New Composite Hydrogel Based on Whey and Gelatin Crosslinked with Copper Sulphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogels
2.3. Swelling Degree
- First order/First Fick’s Law (n~0.5) describes the perfect Fickian model of the swelling process (the slow diffusion of the solvent in the hydrogel matrix).
- Second-order Law (n = 1.0) explains that the mobility of the solvent is much higher than the degree of relaxation of the hydrogel matrix and the amount of solvent diffused is proportional to time.
- The atypical case of sorption (0.5 < n < 1.0) is when the solvent diffusion and the hydrogel matrix relaxation are comparable (the hydrogel matrix does not relax enough for the solvent to penetrate).
2.4. X-ray Diffraction
2.5. FTIR Spectroscopy
2.6. UV-VIS Spectroscopy
2.7. 1H NMR Relaxometry
2.8. Antimicrobial Activity
3. Results and Discussions
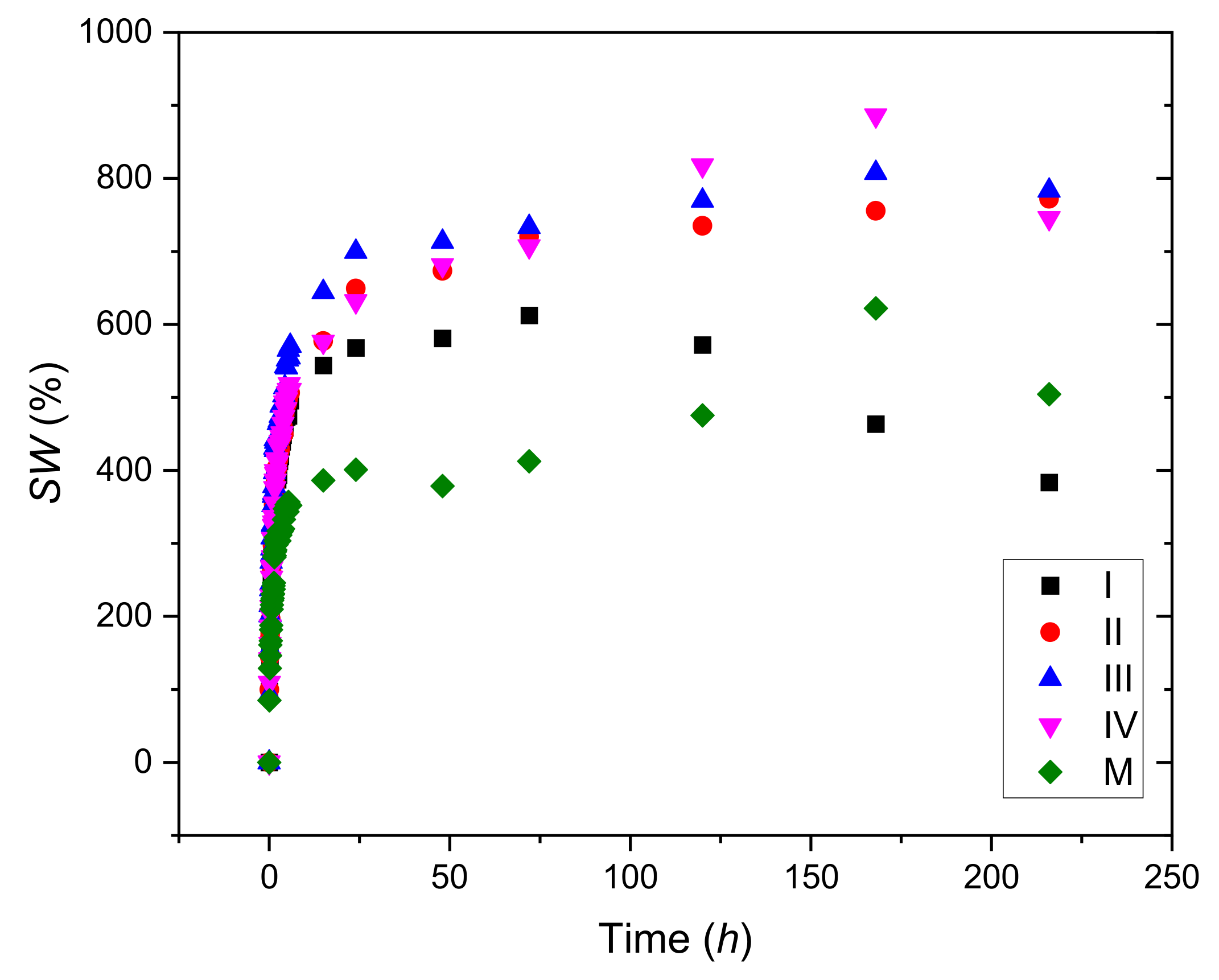
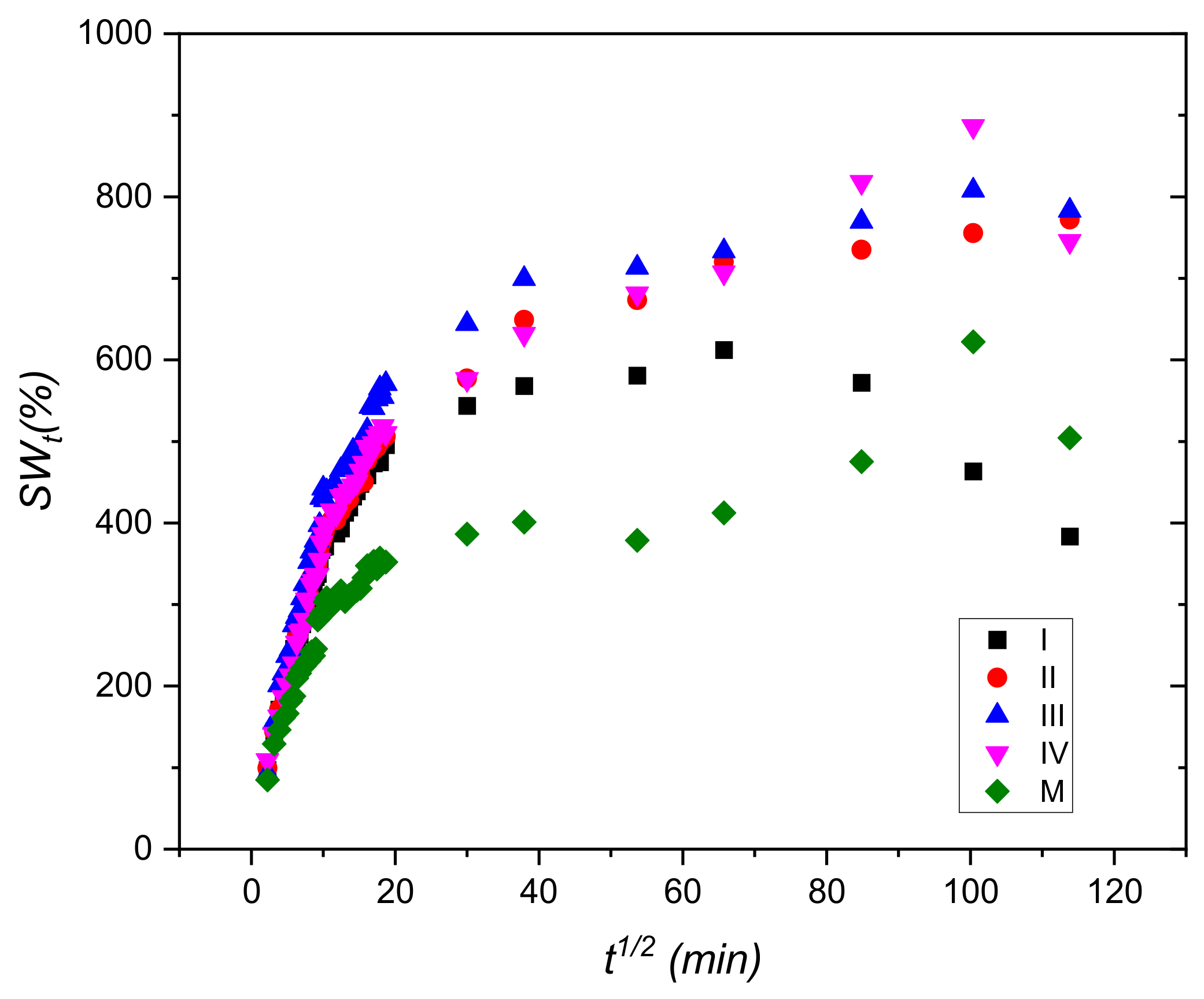
3.1. Swelling Tests
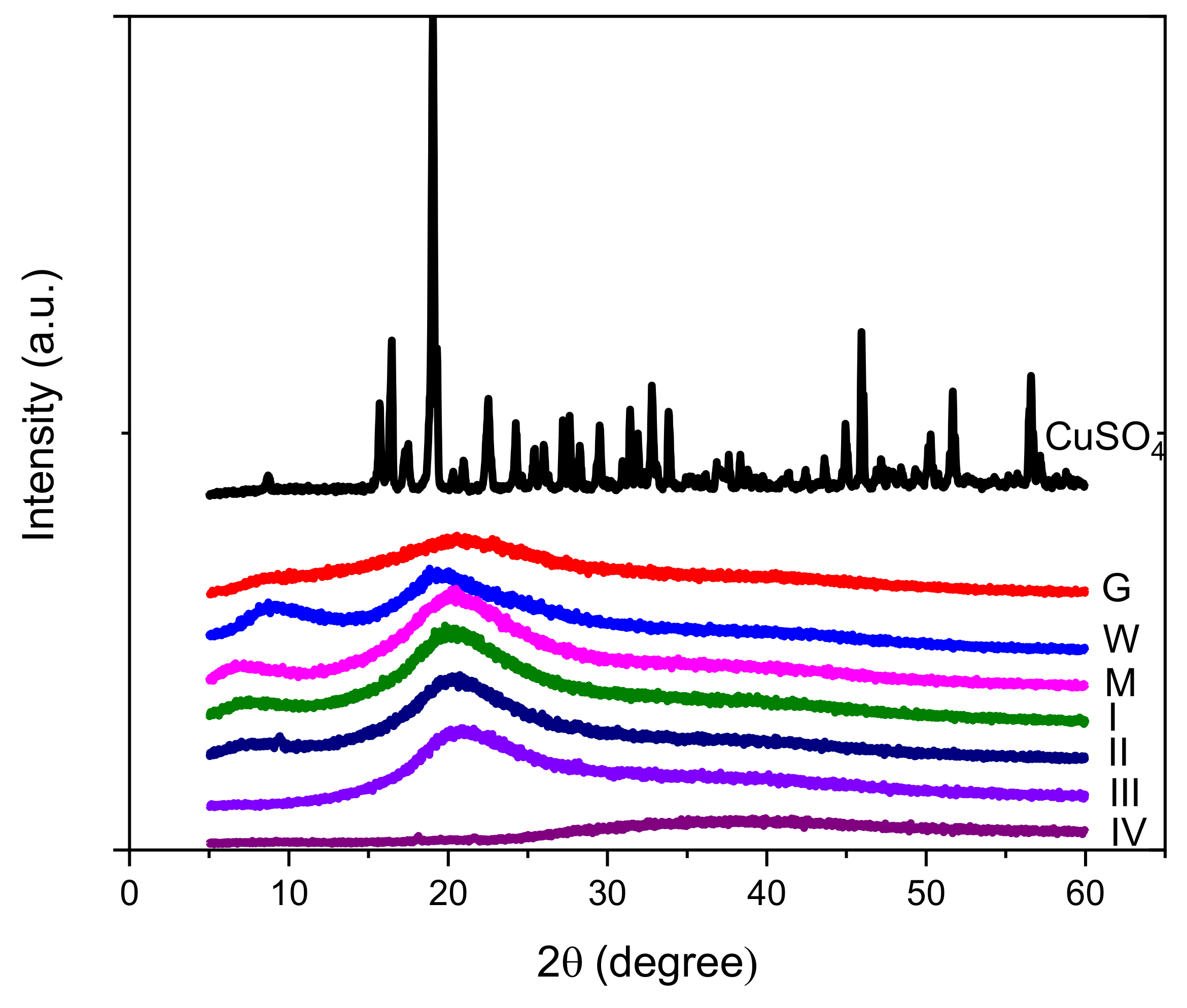
3.2. XRD Diffraction
3.3. Fourier Transform Infrared Spectrometry (FTIR)
3.4. UV-VIS Spectroscopy
3.5. NMR Relaxometry
3.6. Antimicrobial Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and Characterization of the Bionanocomposite Film Based on Whey Protein Biopolymer Loaded with TiO2 Nanoparticles, Cellulose Nanofibers and Rosemary Essential Oil. Ind. Crop. Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Pawde, S.M.; Deshmukh, K. Characterization of Polyvinyl Alcohol/Gelatin Blend Hydrogel Films for Biomedical Applications. J. Appl. Polym. Sci. 2008, 109, 3431–3437. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.-D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active Edible Packaging Based on Milk Proteins: A Route to Carry and Deliver Nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of Ecofriendly Bionanocomposite: Whey Protein Isolate/Pullulan Films with Nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Wu, J.; A’yun, Q.; Van der Meeren, P. Maillard Conjugation as an Approach to Improve Whey Proteins Functionality: A Review of Conventional and Novel Preparation Techniques. Trends Food Sci. Technol. 2019, 91, 1–11. [Google Scholar] [CrossRef]
