Highly Efficient Bioconversion of trans-Resveratrol to δ-Viniferin Using Conditioned Medium of Grapevine Callus Suspension Cultures
Abstract
:1. Introduction
2. Results and Discussion
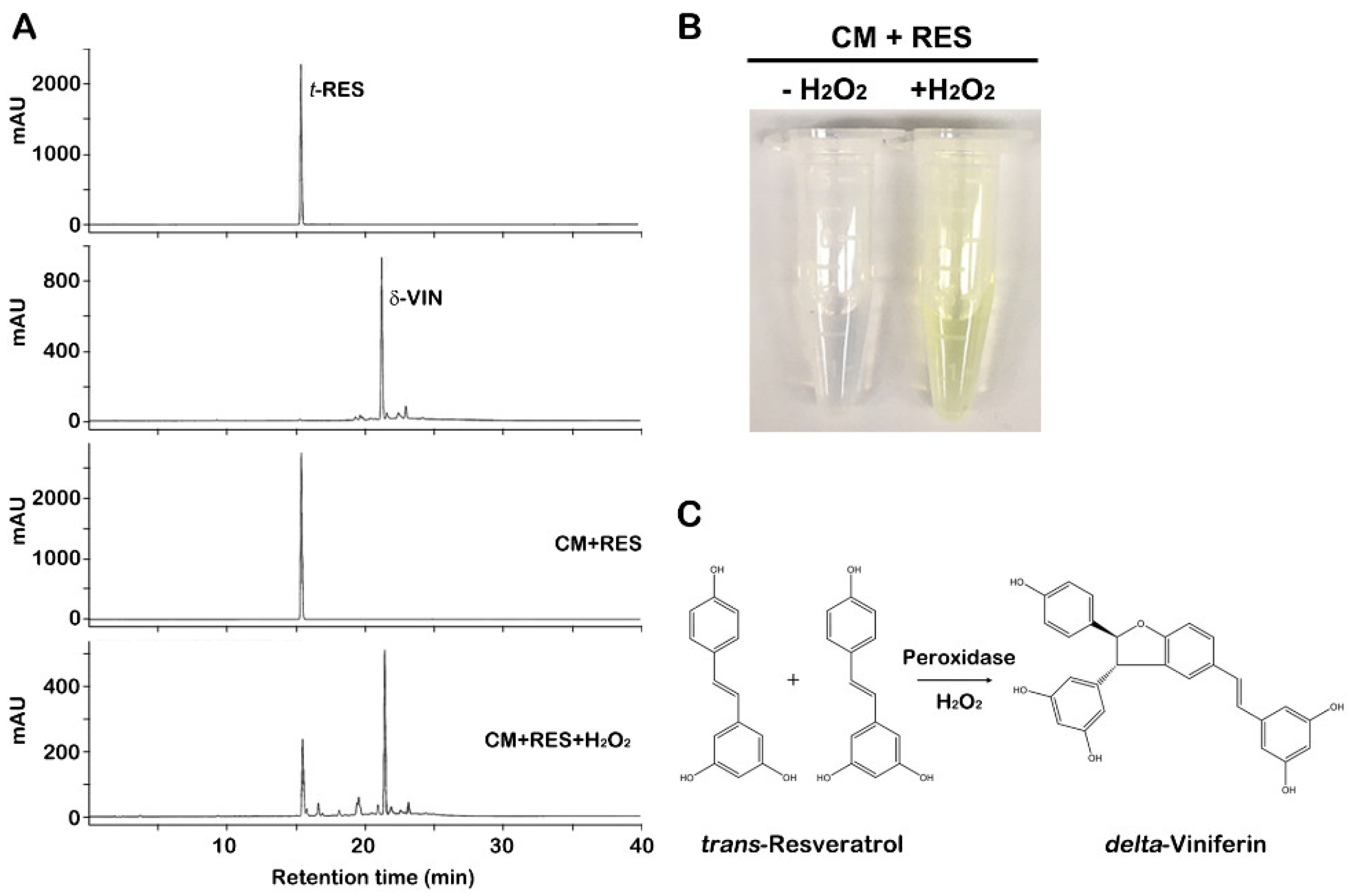
2.1. Bioconversion of trans-Resveratrol to δ-Viniferin through CM of Grapevine Callus Suspension Cultures
2.2. Effect of CM Obtained at Different Time Points of Callus Suspension Cultures on Bioconversion Efficiency
2.3. Optimization of Bioconversion Conditions for trans-Resveratrol to δ-Viniferin
2.4. Bioconversion of trans-Resveratrol through CM from Different Callus Suspension Cultures
3. Materials and Methods
3.1. Plant Materials and Callus Culture Conditions
3.2. Preparation of CM from Plant Callus Suspension Cultures
3.3. Bioconversion of trans-Resveratrol Using CM
3.4. δ-Viniferin Analysis and Quantification
3.5. Optimization of trans-Resveratrol Bioconversion
3.6. Crude Peroxidase Activity Assay and Native Polyacrylamide Gel Electrophoresis (PAGE)
- ΔOD/min = increase in absorbance per minute (min−1)
- RmV = reaction mixture volume (mL)
- EV = enzyme extract volume
- dF = dilution factor
- ε470 = molar absorptivity of tetraguaiacol at 470 nm (mL μ−1 cm−1)
3.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anekonda, T. Resveratrol-A Boon for treating Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.; Tang, X.; Kim, K.; Kopelovich, L.; Bickers, D.; Kim, A. Resveratrol: A Review of Pre-clinical Studies for Human Cancer Prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.; Bomser, J.; Min, D. Bioactivity of Resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- He, S. From Resveratrol to Its Derivatives: New Sources of Natural Antioxidant. Curr. Med. Chem. 2012, 20, 1005–1017. [Google Scholar]
- Ficarra, S.; Ester, T.; Pirolli, D.; Russo, A.; Barreca, D.; Galtieri, A.; Giardina, B.; Gavezzotti, P.; Riva, S.; De Rosa, M.C. Insights into the properties of the two enantiomers of trans-δ-viniferin, a resveratrol derivative: Antioxidant activity, biochemical and molecular modeling studies of its interactions with hemoglobin. Mol. BioSyst. 2016, 12, 1276–1286. [Google Scholar] [CrossRef]
- Yu, B.; Xie, R.; Jin, L.; Tian, X.; Niu, Y.; Ma, T.; Yang, H. trans-δ-Viniferin inhibits Ca2+-activated Cl− channels and improves diarrhea symptoms. Fitoterapia 2019, 139, 104367. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, T.; Fan, B.; Yang, L.; Han, C.; Luo, J.; Kong, L. Protective effect of trans-δ-viniferin against high glucose-induced oxidative stress in human umbilical vein endothelial cells through the SIRT1 pathway. Free Radic. Res. 2015, 50, 1–42. [Google Scholar] [CrossRef]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Merillon, J.-M.; Cluzet, S. Comparative Analyses of Stilbenoids in Canes of Major Vitis vinifera L. Cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef]
