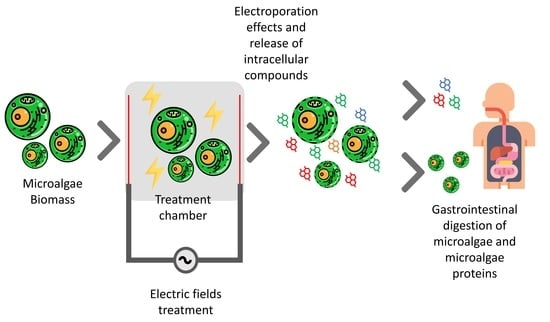
Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass
Abstract
:1. Introduction
1.1. Historical Overview
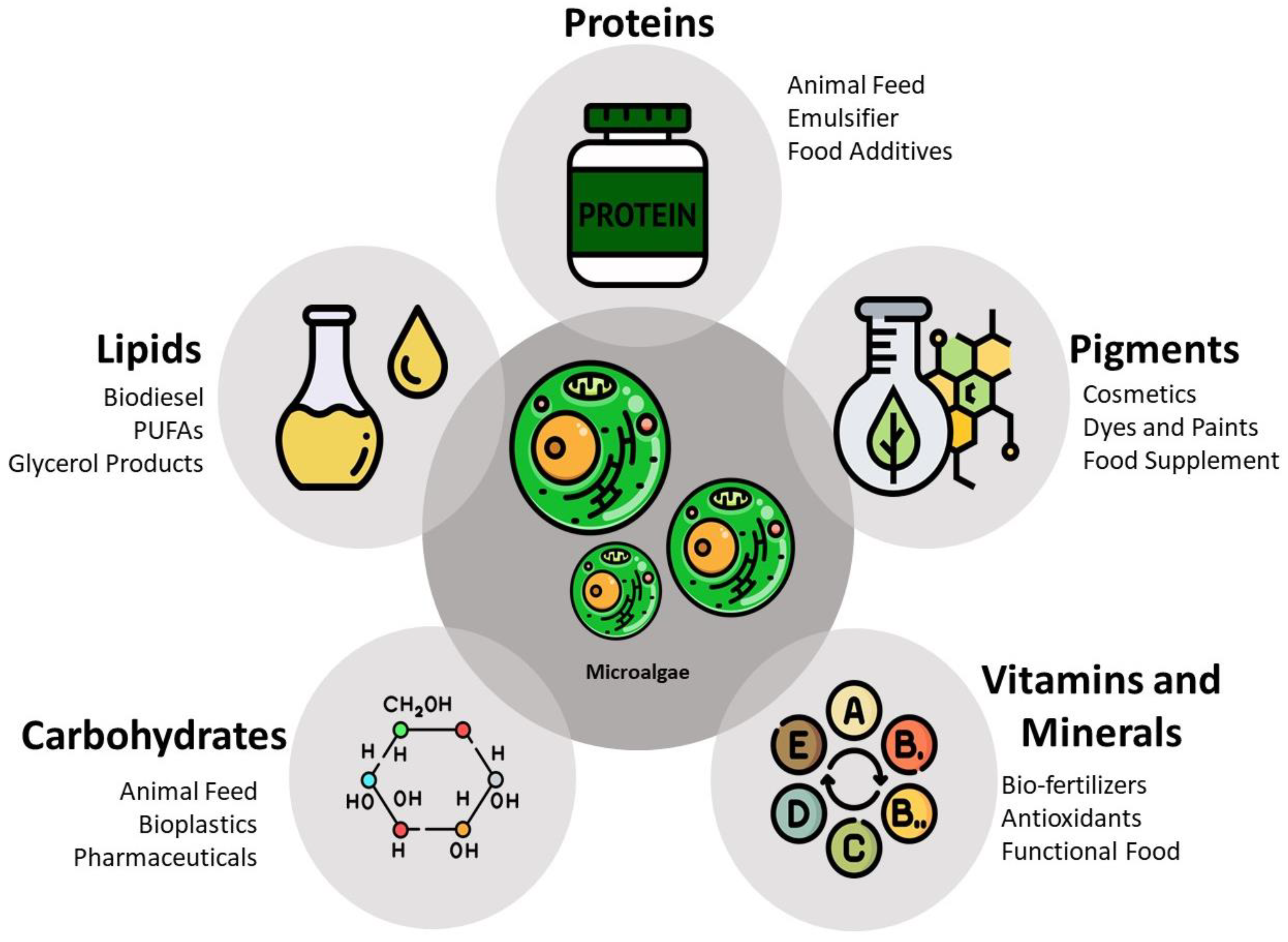
1.2. Sustainable Production and Industrial Applications
1.3. A Promising Protein Source
1.4. Main Challenge
2. Microalgae Protein Content
| Protein Sources | Protein Content (% per Dry Weight) | Reference |
|---|---|---|
| Microalgae | ||
| Chlorella vulgaris | 51–58 | [4,19] |
| Arthrospira platensis | 55.8/46–63 | [19,22] |
| Arthrospira maxima | 60–71 | [3,22] |
| Euglena gracilis | 30–47 | [7,22] |
| Dunaliela salina | 57 | [2,4] |
| Porphyridium cruentum | 28–39 | [2,29] |
| Tetraselmis chuii | 35–40 | [21,30] |
| Galdieria sulphuraria | 26–32 | [20] |
| Macroalgae | ||
| Ulva lactuca | 12–20 | [12,31] |
| Palmaria palmata | 9.8–18.8 | [12,32] |
| Insects | ||
| Crickets (Acheta domesticus) | 60–75 | [33] |
| Flies (Musca domestica) | 55–70 | |
| Conventional sources | ||
| Soy | 37 | [2] |
| Meat | 42 | |
| Egg | 47 | |
| Milk | 26 | |
| Rice | 8 | |
3. Processing of Microalgae Biomass
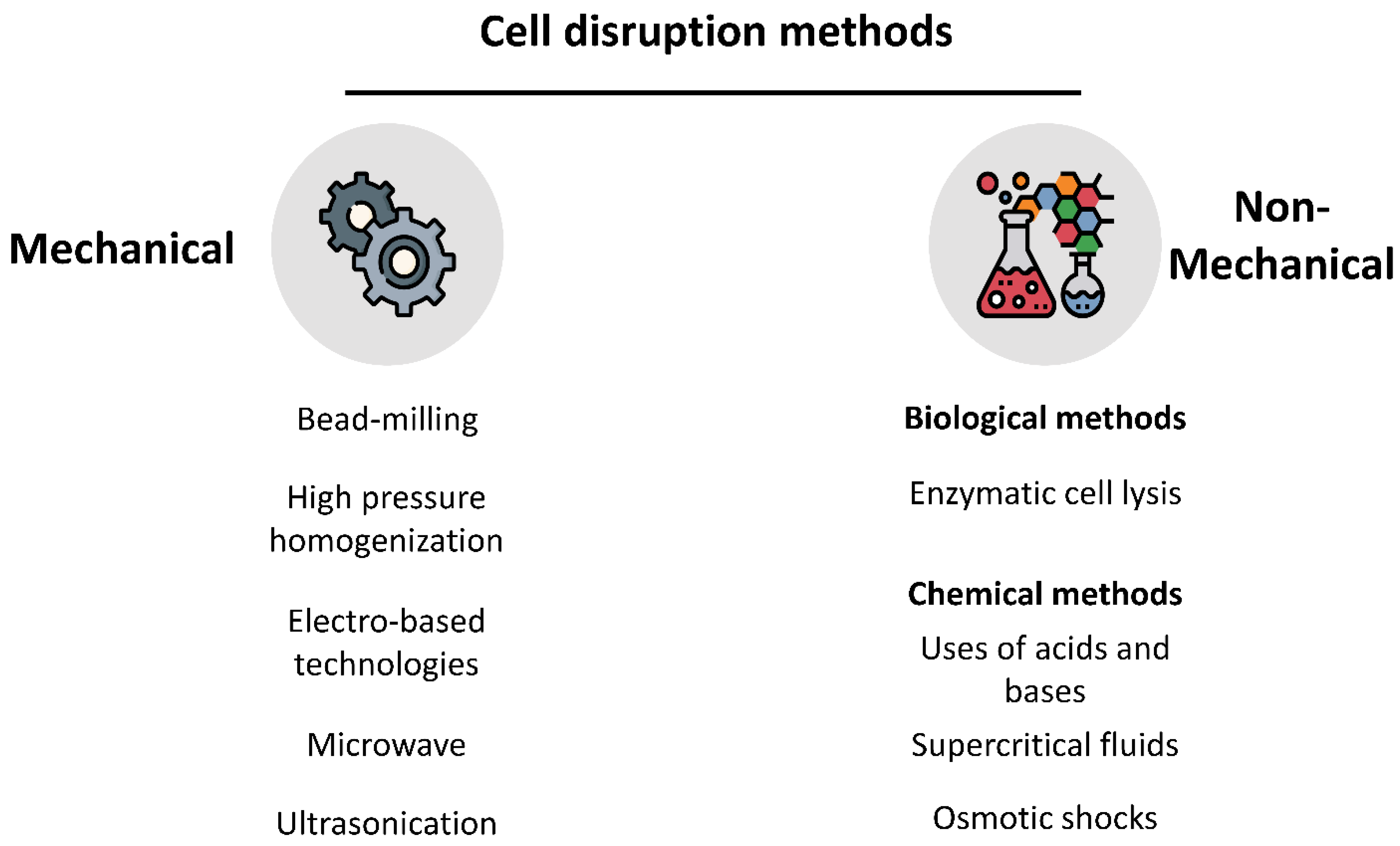
3.1. Integrity of the Cell Wall and Membrane
3.2. Relationship between the Processing Method and Quality of the Intracellular Products
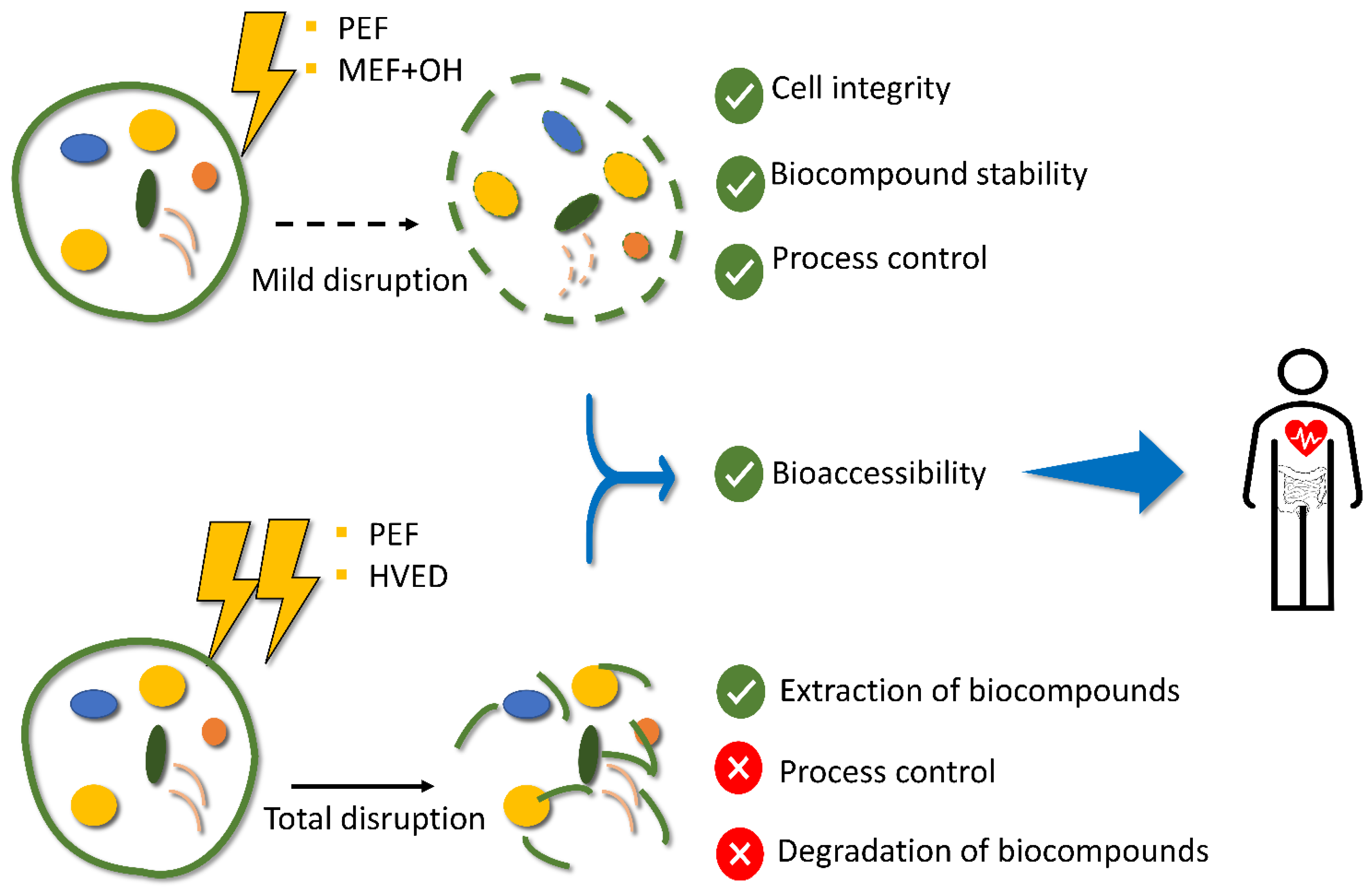
3.3. Impact of Electric Fields on the Cell Integrity
3.3.1. PEF
3.3.2. HVED
3.3.3. MEF
3.3.4. OH
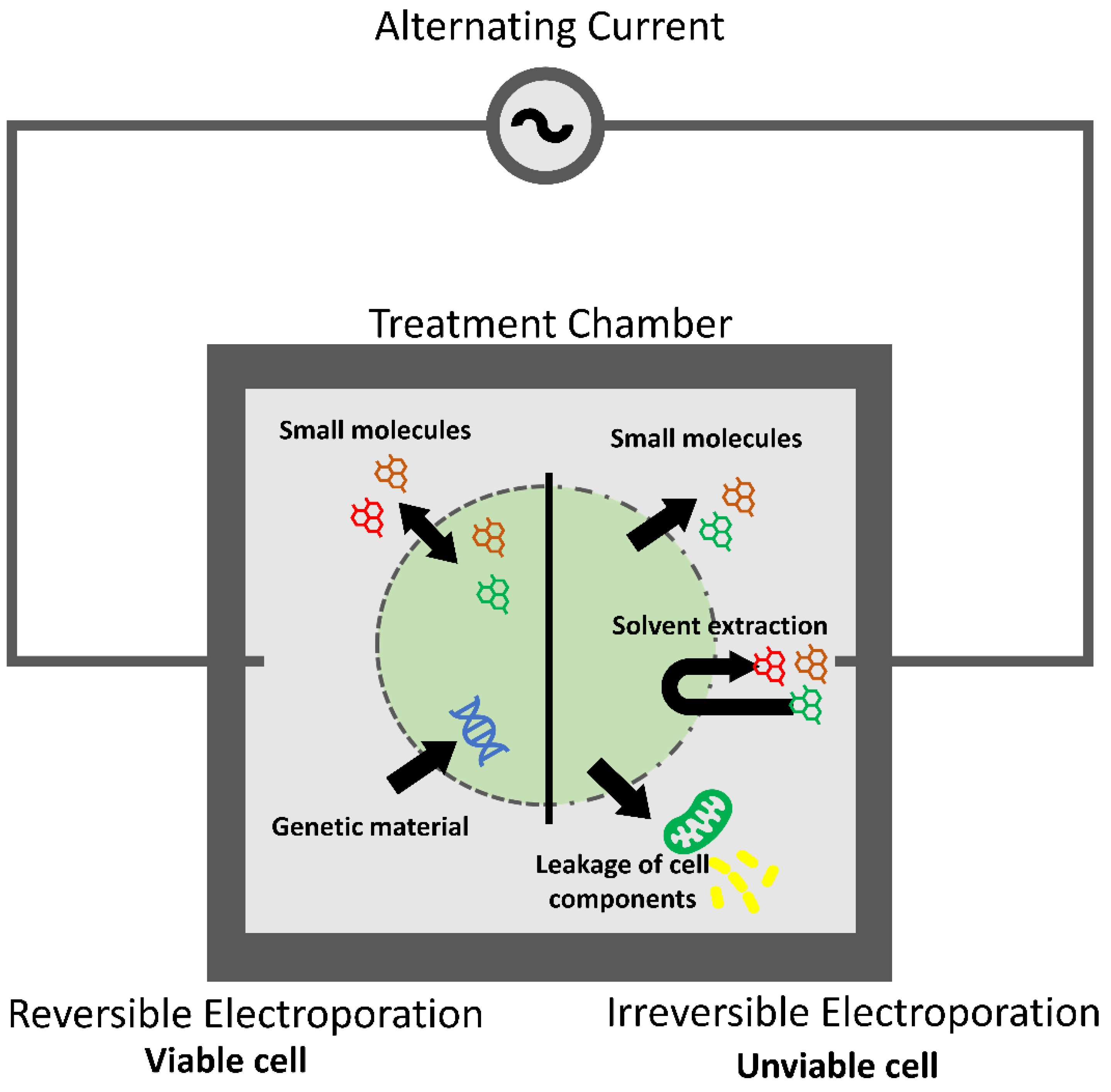
3.3.5. DC Methods
4. Gastrointestinal Digestion of Microalgae Biomass
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barsanti, L.; Gualtieri, P. Is Exploitation of Microalgae Economically and Energetically Sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A Novel Ingredient in Nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential Application of Microalga Spirulina platensis as a Protein Source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.; Kim, J.-D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Gantar, M.; Svirčev, Z. Microalgae and Cyanobacteria: Food for Thought. J. Phycol. 2008, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal Proteins: Production Strategies and Nutritional and Functional Properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P. The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Gonzalez, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, H.; Eppink, M.; Kleinegris, D.; Wijffels, R.; Barbosa, M.J. Towards Industrial Products from Microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef] [Green Version]
- da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant Secondary Metabolites: Biosynthesis, Classification, Function and Pharmacological Properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and Downstream Processing of Microalgae and Cyanobacteria to Generate Protein-Based Technofunctional Food Ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 2961–2989. [Google Scholar] [CrossRef]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as Human Food: Chemical and Nutritional Characteristics of the Thermo-Acidophilic Microalga Galdieria Sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and Characterization of Soluble Protein from the Green Microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathe, S.K. Chemistry and Implications of Antinutritional Factors in Dry Beans and Pulses. In Dry Beans and Pulses Production, Processing and Nutrition; Wiley: Hoboken, NJ, USA, 2012; pp. 359–377. [Google Scholar]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food Proteins from Animals and Plants: Differences in the Nutritional and Functional Properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Bertsch, P.; Böcker, L.; Mathys, A.; Fischer, P. Proteins from Microalgae for the Stabilization of Fluid Interfaces, Emulsions, and Foams. Trends Food Sci. Technol. 