- Popescu, V.; Molea, A.; Moldovan, M.; Lopes, P.M.; Mazilu Moldovan, A.; Popescu, G.L. The Influence of Enzymatic Hydrolysis of Whey Proteins on the Properties of Gelatin-Whey Composite Hydrogels. Materials 2021, 14, 3507. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A Novel Hydrogel Based on Renewable Materials for Agricultural Application. Int. J. Polym. Sci. 2020, 2020, e8363418. [Google Scholar] [CrossRef]
- Čechmánková, J.; Skála, J.; Sedlařík, V.; Duřpeková, S.; Drbohlav, J.; Šalaková, A.; Vácha, R. The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products. Sustainability 2021, 13, 10701. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Coltelli, M.B.; Lazzeri, A. Recyclability of PET/WPI/PE Multilayer Films by Removal of Whey Protein Isolate-Based Coatings with Enzymatic Detergents. Materials 2016, 9, 473. [Google Scholar] [CrossRef] [Green Version]
- Djelad, A.; Mokhtar, A.; Khelifa, A.; Bengueddach, A.; Sassi, M. Alginate-Whey an Effective and Green Adsorbent for Crystal Violet Removal: Kinetic, Thermodynamic and Mechanism Studies. Int. J. Biol. Macromol. 2019, 139, 944–954. [Google Scholar] [CrossRef]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Alkaline Subcritical Water Gasification of Dairy Industry Waste (Whey). Bioresour. Technol. 2011, 102, 6331–6335. [Google Scholar] [CrossRef] [PubMed]
- Okajima, I.; Shimoyama, D.; Sako, T. Gasification and Hydrogen Production from Food Wastes Using High Pressure Superheated Steam in the Presence of Alkali Catalyst. J. Chem. Eng. Jpn. 2007, 40, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Tomczyńska-Mleko, M.; Kamysz, E.; Sikorska, E.; Puchalski, C.; Mleko, S.; Ozimek, L.; Kowaluk, G.; Gustaw, W.; Wesołowska-Trojanowska, M. Changes of Secondary Structure and Surface Tension of Whey Protein Isolate Dispersions upon PH and Temperature. Czech J. Food Sci. 2014, 32, 82–89. [Google Scholar] [CrossRef]
- Zand-Rajabi, H.; Madadlou, A. Caffeine-Loaded Whey Protein Hydrogels Reinforced with Gellan and Enriched with Calcium Chloride. Int. Dairy J. 2016, 56, 38–44. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ko, S.; Xiao, L. Use of Whey Proteins for Encapsulation and Controlled Delivery Applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Mayorova, O.A.; Jolly, B.C.; Verkhovskii, R.A.; Plastun, V.O.; Sindeeva, O.A.; Douglas, T.E. PH-Sensitive Dairy-Derived Hydrogels with a Prolonged Drug Release Profile for Cancer Treatment. Materials 2021, 14, 749. [Google Scholar] [CrossRef]