- Xavier, V.; Bornet, A.; Vanderlinde, R.; Valls, J.; Richard, T.; Delaunay, J.-C.; Merillon, J.-M.; Teissedre, P.-L. Determination of stilbenes (δ-Viniferin, trans-Astringin, trans-Piceid, cis-And trans-Resveratrol, ε-Viniferin) in Brazilian wines. J. Agric. Food Chem. 2005, 53, 5664–5669. [Google Scholar]
- Douillet-Breuil, A.-C.; Jeandet, P.; Adrian, M.; Bessis, R. Changes in the Phytoalexin Content of Various Vitis Spp. in Response to Ultraviolet C Elicitation. J. Agric. Food Chem. 1999, 47, 4456–4461. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.; Tabacchi, R.; Gindro, K.; Viret, O. δ-Viniferin, a Resveratrol Dehydrodimer: One of the Major Stilbenes Synthesized by Stressed Grapevine Leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef] [PubMed]
- Timperio, A.; D’Alessandro, A.; Fagioni, M.; Magro, P.; Zolla, L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol. Biochem. 2011, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Kim, K.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stirred bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Santamaria, A.; Innocenti, M.; Mulinacci, N.; Melani, F.; Valletta, A.; Sciandra, I.; Pasqua, G. Enhancement of Viniferin Production in Vitis vinifera L. cv. Alphonse Lavallée Cell Suspensions by Low-Energy Ultrasound Alone and in Combination with Methyl Jasmonate. J. Agric. Food Chem. 2012, 60, 11135–11142. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Park, S.; Park, S.-C.; Kim, S.; Kim, T.; Lee, J.; Kim, S.; Ryu, Y.; Jeong, J.; Kim, C. Induced extracellular production of stilbenes in grapevine cell culture medium by elicitation with methyl jasmonate and stevioside. Bioresour. Bioprocess. 2020, 7, 38. [Google Scholar] [CrossRef]
- Takaya, Y.; Terashima, K.; Ito, J.; He, Y.-H.; Tateoka, M.; Yamaguchi, N.; Niwa, M. Biomimic transformation of resveratrol. Tetrahedron 2005, 61, 10285–10290. [Google Scholar] [CrossRef]
- Lilly, M.D. Advances in biotransformation processes. Chem. Eng. Sci. 1994, 72, 27–34. [Google Scholar]
- Kennes, C. Bioconversion Processes. Fermentation 2018, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Wilkens, A.; Paulsen, J.; Wray, V.; Winterhalter, P. Structures of Two Novel Trimeric Stilbenes Obtained by Horseradish Peroxidase Catalyzed Biotransformation of trans -Resveratrol and (−)-ε-Viniferin. J. Agric. Food Chem. 2010, 58, 6754–6761. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhu, X.; Li, S.; Wang, Z.; Wang, L.; Li, Z.; Chen, G. Using Laccases in the Nanoflower to Synthesize Viniferin. Catalysts 2017, 7, 188. [Google Scholar] [CrossRef] [Green Version]
- Nicotra, S.; Cramarossa, M.; Mucci, A.; Pagnoni, U.M.; Riva, S.; Forti, L. Biotransformation of resveratrol: Synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron 2004, 60, 595–600. [Google Scholar] [CrossRef]