2021, 108, 326–342. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and Fractionation of Microalgae-Based Protein Products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Madsen, M. Algal Proteins. In Handbook of Food Proteins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 353–394. [Google Scholar]
- Mantecón, L.; Moyano, R.; Cameán, A.M.; Jos, A. Safety Assessment of a Lyophilized Biomass of Tetraselmis Chuii (TetraSOD®) in a 90 Day Feeding Study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef]
- Mansour, A.T.; Ashour, M.; Alprol, A.E.; Alsaqufi, A.S. Aquatic Plants and Aquatic Animals in the Context of Sustainability: Cultivation Techniques, Integration, and Blue Revolution. Sustainability 2022, 14, 3257. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Harnedy, P.A.; FitzGerald, R.J.; Oliveira, M.B.P.P. Macroalgal-Derived Protein Hydrolysates and Bioactive Peptides: Enzymatic Release and Potential Health Enhancing Properties. Trends Food Sci. Technol. 2019, 93, 106–124. [Google Scholar] [CrossRef]
- Roos, N. Insects and Human Nutrition. In Edible Insects in Sustainable Food Systems; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–91. [Google Scholar]
- Kinsella, J.E.; Melachouris, N. Functional Properties of Proteins in Foods: A Survey. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Chronakis, I.S. Gelation of Edible Blue-Green Algae Protein Isolate (Spirulina platensis Strain Pacifica): Thermal Transitions, Rheological Properties, and Molecular Forces Involved. J. Agric. Food Chem. 2001, 49, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Hinrichs, J.; Goff, H.D.; Weiss, J. Heat-Induced Gel Formation of a Protein-Rich Extract from the Microalga Chlorella sorokiniana. Innov. Food Sci. Emerg. Technol. 2019, 56, 102176. [Google Scholar] [CrossRef]
- Morris, H.J.; Almarales, A.; Carrillo, O.; Bermúdez, R.C. Utilisation of Chlorella vulgaris Cell Biomass for the Production of Enzymatic Protein Hydrolysates. Bioresour. Technol. 2008, 99, 7723–7729. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Wu, T.-K.; Fang, T.J. Antioxidant Properties of a New Antioxidative Peptide from Algae Protein Waste Hydrolysate in Different Oxidation Systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of Microalgae for Food and Fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of Microalgae Cell Wall Characteristics on Protein Extractability and Determination of Nitrogen-to-Protein Conversion Factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Phong, W.N.; Show, P.L.; Le, C.F.; Tao, Y.; Chang, J.-S.; Ling, T.C. Improving Cell Disruption Efficiency to Facilitate Protein Release from Microalgae Using Chemical and Mechanical Integrated Method. Biochem. Eng. J. 2018, 135, 83–90. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae Disruption Techniques for Product Recovery: Influence of Cell Wall Composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Safi, C.; Ursu, A.V.; Laroche, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Aqueous Extraction of Proteins from Microalgae: Effect of Different Cell Disruption Methods. Algal Res. 2014, 3, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Corre, G.; Templier, J.; Largeau, C.; Rousseau, B.; Berkaloff, C. Influence of Cell Wall Composition on the Resistance of Two Chlorella Species (Chlorophyta) to Detergents. J. Phycol. 1996, 32, 584–590. [Google Scholar] [CrossRef]