- Betz, M.; Hörmansperger, J.; Fuchs, T.; Kulozik, U. Swelling Behaviour, Charge and Mesh Size of Thermal Protein Hydrogels as Influenced by PH during Gelation. Soft Matter 2012, 8, 2477–2485. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Ndwabu, S. Evaluation of Whey Protein Isolate-Graft-Carbopol-Polyacrylamide PH-Sensitive Composites for Controlled Release of Pamidronate. Polym. Bull. 2017, 74, 5129–5144. [Google Scholar] [CrossRef]
- Ozel, B.; Aydin, O.; Oztop, M.H. In Vitro Digestion of Polysaccharide Including Whey Protein Isolate Hydrogels. Carbohydr. Polym. 2020, 229, 115469. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold Gelation of Curcumin Loaded Whey Protein Aggregates Mixed with K-Carrageenan: Impact of Gel Microstructure on the Gastrointestinal Fate of Curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Asdagh, A.; Sani, I.K.; Pirsa, S.; Amiri, S.; Shariatifar, N.; Eghbaljoo–Gharehgheshlaghi, H.; Shabahang, Z.; Taniyan, A. Production and Characterization of Nanocomposite Film Based on Whey Protein Isolated/Copper Oxide Nanoparticles Containing Coconut Essential Oil and Paprika Extract. J. Polym. Environ. 2021, 29, 335–349. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-Mechanical, Rheological, Structural and Antimicrobial Properties of Bionanocomposite Films Based on Fish Skin Gelatin and Silver-Copper Nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Zhong, T.; Oporto, G.S.; Jaczynski, J. Antimicrobial food packaging with cellulose-copper nanoparticles embedded in thermoplastic resins. In Food Preservation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 671–702. ISBN 978-0-12-804303-5. [Google Scholar]
- Shankar, S.; Jaiswal, L.; Rhim, J.-W. Chapter 27—Gelatin-based nanocomposite films: Potential use in antimicrobial active packaging. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 339–348. ISBN 978-0-12-800723-5. [Google Scholar]
