Formulation of Isopropyl Isothiocyanate Loaded Nano Vesicles Delivery Systems: In Vitro Characterization and In Vivo Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation of Isopropyl Isothiocyanate (IPI) Vesicles
2.2.2. Formulation of Chitosan Coated Isopropyl Isothiocyanate (IPI) Vesicles
2.2.3. Vesicle Characterization
2.2.4. Encapsulation Efficiency
2.2.5. Drug Release
2.2.6. Permeation Study
2.2.7. Mucoadhesive Efficiency
2.2.8. In Vivo Study
Animals
2.2.9. Anti-Platelet and Anti-Thrombotic Study
In Vivo Tail Bleeding Time
In Vitro Anti-Thrombin Activity
2.2.10. Platelet Aggregation Assay
2.2.11. Carrageenan-Induced Tail Vein Thrombosis
2.2.12. Statistical analysis
3. Results and Discussion
3.1. Vesicle Characterization
3.2. Encapsulation Efficiency
3.3. Drug Release
3.4. Mucoadhesive Efficiency
3.5. Permeation Study
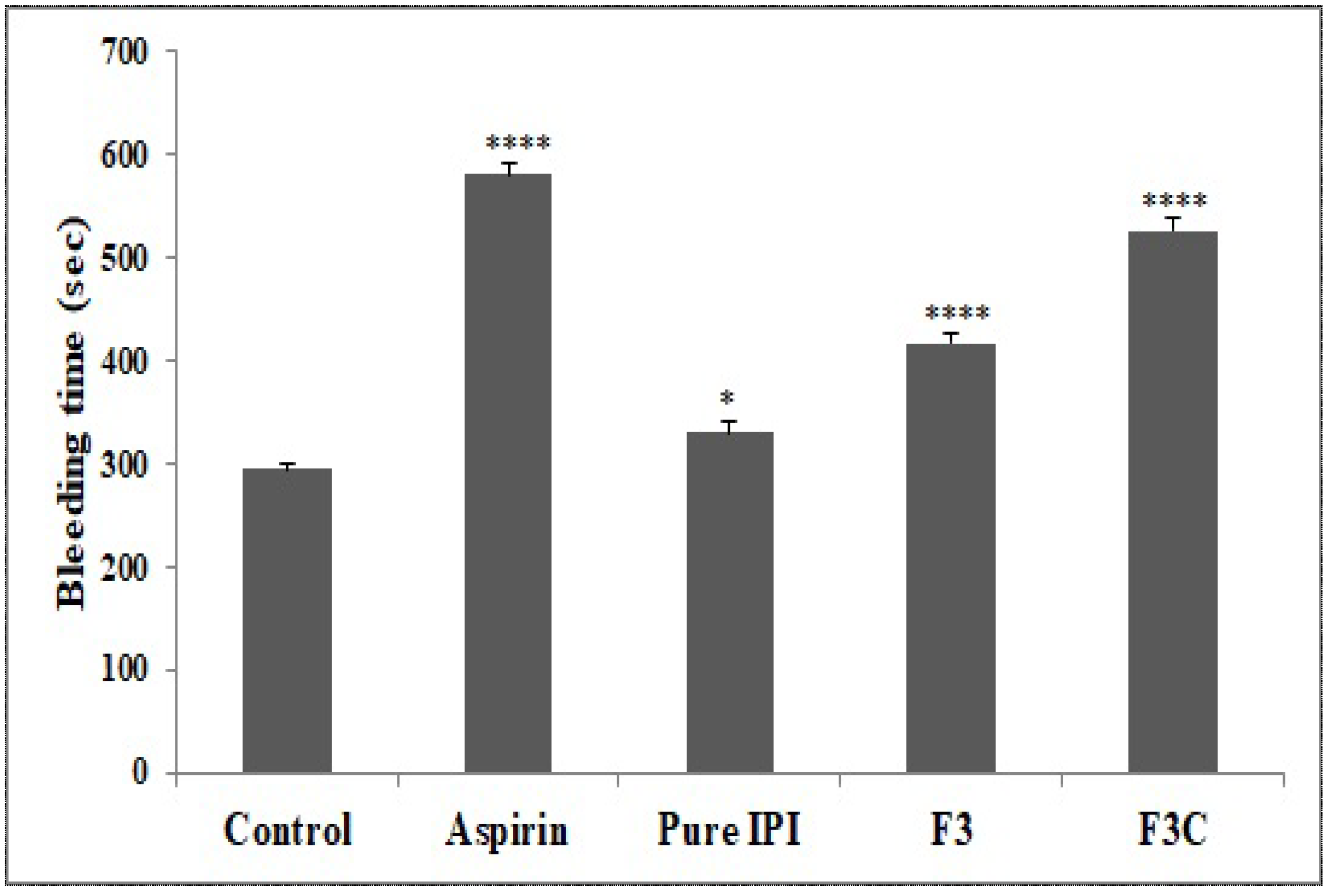
3.6. In Vivo Tail Bleeding Time
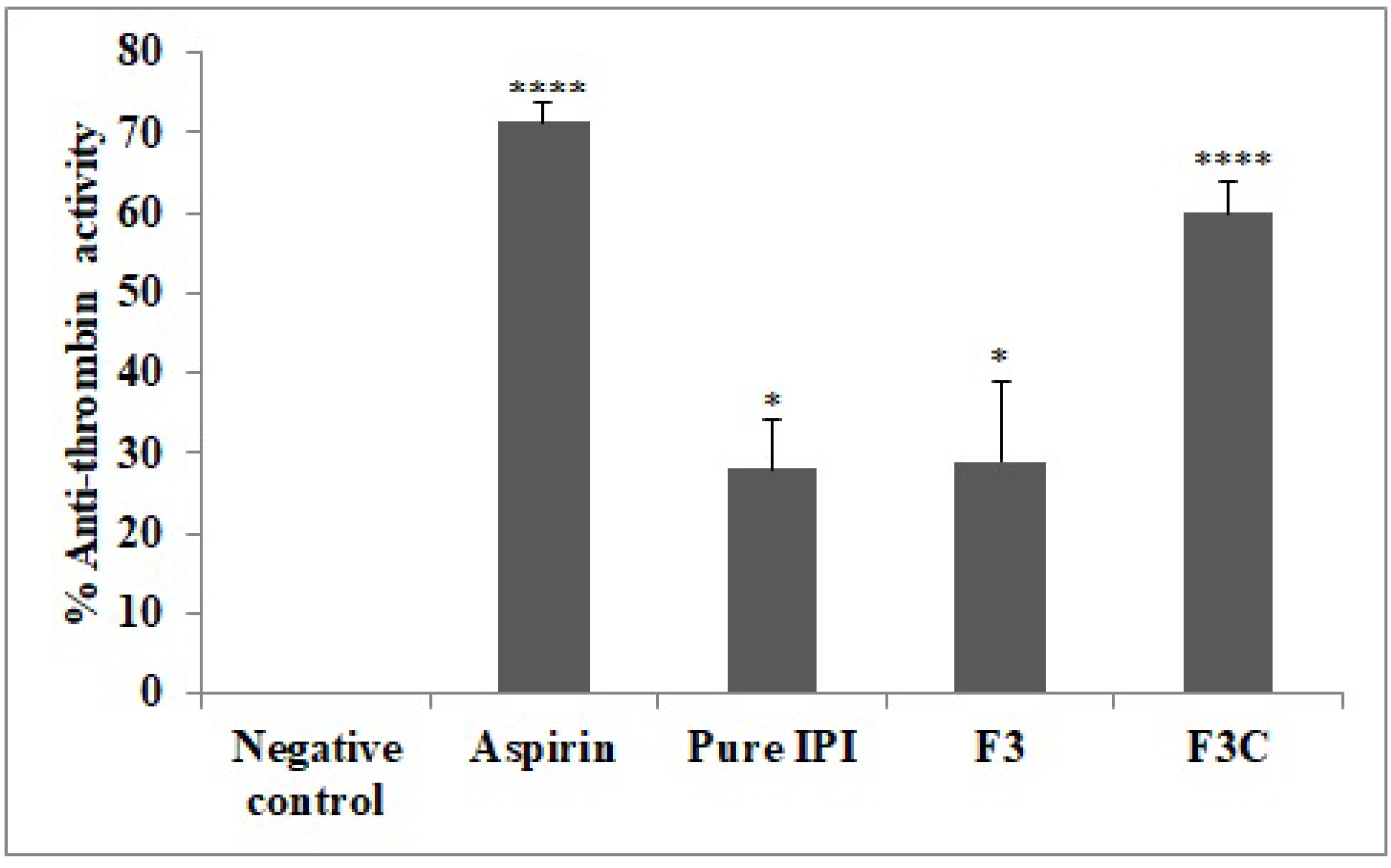
3.7. Anti-Thrombin Activity
3.8. Platelet Aggregation Assay
3.9. Carageenan Induced Tail Vein Thrombosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.; Do, H.J.; Oh, T.W.; Kim, K.-Y.; Kim, T.H.; Ma, J.Y.; Park, K.-I. Antiplatelet and Antithrombotic Activity of a Traditional Medicine, Hwangryunhaedok-Tang. Front. Pharmacol. 2019, 9, 1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redovnikovic, I.R.; Glivetic, T.; Delonga, K.; VorkapicFurac, J. Glucosinolates and their potential role in plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Lee, D.-S.; Kim, T.-H.; Jung, Y.-S. Inhibitory Effect of Allyl Isothiocyanate on Platelet Aggregation. J. Agric. Food Chem. 2014, 62, 7131–7139. [Google Scholar] [CrossRef]
- Gillespie, S.; Holloway, P.M.; Becker, F.; Rauzi, F.; Vital, S.A.; Taylor, K.A.; Stokes, K.Y.; Emerson, M.; Gavins, F.N.E. The isothiocyanate sulforaphane modulates platelet function and protects against cerebral thrombotic dysfunction. Br. J. Pharmacol. 2018, 175, 3333–3346. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, H.L.; Prashith Kekuda, T.R.; Valleesha, N.C.; Sudharshan, S.J.; Chinmaya, A. Screening for cytotoxic activity of methanol extract of Putranjiva roxburghii wall. Phcog. J. 2010, 2, 335–337. [Google Scholar] [CrossRef]