- Domozych, D.S.; Ciancia, M.; Fangel, J.U.; Mikkelsen, M.D.; Ulvskov, P.; Willats, W.G.T. The Cell Walls of Green Algae: A Journey through Evolution and Diversity. Front. Plant Sci. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and Composition of the Nannochloropsis gaditana Cell Wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, J.; Stolarczyk, A.; Zych, M.; Malec, P.; Burczyk, J. The Cell-Wall Glycoproteins of the Green Alga Scenedesmus obliquus. The Predominant Cell-Wall Polypeptide of Scenedesmus obliquus Is Related to the Cell-Wall Glycoprotein Gp3 of Chlamydomonas reinhardtii. Plant Sci. 2014, 215–216, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-K.; Hsieh, C.-C.; Hsu, J.-J.; Yang, Y.-K.; Chou, H.-N. Preventive Effects of Spirulina Platensis on Skeletal Muscle Damage under Exercise-Induced Oxidative Stress. Eur. J. Appl. Physiol. 2006, 98, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.-Y. Understanding the Effect of Cell Disruption Methods on the Diffusion of Chlorella vulgaris Proteins and Pigments in the Aqueous Phase. Algal Res. 2015, 8, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Buchmann, L.; Brändle, I.; Haberkorn, I.; Hiestand, M.; Mathys, A. Pulsed Electric Field Based Cyclic Protein Extraction of Microalgae towards Closed-Loop Biorefinery Concepts. Bioresour. Technol. 2019, 291, 121870. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Influence of Processing Parameters on Disintegration of Chlorella Cells in Various Types of Homogenizers. Appl. Microbiol. Biotechnol. 2008, 81, 431–440. [Google Scholar] [CrossRef]
- Canelli, G.; Neutsch, L.; Carpine, R.; Tevere, S.; Giuffrida, F.; Rohfritsch, Z.; Dionisi, F.; Bolten, C.J.; Mathys, A. Chlorella vulgaris in a Heterotrophic Bioprocess: Study of the Lipid Bioaccessibility and Oxidative Stability. Algal Res. 2020, 45, 101754. [Google Scholar] [CrossRef]
- Ratledge, C.; Streekstra, H.; Cohen, Z.; Fichtali, J. Downstream Processing, Extraction, and Purification of Single Cell Oils. In Single Cell Oils; Elsevier: Amsterdam, The Netherlands, 2010; pp. 179–197. [Google Scholar]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of Bead Milling Parameters for the Cell Disruption of Microalgae: Process Modeling and Application to Porphyridium Cruentum and Nannochloropsis oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the Relative Efficiency of Two- vs. One-Stage Production of Astaxanthin by the Green Alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of Different Cell Disruption Processes on Encysted Cells of Haematococcus pluvialis: Effects on Astaxanthin Recovery and Implications for Bio-Availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Morineau-Thomas, O.; Jaouen, P.; Legentilhomme, P. The Role of Exopolysaccharides in Fouling Phenomenon during Ultrafiltration of Microalgae (Chlorella sp. and Porphyridium purpureum): Advantage of a Swirling Decaying Flow. Bioprocess Biosyst. Eng. 2002, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Mamontov, A.; Weinstein, J. Sulfation of Extracellular Polysaccharides of Red Microalgae: Preparation, Characterization and Properties. J. Biochem. Biophys. Methods 2002, 50, 179–187. [Google Scholar] [CrossRef]
