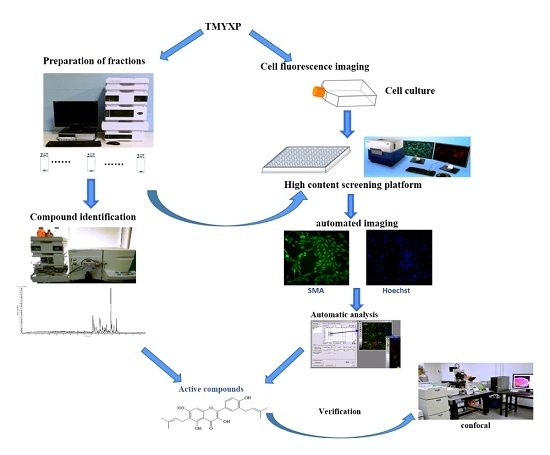
A High Content Screening Assay to Identify Compounds with Anti-Epithelial-Mesenchymal Transition Effects from the Chinese Herbal Medicine Tong-Mai-Yang-Xin-Wan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of the HCS Assay for Screening Compounds with Anti-EMT Effects
2.2. Chemical Composition and Anti-EMT Activity of Active Components
2.3. Validation of Anti-EMT Activities of Active Compounds by Confocal Microscopy
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Cell Cultures and HCS Assays
3.3. Preparation of Standard Compounds from TMYX
3.4. Screening of Anti-EMT Compounds from TMYX
3.5. Identification of Active Components by LC-MS
3.6. Validation of Active Compounds with Anti-EMT Effects by Confocal Microscopy
3.7. Z’ Factor
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Impellizzeri, D.; Esposito, E.; Attley, J.; Cuzzocrea, S. Targeting inflammation: New therapeutic approaches in chronic kidney disease (CKD). Pharmacol. Res. 2014, 81, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, D.M. Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatr. Nephrol. 2009, 24, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; van Lente, F.; Levey, A.S. Prevalence of chronic kidney disease in the united states. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Lysaght, M.J. Maintenance dialysis population dynamics: Current trends and long-term implications. J. Am. Soc. Nephrol. 2002, 13, S37–S40. [Google Scholar] [PubMed]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Sewaralthahab, K.; Balk, E.M.; Jaber, B.L.; Madias, N.E. Short- and long-term effects of alkali therapy in chronic kidney disease: A systematic review. Am. J. Nephrol. 2012, 35, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Lv, J.; Perkovic, V.; Yang, L.; Zhao, N.; Jardine, M.J.; Cass, A.; Zhang, H.; Wang, H. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: A systematic review and meta-analysis. Eur. Heart J. 2013, 34, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Craig, J.C.; Navaneethan, S.D.; Tonelli, M.; Pellegrini, F.; Strippoli, G.F. Benefits and harms of statin therapy for persons with chronic kidney disease: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Choi, H.S.; Seo, E.K.; Kang, D.H.; Oh, E.S. Baicalin and baicalein inhibit transforming growth factor-beta1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem. Biophys. Res. Commun. 2015, 458, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.H.; Lu, H.; Wu, C.Z.; Liang, Y.; Wang, S.L.; Lin, C.C.; Chen, B.C.; Xia, P. Resveratrol inhibits epithelial-mesenchymal transition and renal fibrosis by antagonizing the hedgehog signaling pathway. Biochem. Pharmacol. 2014, 92, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Q.; Wu, W.Z.; Sheng, M.X.; Yang, S.L.; Tan, J.M. Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol. Med. Rep. 2013, 7, 1453–1457. [Google Scholar] [PubMed]