- Mishra, D.; Garg, M.; Dubey, V.; Jain, S.; Jain, N.K. Elastic liposomes mediated transdermal delivery of an anti-hypertensive agent: Propranolol hydrochloride. J. Pharm. Sci. 2007, 96, 145–155. [Google Scholar] [CrossRef]
- Bendas, E.R.; Tadros, M.I. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS Pharm Sci Tech. 2007, 8, E107. [Google Scholar] [CrossRef]
- Abidin, L.; Mujeeb, M.; Imam, S.S.; Aqil, M.; Khurana, D. Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: Quality evaluation and anti-arthritic assessment. Drug Deliv. 2016, 23, 1069–1074. [Google Scholar] [CrossRef]
- Imam, S.S.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design-based proniosome for accentuated transdermal delivery of risperidone: In vitro characterization and in vivo pharmacokinetic study. Drug Deliv. 2015, 22, 1059–1070. [Google Scholar] [CrossRef]
- Khan, K.; Aqil, M.; Imam, S.S.; Ahad, A.; Moolakkadath, T.; Sultana, Y.; Mujeeb, M. Ursolic acid loaded intra nasal nano lipid vesicles for brain tumour: Formulation, optimization, in-vivo brain/plasma distribution study and histopathological assessment. Biomed. Pharmacother. 2018, 106, 1578–1585. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, T.; Zhao, Y.; Song, H.; Zhang, L.; Wu, X.; Lu, B. Chitosan-coated liposomes as delivery systems for improving the stability and oral bioavailability of acteoside. Food Hydrocoll. 2018, 83, 17–24. [Google Scholar] [CrossRef]
- Gregoriadis, G.; Florence, A.T. Liposomes in drug delivery: Clinical, diagnostic and ophthalmic potential. Drugs 1993, 45, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.E.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Tang, L.; Yang, X.; Hwang, K.; Wang, W.; Yin, Q.; Wong, N.Y.; Dobrucki, W.; Yasui, N.; Katzenellenbogen, J.A.; et al. Selective delivery of an anticancer drug with aptamer-functionalized liposomes to breast cancer cells in vitro and in vivo. J. Mater. Chem. B 2013, 1, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hu, C.-M.J.; Fang, R.H.; Zhang, L. Liposome-like nanostructures for drug delivery. J. Mater. Chem. B 2013, 1, 6569–6585. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J. Mater. Chem. B 2012, 1, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Mizrahy, S.; Raz, S.R.; Hasgaard, M.; Liu, H.; Soffer-Tsur, N.; Cohen, K.; Dvash, R.; Landsman-Milo, D.; Bremer, M.G.; Moghimi, S.M.; et al. Hyaluronan-coated nanoparticles: The influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J. Control. Release 2011, 156, 231–238. [Google Scholar] [CrossRef]
- Fakhria, A.; Gilani, S.J.; Imam, S.S. Chandrakala Formulation of thymoquinone loaded chitosan nano vesicles: In-vitro evaluation and in-vivo anti-hyperlipidemic assessment. J. Drug Deliv. Sci. Technol. 2019, 50, 339–346. [Google Scholar] [CrossRef]
- Channarong, S.; Chaicumpa, W.; Sinchaipanid, N.; Mitrevej, A. Development and Evaluation of Chitosan-Coated Liposomes for Oral DNA Vaccine: The Improvement of Peyer’s Patch Targeting Using a Polyplex-Loaded Liposomes. AAPS Pharm. Sci. Tech. 2010, 12, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Mandracchia, D.; Tripodo, G.; Cometa, S.; Cellamare, S.; De Giglio, E.; Klepetsanis, P.; Antimisiaris, S. Protection of dopamine towards autoxidation reaction by encapsulation into non-coated- or chitosan- or thiolated chitosan-coated-liposomes. Colloids Surf. B: Biointerfaces 2018, 170, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Ahad, A.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: In-vitro characterization, and in-vivo appraisal. Mater. Sci. Eng. C 2017, 75, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.; Singhvi, G. Multifunctional Nanocrystals for Cancer Therapy: A Potential Nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 91–116. ISBN 9780128165058. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Akhtar, M.; Imam, S.S.; Afroz, A.M.; Najmi, A.K.; Mujeeb, M.; Aqil, M. Neuroprotective study of Nigella sativa-loaded oral provesicular lipid formulation: In vitro and ex vivo study. Drug. Deliv. 2014, 21, 487–494. [Google Scholar] [CrossRef]
- Pawar, H.; Douroumis, D.; Boateng, J. Preparation and optimization of PMAA–chitosan–PEG nanoparticles for oral drug delivery. Colloids Surf. B Biointerfaces 2012, 90, 102–108. [Google Scholar] [CrossRef]