- Shibata, S.; Arimura, S.; Ishikawa, T.; Awai, K. Alterations of Membrane Lipid Content Correlated with Chloroplast and Mitochondria Development in Euglena gracilis. Front. Plant Sci. 2018, 9, 370. [Google Scholar] [CrossRef] [Green Version]
- Barsanti, L.; Gualtieri, P. Anatomy of Euglena gracilis. In Handbook of Algal Science, Technology and Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 61–70. [Google Scholar]
- Wang, Y.; Seppänen-Laakso, T.; Rischer, H.; Wiebe, M.G. Euglena gracilis Growth and Cell Composition under Different Temperature, Light and Trophic Conditions. PLoS ONE 2018, 13, e0195329. [Google Scholar] [CrossRef] [Green Version]
- El-Baz, F.K.; Aly, H.F.; Salama, A.A.A. Toxicity Assessment of the Green Dunaliella salina Microalgae. Toxicol. Rep. 2019, 6, 850–861. [Google Scholar] [CrossRef]
- Morais Junior, W.G.; Gorgich, M.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Microalgae for Biotechnological Applications: Cultivation, Harvesting and Biomass Processing. Aquaculture 2020, 528, 735562. [Google Scholar] [CrossRef]
- Lari, Z.; Ahmadzadeh, H.; Hosseini, M. Cell Wall Disruption: A Critical Upstream Process for Biofuel Production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 21–35. [Google Scholar]
- de Carvalho, J.C.; Magalhães, A.I.; de Melo Pereira, G.V.; Medeiros, A.B.P.; Sydney, E.B.; Rodrigues, C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; Soccol, V.T.; Soccol, C.R. Microalgal Biomass Pretreatment for Integrated Processing into Biofuels, Food, and Feed. Bioresour. Technol. 2020, 300, 122719. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, J.-S.; Lee, D.-J. Dewatering and Drying Methods for Microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.-R.; Pack, S.P. Recent Progress in Flocculation, Dewatering, and Drying Technologies for Microalgae Utilization: Scalable and Low-Cost Harvesting Process Development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent Developments in High-Quality Drying of Vegetables, Fruits, and Aquatic Products. Crit. Rev. Food Sci. Nutr. 2017, 57, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Defraeye, T.; Martynenko, A. Electrohydrodynamic Drying of Multiple Food Products: Evaluating the Potential of Emitter-Collector Electrode Configurations for Upscaling. J. Food Eng. 2019, 240, 38–42. [Google Scholar] [CrossRef]
- Defraeye, T.; Martynenko, A. Electrohydrodynamic Drying of Food: New Insights from Conjugate Modeling. J. Clean. Prod. 2018, 198, 269–284. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; dos Reis Coimbra, J.S.; de Oliveira Leite, M.; Albino, L.F.T.; Martins, M.A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell Disruption for Microalgae Biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild Disintegration of the Green Microalgae Chlorella vulgaris Using Bead Milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Medeiros, A.B.P.; Letti, L.A.J.; Kirnev, P.C.S.; Soccol, C.R. Cell Disruption and Isolation of Intracellular Products. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 807–822. [Google Scholar]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef]