- Pan, R.-H.; Xie, F.-Y.; Chen, H.-M.; Xu, L.-Z.; Wu, X.-C.; Xu, L.-L.; Yao, G. Salvianolic acid b reverses the epithelial-to-mesenchymal transition of hk-2 cells that is induced by transforming growth factor-β. Arch. Pharm. Res. 2011, 34, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ya, G.; Xu, L.Z.; Xu, X.C.; Xu, L.L.; Yang, J.W.; Chen, H.M. Preventive effects of salvianolic acid b on transforming growth factor-beta 1-induced epithelial-to-mesenchymal transition of human kidney cells. Biol. Pharm. Bull. 2009, 32, 882–886. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Tao, Y.-Y.; Yuan, J.-L.; Shen, L.; Liu, C.-H. Salvianolic acid b prevents epithelial-to-mesenchymal transition through the tgf-β1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Liu, Y.; Chen, Q.; Fu, W.; Wang, H.; Cai, H.; Peng, W.; Zhang, X. Curcumin inhibits transforming growth factor-β1-induced emt via pparγ pathway, not smad pathway in renal tubular epithelial cells. PLoS ONE 2013, 8, e58848. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Liu, F.; Sun, L.; Li, J.; Duan, S.; Liu, H.; Peng, Y.; Xiao, L.; Liu, Y. Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition. PLoS ONE 2013, 8, e66356. [Google Scholar] [CrossRef] [PubMed]
- Zanella, F.; Lorens, J.B.; Link, W. High content screening: Seeing is believing. Trends Biotechnol. 2010, 28, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, S.C.; Pal, S.; Han, E.; Song, J.M. High-content screening of drug-induced cardiotoxicity using quantitative single cell imaging cytometry on microfluidic device. Lab Chip 2011, 11, 104–114. [Google Scholar] [CrossRef] [PubMed]
- O’brien, P.; Irwin, W.; Diaz, D.; Howard-Cofield, E.; Krejsa, C.; Slaughter, M.; Gao, B.; Kaludercic, N.; Angeline, A.; Bernardi, P. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar]
- Cautain, B.; de Pedro, N.; Garzon, V.M.; de Escalona, M.M.; Menendez, V.G.; Tormo, J.R.; Martin, J.; El Aouad, N.; Reyes, F.; Asensio, F.; et al. High-content screening of natural products reveals novel nuclear export inhibitors. J. Biomol. Screen 2014, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Carmona, A.; Castell, J.V.; Gómez-Lechón, M.J.; Donato, M.T. High-content screening of drug-induced mitochondrial impairment in hepatic cells: Effects of statins. Arch. Toxicol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.; Traenkle, B.; Rothbauer, U. Real-time analysis of epithelial-mesenchymal transition using fluorescent single-domain antibodies. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Huang, Y.; Chen, Z.; Chen, Y.; Wang, Y.; Wang, Y. Rapid identification of anti-inflammatory compounds from tongmai yangxin pills by liquid chromatography with high-resolution mass spectrometry and chemometric analysis. J. Separ. Sci. 2015, 38, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Sams-Dodd, F. Target-based drug discovery: Is something wrong? Drug Discov. Today 2005, 10, 139–147. [Google Scholar] [CrossRef]
- Butcher, E.C. Can cell systems biology rescue drug discovery? Nat. Rev. Drug Discov. 2005, 4, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Wang, W.X.; Guo, H.Z.; Zhou, D.F. Antidepressant-like effect of liquiritin from glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav. Brain Res. 2008, 194, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Chen, X.Q.; Wang, W.; Zhang, Y.T.; Yang, Z.Y.; Jin, Y.; Ge, H.M.; Li, E.G.; Yang, G. Glycyrrhizic acid as the antiviral component of glycyrrhiza uralensis fisch against coxsackievirus a16 and enterovirus 71 of hand foot and mouth disease. J. Ethnopharmacol. 2013, 147, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, W.; Wang, Q.; Liu, P.; Deng, Y.; Lan, T.; Zhang, X.; Qiu, B.; Ning, H.; Huang, H. Emodin suppresses cell proliferation and fibronectin expression via p38mapk pathway in rat mesangial cells cultured under high glucose. Mol. Cell Endocrinol. 