- Sarwar, N.; Humayoun, U.B.; Kumar, M.; Zaidi, S.F.A.; Yoo, J.H.; Ali, N.; Jeong, D.I.; Lee, J.H.; Yoon, D.H. Citric Acid Mediated Green Synthesis of Copper Nanoparticles Using Cinnamon Bark Extract and Its Multifaceted Applications. J. Clean. Prod. 2021, 292, 125974. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Effect of Copper Salts and Reducing Agents on Characteristics and Antimicrobial Activity of Copper Nanoparticles. Mater. Lett. 2014, 132, 307–311. [Google Scholar] [CrossRef]
- Kumar, N.; Upadhyay, L.S.B. Facile and Green Synthesis of Highly Stable L-Cysteine Functionalized Copper Nanoparticles. Appl. Surf. Sci. 2016, 385, 225–233. [Google Scholar] [CrossRef]
- Jahan, I.; Erci, F.; Isildak, I. Facile Microwave-Mediated Green Synthesis of Non-Toxic Copper Nanoparticles Using Citrus Sinensis Aqueous Fruit Extract and Their Antibacterial Potentials. J. Drug Deliv. Sci. Technol. 2021, 61, 102172. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Gelatin-Based Film Integrated with Copper Sulfide Nanoparticles for Active Packaging Applications. Appl. Sci. 2021, 11, 6307. [Google Scholar] [CrossRef]
- Mohamed, E.A. Green Synthesis of Copper & Copper Oxide Nanoparticles Using the Extract of Seedless Dates. Heliyon 2020, 6, e03123. [Google Scholar] [CrossRef] [Green Version]
- Chemistry International; Amer, M.; Awwad, A. Green Synthesis of Copper Nanoparticles by Citrus Limon Fruits Extract, Characterization and Antibacterial Activity. Chem. Int. 2021, 7, 1–8. [Google Scholar]
- Kang, N.; Hua, J.; Gao, L.; Zhang, B.; Pang, J. The Interplay between Whey Protein Fibrils with Carbon Nanotubes or Carbon Nano-Onions. Materials 2021, 14, 608. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary Structure of Food Proteins by Fourier Transform Spectroscopy in the Mid-Infrared Region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Maus, A.; Hertlein, C.; Saalwachter, K. A Robust Proton NMR Method to Investigate Hard/Soft Ratios, Crystallinity, and Component Mobility in Polymers. Macromol. Chem. Phys. 2006, 207, 1150–1158. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/macp.200600169?casa_token=QWibzMu_AvoAAAAA:q3PjJH4WTCh0aeyVIDESqZvhku7-94ANYR2WKHfzTll2JaDBfboMvsX38cVEKxpSMmM-HAMu9aAs28w (accessed on 6 February 2022). [CrossRef]
