Performance Comparison of LMNO Cathodes Produced with Pullulan or PEDOT:PSS Water-Processable Binders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Electrodes
2.2. Chemical–Physical Analyses
2.3. Lithium Metal/LMNO Coin Cell Prototype Assembly
2.4. Electrochemical Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SET-Plan ACTION n°7. Declaration of Intent “Become Competitive in the Global Battery Sector to Drive E-Mobility Forward”. Available online: https://setis.ec.europa.eu/system/files/2021-05/action7_declaration_of_intent_0.pdf (accessed on 20 February 2022).
- Deliverable 3.6. Strategic Research Agenda for Batteries. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/batteries_europe_strategic_research_agenda_december_2020__1.pdf (accessed on 24 February 2022).
- Liang, G.; Peterson, V.K.; See, K.W.; Guo, Z.; Pang, W.K. Developing high-voltage spinel LiNi0.5Mn1.5O4 cathodes for high-energy-density lithium-ion batteries: Current achievements and future prospects. J. Mater. Chem. A 2020, 8, 15373–15398. [Google Scholar] [CrossRef]
- Xu, J.; Dou, S.; Liu, H.; Dai, L. Cathode materials for next generation lithium ion batteries. Nano Energy 2013, 2, 439–442. [Google Scholar] [CrossRef]
- European Battery Alliance. Available online: https://ec.europa.eu/growth/industry/strategy/industrial-alliances/european-battery-alliance_it (accessed on 24 February 2022).
- Bresser, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative Binders for sustainable electrochemical energy storage–the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- REGULATION (EC) No 1907/2006 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1907&from=EN] (accessed on 24 February 2022).