- Szewczuk, L.; Lee, S.H.; Blair, I.; Penning, T. Viniferin Formation by COX-1: Evidence for Radical Intermediates during Co-oxidation of Resveratrol. J. Nat. Prod. 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Esteso, M.J.; Sellés-Marchart, S.; Vera-Urbina, J.C.; Pedreño, M.; Bru, R. Changes of defense proteins in the extracellular proteome of grapevine (Vitis vinifera cv. Gamay) cell cultures in response to elicitors. J. Proteom. 2009, 73, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Sakurai, M.; Seki, M.; Furusaki, S. Effects of conditioning factor on anthocyanin production in strawberry suspension cultures. J. Sci. Food Agric. 1994, 66, 381–388. [Google Scholar] [CrossRef]
- Sakurai, M.; Ozeki, Y.; Mori, T. Induction of anthocyanin accumulation in rose suspension-cultured cells by conditioned medium of strawberry suspension cultures. Plant Cell Tissue Organ Cult. 1997, 50, 211–214. [Google Scholar] [CrossRef]
- Bermudez, M.; Sendón-Lago, J.; Eiró, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.; Macia, M.; Lamelas, M.; Saa, J.; et al. Corneal Epithelial Wound Healing and Bactericidal Effect of Conditioned Medium From Human Uterine Cervical Stem Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef]
- Vizoso, F.; Eiró, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- Gindro, K.; Schnee, S.; Righi, D.; Marcourt, L.; Nejad Ebrahimi, S.; Codina, J.; Voinesco, F.; Michellod, E.; Wolfender, J.-L.; Queiroz, E. Generation of Antifungal Stilbenes Using the Enzymatic Secretome of Botrytis cinerea. J. Nat. Prod. 2017, 80, 887–898. [Google Scholar] [CrossRef]
- Cho, W.; Chen, X.; Chu, H.; Rim, Y.; Kim, S.; Kim, S.; Kim, S.-W.; Park, Z.-Y.; Kim, J.-Y. Proteomic analysis of the secretome of rice calli. Physiol. Plant. 2009, 135, 331–341. [Google Scholar] [CrossRef]
- Gupta, S.; Wardhan, V.; Verma, S.; Gayali, S.; Rajamani, U.; Datta, A.; Chakraborty, S.; Chakraborty, N. Characterization of the Secretome of Chickpea Suspension Culture Reveals Pathway Abundance and the Expected and Unexpected Secreted Proteins. J. Proteome Res. 2011, 10, 5006–5015. [Google Scholar] [CrossRef]
- Moreno, V.O.A.; Vazquez-Duhalt, R. Peroxidase activity in calluses and cell suspension cultures of radish Raphanus sativus var. Cherry Bell. Plant Cell Tissue Organ Cult. 1989, 18, 321–327. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, Y.; Cho, E.; Kwak, S.; Kwon, S.; Bae, J.; Lee, B.; Meen, B.; Huh, G.-H. Alterations in intracellular and extracellular activities of antioxidant enzyme during suspension culture of sweetpotato. Phytochemistry 2004, 65, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Pyun, J.; Jeong, Y.; Park, S.; Kim, S.; Kim, Y.-H.; Lee, J.; Kim, C.; Jeong, J. Overexpression of VlPRX21 and VlPRX35 genes in Arabidopsis plants leads to bioconversion of trans-resveratrol to δ-viniferin. Plant Physiol. Biochem. 2021, 162, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Sesto, P.A.; van Huystee, R. Purification and yield of a cationic peroxidase from a peanut suspension culture. Plant Sci. 1989, 61, 163–168. [Google Scholar] [CrossRef]