- Yoon, E.-K.; Ku, S.-K.; Lee, W.; Kwak, S.; Kang, H.; Jung, B.; Bae, J.-S. Antitcoagulant and antiplatelet activities of scolymoside. BMB Rep. 2015, 48, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Osunsanmi, F.O.; Zaharare, G.E.; Oyinloye, B.E.; Mosa, R.A.; Ikhile, M.I.; Shode, F.O.; Ogunyinku, I.B.; Opoku, A.R. Antithrombotic, anticoagulant and antiplatelet activity of betulinic acid and 3β-acetoxybetulinic acid from Melaleuca bracteata ‘Revolution Gold’ (Myrtaceae) Muell leaf. Trop. J. Pharm. Res. 2018, 17, 1983–1989. [Google Scholar] [CrossRef] [Green Version]
- Amirou, A.; Bnouham, M.; Legssyer, A.; Ziyyat, A.; Aziz, M.; Berrabah, M.; Mekhfi, H. Effects of Juglans regia Root Bark Extract on Platelet Aggregation, Bleeding Time, and Plasmatic Coagulation: In Vitro and Ex Vivo Experiments. Evid.-Based Complement. Altern. Med. 2018, 2018, 7313517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kala, C.; Khan, N.A. Isolation and characterization of isopropyl isothiocyanate isolated from seeds of Drypetes roxburghii wall and its anti-platelet and anti-thrombotic activity. Sci. Afr. 2020, 10, e00658. [Google Scholar] [CrossRef]
- Kumar, S.H.; Pushpa, A. Thrombolytic Potential of Punica Granatum—A Study in the Rat Model. Int. J. Pharm. Sci. Res. 2016, 7, 3348–3354. [Google Scholar]
- Zaman, R.; Mohammad, P.; Jakaria, M.; Sayeed, M.A.; Minhajul, I. In vitro Clot Lysis Activity of Different Extracts of Mangifera sylvatica Roxb. Leaves. Res. J. Med. Plant. 2015, 9, 135–140. [Google Scholar]
- Telange, D.R.; Patil, A.T.; Pethe, A.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef]
- Han, H.-K.; Jung, I.-W.; Lee, B.-J. Effective mucoadhesive liposomal delivery system for risedronate: Preparation and in vitro/in vivo characterization. Int. J. Nanomed. 2014, 9, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of Lipid-Based Encapsulation on the Bioaccessibility and Bioavailability of Phenolic Compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Shao, B.; Cui, C.; Ji, H.; Tang, J.; Wang, Z.; Liu, H.; Qin, M.; Li, X.; Wu, L. Enhanced oral bioavailability of piperine by selfemulsifying drug delivery systems: In-vitro, in-vivo and in-situ intestinal permeability studies. Drug Deliv. 