- Wood, D.L.; Quass, J.D.; Li, J.; Ahmed, S.; Ventola, D.; Daniel, C. Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP. Dry. Technol. 2018, 36, 234–244. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, T.; Ge, D.; Wood III, D.L.; Li, Z. Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System. iScience 2020, 23, 101081. [Google Scholar] [CrossRef] [PubMed]
- Brilloni, A.; Poli, F.; Spina, G.E.; Samorì, C.; Guidi, E.; Gualandi, C.; Maisuradze, M.; Giorgetti, M.; Soavi, F. Easy recovery of Li-ion cathode powders by the use of water-processable binders. Electrochim. Acta, 2022; under review. [Google Scholar]
- Bigoni, F.; De Giorgio, F.; Soavi, F.; Arbizzani, C. New Formulations of High-Voltage Cathodes for Li-Ion Batteries with Water-Processable Binders. ECS Trans. 2016, 73, 249–257. [Google Scholar] [CrossRef]
- Malavolta, L.; Terella, A.; De Giorgio, F.; Arbizzani, C. Improved Adhesion of Nafion™-Coated Separator to Water-Processable LiNi0.5Mn1.5O4 Electrodes. Batteries 2020, 6, 28. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Poli, F.; Momodu, D.; Spina, G.E.; Terella, A.; Mutuma, B.K.; Focarete, M.L.; Manyala, N.; Soavi, F. Pullulan-ionic liquid-based supercapacitor: A novel, smart combination of components for an easy-to-dispose device. Electrochim. Acta 2020, 338, 135872. [Google Scholar] [CrossRef]