- Cuenca, J.; García-Florenciano, E.; Barceló, A. Sequential release of both basic and acidic isoperoxidases to the media of susension cultured cells of Capsicum annuum. Plant Cell Rep. 1989, 8, 471–474. [Google Scholar] [CrossRef]
- Melo, N.; Cabral, J.; Fevereiro, P. Extracellular peroxidases from cell suspension cultures of Vaccinium myrtillus. Purification and characterization of two cationic enzymes. Plant Sci. 1995, 106, 177–184. [Google Scholar] [CrossRef]
- Temoçin, Z.; Yiğitoğlu, M. Studies on the activity and stability of immobilized horseradish peroxidase on poly(ethylene terephthalate) grafted acrylamide fiber. Bioprocess Biosyst. Eng. 2008, 32, 467–474. [Google Scholar] [CrossRef]
- Ambatkar, M.; Mukundan, U. Calcium Salts Enhance Activity and Azo Dye Decolourisation Capacity of Crude Peroxidase from Armoracia rusticana. Am. J. Plant Sci. 2014, 5, 212–218. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Jeong, Y.J.; Park, S.-C.; Kim, S.; Kim, Y.-G.; Shin, G.; Jeong, H.J.; Ryu, Y.B.; Lee, J.; Lee, O.R.; et al. Highly Efficient Bioconversion of trans-Resveratrol to δ-Viniferin Using Conditioned Medium of Grapevine Callus Suspension Cultures. Int. J. Mol. Sci. 2022, 23, 4403. https://doi.org/10.3390/ijms23084403
Park SH, Jeong YJ, Park S-C, Kim S, Kim Y-G, Shin G, Jeong HJ, Ryu YB, Lee J, Lee OR, et al. Highly Efficient Bioconversion of trans-Resveratrol to δ-Viniferin Using Conditioned Medium of Grapevine Callus Suspension Cultures. International Journal of Molecular Sciences. 2022; 23(8):4403. https://doi.org/10.3390/ijms23084403
Chicago/Turabian StylePark, Su Hyun, Yu Jeong Jeong, Sung-Chul Park, Soyoung Kim, Yong-Goo Kim, Gilok Shin, Hyung Jae Jeong, Young Bae Ryu, Jiyoung Lee, Ok Ran Lee, and et al. 2022. "Highly Efficient Bioconversion of trans-Resveratrol to δ-Viniferin Using Conditioned Medium of Grapevine Callus Suspension Cultures" International Journal of Molecular Sciences 23, no. 8: 4403. https://doi.org/10.3390/ijms23084403
APA StylePark, S. H., Jeong, Y. J., Park, S. -C., Kim, S., Kim, Y. -G., Shin, G., Jeong, H. J., Ryu, Y. B., Lee, J., Lee, O. R., Jeong, J. C., & Kim, C. Y. (2022). Highly Efficient Bioconversion of trans-Resveratrol to δ-Viniferin Using Conditioned Medium of Grapevine Callus Suspension Cultures. International Journal of Molecular Sciences, 23(8), 4403. https://doi.org/10.3390/ijms23084403







