Spinal Intradural Hematoma after Spinal Anesthesia in a Young Male Patient: Case Report and Review of the Literature
Abstract
1. Introduction
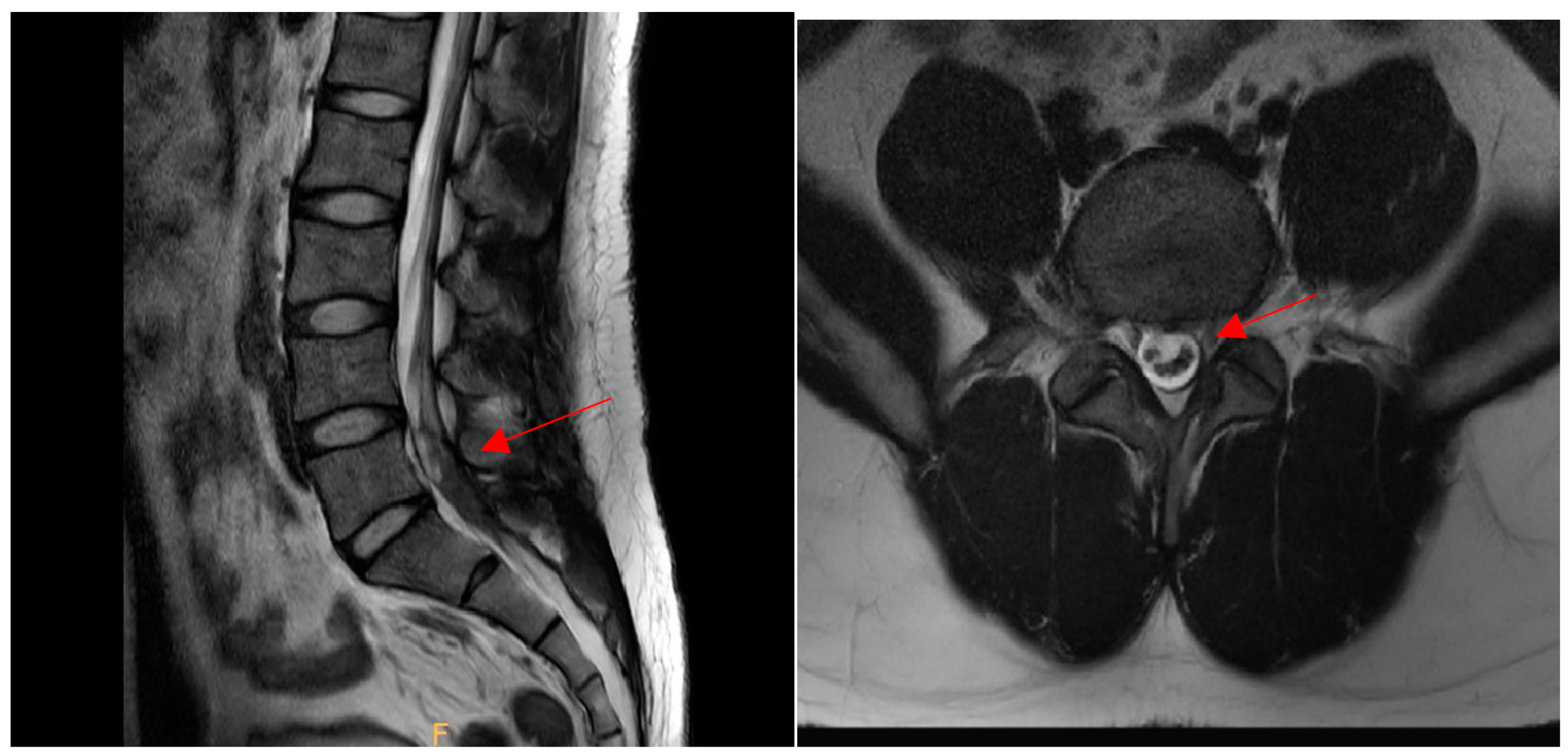
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SIH | Spinal intradural hematoma |
| MRI | Magnetic resonance imaging |
| NRS | Numeric Rating Scale |
References
- Avecillas-Chasin, J.; Matias-Guiu, J.A.; Gómez, G.; Saceda-Gutierrez, J. A case of acute spinal intradural hematoma due to spinal anesthesia. J. Acute Dis. 2015, 4, 341–343. [Google Scholar] [CrossRef][Green Version]
- Domenicucci, M.; Ramieri, A.; Paolini, S.; Russo, N.; Occhiogrosso, G.; Di Biasi, C.; Delfini, R. Spinal subarachnoid hematomas: Our experience and literature review. Acta Neurochir. 2005, 147, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Brand, R.A.; Colleran, G.C.; Broderick, J.M.; Lui, D.F.; Smith, E.M.; Kavanagh, E.C.; Poynton, A.R. Acute Nontraumatic Spinal Intradural Hematoma in a Patient on Warfarin. J. Emerg. Med. 2013, 45, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Maddali, P.; Walker, B.; Fisahn, C.; Page, J.; Diaz, V.; Zwillman, M.E.; Oskouian, R.J.; Tubbs, R.S.; Moisi, M. Subdural Thoracolumbar Spine Hematoma after Spinal Anesthesia: A Rare Occurrence and Literature Review of Spinal Hematomas after Spinal Anesthesia. Cureus 2017, 9, e1032. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Cho, Y.-H.; Lee, D.-H.; Kim, S.-H. Subarachnoid hematoma after spinal anesthesia—A case report. Anesthesia Pain Med. 2018, 13, 154–157. [Google Scholar] [CrossRef]
- Jeon, S.B.; Ham, T.-I.; Kang, M.-S.; Shim, H.-Y.; Park, S.L. Spinal subarachnoid hematoma after spinal anesthesia. Korean J. Anesthesiol. 2013, 64, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.H. Subarachnoid haematoma after spinal anaesthesia mimicking transient radicular irritation: A case report and review. Anaesthesia 2008, 63, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, D.; Murakami, E.; Aizawa, T. Referred pain location depends on the affected section of the sacroiliac joint. Eur. Spine J. 2014, 24, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, D.; Antoniadis, G.; Seeling, W. Spinal hematoma: A literature survey with meta-analysis of 613 patients. Neurosurg. Rev. 2003, 26, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-R.; Kim, S.J.; Cho, Y.J.; Cho, D.S. Spontaneous Resolution of Nontraumatic Acute Spinal Subdural Hematoma. J. Korean Neurosurg. Soc. 2011, 50, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, A.E.; Lalam, R.K.; Masry, W.S.E. Acute spontaneous spinal subdural hematoma presenting as paraplegia: A rare case. Spine 2007, 32, E619–E622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, H.; Ji, Y.; Lu, Q.; Li, X.; Jiang, X. Idiopathic Spinal Subdural Hematoma: Case Report and Review of the Literature. World Neurosurg. 2018, 116, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhong, Z.; Wang, B.; Sun, Q.; Zhong, C.; Bian, L. Coexistence of Spontaneous Spinal and Undiagnosed Cranial Subdual Hematomas. J. Craniofacial Surg. 2015, 26, e118–e119. [Google Scholar] [CrossRef] [PubMed]