- Moraes, T.B.; Monaretto, T.; Colnago, L.A. Rapid and Simple Determination of T1 Relaxation Times in Time-Domain NMR by Continuous Wave Free Precession Sequence. J. Magn. Reson. 2016, 270, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ozel, B.; Kruk, D.; Wojciechowski, M.; Osuch, M.; Oztop, M.H. Water Dynamics in Whey-Protein-Based Composite Hydrogels by Means of NMR Relaxometry. Int. J. Mol. Sci. 2021, 22, 9672. [Google Scholar] [CrossRef]
- Ozel, B.; Uguz, S.S.; Kilercioglu, M.; Grunin, L.; Oztop, M.H. Effect of Different Polysaccharides on Swelling of Composite Whey Protein Hydrogels: A Low Field (LF) NMR Relaxometry Study. J. Food Process Eng. 2017, 40, e12465. [Google Scholar] [CrossRef]
- Deng, R.; Seimys, A.; Mars, M.; Janssen, A.E.M.; Smeets, P.A.M. Monitoring PH and Whey Protein Digestion by TD-NMR and MRI in a Novel Semi-Dynamic in Vitro Gastric Simulator (MR-GAS). Food Hydrocoll. 2022, 125, 107393. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Peña, C.; de la Caba, K.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing Water Repellence and Mechanical Properties of Gelatin Films by Tannin Addition. Bioresour. Technol. 2010, 101, 6836–6842. [Google Scholar] [CrossRef]
- Frazier, S.D.; Srubar, W.V., III. Evaporation-Based Method for Preparing Gelatin Foams with Aligned Tubular Pore Structures. Mater. Sci. Eng. C 2016, 62, 467–473. [Google Scholar] [CrossRef]
- Moldovan, M.; Prodan, D.; Ciotlaus, I.; Popescu, V.; Baldea, I.; Carpa, R.; Sava, S.; Chifor, R.; Badea, M.E. Assessment and Characterization of Some New Photosensitizers for Antimicrobial Photodynamic Therapy (APDT). Materials 2020, 13, 3012. [Google Scholar]
- Fechete, R.; Morar, I.A.; Moldovan, D.; Chelcea, R.I.; Crainic, R.; Nicoară, S.C. Fourier and Laplace-like Low-Field NMR Spectroscopy: The Perspectives of Multivariate and Artificial Neural Networks Analyses. J. Magn. Reson. 2021, 324, 106915. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; Taylor and Francis Group: Abingdon, UK, 2010; pp. 1333–1334. [Google Scholar]
- Carpa, R.; Drăgan-Bularda, M.; Muntean, V. Microbiologie Generală: Lucrări Practice; Presa Universitară Clujeană: Cluj-Napoca, Romania, 2014. [Google Scholar]
