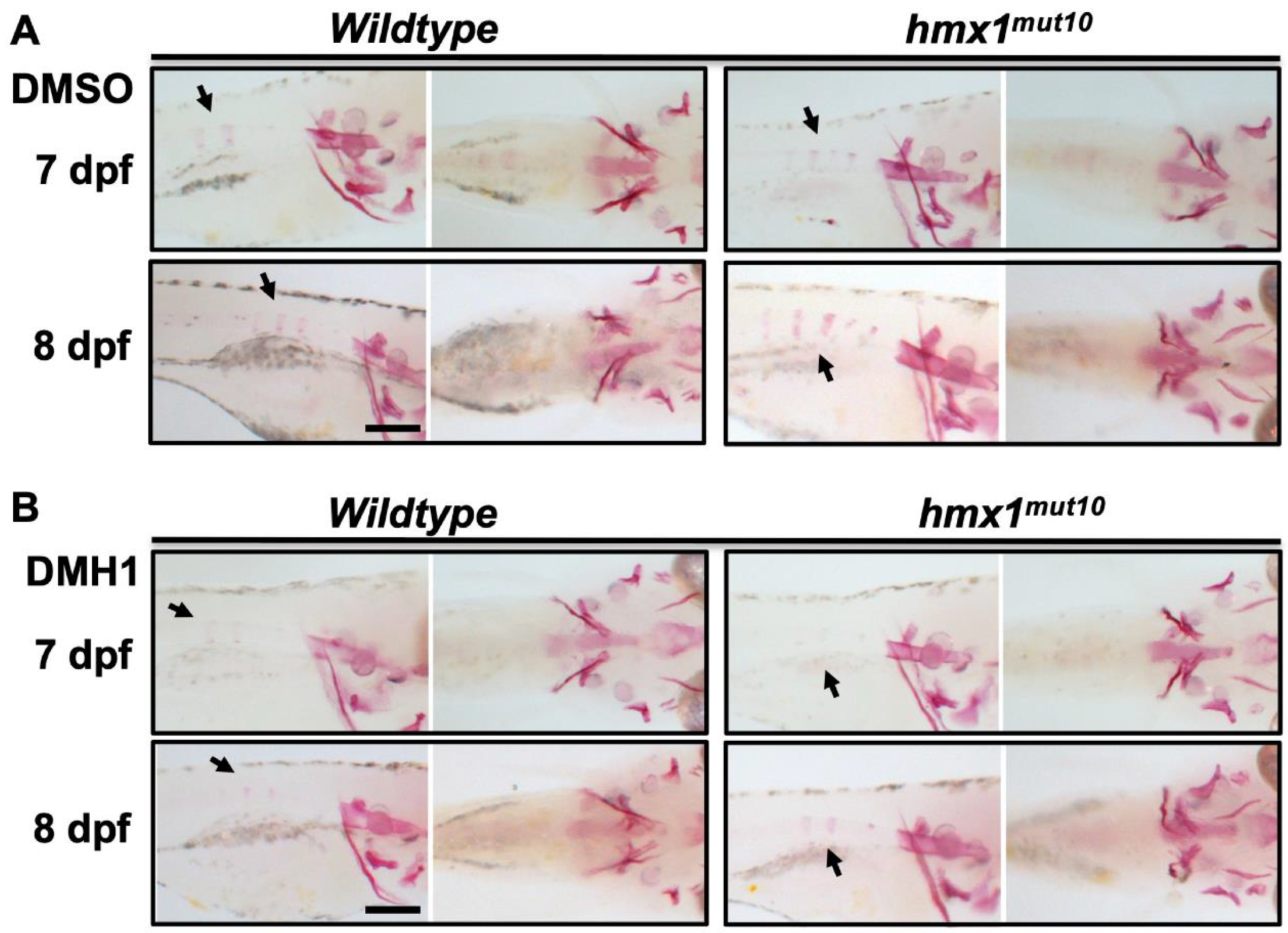
3.5. Inhibition of bmp Signaling at 2 dpf Reduced the Progression of Vertebral Mineralization in Hmx1mut10 Embryos
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schorderet, D.F.; Nichini, O.; Boisset, G.; Polok, B.; Tiab, L.; Mayeur, H.; Raji, B.; de la Houssaye, G.; Abitbol, M.M.; Munier, F.L. Mutation in the human homeobox gene NKX5-3 causes an oculo-auricular syndrome. Am. J. Hum. Genet. 2008, 82, 1178–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, P.W.; Booth, H.A.; Bruford, E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007, 5, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Salam, G.M.H.; Abdel-Hamid, M.S.; Mehrez, M.I.; Kamal, A.M.; Taher, M.B.; Afifi, H.H. Further delineation of the oculoauricular syndrome phenotype: A new family with a novel truncating HMX1 mutation. Ophthalmic Genet. 2018, 39, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Boisset, G.; Schorderet, D.F. Zebrafish hmx1 promotes retinogenesis. Exp. Eye Res. 2012, 105, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Munroe, R.J.; Prabhu, V.; Acland, G.M.; Johnson, K.R.; Harris, B.S.; O’Brien, T.P.; Welsh, I.C.; Noden, D.M.; Schimenti, J.C. Mouse H6 Homeobox 1 (Hmx1) mutations cause cranial abnormalities and reduced body mass. BMC Dev. Biol. 2009, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Quina, L.A.; Tempest, L.; Hsu, Y.W.; Cox, T.C.; Turner, E.E. Hmx1 is required for the normal development of somatosensory neurons in the geniculate ganglion. Dev. Biol. 2012, 365, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Shahi, M.; Peymani, A.; Sahmani, M. Regulation of Bone Metabolism. Rep. Biochem. Mol. Biol. 2017, 5, 73–82. [Google Scholar]
- Bird, N.C.; Mabee, P.M. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev. Dyn. 2003, 228, 337–357. [Google Scholar] [CrossRef]
- Mork, L.; Crump, G. Zebrafish Craniofacial Development: A Window into Early Patterning. Curr. Top. Dev. Biol. 2015, 115, 235–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, J.C.; Nichols, J.T.; Yan, Y.L.; Leonard, I.F.; BreMiller, R.A.; Amacher, S.L.; Postlethwait, J.H.; Kimmel, C.B. Pharyngeal morphogenesis requires fras1-itga8-dependent epithelial-mesenchymal interaction. Dev. Biol. 2016, 416, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Aceto, J.; Nourizadeh-Lillabadi, R.; Maree, R.; Dardenne, N.; Jeanray, N.; Wehenkel, L.; Alestrom, P.; van Loon, J.J.; Muller, M. Zebrafish Bone and General Physiology Are Differently Affected by Hormones or Changes in Gravity. PLoS ONE 2015, 10, e0126928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, R.; Pezoa, S.A.; Carpio Shull, L.; Hernandez-Lagunas, L.; Niswander, L.A.; Artinger, K.B. Kat2a and Kat2b Acetyltransferase Activity Regulates Craniofacial Cartilage and Bone Differentiation in Zebrafish and Mice. J. Dev. Biol. 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensimon-Brito, A.; Cardeira, J.; Cancela, M.L.; Huysseune, A.; Witten, P.E. Distinct patterns of notochord mineralization in zebrafish coincide with the localization of Osteocalcin isoform 1 during early vertebral centra formation. BMC Dev. Biol. 2012, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branam, A.M.; Hoffman, G.G.; Pelegri, F.; Greenspan, D.S. Zebrafish chordin-like and chordin are functionally redundant in regulating patterning of the dorsoventral axis. Dev. Biol. 2010, 341, 444–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, S.; Halpern, M.E. Patterning the zebrafish axial skeleton requires early chordin function. Nat. Genet. 1999, 23, 442–446. [Google Scholar] [CrossRef]