- Rocha, C.M.R.; Genisheva, Z.; Ferreira-Santos, P.; Rodrigues, R.; Vicente, A.A.; Teixeira, J.A.; Pereira, R.N. Electric Field-Based Technologies for Valorization of Bioresources. Bioresour. Technol. 2018, 254, 325–339. [Google Scholar] [CrossRef] [Green Version]
- Geada, P.; Rodrigues, R.; Loureiro, L.; Pereira, R.; Fernandes, B.; Teixeira, J.A.; Vasconcelos, V.; Vicente, A.A. Electrotechnologies Applied to Microalgal Biotechnology—Applications, Techniques and Future Trends. Renew. Sustain. Energy Rev. 2018, 94, 656–668. [Google Scholar] [CrossRef] [Green Version]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.; Robles Medina, A.; Chisti, Y. Recovery of Microalgal Biomass and Metabolites: Process Options and Economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, T.; Li, J.; Pan, B.; Hu, Q.; Duan, M.; Zhang, X. Study on the Effect of Spray Drying Process on the Quality of Microalgal Biomass: A Comprehensive Biocomposition Analysis of Spray-Dried S. acuminatus Biomass. BioEnergy Res. 2022, 15, 320–333. [Google Scholar] [CrossRef]
- Miranda, M.; Maureira, H.; Rodríguez, K.; Vega-Gálvez, A. Influence of Temperature on the Drying Kinetics, Physicochemical Properties, and Antioxidant Capacity of Aloe Vera (Aloe Barbadensis Miller) Gel. J. Food Eng. 2009, 91, 297–304. [Google Scholar] [CrossRef]
- Defraeye, T.; Martynenko, A. Future Perspectives for Electrohydrodynamic Drying of Biomaterials. Dry. Technol. 2018, 36, 1–10. [Google Scholar] [CrossRef]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, Fractionation and Functional Properties of Proteins from the Microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Chronakis, I.S. Biosolar Proteins from Aquatic Algae. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 39–75. [Google Scholar]
- Hedenskog, G.; Hofsten, A.V. The Ultrastructure of Spirulina platensis—A New Source of Microbial Protein. Physiol. Plant. 1970, 23, 209–216. [Google Scholar] [CrossRef]
- Muñoz, R.; Navia, R.; Ciudad, G.; Tessini, C.; Jeison, D.; Mella, R.; Rabert, C.; Azócar, L. Preliminary Biorefinery Process Proposal for Protein and Biofuels Recovery from Microalgae. Fuel 2015, 150, 425–433. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. PH-Shift Processing of Nannochloropsis Oculata Microalgal Biomass to Obtain a Protein-Enriched Food or Feed Ingredient. Algal Res. 2015, 11, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Nolsøe, H.; Undeland, I. The Acid and Alkaline Solubilization Process for the Isolation of Muscle Proteins: State of the Art. Food Bioprocess Technol. 2009, 2, 1–27. [Google Scholar] [CrossRef]
- Gerde, J.A.; Wang, T.; Yao, L.; Jung, S.; Johnson, L.A.; Lamsal, B. Optimizing Protein Isolation from Defatted and Non-Defatted Nannochloropsis Microalgae Biomass. Algal Res. 2013, 2, 145–153. [Google Scholar] [CrossRef]
- Coustets, M.; Al-Karablieh, N.; Thomsen, C.; Teissié, J. Flow Process for Electroextraction of Total Proteins from Microalgae. J. Membr. Biol. 2013, 246, 751–760. [Google Scholar] [CrossRef] [PubMed]