- Spina, G.E.; Poli, F.; Brilloni, A.; Marchese, D.; Soavi, F. Natural polymers for green supercapacitors. Energies 2020, 13, 3115. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Van At Nguyen, V.A.; Kuss, C. Review—Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Ozerova, V.V.; Stenina, I.A.; Kuz’mina, A.A.; Kulova, T.L.; Yaroslavtsev, A.B. Cathode materials based on lithium iron phosphate/PEDOT composites for lithium-ion batteries. Inorg. Mater. 2020, 56, 648–656. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J. Improvement of the Cycle Performance of LiNi0.5Mn1.5O4 Cathode Active Materials by In-Situ Coating with Poly (3,4-Ethylenedioxythiophene). J. Power Energy Eng. 2017, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Smith, P.M.; Anand, P.; Reeja-Jayan, B. Surface Engineering of a LiMn2O4 Electrode Using Nanoscale Polymer Thin Films via Chemical Vapor Deposition Polymerization. ACS Appl. Mater. Interfaces 2018, 10, 27063–27073. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Weaver, J.L.; Groenenboom, M.; Nakamura, N.; Rus, E.; Anand, P.; Jha, S.K.; Okasinski, J.S.; Dura, J.A.; Reeja-Jayan, B. Tailoring Electrode–Electrolyte Interfaces in Lithium-Ion Batteries Using Molecularly Engineered Functional Polymers. ACS Appl. Mater. Interfaces 2021, 13, 9919–9931. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, J.; Li, L.; Zhang, X.; Luo, R.; Ye, Y.; Chen, R. Surface modification of Li-rich cathode materials for lithium-ion batteries with a PEDOT:PSS conducting polymer. ACS Appl. Mater. Interfaces 2016, 8, 23095–23104. [Google Scholar] [CrossRef]