- Li, H.; Zhao, L.; Chen, X.D.; Mercadé-Prieto, R. Swelling of Whey and Egg White Protein Hydrogels with Stranded and Particulate Microstructures. Int. J. Biol. Macromol. 2016, 83, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Asma, C.; Meriem, E.; Mahmoud, B.; Djaafer, B. Physicochemical Characterization of Gelatin-Cmc Composite Edibles Films from Polyion-Complex Hydrogels. J. Chil. Chem. Soc. 2014, 59, 2279–2283. [Google Scholar] [CrossRef] [Green Version]
- Yakimets, I.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Farhat, I.; Mitchell, J. Mechanical Properties with Respect to Water Content of Gelatin Films in Glassy State. Polymer 2005, 46, 12577–12585. [Google Scholar] [CrossRef]
- Yin, Y.; Ji, X.; Dong, H.; Ying, Y.; Zheng, H. Study of the Swelling Dynamics with Overshooting Effect of Hydrogels Based on Sodium Alginate-g-Acrylic Acid. Carbohydr. Polym. 2008, 71, 682–689. [Google Scholar] [CrossRef]
- Das, M.P.; Suguna, P.R.; Prasad, K.; Vijaylakshmi, J.V.; Renuka, M. Extraction and Characterization of Gelatin: A Functional Biopolymer. Int. J. Pharm. Pharm. Sci. 2017, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Frazier, S.D.; Aday, A.N.; Srubar, W.V. On-Demand Microwave-Assisted Fabrication of Gelatin Foams. Molecules 2018, 23, 1121. [Google Scholar] [CrossRef] [Green Version]
- Gioffre, M.; Torricelli, P.; Panzavolta, S.; Rubini, K.; Bigi, A. Role of PH on Stability and Mechanical Properties of Gelatin Films. J. Bioact. Compat. Polym. 2012, 27, 67–77. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between Triple-Helix Content and Mechanical Properties of Gelatin Films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Ju, Z.; Kilara, A. Aggregation Induced by Calcium Chloride and Subsequent Thermal Gelation of Whey Protein Isolate. J. Dairy Sci. 1998, 81, 925–931. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Liu, F.; Ren, F.; Zhao, G.; Leng, X. Fabrication and Characterization of TiO2/Whey Protein Isolate Nanocomposite Film. Food Hydrocoll. 