- bin Azmi, A.A.; Sankaran, R.; Show, P.L.; Ling, T.C.; Tao, Y.; Munawaroh, H.S.H.; Kong, P.S.; Lee, D.J.; Chang, J.S. Current Application of Electrical Pre-Treatment for Enhanced Microalgal Biomolecules Extraction. Bioresour. Technol. 2020, 302, 122874. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, E.; Barba, F.J. Electrotechnologies Applied to Valorization of By-Products from Food Industry: Main Findings, Energy and Economic Cost of Their Industrialization. Food Bioprod. Processing 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Canelli, G.; Murciano Martínez, P.; Maude Hauser, B.; Kuster, I.; Rohfritsch, Z.; Dionisi, F.; Bolten, C.J.; Neutsch, L.; Mathys, A. Tailored Enzymatic Treatment of Chlorella vulgaris Cell Wall Leads to Effective Disruption while Preserving Oxidative Stability. LWT 2021, 143, 111157. [Google Scholar] [CrossRef]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed Electric Field Assisted Extraction of Intracellular Valuables from Microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective Extraction from Microalgae Nannochloropsis sp. Using Different Methods of Cell Disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Bordetas, A.; Álvarez, I.; Raso, J. Influence of the Treatment Medium Temperature on Lutein Extraction Assisted by Pulsed Electric Fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N. Electric Field Effects on Proteins—Novel Perspectives on Food and Potential Health Implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Enhanced Extraction from Solid Foods and Biosuspensions by Pulsed Electrical Energy. Food Eng. Rev. 2010, 2, 95–108. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L. Continuous Extraction of Phenolic Compounds from Pomegranate Peel Using High Voltage Electrical Discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Nunes, R.; De Biasio, F.; Spigno, G.; Gorgoglione, D.; Teixeira, J.A.; Rocha, C.M.R. Influence of Thermal and Electrical Effects of Ohmic Heating on C-Phycocyanin Properties and Biocompounds Recovery from Spirulina Platensis. LWT 2020, 128, 109491. [Google Scholar] [CrossRef]
- Indiarto, R.; Rezaharsamto, B. A Review on Ohmic Heating and Its Use in Food. Int. J. Sci. Technol. Res. 2020, 9, 485–490. [Google Scholar]
- Pandey, A.; Shah, R.; Yadav, P.; Verma, R.; Srivastava, S. Harvesting of Freshwater Microalgae Scenedesmus sp. by Electro–Coagulation–Flocculation for Biofuel Production: Effects on Spent Medium Recycling and Lipid Extraction. Environ. Sci. Pollut. Res. 2020, 27, 3497–3507. [Google Scholar] [CrossRef]
- Pearsall, R.; Connelly, R.; Fountain, M.; Hearn, C.; Werst, M.; Hebner, R.; Kelley, E. Electrically Dewatering Microalgae. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1578–1583. [Google Scholar] [CrossRef] [Green Version]
- Shuman, T.R.; Mason, G.; Reeve, D.; Schacht, A.; Goodrich, A.; Napan, K.; Quinn, J. Low-Energy Input Continuous Flow Rapid Pre-Concentration of Microalgae through Electro-Coagulation–Flocculation. Chem. Eng. J. 2016, 297, 97–105. [Google Scholar] [CrossRef]
- ’t Lam, G.P.; Postma, P.R.; Fernandes, D.A.; Timmermans, R.A.H.; Vermuë, M.H.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Olivieri, G. Pulsed Electric Field for Protein Release of the Microalgae Chlorella vulgaris and Neochloris oleoabundans. Algal Res. 2017, 24, 181–187. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Amaro, H.M.; Vasconcelos, V.; Guedes, A.C.; Vicente, A.A. Continuous Pressurized Extraction versus Electric Fields-Assisted Extraction of Cyanobacterial Pigments. J. Biotechnol. 2021, 334, 35–42. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Eing, C.; Goettel, M.; Straessner, R.; Gusbeth, C.; Frey, W. Pulsed Electric Field Treatment of Microalgae—Benefits for Microalgae Biomass Processing. IEEE Trans. Plasma Sci. 2013, 41, 2901–2907. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. Applications of Pulsed Electric Fields Technology for the Food Industry. In Pulsed Electric Fields Technology for the Food Industry; Springer: Boston, MA, USA, 2006; pp. 197–221. [Google Scholar]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed Electric Field Assisted Extraction of Nutritionally Valuable Compounds from Microalgae Nannochloropsis spp. Using the Binary Mixture of Organic Solvents and Water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Zhang, R.; Grimi, N.; Marchal, L.; Vorobiev, E. Application of High-Voltage Electrical Discharges and High-Pressure Homogenization for Recovery of Intracellular Compounds from Microalgae Parachlorella kessleri. Bioprocess Biosyst. Eng. 2019, 42, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.F.L.F.; Pereira, R.N.; Martins, R.C.R.C.; Teixeira, J.A.J.A.; Vicente, A.A.A. Moderate Electric Fields Can Inactivate Escherichia Coli at Room Temperature. J. Food Eng. 2010, 96, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Machado, L.; Pereira, R.N.; Vicente, A.A.; Rodrigues, R.M. Unraveling the Nature of Ohmic Heating Effects in Structural Aspects of Whey Proteins—The Impact of Electrical and Electrochemical Effects. Innov. Food Sci. Emerg. Technol. 2021, 74, 102831. [Google Scholar] [CrossRef]
- Pataro, G.; Barca, G.M.J.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A.; Ferrari, G. Quantification of Metal Release from Stainless Steel Electrodes during Conventional and Pulsed Ohmic Heating. Innov. Food Sci. Emerg. Technol. 2014, 21, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Varghese, K.S.; Pandey, M.C.; Radhakrishna, K. Technology, Applications and Modelling of Ohmic Heating: A Review. J. Food Sci. Technol. 2014, 51, 2304–2317. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef] [Green Version]
- Visigalli, S.; Barberis, M.G.; Turolla, A.; Canziani, R.; Berden Zrimec, M.; Reinhardt, R.; Ficara, E. Electrocoagulation–Flotation (ECF) for Microalgae Harvesting—A Review. Sep. Purif. Technol. 2021, 271, 118684. [Google Scholar] [CrossRef]
- Janczyk, P.; Wolf, C.; Souffrant, W.B. Evaluation of Nutritional Value and Safety of the Green Microalgae Chlorella vulgaris Treated with Novel Processing Methods. Arch Zootech 2005, 8, 132–147. [Google Scholar]
- Mantovani, R.A.; Pinheiro, A.C.; Vicente, A.A.; Cunha, R.L. In Vitro Digestion of Oil-in-Water Emulsions Stabilized by Whey Protein Nanofibrils. Food Res. Int. 2017, 99, 790–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, A.C.; Coimbra, M.A.; Vicente, A.A. In Vitro Behaviour of Curcumin Nanoemulsions Stabilized by Biopolymer Emulsifiers—Effect of Interfacial Composition. Food Hydrocoll. 2016, 52, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen Content, Dietary Fiber, and Digestibility in Algal Food Products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef] [Green Version]
| Microalgae Genus | Chemical Composition of the Cell Wall | Resistance | Reference |
| Chlorella/Chloroidium |
| + | [17,42,44,45] |
| Tetraselmis |
| − | [42] |
| Nannochloropsis |
| ++ | [42,46] |
| Scenedesmus/Tetradesmus |
| + | [42,47] |
| Arthrospira |
| − | [23,43,48] |
| Technique Applied | Principle of the Technique | Effects on the Biomass Treated | Reference |
|---|---|---|---|
| Drying process | |||
| Solar | Direct solar energy for biomass drying. |
| [65,66] |