2015, 22, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, A.M.; Rassol, B.K.A. Optimized Mucoadhesive Coated Niosomes as a Sustained Oral Delivery System of Famotidine. AAPS PharmSciTech 2017, 8, 3064–3075. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Zhu, L.; Gan, Q.; Le, X. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Guo, S.; Liu, C.; Yang, C.; Dou, J.; Li, L.; Zhai, G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 24–30. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef]
- Sachs, U.J.; Nieswandt, B. In Vivo Thrombus Formation in Murine Models. Circ. Res. 2007, 100, 979–991. [Google Scholar] [CrossRef]
- Soslau, G.; Class, R.; Morgan, D.A.; Foster, C.; Lord, S.T.; Marchese, P.; Ruggeri, Z.M. Unique Pathway of Thrombin-induced Platelet Aggregation Mediated by Glycoprotein Ib. J. Biol. Chem. 2001, 276, 21173–21183. [Google Scholar] [CrossRef] [Green Version]
- Chistokhodova, N.; Nguyen, C.; Calvino, T.; Kachirskaia, I.; Cunningham, G.; Miles, D.H. Antithrombin activity of medicinal plants from central Florida. J. Ethnopharmacol. 2002, 81, 277–280. [Google Scholar] [CrossRef]
- Hagimori, M.; Kamiya, S.; Yamaguchi, Y.; Arakawa, M. Improving frequency of thrombosis by altering blood flow in the carrageenan-induced rat tail thrombosis model. Pharmacol. Res. 2009, 60, 320–323. [Google Scholar] [CrossRef]
- Kishore, K. In-vitro and In-vivo Screening Methods for Antithrombotic Agents. Am. J. Phytomed. Clin. Ther. 2013, 1, 497–506. [Google Scholar]
- Stalker, T.J. Mouse models of platelet function in vivo. Platelets 2020, 31, 415–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Code | Cholesterol (%) | Span 20 (%) | Span 40 (%) | Span 60 (%) | Span 80 (%) | Phospholipid (%) | Chitosan (w/v) |
|---|---|---|---|---|---|---|---|
| F1 | 10 | 10 | 0 | 0 | 0 | 80 | 0 |
| F2 | 10 | 0 | 10 | 0 | 0 | 80 | 0 |
| F3 | 10 | 0 | 0 | 10 | 0 | 80 | 0 |
| F4 | 10 | 0 | 0 | 0 | 10 | 80 | 0 |
| F3C | 10 | 0 | 0 | 10 | 0 | 80 | 0.15 |
| Code | Surfactant | Size(nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) | Drug Release (%) |
|---|---|---|---|---|---|---|
| F1 | span 20 | 270 ± 6.2 | 0.13 ± 0.05 | −21.5 ± 1.5 | 68.4 ± 4.1 | 91.2 ± 4.9 |
| F2 | span 40 | 311 ± 5.5 | 0.24 ± 0.04 | −26.4 ± 1.7 | 70.6 ± 4.6 | 86.3 ± 3.2 |