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Hemalatha, K.; Somashekarappa, H.; Somashekar, R. Micro-Structure, Ac Conductivity and Spectroscopic Studies of Cupric Sulphate Doped PVA/PVP Polymer Composites. Adv. Mater. Phys. Chem. 2015, 5, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Fechete, R.; Demco, D.E.; Zhu, X.; Tillmann, W.; Möller, M. Water States and Dynamics in Perfluorinated Ionomer Membranes by 1H One- and Two-Dimensional NMR Spectroscopy, Relaxometry, and Diffusometry. Chem. Phys. Lett. 2014, 597, 6–15. [Google Scholar] [CrossRef]
- Cioica, N.; Fechete, R.; Filip, C.; Cozar, I.B.; Cota, C.; Nagy, E.M. Nmr and Sem investigation of extruded native corn starch with plasticizers. Rom. J. Phys. 2015, 60, 512–520. [Google Scholar]
- Klinkajon, W.; Supaphol, P. Novel Copper (II) Alginate Hydrogels and Their Potential for Use as Anti-Bacterial Wound Dressings. Biomed. Mater. 2014, 9, 045008. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Carrageenan-Based Hydrogels and Films: Effect of ZnO and CuO Nanoparticles on the Physical, Mechanical, and Antimicrobial Properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
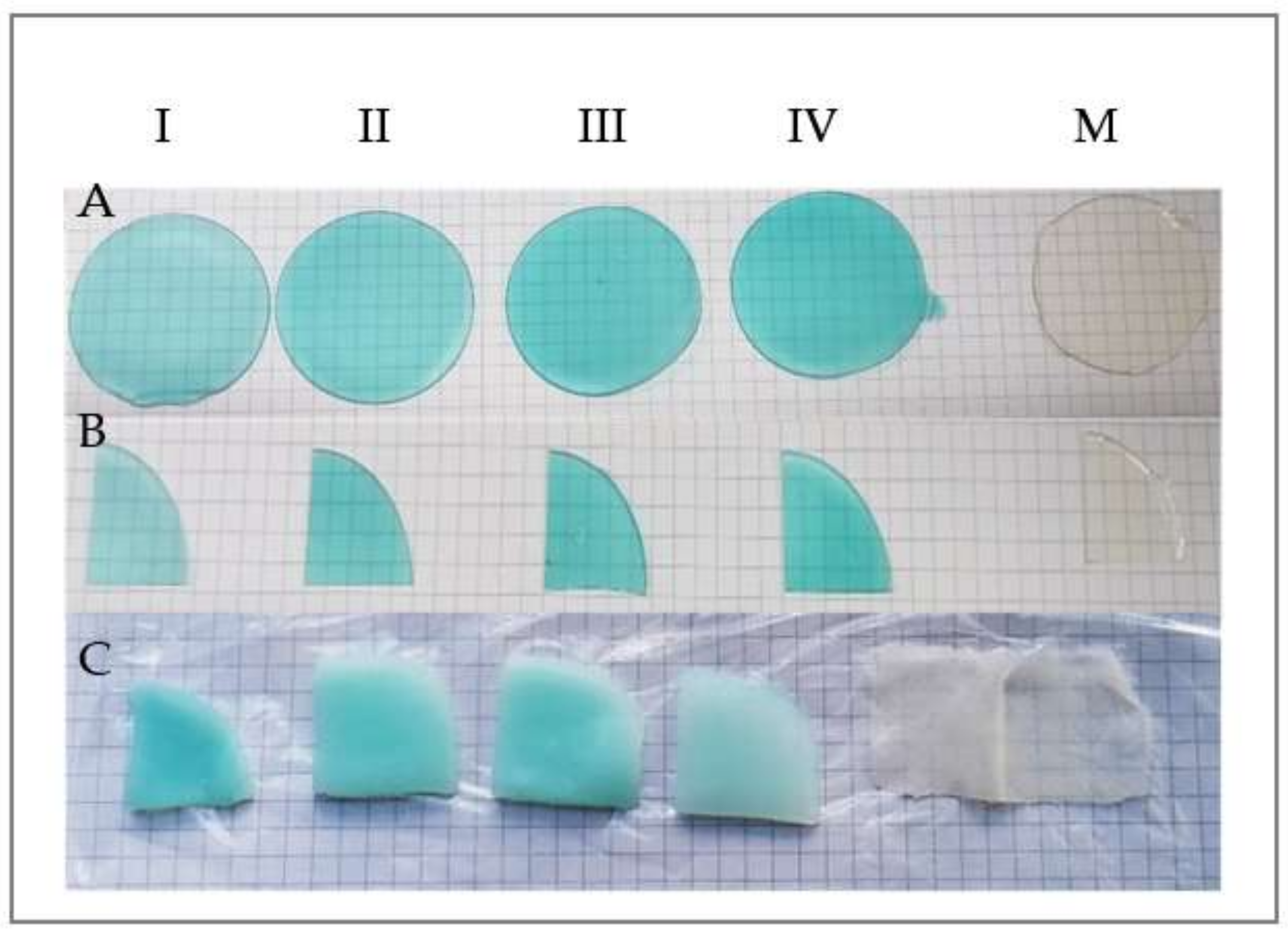





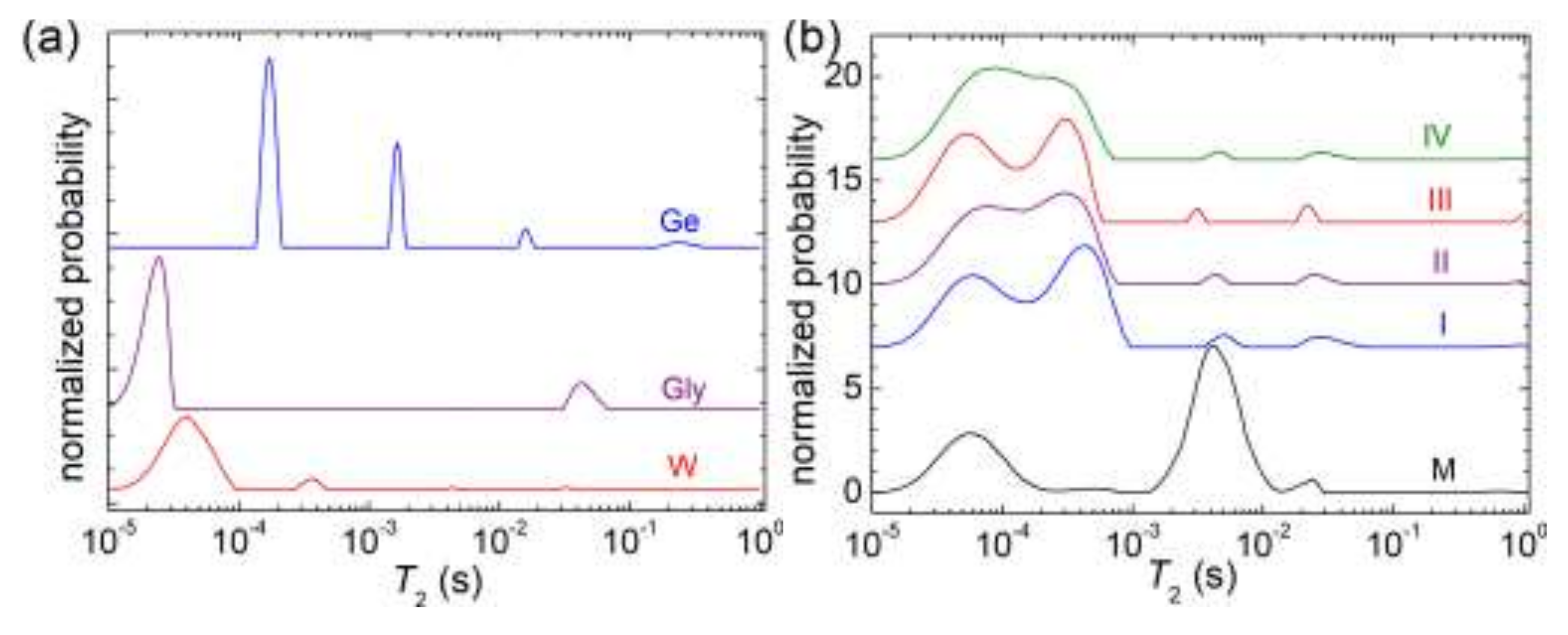
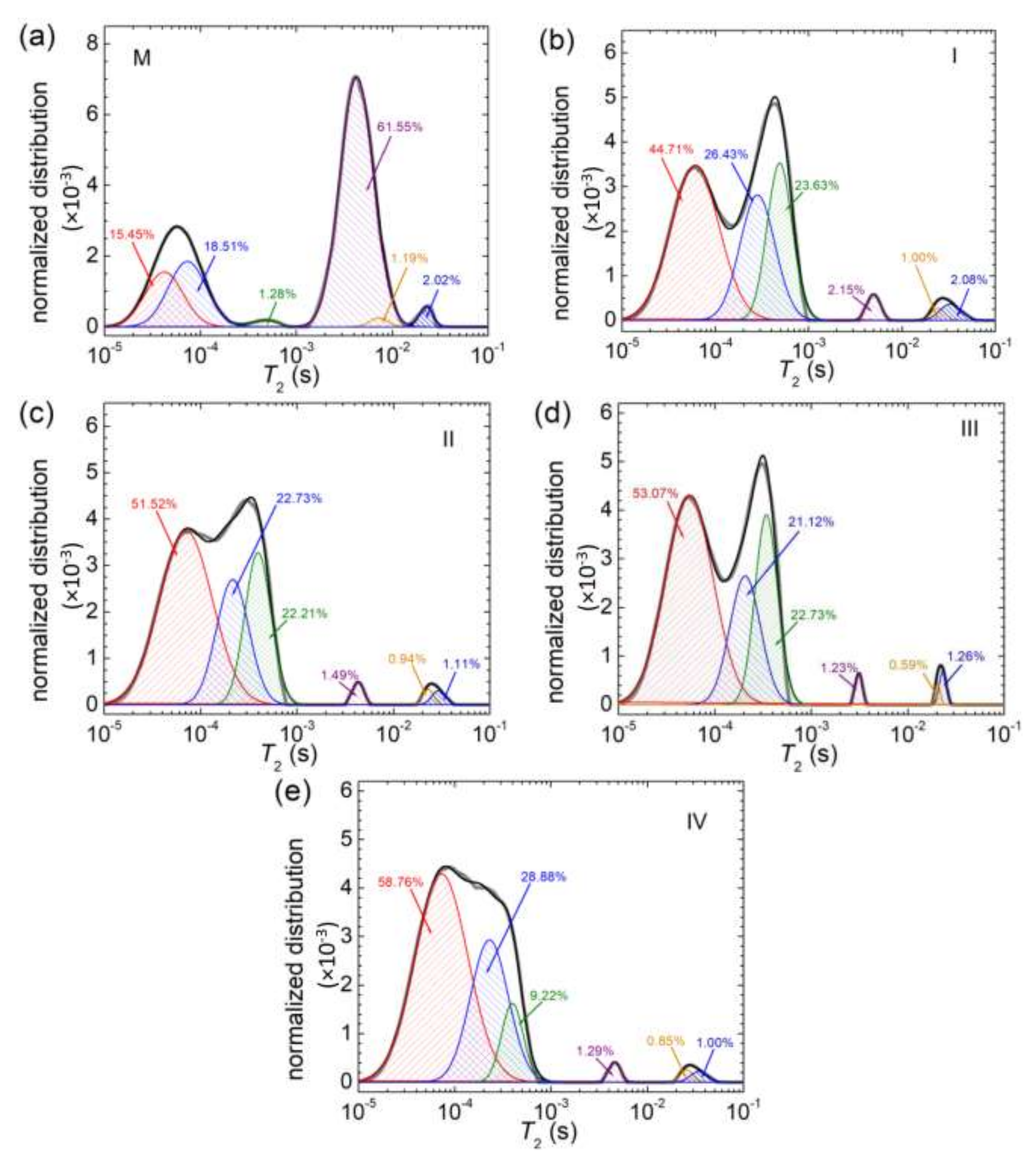
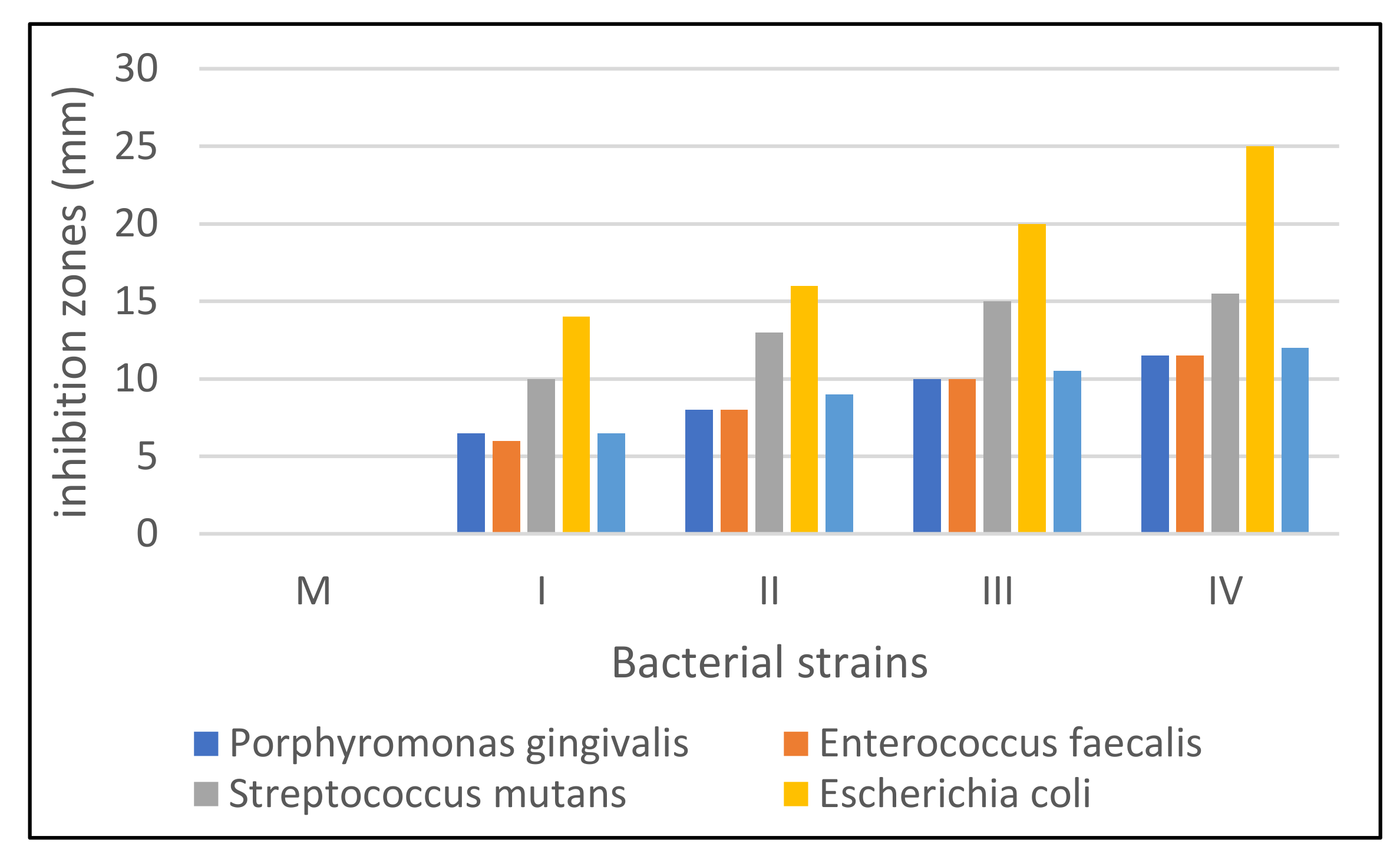
| Sample | M | I | II | III | IV |
|---|---|---|---|---|---|
| Copper sulphate concentration (%) * | 0 | 0.39 | 0.55 | 0.70 | 0.86 |
| Code Sample | Slope | SWe (Calculated) (%) | SWe Experimental (%) | Intercept | K×105 (1/min) ×105 |
|---|---|---|---|---|---|
| I | 0.00229 | 434.78 | 383.55 | 0.1244 | 4.25 |
| II | 0.00131 | 769.23 | 772.03 | 0.1644 | 1.03 |
| III | 0.00126 | 769.23 | 783.20 | 0.1331 | 1.27 |
| IV | 0.00125 | 769.23 | 745.63 | 0.1661 | 1.02 |
| M | 0.00191 | 526.31 | 504.39 | 0.2653 | 1.36 |
| Sample | 2θ (Degree) | Distance between Turns (nm) | 2θ (Degree) | Distance between Chains (nm) |
|---|---|---|---|---|
| G | 9.89 | 0.894 | 20.21 | 0.439 |
| W | 9.02 | 0.979 | 19.85 | 0.447 |
| M | 7.10 | 1.244 | 20.37 | 0.436 |
| I | 7.45 | 1.186 | 20.39 | 0.435 |
| II | 7.89 | 1.186 | 20.44 | 0.434 |
| III | - | - | 21.07 | 0.421 |
| IV | - | - | 36.91 | 0.243 |
| Glycerin | Gelatin | Whey | I | II | III | IV | Attributions |
|---|---|---|---|---|---|---|---|
| (cm−1) | |||||||