2009, 307, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Noor, M.; Wong, Y.F.; Hylands, P.J.; Simmonds, M.S.; Xu, Q.; Jiang, D.; Hendry, B.M.; Xu, Q. In vitro anti-fibrotic activities of herbal compounds and herbs. Nephrol. Dial. Transplant. 2009, 24, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through mirna-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Kraehe, P.; Lueders, C.; Shayan, P.; Goel, A.; Shakibaei, M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: Potential role of emt. PLoS ONE 2014, 9, e107514. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Lai, Y.-A.; Lin, Y.-C.; Ma, J.-W.; Huang, L.-F.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Curcumin suppresses doxorubicin-induced epithelial-mesenchymal transition via the inhibition of tgf-β and pi3k/akt signaling pathways in triple-negative breast cancer cells. J. Agric. Food Chem. 2013, 61, 11817–11824. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mazumdar, M.; Chakraborty, S.; Manna, A.; Saha, S.; Khan, P.; Bhattacharjee, P.; Guha, D.; Adhikary, A.; Mukhjerjee, S. Curcumin inhibits breast cancer stem cell migration by amplifying the e-cadherin/beta-catenin negative feedback loop. Stem Cell Res. Ther. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds including glycyrrhizic acid, glyasperin A, and licorisoflavan A are available from the authors.






| Peak No. | tR (min) | Molecular Formula | [M − H]−/[M + H]+ | Identification | Source | |
|---|---|---|---|---|---|---|
| Detected | Error (ppm) | |||||
| 1 | 51.26 | C42H62O16 | 821.3956 | 0.3 | Glycyrrhizic acid | Glycyrrhiza uralensis Fisch |
| 2 | 63.83 | C21H20O6 | 367.1180 | 0.9 | Glycycoumarin | Glycyrrhiza uralensis Fisch |
| 3 | 81.07 | C25H26O6 | 421.1639 | −1.6 | Glyasperin A | Glycyrrhiza uralensis Fisch |
| 4 | 72.53 | C15H10O5 | 269.0452 | 2.6 | Emodin | Rheum palmatum L |
| 5 | 87.27 | C27H34O5 | 437.2321 | −0.4 | Licorisoflavan A | Glycyrrhiza uralensis Fisch |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Li, L.; Zhu, X.; Ling, Z.; Feng, J.; Hu, Y.; Wang, Y.; Mou, L.; Wang, Y. A High Content Screening Assay to Identify Compounds with Anti-Epithelial-Mesenchymal Transition Effects from the Chinese Herbal Medicine Tong-Mai-Yang-Xin-Wan. Molecules 2016, 21, 1340. https://doi.org/10.3390/molecules21101340
Liu N, Li L, Zhu X, Ling Z, Feng J, Hu Y, Wang Y, Mou L, Wang Y. A High Content Screening Assay to Identify Compounds with Anti-Epithelial-Mesenchymal Transition Effects from the Chinese Herbal Medicine Tong-Mai-Yang-Xin-Wan. Molecules. 2016; 21(10):1340. https://doi.org/10.3390/molecules21101340
Chicago/Turabian StyleLiu, Ningning, Lailai Li, Xin Zhu, Zhiqiang Ling, Jianguo Feng, Ying Hu, Yi Wang, Lijun Mou, and Yi Wang. 2016. "A High Content Screening Assay to Identify Compounds with Anti-Epithelial-Mesenchymal Transition Effects from the Chinese Herbal Medicine Tong-Mai-Yang-Xin-Wan" Molecules 21, no. 10: 1340. https://doi.org/10.3390/molecules21101340
APA StyleLiu, N., Li, L., Zhu, X., Ling, Z., Feng, J., Hu, Y., Wang, Y., Mou, L., & Wang, Y. (2016). A High Content Screening Assay to Identify Compounds with Anti-Epithelial-Mesenchymal Transition Effects from the Chinese Herbal Medicine Tong-Mai-Yang-Xin-Wan. Molecules, 21(10), 1340. https://doi.org/10.3390/molecules21101340