- Boukobza, M.; Haddar, D.; Boissonet, M.; Merland, J. Spinal Subdural Haematoma: A Study of Three Cases. Clin. Radiol. 2001, 56, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Tomimatsu, T.; Kanagawa, T.; Sawada, K.; Tsutsui, T.; Kimura, T.; Chang, Y.S.; Wasada, K.; Imai, S.; Murata, Y. Spinal subarachnoid hematoma following spinal anesthesia in a patient with HELLP syndrome. Int. J. Obstet. Anesth. 2010, 19, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.; Joo, W.; Chough, J.; Park, H. Spontaneous spinal subdural hematoma concurrent with cranial subdural hematoma. J. Korean Neurosurg. Soc. 2013, 54, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Basaran, R.; Efendioglu, M.; Bölükbaşi, F.H.; Aslan, S.; Isik, N.; Kaner, T. Spinal Intradural Hematoma and Permanent Paraparesis after a Lumboperitoneal Shunt Operation: An Unusual Complication. Asian Spine J. 2014, 8, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Domenicucci, M.; Ramieri, A.; Ciappetta, P.; Delfini, R. Nontraumatic acute spinal subdural hematoma: Report of five cases and review of the literature. J. Neurosurg. 1999, 91 (Suppl. 1), 65–73. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Mochida, J.; Toh, E.; Nishimura, K.; Nomura, T. Nonsurgical treatment of an upper thoracic spinal subdural hemorrhage. Spinal Cord. 2001, 39, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, R.; Noguchi, S.; Uemura, K.; Takada, T.; Matsumura, A. Lumbosacral Subdural Hematoma-Case Report. Neurol. Med. Chir. 2006, 46, 258–261. [Google Scholar] [CrossRef] [PubMed]



| Case Study | Age (Years) | Cause | Hematoma Type | Symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Boukobza et al. (2001) [14] | 54 | Antivitamin K treatment Spontaneous | Subdural (T9-L1) | Lower back pain, saddle pain, urinary retention | Laminectomy (T10-L4) | Complete recovery |
| Mashiko et al. (2006) [20] | 18 | Trauma | Subdural (L5-S2) | Headache, lower back pain | Observation | Complete recovery |
| Lam et al. (2008) [7] | Elderly | Spinal anesthesia | Subarachnoid (L4) | Lower back pain, buttock pain, right leg pain | Conservative treatment | Complete recovery |
| Koyama et al. (2009) [15] | 39 | HELLP (Hemolysis, Elevated Liver enzymes, and Low platelet count) syndrome, Spinal anesthesia | Subarachnoid (L2-S1) | Numbness of the thigh and toes, urinary retention | Conservative treatment | Complete recovery |