- Bae, J.J.; Shin, J.W.; Lee, S.J.; Kim, S.J.; Hong, T.W.; Son, J.T. Effect of PEDOT:PSS (Poly(3,4-ethylenedioxythiophene)—polystyrenesulfonate) Coating on Nanostructured Cobalt-Free LiNi0.9Mn0.1O2 Layered Oxide Cathode Materials for Lithium-Ion Battery. J. Nanosci. Nanotechnol. 2020, 20, 338–343. [Google Scholar] [CrossRef]
- Das, P.R. Conducting Polymers as Functional Binders for Lithium Ion Battery Positive Electrodes. Ph.D. Thesis, Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany, 2016. [Google Scholar]
- Xin, X.; Xue, Z.; Gao, N.; Yu, J.; Liu, H.; Zhang, W.; Xu, J.; Chen, S. Effects of conductivity-enhancement reagents on self-healing properties of PEDOT:PSS films. Synth. Met. 2020, 268, 116503. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.H.; King, P.J.; Zhang, C.J.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A Commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Lee, J.A.; Vosgueritchian, M.; Tee, B.C.-K.; Bolander, J.A.; Bao, Z. Electronic Properties of Transparent Conductive Films of PEDOT:PSS on Stretchable Substrates. Chem. Mater. 2012, 24, 373–382. [Google Scholar] [CrossRef]
- Lee, H.D.; Jung, G.J.; Lee, H.S.; Kim, T.; Byun, J.D.; Suh, K.S. Improved stability of lithium-ion battery cathodes using conducting polymer binders. Sci. Adv. Mater. 2016, 8, 84–88. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur. J. Pharm. Sci. 2017, 96, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Xiao, Q.; Lim, L.T. Preparation and properties of pullulan–alginate–carboxymethylcellulose blend films. Food Res. Int. 2008, 41, 1007–1014. [Google Scholar] [CrossRef]