- Laux, D.W.; Febbo, J.A.; Roman, B.L. Dynamic analysis of BMP-responsive smad activity in live zebrafish embryos. Dev. Dyn. 2011, 240, 682–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomreinke, A.P.; Soh, G.H.; Rogers, K.W.; Bergmann, J.K.; Blassle, A.J.; Muller, P. Dynamics of BMP signaling and distribution during zebrafish dorsal-ventral patterning. eLife 2017, 6, e25861. [Google Scholar] [CrossRef]
- Windhausen, T.; Squifflet, S.; Renn, J.; Muller, M. BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production. Molecules 2015, 20, 7586–7601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef] [Green Version]
- van der Meulen, T.; Kranenbarg, S.; Schipper, H.; Samallo, J.; van Leeuwen, J.L.; Franssen, H. Identification and characterisation of two runx2 homologues in zebrafish with different expression patterns. Biochim. Biophys. Acta 2005, 1729, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Afzal, F.; Bae, J.S.; Gutierrez, S.; Zaidi, K.; Pratap, J.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2-SMAD complex to promote osteoblast differentiation. Cells Tissues Organs 2009, 189, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R., Jr.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Dudas, M.; Sridurongrit, S.; Nagy, A.; Okazaki, K.; Kaartinen, V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech. Dev. 2004, 121, 173–182. [Google Scholar] [CrossRef]
- Asharani, P.V.; Keupp, K.; Semler, O.; Wang, W.; Li, Y.; Thiele, H.; Yigit, G.; Pohl, E.; Becker, J.; Frommolt, P.; et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am. J. Hum. Genet. 2012, 90, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Amendt, B.A.; Sutherland, L.B.; Russo, A.F. Transcriptional antagonism between Hmx1 and Nkx2.5 for a shared DNA-binding site. J. Biol. Chem. 1999, 274, 11635–11642. [Google Scholar] [CrossRef] [Green Version]
- Boulling, A.; Wicht, L.; Schorderet, D.F. Identification of HMX1 target genes: A predictive promoter model approach. Mol. Vis. 2013, 19, 1779–1794. [Google Scholar] [PubMed]
- El Fersioui, Y.; Pinton, G.; Allaman-Pillet, N.; Schorderet, D.F. Hmx1 regulates urfh1 expression in the craniofacial region in zebrafish. PLoS ONE 2021, 16, e0245239. [Google Scholar] [CrossRef]
- Marcelli, F.; Boisset, G.; Schorderet, D.F. A dimerized HMX1 inhibits EPHA6/epha4b in mouse and zebrafish retinas. PLoS ONE 2014, 9, e100096. [Google Scholar] [CrossRef] [Green Version]
- Alestrom, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2019, 54, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, D.A.; Brunet, L.J.; Khokha, M.K.; Economides, A.N.; Harland, R.M. Cooperative activity of noggin and gremlin 1 in axial skeleton development. Development 2011, 138, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Stottmann, R.W.; Berrong, M.; Matta, K.; Choi, M.; Klingensmith, J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev. Biol. 2006, 295, 647–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakin, L.; Chang, E.Y.; Plouhinec, J.L.; De Robertis, E.M. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev. Biol. 2010, 347, 204–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Wang, X.L.; Zettervall, S.L.; Cai, Y.; Guzman, R.J. Dorsomorphin homologue 1, a highly selective small-molecule bone morphogenetic protein inhibitor, suppresses medial artery calcification. J. Vasc. Surg. 2017, 66, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.L.; Urquhart, J.; Lovell, S.C.; Biswas, S.; Parry, N.R.; Schorderet, D.F.; Lloyd, I.C.; Clayton-Smith, J.; Black, G.C. Abrogation of HMX1 function causes rare oculoauricular syndrome associated with congenital cataract, anterior segment dysgenesis, and retinal dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Wijgerde, M.; Karp, S.; McMahon, J.; McMahon, A.P. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev. Biol. 2005, 286, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, D.A.; Monica, S.D.; Harland, R.M. Follistatin interacts with Noggin in the development of the axial skeleton. Mech. Dev. 2014, 131, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Noel, D.; Gazit, D.; Bouquet, C.; Apparailly, F.; Bony, C.; Plence, P.; Millet, V.; Turgeman, G.; Perricaudet, M.; Sany, J.; et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells 2004, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.; Schafer, H.; Fliegauf, M.; Otto, F. Identification of novel genes of the bone-specific transcription factor Runx2. J. Bone Miner. Res. 2004, 19, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Tsuji, K.; Cox, K.; Harfe, B.D.; Rosen, V.; Tabin, C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006, 2, e216. [Google Scholar] [CrossRef] [Green Version]
- Choi, T.Y.; Khaliq, M.; Tsurusaki, S.; Ninov, N.; Stainier, D.Y.R.; Tanaka, M.; Shin, D. Bone morphogenetic protein signaling governs biliary-driven liver regeneration in zebrafish through tbx2b and id2a. Hepatology 2017, 66, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Esser, J.S.; Steiner, R.E.; Deckler, M.; Schmitt, H.; Engert, B.; Link, S.; Charlet, A.; Patterson, C.; Bode, C.; Zhou, Q.; et al. Extracellular bone morphogenetic protein modulator BMPER and twisted gastrulation homolog 1 preserve arterial-venous specification in zebrafish blood vessel development and regulate Notch signaling in endothelial cells. FEBS J. 2018, 285, 1419–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Ho, J.N.; Lewis, J.A.; Karim, K.A.; Daniels, R.N.; Gentry, P.R.; Hopkins, C.R.; Lindsley, C.W.; Hong, C.C. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem. Biol. 2010, 5, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Neely, M.D.; Litt, M.J.; Tidball, A.M.; Li, G.G.; Aboud, A.A.; Hopkins, C.R.; Chamberlin, R.; Hong, C.C.; Ess, K.C.; Bowman, A.B. DMH1, a highly selective small molecule BMP inhibitor promotes neurogenesis of hiPSCs: Comparison of PAX6 and SOX1 expression during neural induction. ACS Chem. Neurosci. 2012, 3, 482–491. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Fersioui, Y.; Pinton, G.; Allaman-Pillet, N.; Schorderet, D.F. Premature Vertebral Mineralization in hmx1-Mutant Zebrafish. Cells 2022, 11, 1088. https://doi.org/10.3390/cells11071088
El Fersioui Y, Pinton G, Allaman-Pillet N, Schorderet DF. Premature Vertebral Mineralization in hmx1-Mutant Zebrafish. Cells. 2022; 11(7):1088. https://doi.org/10.3390/cells11071088
Chicago/Turabian StyleEl Fersioui, Younes, Gaëtan Pinton, Nathalie Allaman-Pillet, and Daniel F. Schorderet. 2022. "Premature Vertebral Mineralization in hmx1-Mutant Zebrafish" Cells 11, no. 7: 1088. https://doi.org/10.3390/cells11071088