| Moon et al. (2013) [16] | 39 | Chronic cranial subdural hematoma | Subdural (L4-S2) | Headache, lower back pain, pain radiating down both legs | Evacuation of cranial hematoma | Complete recovery |
| Bruce–Brand et al. (2013) [3] | 76 | Warfarin treatment Spontaneous | Intradural (L1-L4) | Flaccid paraparesis in both legs, sensory weakness below T12 | Laminectomy (T12-L4), intradural hematoma evacuation | Remaining motor weakness (ASIA grade C) |
| Jeon et al. (2013) [6] | 33 | Unknown Spinal anesthesia | Subarachnoid (L5) | Lower back pain, numbness of the lower limbs | Observation | Complete recovery |
| Basaran et al. (2014) [17] | 27 | Lumboperitoneal shunt operation | Intradural (L2-3) | Urinary and fecal incontinence with paraparesis | L2-L3 total laminectomy | Permanent paraparesis |
| Avecillas-Chasin et al. (2015) [1] | 79 | Spinal anesthesia | Intradural (L1-L3) | Motor and sensory weakness | Laminectomy (L1-L3), intradural hematoma evacuation | Unable to walk without assistance |
| Cui et al. (2015) [13] | 45 | Unknown | Subdural (L4-S3) | Saddle pain and dysuria | Hematoma evacuation | Complete recovery |
| Jang et al. (2017) [5] | 59 | Unknown | Subarachnoid (L3-L4) | Headache, nausea, vomiting, neck stiffness, lower back pain | Laminectomy (L3-4), hematoma evacuation | Complete recovery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.Y.; Ahn, J.M.; Chung, J.H.; Kim, N.S.; Seo, Y.H.; Jung, H.S.; Chun, H.R.; Kim, W.J.; Park, C.H.; Choi, J.S.; et al. Spinal Intradural Hematoma after Spinal Anesthesia in a Young Male Patient: Case Report and Review of the Literature. Int. J. Environ. Res. Public Health 2022, 19, 4845. https://doi.org/10.3390/ijerph19084845
Ji JY, Ahn JM, Chung JH, Kim NS, Seo YH, Jung HS, Chun HR, Kim WJ, Park CH, Choi JS, et al. Spinal Intradural Hematoma after Spinal Anesthesia in a Young Male Patient: Case Report and Review of the Literature. International Journal of Environmental Research and Public Health. 2022; 19(8):4845. https://doi.org/10.3390/ijerph19084845
Chicago/Turabian StyleJi, Jae Young, Jae Min Ahn, Jin Hun Chung, Nan Seol Kim, Yong Han Seo, Ho Soon Jung, Hea Rim Chun, Woo Jong Kim, Chan Ho Park, Jeong Soo Choi, and et al. 2022. "Spinal Intradural Hematoma after Spinal Anesthesia in a Young Male Patient: Case Report and Review of the Literature" International Journal of Environmental Research and Public Health 19, no. 8: 4845. https://doi.org/10.3390/ijerph19084845
APA StyleJi, J. Y., Ahn, J. M., Chung, J. H., Kim, N. S., Seo, Y. H., Jung, H. S., Chun, H. R., Kim, W. J., Park, C. H., Choi, J. S., Jung, H. C., & Park, J. S. (2022). Spinal Intradural Hematoma after Spinal Anesthesia in a Young Male Patient: Case Report and Review of the Literature. International Journal of Environmental Research and Public Health, 19(8), 4845. https://doi.org/10.3390/ijerph19084845