- Schönherr, K.; Schumm, B.; Hippauf, F.; Lissy, R.; Althues, H.; Leyens, C.; Kaskel, S. Liquid lithium metal processing into ultrathin metal anodes for solid state batteries. Chem. Eng. J. Adv. 2022, 9, 100218. [Google Scholar] [CrossRef]
- Heubner, C.; Voigt, K.; Marcinkowski, P.; Reuber, S.; Nikolowski, K.; Schneider, M.; Partsch, M.; Michaelis, A. From Active Materials to Battery Cells: A Straightforward Tool to Determine Performance Metrics and Support Developments at an Application-Relevant Level. Adv. Energy Mater. 2021, 11, 2102647. [Google Scholar] [CrossRef]

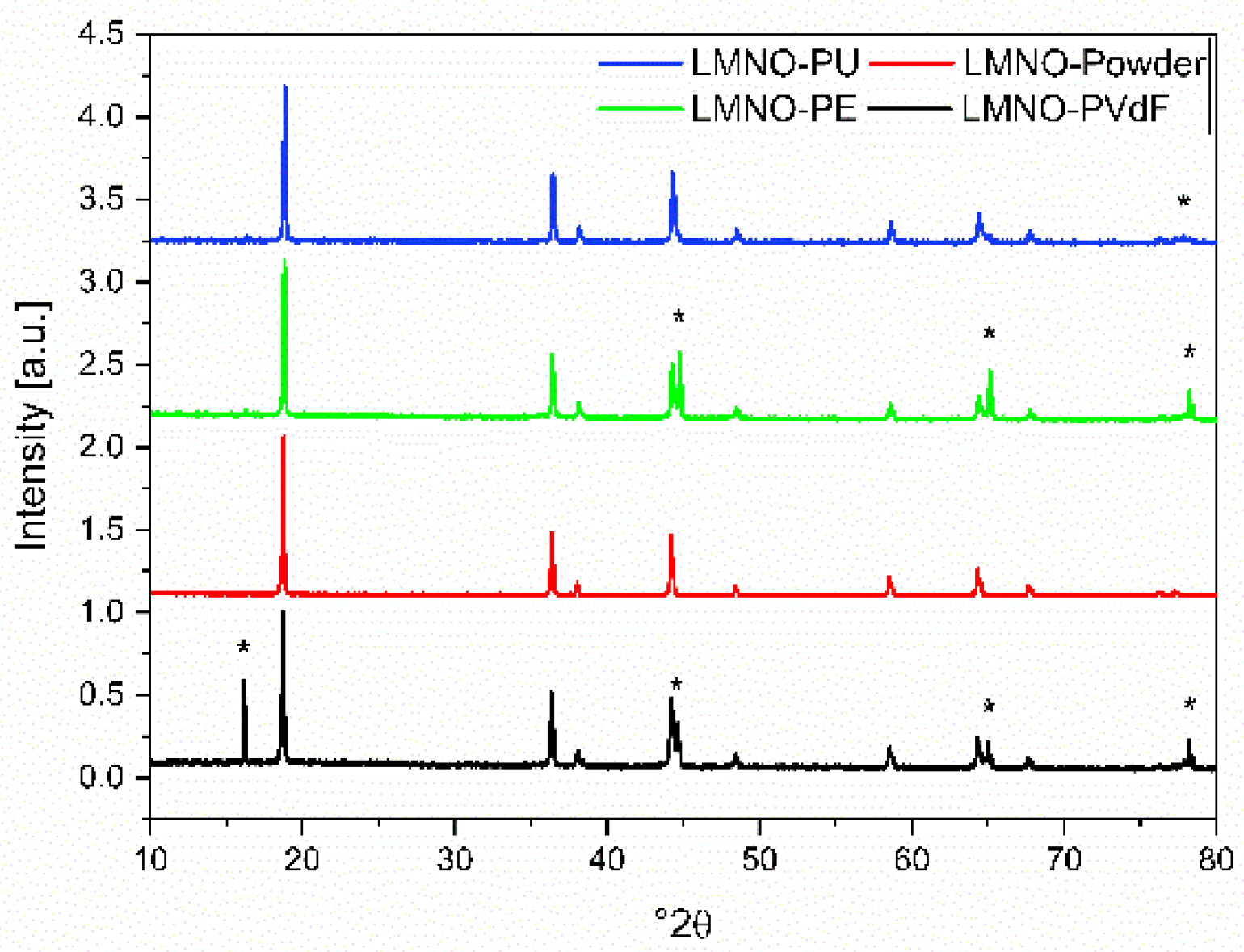

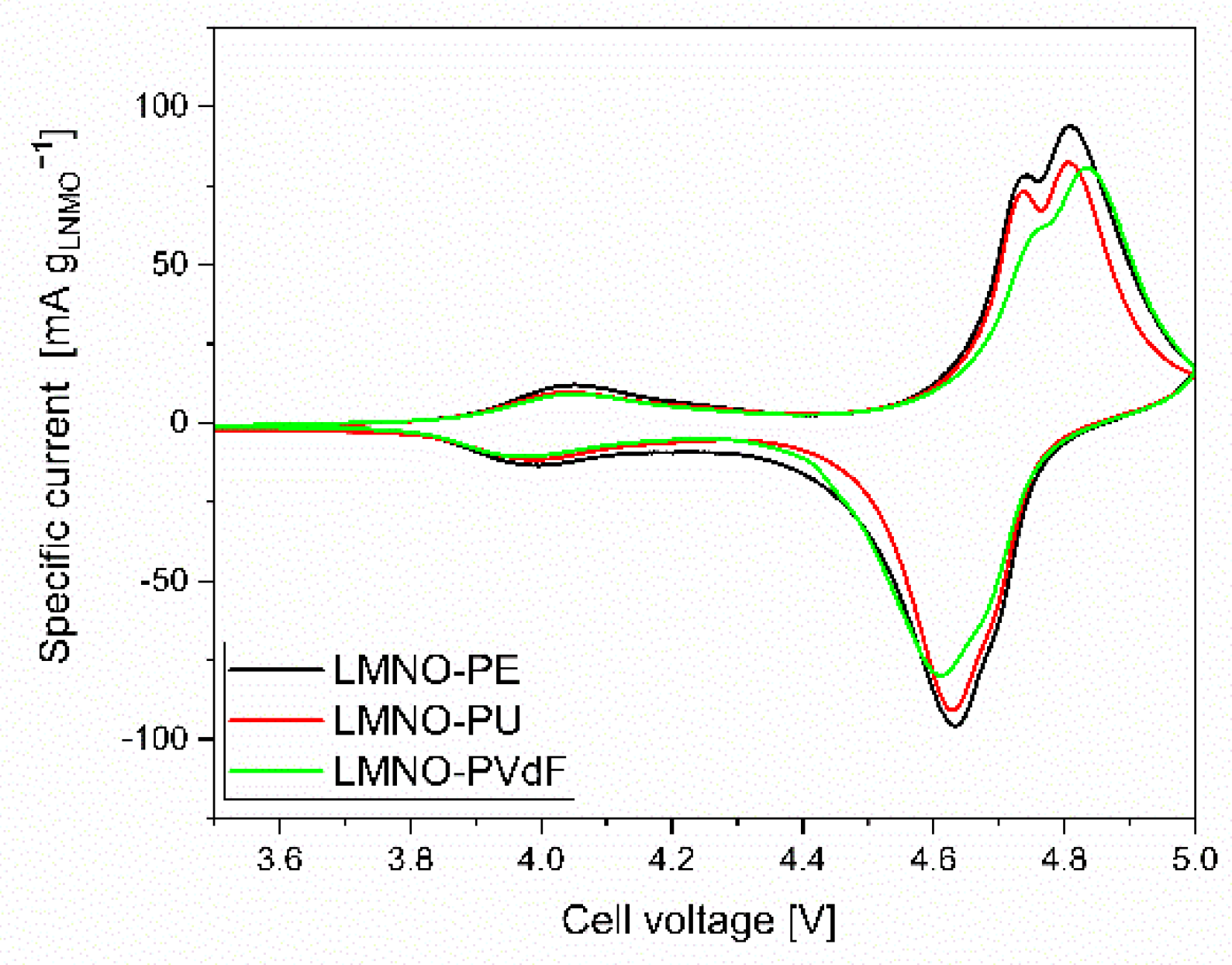


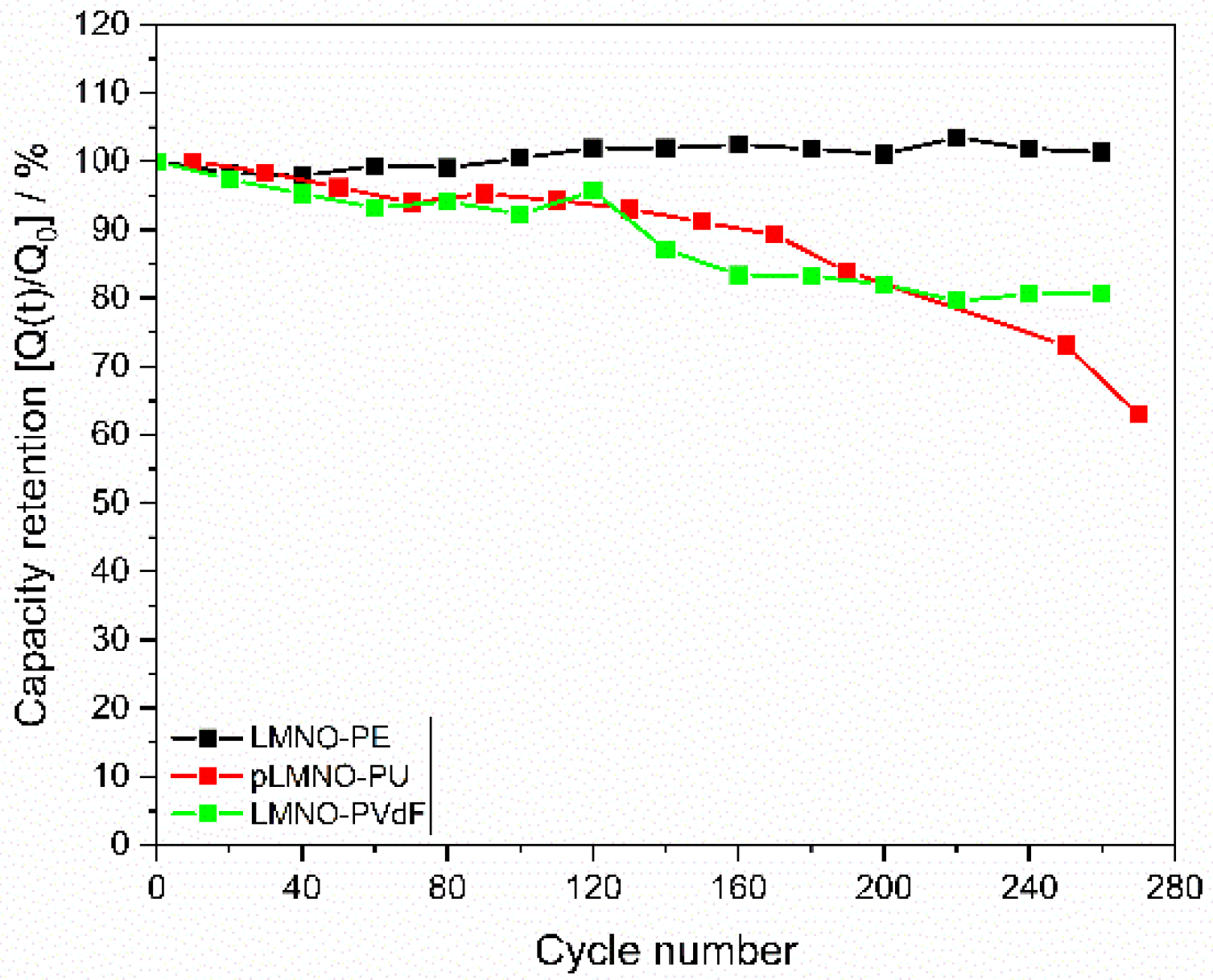
| Electrode Name | Binder | Composite Mass Loading (mg cm−2) | LMNO Mass Loading (mg cm−2) |
|---|---|---|---|
| LMNO-PU | Pullulan | 2.1 | 1.8 |
| LMNO-PE | PEDOT:PSS | 3.8 | 3.2 |
| LMNO-PVdF | PVdF | 3.7 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brilloni, A.; Marchesini, F.; Poli, F.; Petri, E.; Soavi, F. Performance Comparison of LMNO Cathodes Produced with Pullulan or PEDOT:PSS Water-Processable Binders. Energies 2022, 15, 2608. https://doi.org/10.3390/en15072608
Brilloni A, Marchesini F, Poli F, Petri E, Soavi F. Performance Comparison of LMNO Cathodes Produced with Pullulan or PEDOT:PSS Water-Processable Binders. Energies. 2022; 15(7):2608. https://doi.org/10.3390/en15072608
Chicago/Turabian StyleBrilloni, Alessandro, Francesco Marchesini, Federico Poli, Elisabetta Petri, and Francesca Soavi. 2022. "Performance Comparison of LMNO Cathodes Produced with Pullulan or PEDOT:PSS Water-Processable Binders" Energies 15, no. 7: 2608. https://doi.org/10.3390/en15072608