| Microwave Drying | Uses microwave heating for biomass drying. |
| [65] |
| Spray-drying | Warm liquid and air carry the water. |
| [63,65,67] |
| Convective Drying | Convective hot air to remove the water, such as an oven. |
| [65,67] |
| Lyophilization (Freeze-drying) | Heat frozen biomass, leading to water removal through sublimation. |
| [65,67,68,69] |
| Electrohydrodynamic Drying (EHD) | Application of high voltage difference, this leads to the generation of airflow between the electrode and the plate where the sample is placed. It does not need any type of heat for the drying process. |
| [69,70,71] |
| Cell Disruption Methods | |||
| Bead Mill | Shear stress between the beads and the cells in the sample. |
| [51,72,73,74] |
| High-pressure Homogenization (HPH) | The cells are submitted to intense shear stress, turbulence, and cavitation, resulting in damage to the cell wall and membrane. The pressure transforms into steep velocity. It also has the advantage of being used without the drying step. |
| [12,66,73,75] |
| Enzymatic Hydrolysis | Takes advantage of using enzymes that can affect structural components of the cell wall and membrane by weakening or even dissolving them. |
| [72,73,75,76] |
| Ultrasonication | Ultrasonic waves propagating through a certain sample, produce some microbubbles which when expanded create violent shockwaves, thus damaging the cells. |
| [12,43,73,75] |
| Microwave | The cell wall disruption is caused by the evaporation of the cell water. |
| [12,72,73] |
| Pulsed Electric Fields (PEF) | The application of an electric field will affect the transmembrane potential of the cell, causing an electroporation or electropermeabilization effect. This will lead to the release of the intracellular compounds. |
| [50,72,73,77,78] |
| Electric-Based Technology | Electric Field Strength | Time | Frequencies Used | Operation Temperature | Main Effects | Reference |
| Pulsed Electric Fields (PEF) |
| 0.01 μs to 2.4 ms | 1 Hz to 2000 Hz | 10 °C to 60 °C |
| [78,95,96,97,98] |
| High Voltage Electric Discharge (HVED) | 10 kV/cm to 100 kV/cm | 0.01 µs to 10 µs | Up to 1000 Hz | 20 °C to 60 °C |
| [77,78,93,99,100] |
| Moderate Electric Fields (MEF) | <1000 V/cm | No limit | 0.06 kHz to 25 kHz |
|
| [77,78,98,101,102] |
| Direct Current (DC) |
| No limit | 0 Hz | Temperature not relevant |
| [78,103,104,105] |
| Microalgae Species | Electric-Based Treatment | Effects Caused in the Microalgae | Reference |
| Auxenochlorella protothecoides | PEF |
| [95] |
| Nannochloropsis sp. | PEF |
| [96] |
| Nannochloropsis sp. | HVED |
| [96] |
| Chlorella vulgaris | PEF |
| [97,106] |
| Arthrospira platensis | MEF (with OH effects) |
| [101] |
| Neochloris oleoabundans | PEF |
| [106] |
| Cyanobium sp. | MEF (with OH effects) |
| [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80-102. https://doi.org/10.3390/biomass2020006
Machado L, Carvalho G, Pereira RN. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass. 2022; 2(2):80-102. https://doi.org/10.3390/biomass2020006
Chicago/Turabian StyleMachado, Luís, Gonçalo Carvalho, and Ricardo N. Pereira. 2022. "Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass" Biomass 2, no. 2: 80-102. https://doi.org/10.3390/biomass2020006
APA StyleMachado, L., Carvalho, G., & Pereira, R. N. (2022). Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass, 2(2), 80-102. https://doi.org/10.3390/biomass2020006