| F3 | span 60 | 298 ± 5.1 | 0.33 ± 0.07 | −18.7 ± 1.8 | 86.2 ± 5.3 | 89.5 ± 3.5 |
| F4 | span 80 | 352 ± 5.9 | 0.36 ± 0.06 | −24.5 ± 2.4 | 74.6 ± 3.5 | 81.3 ± 3.3 |
| F3C | span 60 | 379 ± 4.5 | 0.32 ± 0.08 | +23.5 ± 2.8 | 77.3 ± 4.1 | 63.2 ± 3.9 |
| S. No | Treatment and Dose (BW) | Parameters | ||
|---|---|---|---|---|
| Tail Length (cm) | Thrombus Length (cm) 24 h | Thrombus Length (cm) 48 h | ||
| 1 | Negative control | 13.83 ± 0.3 | 12.51 ± 0.4 | 12.7 ± 0.4 |
| 2 | Aspirin (50 mg/kg) | 14.05 ± 0.3 ns | 10.6 ± 0.4 *** | 9.73 ± 0.3 **** |
| 3 | Pure IPI (20 mg/kg) | 13.95 ± 0.2 ns | 12.01 ± 0.3 ns | 11.89 ± 0.2 ns |
| 4 | F3 (20 mg/kg) | 13.96 ± 0.3 ns | 11.76 ± 0.2 ns | 10.98 ± 0.3 ** |
| 5 | F3C (20 mg/kg) | 13.83 ± 0.3 ns | 9.4 ± 0.2 **** | 8.95 ± 0.2 **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kala, C.; Asif, M.; Gilani, S.J.; Imam, S.S.; Khan, N.A.; Taleuzzaman, M.; Zafar, A.; Ahmed, M.M.; Alshehri, S.; Ghoneim, M.M. Formulation of Isopropyl Isothiocyanate Loaded Nano Vesicles Delivery Systems: In Vitro Characterization and In Vivo Assessment. Molecules 2022, 27, 2876. https://doi.org/10.3390/molecules27092876
Kala C, Asif M, Gilani SJ, Imam SS, Khan NA, Taleuzzaman M, Zafar A, Ahmed MM, Alshehri S, Ghoneim MM. Formulation of Isopropyl Isothiocyanate Loaded Nano Vesicles Delivery Systems: In Vitro Characterization and In Vivo Assessment. Molecules. 2022; 27(9):2876. https://doi.org/10.3390/molecules27092876
Chicago/Turabian StyleKala, Chandra, Mohammad Asif, Sadaf Jamal Gilani, Syed Sarim Imam, Najam Ali Khan, Mohamad Taleuzzaman, Ameeduzzafar Zafar, Mohammed Muqtader Ahmed, Sultan Alshehri, and Mohammed M. Ghoneim. 2022. "Formulation of Isopropyl Isothiocyanate Loaded Nano Vesicles Delivery Systems: In Vitro Characterization and In Vivo Assessment" Molecules 27, no. 9: 2876. https://doi.org/10.3390/molecules27092876
APA StyleKala, C., Asif, M., Gilani, S. J., Imam, S. S., Khan, N. A., Taleuzzaman, M., Zafar, A., Ahmed, M. M., Alshehri, S., & Ghoneim, M. M. (2022). Formulation of Isopropyl Isothiocyanate Loaded Nano Vesicles Delivery Systems: In Vitro Characterization and In Vivo Assessment. Molecules, 27(9), 2876. https://doi.org/10.3390/molecules27092876