| 3285.58 | 3272.82 | 3272.4 | 3291.67 | 3290.14 | 3291.20 | 3291.41 | Amide A, tensile vibrations NH [14,21,41], hydrogen bonds [14,54] |
| - | 1630.62 | 1631.72 | 1633.51 | 1631.75 | 1633.32 | 1632.03 | Amide I, C=O and NH vibrations [14,21,22,41], H bonds coupled with COO− [14,22,54] |
| - | 1527.47 | 1515.90 | 1550.56 | 1548.66 | 1549.63 | 1548.64 | Amide II, CN vibrations/stretching, NH bending [14,22] |
| 1413.81 | 1445.43 | 1446.41 | 1450.33 | 1450.35 | 1451.20 | 1450.27 | Amide II, CN vibrations, NH bending [21] |
| - | 1080.02 | 1076.17 | 1093.52 | 1095.46 | 1095.43 | 1095.73 | Amide III CN and NH vibrations [6,21] |
| Bacterial Strain | Sample Code | ||||
|---|---|---|---|---|---|
| M | I | II | III | IV | |
| Porphyromonas gingivalis | 0 | 6.5 | 8 | 10 | 11.5 |
| Enterococcus faecalis | 0 | 6 | 8 | 10 | 11.5 |
| Streptococcus mutans | 0 | 10 | 13 | 15 | 15.5 |
| Escherichia coli | 0 | 14 | 16 | 20 | 25 |
| Staphylococcus aureus | 0 | 6.5 | 9 | 10.5 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purcea Lopes, P.M.; Moldovan, D.; Moldovan, M.; Carpa, R.; Saroşi, C.; Păşcuţă, P.; Mazilu Moldovan, A.; Fechete, R.; Popescu, V. New Composite Hydrogel Based on Whey and Gelatin Crosslinked with Copper Sulphate. Materials 2022, 15, 2611. https://doi.org/10.3390/ma15072611
Purcea Lopes PM, Moldovan D, Moldovan M, Carpa R, Saroşi C, Păşcuţă P, Mazilu Moldovan A, Fechete R, Popescu V. New Composite Hydrogel Based on Whey and Gelatin Crosslinked with Copper Sulphate. Materials. 2022; 15(7):2611. https://doi.org/10.3390/ma15072611
Chicago/Turabian StylePurcea Lopes, Pompilia Mioara, Dumitriţa Moldovan, Marioara Moldovan, Rahela Carpa, Codruţa Saroşi, Petru Păşcuţă, Amalia Mazilu Moldovan, Radu Fechete, and Violeta Popescu. 2022. "New Composite Hydrogel Based on Whey and Gelatin Crosslinked with Copper Sulphate" Materials 15, no. 7: 2611. https://doi.org/10.3390/ma15072611
APA StylePurcea Lopes, P. M., Moldovan, D., Moldovan, M., Carpa, R., Saroşi, C., Păşcuţă, P., Mazilu Moldovan, A., Fechete, R., & Popescu, V. (2022). New Composite Hydrogel Based on Whey and Gelatin Crosslinked with Copper Sulphate. Materials, 15(7), 2611. https://doi.org/10.3390/ma15072611








